Using a proteomics approach, the authors examined whether class 1 UV-blocking contact lenses protect against UVB radiation–induced damage in a human lens epithelial cell line (HLE B-3) and postmortem human lenses.
Abstract
Purpose.
To determine whether class 1 UV-blocking contact lenses protect against UVB radiation–induced damage in a human lens epithelial cell line (HLE B-3) and postmortem human lenses using a proteomics approach.
Methods.
HLE B-3 cells were exposed to 6.4 mW/cm2 UVB radiation at 302 nm for 2 minutes (768 mJ/cm2) with or without covering by senofilcon A class 1 UV-blocking contact lenses or lotrafilcon A non–UV-blocking (lotrafilcon A has some UV-blocking ability, albeit minimal) contact lenses. Control cells were not exposed to UVB radiation. Four hours after treatment, cells were analyzed by two-dimensional difference gel electrophoresis and tandem mass spectrometry, and changes in protein abundance were quantified. F-actin and microtubule cytoskeletons were examined by fluorescence staining. In addition, human donor lenses were exposed to UVB radiation at 302 nm for 4 minutes (1536 mJ/cm2). Cortical and epithelial cell proteins were scraped from lens surfaces and subjected to the same protein analyses.
Results.
Senofilcon A lenses were beneficial for protecting HLE B-3 cells against UVB radiation–induced changes in caldesmon 1 isoform, lamin A/C transcript variant 1, DEAD (Asp-Glu-Ala-Asp) box polypeptide, β-actin, glyceraldehyde 3-phosphate dehydrogenase (G3PDH), annexin A2, triose phosphate isomerase, and ubiquitin B precursor. These contact lenses also prevented actin and microtubule cytoskeleton changes typically induced by UVB radiation. Conversely, non–UV-blocking contact lenses were not protective. UVB-irradiated human lenses showed marked reductions in αA-crystallin, αB-crystallin, aldehyde dehydrogenase 1, βS-crystallin, βB2-crystallin, and G3PDH, and UV-absorbing contact lenses significantly prevented these alterations.
Conclusions.
Senofilcon A class 1 UV-blocking contact lenses largely prevented UVB-induced changes in protein abundance in lens epithelial cells and in human lenses.
Because of its location along the optical axis of the eye, the lens is chronically exposed to intermittent solar near-ultraviolet (UV) radiation, which is composed of UVB (290–320 nm) and UVA (320–400 nm) radiation. All UV wavelengths <297 nm are absorbed by the cornea, which then transmits increasing amounts of longer UV wavelengths to the lens. The solar UV radiation that reaches the Earth's surface typically contains only 3% UVB, although geographic, physical, and meteorological factors influence this value. Environmental radiation that reaches the lens epithelium contains 3% to 8% UVB and 40% to 60% UVA.1,2 Approximately 0.0006 to 0.005 mW/cm2 UVB and 100 to 1000 mW/cm2 UVA radiation are transmitted to the human lens epithelium and cortical fiber cells. Even at these low levels, UV radiation can adversely affect the lens after cumulative exposure over many decades.3–5
Lens epithelial cells are a likely target for UVB damage because they are the first cells in the lens to be exposed to UV radiation.6 Epithelial cells, which serve key transport functions for the entire lens, are key sites of enzyme systems that protect the lens from oxidative stress. Exposure of cultured cells to UVB radiation induces DNA damage and repair and triggers alterations in the synthesis of specific proteins.7–9 Lens fiber cell proteins have a long lifespan because of limited protein turnover. Tryptophan residues within lens proteins absorb UVB radiation, and these proteins also accumulate chromophores, such as the singlet oxygen-producing chromophore N-formylkynurenine (N-FK), which absorbs UVB radiation from the environment and produces reactive oxygen species.5,10,11 In addition, UVB and UVA radiation is absorbed by benign UV filters present in human lenses such as 3-hydroxykynurenine glucoside, which do not themselves have photosensitizing properties. The function of these UV filters, which are present even before birth, is to reduce blue light scatter and protect the retina from UV radiation.12 With age, these benign UV filters become bound to lens proteins and then function as photosensitizers.12–14 Thus, the lens is particularly susceptible to the long-term effects of stressors such as environmental near-UV radiation. Near-UV radiation is a risk factor for cataract formation,15,16 and UVB irradiation of animal lenses in vivo results in cataract formation.17,18 After 1 day of UVB exposure, apoptotic bodies were detected in both central and equatorial lens epithelia of rat lenses.17 After a 1-week latency period, abnormal fiber cells were detected. Several weeks later, the epithelium recovered completely whereas the lens fibers, although mostly repaired, still contained some damage. These findings suggest that disturbances in fiber cell spatial order correlate with initial damage to the lens epithelium.17
The role of different wavelengths of near-UV radiation on the etiology of cortical cataracts varies widely among species.19 Mouse lenses mainly absorb UVB, whereas guinea pig and rabbit lenses also contain chromophores that absorb UVA. UV absorption by human lenses increases substantially with age. A window of transmission at 320 nm occurs in very young human lenses, but this window is no longer present by middle age. Older human lenses absorb all UVA and blue light wavelengths, filtering them from the retina. In this case, lens absorption extends to wavelengths in the visible region up to 550 nm. Thus, although UVB may have a higher potential to cause damage, the amount of UVA radiation reaching the human lens is two orders of magnitude greater.2,19
The clinical and protective effects of UV (UVB and UVA)-blocking contact lenses (Acuvue Oasys with HydraClear Plus Technology; Vistakon, Johnson & Johnson Vision Care, Inc., Jacksonville, FL) on lens aging and cataract formation are unknown. Recently, UV-blocking contact lenses were shown to protect the rabbit cornea and lens from UVB-induced activation of matrix metalloproteinases and decreases in ascorbate levels of the aqueous humor.20 Quantification of the protective effects of these UV-absorbing polymers against the biological effects of UVB radiation in human lens epithelium and lens fiber cells is important for understanding mechanisms of protecting the human eye from aging and cataractogenesis.21,22 Although sunglasses filter UVB transmission to the lens, they do not protect against peripheral light focusing.23 In contrast, UVB-blocking contact lenses provide complete protection from peripheral light focusing of UVB radiation.21,22 As ozone depletion increases the amount of UVB radiation that reaches the Earth's surface, early assessment of the protective effects of UV-blocking contact lenses is invaluable.3,6,23
Although global gene expression changes have been observed in UVA-exposed lens epithelial cells,24 alterations in protein expression in human lens epithelial cells and postmortem human lenses after UVB radiation have not been studied. We have studied the effects of UVB radiation on a human lens epithelial cell line (HLE B-3) and postmortem human lenses to observe changes in protein abundance. Our objective was to use a proteomics approach to identify alterations in protein abundance that accompany the mechanisms associated with UVB-induced cataract formation. We hypothesized that UV-blocking contact lenses can ameliorate such changes and that changes in the human lens proteins of cortical fiber cells are prevented or inhibited by UV-blocking contact lenses. To test these hypotheses, we compared the contact lens Acuvue Oasys (UV-blocking Acuvue Oasys senofilcon A; Vistakon), the non–UV-blocking contact lens Focus Night and Day (lotrafilcon A; CIBA Vision, Duluth, GA), and a control (no contact lens). Both contact lens types contain silicone hydrogel materials. Senofilcon is associated with significant UV-blocking activity (US Food and Drug Administration class 1 blocker that absorbs 99% of UVB and 90% of UVA).25 Lotrafilcon absorbs only 30% of incident UVB and 15% of UVA,25 although both these contact lenses have high oxygen permeability.
Methods
Cells and Lenses
An extended lifespan human lens epithelial cell line (HLE B-3) that was generated in our laboratory26 and characterized previously7,24,27,28 was used in these studies from passages 8 to 10. Postmortem human lenses were obtained from the National Disease Research Interchange (Philadelphia, PA). These lenses were not associated with any personal identification information.
UVB Irradiation
A 1000-W, ozone-free, mercury-xenon arc lamp was used for UVB irradiation. Infrared radiation was filtered using a water filter. Monochromatic radiation at 302 nm was obtained using a 0.25-m monochromator with a 1200-line/mm grating blazed at 280 nm. A beam turner was used to turn the beam by 90° to irradiate the samples at a distance of 16 cm. The bandwidth was 5.1 nm.4,28,29 To prevent residual lower wavelength UVB radiation from reaching the cells, a filter (CS0–53, 1 mm; Corning Inc., Corning, NY) was placed on top of the tissue culture plate according to previously described methods.4 Irradiance was routinely measured with a calibrated radiometer (IL1700; International Light Technologies, Peabody, MA).4 Cell cultures were exposed to 6.4 mW/cm2 of 302 nm radiation for 2 minutes. Human lenses were exposed to 6.4 mW/cm2 of 302 nm radiation for 4 minutes.
Irradiation of Cell Cultures for Confocal Microscopy
HLE B-3 cells were plated in 24-well tissue culture plates containing glass coverslips. Cells were cultured in Eagle's minimum essential medium (Sigma-Aldrich, St. Louis, MO) containing 20% fetal bovine serum (Sigma) and 50 μg/mL gentamicin (Sigma) as described previously.26 The culture medium was removed and replaced with phosphate-buffered saline containing Ca2+ and Mg2+. In each UVB irradiation experiment, a well of one plate was covered with a senofilcon A (Acuvue Oasys; Vistakon) class 1 UV-blocking contact lens, a well of a second plate was covered with a lotrafilcon A (Focus Night and Day) non–UV-blocking contact lens, and a third plate remained uncovered. A fourth plate was not exposed to UVB radiation as a control. Cells were fixed, permeabilized, and labeled with fluorescein phalloidin to examine changes in the F-actin cytoskeleton.30 Cells were also colabeled with a β-tubulin antibody and an Alexa-568–labeled secondary antibody to allow visualization of the microtubule cytoskeleton.31 Cells were examined by confocal microscopy.31 Four random microscopic fields were examined under each condition.
Irradiation of Cell Cultures for Proteomic Analysis
HLE B-3 cells were plated in 24-well tissue culture plates and covered with a filter (CS 0–53; Corning Inc.) to separate out residual shorter wavelengths (<300 nm) before irradiation. Cells were irradiated as described with or without senofilcon A class 1 UV-blocking contact lenses (Acuvue Oasys; Vistakon) or non–UV-blocking contact lenses placed on top of the Corning filter. The total fluence was 768 mJ/cm2, which is equivalent to 42 hours of maximum sunlight exposure of human lens epithelial cells.2,6 Cells were incubated for 4 hours after UVB exposure, lysed, and assessed by proteomic analysis.
Irradiation of Human Lenses
Two 17-year-old and two 62-year-old human lenses were used in this study. Lenses removed from the eyes were received on ice and appeared to be transparent. The lenses were used <48 hours after death. Human lenses were dissected into halves with a sharp blade, and the four sections were each placed in one well of four 24-well tissue culture plates. The anterior side of the lens faced the UVB beam, which was evenly spread over the entire lens surface. The lens sections were moistened with a drop of PBS to prevent drying. Before UVB irradiation, each plate was covered with a filter (CS 0–53; Corning Inc.). In each experiment, the well of one plate was covered with the UV-blocking contact lens, the well of another plate was covered with the non–UV-blocking contact lens, and a third plate remained uncovered. These three plates were exposed to UVB radiation. In addition, a fourth plate was not treated with UV radiation. To prevent any leakage of UVB radiation around the contact lenses, three contact lenses were placed adjacent to each other to completely cover the top of the culture well containing the tissue. Lenses were irradiated with monochromatic UVB radiation at 302 nm (6.4 mW/cm2) for 4 minutes. The total fluence was 1536 mJ/cm2, which is equivalent to 85 hours of maximum sunlight exposure of the lens.2,6
Two-Dimensional Difference Gel Electrophoresis
Because UVB radiation is absorbed mainly by the epithelium and outer cortical fiber cells, the outer epithelial and cortical fractions of the human lenses were dissected and placed in lysis buffer containing 30 mM Tris-HCl (Bio-Rad, Hercules, CA), 2 M thiourea (Sigma-Aldrich), 7 M urea (Bio-Rad), 4% CHAPS (Bio-Rad), and 1× complete protease inhibitor cocktail tablets (Roche, Indianapolis, IN), pH 8.5. Lens proteins (50 μg) were labeled with 400 pmol Cy2, Cy3, or Cy5. Pools were prepared by mixing equal protein quantities from each sample after dye labeling. Two-dimensional difference gel electrophoresis (2D-DIGE) was performed at the Proteomics Core Laboratory according to published methods.32 Briefly, samples were equilibrated onto immobilized pH gradient strips at 100 V and subjected to isoelectric focusing using a maximum of 10,000 focusing volts (Protean IEF cell; Bio-Rad). After focusing, proteins were reduced with Tris(2-carboxyethyl) phosphine hydrochloride (TCEP, 10 mM) and alkylated with iodoacetamide (20 mM). The IPG strip was then layered on a 10% to 20% polyacrylamide gel, and proteins were separated by SDS-PAGE. Samples were imaged (Typhoon 9400 Imager; GE Healthcare, Piscataway, NJ) using specific excitation and emission wavelengths for Cy2 (488 and 522 nm), Cy3 (520 and 580 nm), and Cy5 (620 and 670 nm). Control and experimental samples were labeled with blue, green or red fluorescent dyes and run on the same 2D gel.33,34 Image analysis was performed to assess differences between contact lens-protected and unprotected UVB-irradiated human lenses. Individual protein spots that showed differential intensities between covered and uncovered lenses were excised from the gel and analyzed by mass spectroscopy.
Mass Spectrometric Analysis
Single- or multi-gel analyses were used to determine changes in protein abundance after UVB exposure. Single-gel analysis was performed to compare the following conditions: control and UVB-exposed HLE B-3 cells (Table 1, Fig. 1); control, UVB, and UVB + UV-blocking contact lens–covered HLE B-3 samples (Table 2, Figs. 1, 2); and control, UVB, and UVB + UV-blocking contact lens–covered human lenses from a 62-year-old patient (see Table 5). Multi-gel analysis was performed with a pooled internal standard. This approach was used to compare control, UVB-exposed, UVB + UV-blocking contact lens, and UVB + non–UV-blocking contact lens samples of HLE B-3 cells (Table 3; see Fig. 4) with the 17-year-old human lenses (Table 4). Multi-gel comparisons were performed using different combinations of sample sets. The control sample was labeled with Cy3, and irradiated samples were labeled with Cy5. A pool of all samples was labeled with Cy2 and served as a standard common to each gel. The pooled standard, the control, and one test sample were combined and run on each gel. Images were generated and compared within each 2D gel using image analysis software (DeCyder version 6.5; GE Healthcare). Differential in-gel analysis was used to normalize and compare quantitative differences between images from each gel. Pairwise analysis of proteins across different physical gels was performed using the biological variation analysis (BVA) module to quantify relative differences between the samples.32
Table 1.
Mascot Search Results for HLE B-3 Cells (Control vs. UVB Exposed)
| Spot | Protein | Accession No. (Gi) | Assigned Spectra (n) | MW (kDa) | Fold-Change |
||
|---|---|---|---|---|---|---|---|
| C1 vs. C2 | C1 vs. UVB | C2 vs. UVB | |||||
| 2348 | Heat shock protein 90 kDa-α | 154146191 | 9 | 85 | −1.24 | −2.2 | −1.8 |
| Heat shock 90 kDa protein 1-β | 20149594 | 7 | 83 | ||||
| 80 K-H protein | 182855 | 2 | 59 | ||||
| 2562 | Caldesmon 1 isoform 5 | 15149465 | 22 | 61 | 1.06 | 2.17 | 2.02 |
| Inner membrane protein mitochondrial isoform 1 | 154354964 | 15 | 84 | ||||
| Ezrin | 46249758 | 5 | 69 | ||||
| Procollagen-lysine 2-oxoglutarate 5-dioxygenase 3 | 4504517 | 4 | 85 | ||||
| ZYX protein | 33870614 | 3 | 62 | ||||
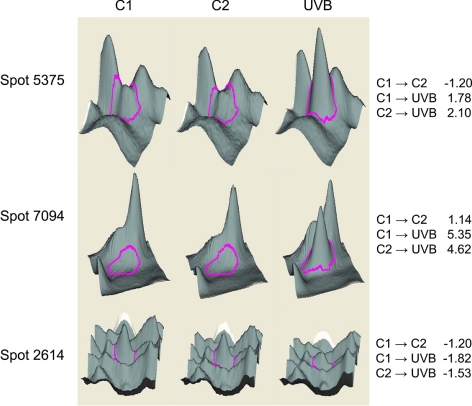
| 2614 | LaminA/C isoform | 27436946 | 41 | 74 | −1.2 | −1.82 | −1.53 |
| DEAD/H (Asp-Glu-Ala-Asp/His) box polypeptide 3 | 62087546 | 16 | 73 | ||||
| Glycosyltransferase 25 domain cont. 1 | 31377697 | 8 | 72 | ||||
| 2771 | Myristoylated alanine-rich protein kinase C substrate | 153070260 | 7 | 32 | −1.27 | −2.78 | −2.22 |
| Protein phosphatase 1G (6) | 405999 | 6 | 59 | ||||
| 3880 | β-Actin | 14250401 | 13 | 41 | 1.01 | 2.25 | 2.19 |
| HNRPF protein | 16876910 | 12 | 46 | ||||
| Eukaryotic translation initiation factor 4A isoform 1 | 4503529 | 9 | 46 | ||||
| Nuclear distribution gene C homolog | 5729953 | 3 | 38 | ||||
| Vimentin | 62414289 | 3 | 54 | ||||
| TXNDC5 protein | 30354488 | 3 | 40 | ||||
| 5375 | Triosephosphate isomerase | 136066 | 7 | 27 | −1.2 | 1.78 | 2.1 |
| Hsp27 1 | 4504517 | 5 | 23 | ||||
| Endoplasmic reticulum protein 29 isoform 1 precursor | 5803013 | 3 | 29 | ||||
| 6-Phosphogluconolactonase | 6912586 | 2 | 28 | ||||
| 5378 | Hsp27 | 4504517 | 2 | 23 | −1.08 | 2.36 | 2.53 |
| 5380 | Hsp27 | 4504517 | 17 | 23 | −1.23 | −1.75 | −1.44 |
| Peroxiredoxin 6 | 4758638 | 13 | 25 | ||||
| Triosephosphate isomerase | 136066 | 7 | 27 | ||||
| 5389 | Peroxiredoxin 6 | 4758638 | 11 | 25 | −1.39 | −2.38 | −1.73 |
| CLE | 55613379 | 9 | 28 | ||||
| Triosephosphate isomerase | 136066 | 7 | 27 | ||||
| Hsp27 | 4504517 | 5 | 23 | ||||
| Phosphoglycerate mutase | 387016 | 3 | 29 | ||||
| Endoplasmic reticulum protein 29 isoform 1 | 5803013 | 2 | 29 | ||||
| 5920 | Myosin regulatory light chain MRCL2 | 15809016 | 5 | 20 | −2.57 | −4.73 | −1.86 |
| 7094 | S100 calcium-binding protein A4 | 4506765 | 9 | 12 | 1.14 | 5.35 | 4.62 |
Two controls (C1 and C2) and one UVB sample were compared on the same gel. All the entries had 100% identification probability except for 6-phosphogluconolactonase, which had a 98% identification probability, and TXDNC5, which had 99% protein identification probability.
Figure 1.
Analysis of changes in lens epithelial cell proteins in unirradiated and UVB-irradiated cells. The 3D data sets for representative proteins in two control (C1 and C2) and one UVB-exposed HLE B-3 cultures are shown. See Table 1 for proteins contained in each protein spot. Right: fold changes between each sample.
Table 2.
Mascot Search Results for HLE B-3 Cells (Control vs. UVB vs. UVB + UV-Blocking Senofilcon A Class 1 Contact Lenses)
| Spot | Protein | Accession No. (Gi) | Assigned Spectra (n) | MW (kDa) | Fold-Change |
|
|---|---|---|---|---|---|---|
| C vs. UVB | C vs. UVB + UV-Blocking Contact Lenses | |||||
| 1972 | Heat shock protein gp96 precursor | 15010550 | 4 | 90 | −2.88 | −1.61 |
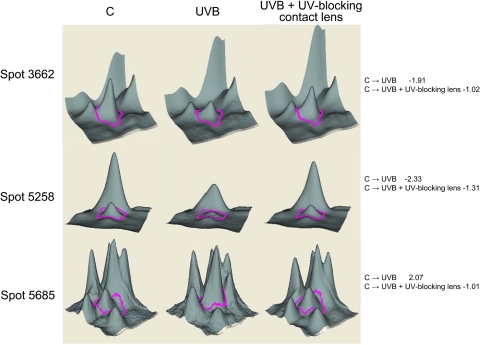
| 3662 | β-Actin | 14250401 | 17 | 41 | −1.91 | −1.02 |
| HNR PF protein | 16876910 | 3 | 46 | |||
| Eukaryotic translation initiation factor | 4503529 | 3 | 46 | |||
| UAP56 | 61679617 | 2 | 44 | |||
| 5258 | Peroxiredoxin 6 | 4758638 | 16 | 25 | −2.33 | −1.31 |
| Hsp27 | 4504517 | 15 | 23 | |||
| Triosephosphate isomerase | 136066 | 2 | 27 | |||
| 6348 | Histone cluster 1 h2bd | 10800138 | 4 | 14 | −1.98 | −1.74 |
| 3829 | β-Actin | 14250401 | 3 | 41 | 4.12 | 1.17 |
| 67 kDa laminin receptor | 250127 | 3 | 33 | |||
| Vimentin | 62414289 | 2 | 54 | |||
| 4115 | Vimentin | 6241428944 | 7 | 54 | 2.71 | 1.14 |
| Ribosomal protein SA | 890755 | 4 | 33 | |||
| Nucleophosmin 1 isoform 1 | 10835063 | 14 | 33 | |||
| 5685 | RING12 | 36059 | 3 | 23 | 2.07 | 1.01 |
For all proteins, the identification probabilities were 100%.
Figure 2.
Analysis of changes in lens epithelial cell proteins induced by UVB exposure in the absence and presence of UV-blocking contact lenses. The 3D data sets for representative proteins are shown. Quantitative image analysis data for identified proteins are shown in Table 2. Right: fold changes between each sample.
Table 5.
Mascot Search Results for Human Lenses from a 62-Year-Old Donor Exposed to UVB Radiation in the Presence and Absence of UV-Blocking Contact Lenses
| Spot | Protein | Accession No. | Assigned Spectra (n) | MW (kDa) | Fold-Change |
|
|---|---|---|---|---|---|---|
| C vs. UVB | C vs. UVB + UV-Blocking Contact Lens | |||||
| 4612 | Sorbitol dehydrogenase | 1583520 | 4 | 38 | −7.49 | −2.94 |
| αB-crystallin | 4503057 | 3 | 20 | |||
| Glyceraldehyde 3 phosphate dehydrogenase | 31645 | 3 | 36 | |||
| βS-crystallin | 258660 | 2 | 21 | |||
| 4614 | Glyceraldehyde 3 phosphate dehydrogenase | 31645 | 4 | 36 | −6.91 | −2.93 |
Single-gel comparison of protein spots in control and UVB-exposed human lenses from a 62-year-old donor. Note that this analysis was consistent with several proteins that changed in abundance upon exposure to UVB in the young human lenses: αB-crystallin, glyceraldehyde 3 phosphate dehydrogenase, and βS-crystallin. Identification probability was 100% for all proteins except βS-crystallin, which was 99%.
Table 3.
Mascot Search Results for HLE B-3 cells (Control vs. UVB-Treated vs. UVB-Treated in the Presence of UV-Blocking Senofilcon A Class 1 or Non-UV-Blocking Lotrafilcon Contact Lenses)
| Spot | Protein | Accession No. (Gi) | Assigned Spectra (n) | MW (kDa) | Fold-Change |
||
|---|---|---|---|---|---|---|---|
| C vs. UVB | C vs. UVB + UV-Blocking Contact Lenses | C vs. UVB + Non–UV-Blocking Contact Lenses | |||||
| 769 | Prepro-α1(1)collagen | 1418928 | 9 | 139 | −2.86 | −2.50 | −1.92 |
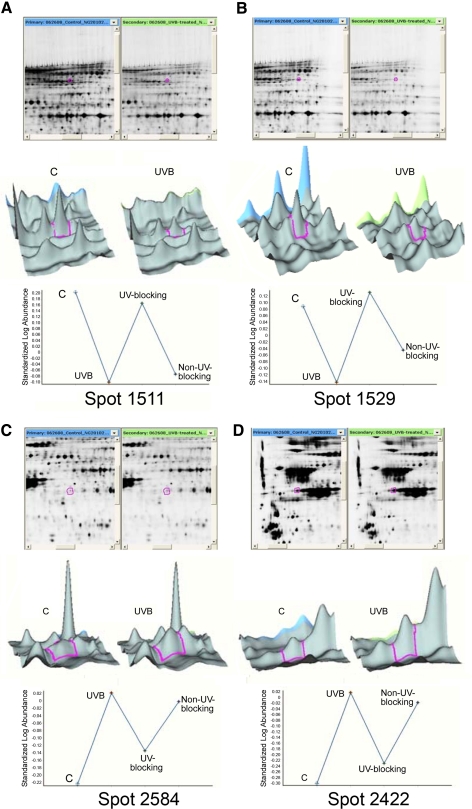
| 1511 | Caldesmon 1 isoform 5 | 15149465 | 39 | 61 | −2.01 | −1.09 | −1.89 |
| Ezrin | 46249758 | 19 | 69 | ||||
| Zyx protein | 33869857 | 5 | 50 | ||||
| 1529 | Lamin A/C transcript variant 1 | 57014043 | 45 | 74 | −1.70 | 1.10 | −1.36 |
| DEAD box polypeptide | 13514809 | 4 | 73 | ||||
| Glycosyltransferase 25 domain containing 1 | 31377697 | 2 | 72 | ||||
| 2284 | Eukaryotic translation initiation factor | 16198386 | 3 | 46 | −1.85 | −1.24 | −1.97 |
| β-Actin | 4501885 | 2 | 42 | ||||
| 2422 | β-Actin | 14350401 | 19 | 42 | 2.09 | 1.18 | 1.93 |
| α-Actin | 4501881 | 2 | 42 | ||||
| 2580 | G3PDH | 31645 | 14 | 36 | 1.95 | 1.5 | 1.92 |
| Annexin A2 | 16306978 | 13 | 39 | ||||
| Methionine adenosyltransferase II | 11034825 | 3 | 38 | ||||
| 2584 | Annexin A2 | 16306978 | 18 | 39 | 1.75 | 1.23 | 1.65 |
| TALDO1 protein | 48257056 | 8 | 37 | ||||
| Annexin I | 4502101 | 3 | 39 | ||||
| Calponin 3 | 4502923 | 2 | 36 | ||||
| Chain B structure of human Dcps | 62738482 | 2 | 39 | ||||
| 3086 | Triose phosphate isomerase | 17389815 | 12 | 27 | 2.08 | 1.57 | 2.11 |
| GTP-binding protein | 4092054 | 2 | 24 | ||||
| 3105 | Heat shock 27 kDa protein 1 | 4504517 | 9 | 23 | 2.27 | 1.68 | 2.28 |
| Triose phosphate isomerase | 17389815 | 3 | 27 | ||||
| 4077 | Ubiquitin B precursor | 11024714 | 10 | 26 | 2.08 | 1.04 | 1.40 |
| 4162 | Vimentin | 62414289 | 14 | 54 | 1.83 | 1.08 | 1.51 |
| Nucleophosmin 1 | 10835063 | 8 | 33 | ||||
| β-Actin | 14250401 | 7 | 41 | ||||
| Lamin-binding protein | 1125065 | 3 | 14 | ||||
For all proteins, the identification probabilities were 100%.
Figure 4.
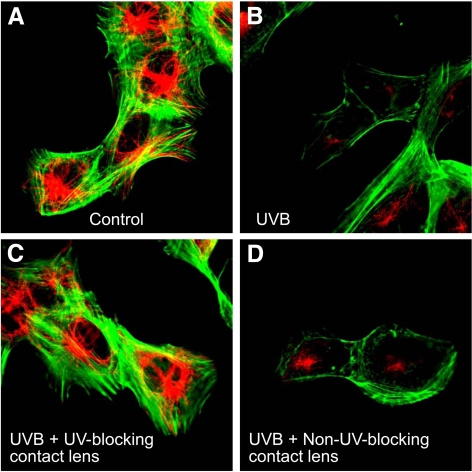
Confocal microscopic analysis of F-actin and β-tubulin staining in HLE B-3 cells exposed to UVB radiation. HLE B-3 cells were exposed to 768 mJ/cm2 of UVB radiation at 302 nm and then incubated in culture medium for 4 hours. Cells were then fixed, stained with fluorescein phalloidin to visualize actin (green), and immunostained with an antibody to β-tubulin and Alexa 568–labeled secondary antibody (red). Note that UVB exposure reduced the F-actin and β-tubulin signals (B) compared with controls with no UVB exposure (A). This decrease was prevented by senofilcon A class 1 UV-blocking contact lenses (C) but not by non–UV-blocking lotrafilcon A contact lenses (D).
Table 4.
Mascot Search Results for Human Lenses from a 17-Year-Old Donor Exposed to UVB Radiation in the Presence and Absence of Contact Lenses
| Spot | Protein | Accession No. | Assigned Spectra (n) | MW (kDa) | Fold-Change |
||
|---|---|---|---|---|---|---|---|
| C vs. UVB | C vs. UVB + UV-Blocking | C vs. UVB + Non–UV-Blocking | |||||
| 602 | Aldehyde dehydrogenase 1 | 2183299 | 8 | 55 | −2.14 | −1.53 | −2.09 |
| αA-crystallin | 4503055 | 3 | 20 | ||||
| βB1-crystallin | 4503061 | 2 | 28 | ||||
| βS-crystallin | 258660 | 2 | 21 | ||||
| αB-crystallin | 4503057 | 2 | 20 | ||||
| 608 | Aldehyde dehydrogenase 1 | 2183299 | 11 | 55 | −1.84 | −1.53 | −1.93 |
| αA-crystallin | 4503055 | 7 | 20 | ||||
| βS-crystallin | 258660 | 5 | 21 | ||||
| βB2-crystallin | 4503063 | 2 | 23 | ||||
| 615 | Aldehyde dehydrogenase 1 | 2183299 | 12 | 55 | −1.73 | −1.48 | −1.87 |
| αA-crystallin | 4503055 | 5 | 20 | ||||
| βS-crystallin | 258660 | 5 | 21 | ||||
| αB-crystallin | 4503057 | 2 | 20 | ||||
| βB2-crystallin | 4503063 | 2 | 23 | ||||
| βA3-crystallin | 12056461 | 2 | 25 | ||||
| 627 | Aldehyde dehydrogenase 1 | 2183299 | 10 | 55 | −1.63 | −1.30 | −1.69 |
| αA-crystallin | 4503055 | 7 | 20 | ||||
| βS-crystallin | 258660 | 5 | 21 | ||||
| αB-crystallin | 4503057 | 4 | 20 | ||||
| βB2-crystallin | 4503063 | 2 | 23 | ||||
| 1082 | Glyceraldehyde 3 phosphate dehydrogenase | 31645 | 11 | 36 | −1.37 | −1.16 | −1.32 |
| αA-crystallin | 4503055 | 5 | 20 | ||||
| γC-crystallin | 10518338 | 5 | 21 | ||||
| βS-crystallin | 258660 | 4 | 21 | ||||
| 1453 | βB1-crystallin | 4503061 | 20 | 28 | −3.38 | −2.37 | −3.29 |
| αA-crystallin | 4503055 | 7 | 20 | ||||
| αB-crystallin | 4503057 | 5 | 20 | ||||
| βB2-crystallin | 4503063 | 3 | 23 | ||||
| βS-crystallin | 258660 | 2 | 21 | ||||
| 2075 | αA-crystallin | 4503055 | 8 | 20 | −2.14 | −1.63 | −1.99 |
| βS-crystallin | 258660 | 2 | 21 | ||||
| 2230 | αA-crystallin | 4503055 | 4 | 20 | −2.86 | −1.09 | −2.03 |
| 2450 | αA-crystallin | 4503055 | 8 | 20 | −3.19 | −2.07 | −3.67 |
| βS-crystallin | 258660 | 2 | 21 | ||||
For all proteins, the identification probabilities were greater than 95%. Only those proteins identified by two or more peptides are included. The lenses from a 17-year-old human donor were cut in half, and the halves were used as unexposed controls or controls exposed to UVB radiation with or without contact lenses. Pool-based analysis of the protein abundances was performed.
Analysis of Pool-Based Data
Pool-based experiments involved a pool of proteins from all samples in the experiment to provide a common comparator for each sample. Because the pool is identical on each gel, the fold-change “difference” for a spot in the pool image is 1.0 (representing no change) when comparing pool images from any two gels. This designation allowed us to compare protein amounts for spots in UVB or UVB/UV-blocking contact lens samples to the pool on the same gel and to determine relative amounts of protein. Although UVB treatment and UVB treatment/UV-blocking contact lens samples were on different gels, their fold-change values were determined in comparison with the pooled sample, which was run on each gel. Because the pool from one gel is identical with the pool from the other, the UVB treatment and UVB treatment/UV-blocking contact lens fold-change values can be compared directly.
Database Searching
Mass spectra were acquired using nano-LC-MS as previously described.35 The tandem spectra were used to search protein databases with Mascot software (Matrix Science, London, UK; version 2.1.1.0). The nr_20080708 database (selected for Homo sapiens, 127,310 entries) was searched using trypsin as the endoprotease, a parent mass tolerance of 20 ppm, and a fragment ion mass tolerance of 0.7 Da. The iodoacetamide derivative of cysteine was specified in Mascot as a fixed modification and oxidized methionine residues as a variable modification. Additional data processing details are described in Mendelsohn et al.32
Criteria for Protein Identification
Scaffold (version Scaffold_3_01_00; Proteome Software Inc., Portland, OR) was used to qualify MS/MS-based peptide and protein identification.36 Protein identification was accepted if identity could be established at >95% probability and contained at least two identified peptides. Protein probabilities were assigned by the Protein Prophet algorithm.37 Proteins that contained similar peptides but were not differentiated based on MS/MS analysis alone were grouped to satisfy the principles of parsimony.
Knowledge-Based Network Analysis
After false-positive analysis (Protein Prophet) and removal of contaminants (e.g., keratins), proteins listed in Tables 1 to 5 (identified by NCBI Gi numbers) were entered into Ingenuity Pathways (www.ingenuity.com) (IPA, version 8.8; Redwood City, CA) as an *.xls file. Fold changes represent proteins with increased (positive numbers) or decreased (negative numbers) expression in UV-exposed versus control samples.
The software mapped 99 of 118 Gi numbers, corresponding to 99 gene symbols. Duplicate names corresponding to the same gene were eliminated. Ingenuity was set to generate up to 25 networks containing up to 35 members each, with no additional restrictions. Biological networks and pathways were generated from the input data (“focus genes”) and gene objects in the Ingenuity Pathways Knowledge Base. Interaction networks generated using this method show proteins with positive fold change as shades of red and those with negative fold change as shades of green. Increased magnitude of change is indicated by a more intense color.
Results
HLE B-3 Cells
Cells appeared to have a normal morphology at 4 hours after UVB exposure. The effects of UVB radiation on the HLE B-3 proteome had not been analyzed previously. Our objective was to determine the protective effects of UV-absorbing contact lenses against UVB-induced changes in protein expression in HLE B-3 cells. We first analyzed the effect of UVB radiation on protein abundances in HLE B-3 cells (Table 1). Two controls (C1 and C2) and one UVB-exposed sample were run on a single gel (Supplementary Fig. S1, http://www.iovs.org/lookup/suppl/doi:10.1167/iovs.11-7633/-/DCSupplemental). Analysis identified changes in the abundance of several proteins after UVB exposure, and the 3D data sets for some of these protein spots are shown in Figure 1. We next determined the effects of class 1 UV-blocking contact lenses on proteins that showed increased or decreased abundance after UVB exposure. Control cells, UVB-exposed cells, and cells exposed to UVB through the UV-blocking contact lenses were run on the same gel. Analysis of this gel identified changes in expression of several proteins after UVB exposure. Specifically, protein spot 3662 (containing β-actin, HNR PF protein, and eukaryotic translational factor), spot 5258 (peroxiredoxin, Hsp27, and triose phosphate isomerase), and spot 5685 (RING12) were altered. The class 1 UV-blocking contact lenses provided partial protection against alterations in these proteins after irradiation (Table 2, Fig. 2).
Next, we compared the protective effects of UV-blocking and non–UV-blocking contact lenses on the UVB-induced effects on protein expression in HLE B-3 cells. The four samples (control, UVB, UVB + UV-blocking contact lens, and UVB + non–UV-blocking contact lens) were pooled and run on each of four 2D gels as a standard. For each protein spot, we determined the ratio of the experimental sample to the pooled sample (control vs. pooled, UVB vs. pooled, UVB + UV-blocking contact lens vs. pooled, and UVB + non–UV-blocking contact lens vs. pooled). Protein spots that changed >1.7-fold in this comparison were considered significant, indicating a 95% confidence interval (that is, 95% of the fold change in protein spots was <1.7). Changes in protein abundance after UVB irradiation were of lower magnitude in samples irradiated through UV-blocking contact lenses. The 3D data sets for a few representative spots are shown in Figure 3. Mass spectroscopy-based proteomic analyses of these proteins are presented in Table 3. Protein spot 2422 (β-actin) showed a 2.09-fold increase between the control cells and the UVB-treated cells. This difference decreased to nearly 1, representing no change, when UV-blocking contact lenses were used (control vs. UVB + UV-blocking contact lenses = 1.18) but did not decrease substantially when non–UV-blocking contact lenses were used (C vs. UVB + non–UV-blocking contact lenses = 1.93). The abundance of spot 2580 (G3PDH and annexin A2) and spot 2584 (annexin A2) increased 1.95- and 1.75-fold, respectively, after UVB irradiation. In these cases, only UV-blocking contact lenses protected the cells from these changes, whereas non–UV-blocking contact lens lenses provided no protection (Table 3). Similarly, spot 3086 (triose phosphate isomerase), spot 4077 (ubiquitin B precursor), and spot 4162 (vimentin, nucleophosmin 1, and β-actin) increased 2.08-, 2.08-, and 1.83-fold, respectively, with UVB treatment. The alterations in spot 4162 (vimentin, nucleophosmin 1, and β-actin) were not protected by non–UV-blocking contact lenses but were somewhat protected by UV-blocking contact lenses. The changes in spot 4077 (ubiquitin B precursor) and spot 3086 (triose phosphate isomerase) were almost completely protected by UV-blocking contact lenses but were not protected by the non–UV-blocking contact lenses. For all proteins, the UV-blocking contact lenses had a greater protective effect than the non–UV-blocking contact lenses. Among the proteins that showed decreased abundance with UVB treatment, spot 1511 (caldesmon 1, ezrin, and zyx protein) decreased 2.01-fold with UVB radiation, and this change was prevented by the UV-blocking contact lenses, whereas no protection was observed with the non–UV-blocking contact lenses. Spot 1529 (lamin A/C transcript variant 1 and other proteins; see Table 3) decreased 1.7-fold after UVB radiation, and this alteration was completely abrogated by the UV-blocking contact lenses but not by the non–UV-blocking contact lenses. Spot 2284 (eukaryotic translational initiation factor and β-actin) decreased 1.85-fold in the presence of UVB radiation; this change was partially protected by UV-blocking contact lenses but was not protected by non–UV-blocking contact lenses. Although spot 769 (prepro α [1] collagen) decreased 2.86-fold after UVB exposure, the changes in this protein spot were not significantly protected by either lens type, although non–UV-blocking contact lenses protected slightly better protection than did UV-blocking contact lenses.
Figure 3.
Analysis of changes in lens epithelial cell proteins induced by UVB exposure in the absence of contact lenses and in the presence of UV-blocking and non–UV-blocking contact lenses. HLE B-3 cells were exposed to 768 mJ/cm2 UVB radiation at 302 nm and then incubated in culture medium for 4 hours. Proteins were separated by 2D-DIGE and subjected to MS/MS analysis. Quantitative image analysis data for identified proteins are shown in Table 3. Top: image of the gel with the protein spot encircled (red); middle: 3D data representation; bottom: quantitation of the proteins contained within the spot. (A, B) Spots 1511 and 1529 represent two of the protein spots that decreased with UVB irradiation. (C, D) Spots 2584 and 2422 represent two of the protein spots that increased with UVB irradiation.
Staining with fluorescein phalloidin, which labels the F-actin cytoskeleton, and β-tubulin immunofluorescence, which labels the microtubule skeleton, was examined in untreated cells, cells exposed to UVB, cells exposed to UVB in the presence of UV-blocking senofilcon A lenses, and cells exposed to UVB in the presence of non–UV-blocking lotrafilcon lenses (Fig. 4). F-actin fluorescence was strong in control cells but decreased substantially when cells were exposed to UVB radiation. The UV-blocking senofilcon A contact lenses completely prevented changes in F-actin staining after UVB radiation, whereas non–UV-blocking lotrafilcon lenses did not prevent the decrease in intensity of F-actin fluorescence. Similarly, the microtubule cytoskeleton visualized by immunofluorescence staining for tubulin (red) was strong in control cells but decreased substantially when cells were exposed to UVB radiation. UV-blocking senofilcon A contact lenses completely prevented the changes in microtubule staining after UVB radiation. Non–UV-blocking lotrafilcon contact lenses, however, were less protective against the loss of β-tubulin immunofluorescence than the UV-blocking senofilcon A lenses. These results indicate that UVB radiation reduces β-tubulin staining, and this effect is protected by UV-blocking contact lenses.
Human Lenses
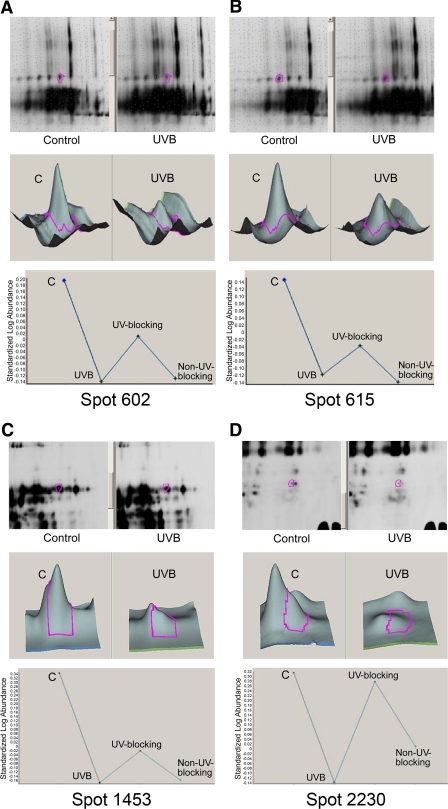
The HLE B-3 cell line is a transformed cell line containing low expression levels of crystallins.26,27 Given that crystallins are expressed in lens epithelial cells and are the major proteins of the fiber cells, human lenses are a better model with which to study UVB effects on the lens and especially to determine whether changes in crystallins occur with UVB exposure. We next determined the effects of the senofilcon A class 1 UV-blocking contact lenses on UVB-induced changes in protein expression in a pair of 17-year-old human lens by proteomic analysis. To control for the biological variation inherent in human samples, we used the multiplexing attribute of 2D-DIGE to perform an experiment with a pooled sample as an internal standard to normalize protein abundance across multiple gels. The following four experimental conditions were used: no UVB exposure (control), UVB exposure without contact lenses (UVB), UVB exposure in the presence of senofilcon A class 1 UV-blocking contact lenses (UVB + UV-blocking contact lenses), and UVB exposure in the presence of lotrafilcon A non–UV-blocking contact lenses (UVB + non–UV-blocking contact lenses). We conducted BVA analysis of these gels. Analysis was restricted to spots that were visualized in all three gels and that displayed statistically significant variation (P < 0.05; Supplementary Fig. S2, http://www.iovs.org/lookup/suppl/doi:10.1167/iovs.11-7633/-/DCSupplemental). Using these criteria, nine spots that changed in intensity after 4 minutes of UVB exposure relative to no-UVB controls were identified (Table 4). These spots were subjected to analysis by MS/MS. Marked reductions in αA-crystallin, αB-crystallin, aldehyde dehydrogenase 1, glyceraldehyde 3-phosphate dehydrogenase (G3PDH), βS-crystallin, and βB2-crystallin were observed. These decreases were significantly prevented by class 1 UV-blocking contact lenses but not by non–UV-blocking lotrafilcon A contact lenses (Table 4, Fig. 5). Spots 602, 608, 615, and 627 contained αA-crystallin or αB-crystallin, β-crystallins, and ALDH1 (55 kDa). Spot 1082 contained G3PDH (36 kDa), αA-crystallin, βS-crystallin, and γC-crystallin. Spots 602, 608, 615, 627, and 1082 decreased in abundance with UVB irradiation. We next performed proteomic experiments on an additional pair of human lenses. Table 5 shows the decrease in abundance of two protein spots of 62-year-old human lenses after UVB irradiation. The decrease in abundance of these proteins was markedly prevented in the presence of UV-blocking contact lenses. Not surprisingly, UVB exposure induced only reductions in protein abundance in postmortem human lenses, which were lysed within 5 minutes after UVB exposure. In contrast, both decreases and increases in protein abundance were observed in cultured human lens epithelial cells because these cells had been incubated for 4 hours after UVB treatment, a sufficient time to allow an increase in protein synthesis.
Figure 5.
Analysis of changes in human lens proteins after UVB exposure in the presence and absence of UV-blocking or non–UV-blocking contact lenses. Proteins that decreased with UVB exposure included spots (A) 602, (B) 615, (C) 1453, and (D) 2230. Other proteins are listed in Table 4. Top: gel with the protein spot encircled (red); middle: 3D data representation; bottom: quantitation of the proteins contained within the spot.
Network Analysis
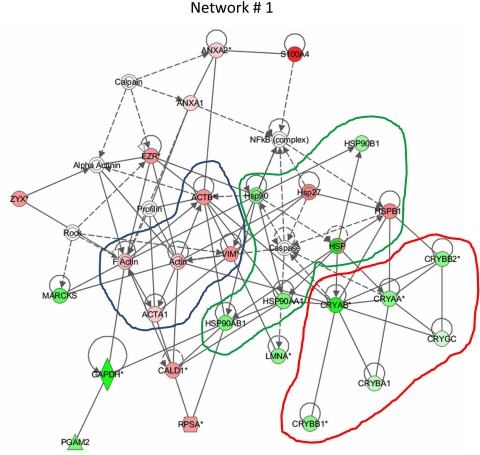
To determine whether specific groups of proteins were affected by UVB exposure of lenses, the proteins listed in Tables 1 to 5 were analyzed using Ingenuity Pathways software. Figure 6 shows that expression of crystallins as a group decreased after UVB radiation, whereas expression of cytoskeletal proteins such as vimentin and actin increased. The intensity of the signal increased as the magnitude of the UVB-induced changes increased. A third network containing the heat shock proteins Hsp90 and Hsp27 was also affected, with UVB radiation increasing the expression of Hsp27 while decreasing the level of Hsp90. Proposed interactions within Ingenuity networks containing the lens proteins that were affected by UV are shown in Supplementary Fig. S3 (http://www.iovs.org/lookup/suppl/doi:10.1167/iovs.11-7633/-/DCSupplemental). Comprehensive information on the proteins analyzed by Ingenuity Pathways software is provided in Supplementary Table S1 (http://www.iovs.org/lookup/suppl/doi:10.1167/iovs.11-7633/-/DCSupplemental).
Figure 6.
Analysis of altered protein networks by Ingenuity Pathway software. Biological networks and pathways generated from input data (control vs. UVB; Tables 1–5) indicate proteins with positive fold increase as shades of red and those with negative fold increase with shades of green. Increased magnitude is indicated by a more intense color. Expression of the cytoskeletal protein network (blue outline) was increased as a group by UVB irradiation. In contrast, expression of crystallins as a group decreased with UVB irradiation (red outline). The green line shows a third network containing heat shock proteins. Expression of heat shock protein Hsp90 decreased, whereas that of the small heat shock protein Hsp27 (HSPB1) was increased by UVB irradiation.
Discussion
Proteomic alterations induced by UVB radiation are of unique interest in the lens and are likely to represent proteins that are most susceptible to UVB radiation. These global analyses may provide insight into perturbations in UVB-induced human cataracts. We have studied the effects of UVB radiation on HLE B-3 cells and postmortem human lenses to observe proteomic changes that represent UVB-induced changes in the abundance of human lens proteins. We have also compared the effects of UV-blocking contact lenses and non–UV-blocking contact lenses and demonstrated remarkable protection from UVB-induced lens protein damage by the UV-blocking contact lenses.
Several important experimental paradigms of this study require further discussion. We used an extended lifespan human lens epithelial cell line in these proteomic analyses,26 and this protocol allowed us to extrapolate some of the findings to human lens epithelial cells. Using these cells also permitted identification of proteins that were upregulated or downregulated by UVB exposure. Unlike primary cultures, however, these cells express only low levels of αA-, αB-, and βB2-crystallin in early passages.26,27,38 Not surprisingly, UVB-induced changes in crystallins, which are known to be expressed in the lens epithelium, were not detected in these cells. Fortunately, the use of these cells allowed us to examine changes in proteins that we might not have otherwise detected in the presence of the abundant crystallins. In addition, the variations in spot intensities could be used to quantify the protective effect of the contact lenses in this study.
Our proteomic analysis of untreated HLE B-3 cells identified many of the proteins reported previously38 in addition to β-actin and caldesmon 1 isoform 5, which were not reported previously. We found that proteins associated with cell death, such as annexin A2, increased in abundance in UVB-exposed cells, and this increase was inhibited by the senofilcon A class 1 UV-blocking contact lenses but not by the lotrafilcon A non–UV-blocking contact lenses (Table 3). Similarly, decreases in caldesmon A and lamin A/C transcript variant 1 were prevented by the class 1 UV-blocking lenses. These proteins have not been identified previously in studies of UVB-induced protein effects in lens epithelial cells. Furthermore, our results showed that two of the spots (2422 and 4162) that were identified as β-actin increased with UVB exposure, whereas a third spot (2284) that contained β-actin and eukaryotic translation initiation factor decreased with UVB (Table 3). This finding suggests that the increased actin spots represent modified forms of the original β-actin (Table 3) and indicates that we likely observed an increase in the modified forms of β-actin in UVB-exposed HLE B-3 cells. The loss of F-actin staining in our confocal microscopy study suggests that polymerization of actin to F-actin polymers decreases after UVB exposure, and our data further indicate that this decrease is prevented by the senofilcon A UV-blocking contact lenses (Fig. 4). Furthermore, staining of microtubules decreased with UVB exposure, and this decrease was also prevented by the senofilcon A UV-blocking contact lenses but not by the lotrafilcon A non–UV-blocking contact lenses. Actin filaments and microtubules are required for cell survival and replication, suggesting that the UV-blocking contact lenses can protect lens epithelial cells from UVB-induced cell death.
The experimental paradigm used in this study necessarily required dissection of the human lens into sections and scraping of the epithelial and cortical proteins from the surface. Although care was taken for the same person do this uniformly, the actual changes in human lens protein abundance induced by UVB exposure and the protective effects of UV-blocking contact lenses may not be exact. Nonetheless, we consistently observed protection by UV-blocking contact lenses in HLE B-3 cells and human lenses (Tables 2, 5; Fig. 2), and higher protection by UV-blocking contact lenses than the non–UV-blocking contact lenses in both experimental systems (Tables 3, 4; Figs. 3, 5). Although we did not always observe 100% protection by the senofilcon A UV-blocking contact lens, this might have been due to the complexity of the methods.
Many of the proteins identified in the human lenses function to maintain the refractive properties of the lens; notably, αA- and αB-crystallin function as molecular chaperones.39–42 The UVB-induced decrease in abundance of αA- and αB-crystallins and the protection against this decrease provided by the senofilcon A class 1 UV-blocking contact lenses are therefore remarkable and, to our knowledge, have not been reported previously for intact human lenses (Tables 4, 5). The decreases in protein abundance observed in this study result from their absorption of 302 nm UVB radiation and its photoproducts, N-FK and related species.9,11,43,44 The reduction in α-crystallins may suggest that the α-crystallins bind to UVB-denatured proteins. Based on cell culture data (Fig. 4), one possible candidate for such interaction is the cytoskeleton.
In addition to these crystallins, G3PDH, aldehyde dehydrogenase 1 (ALDH1), and βB1-crystallin are among the most UVB-sensitive proteins in the human lens (Table 4). Photochemical damage to human lenses has previously been shown to reduce activity of the enzyme G3PDH,45 which functions as an important component of the protective enzyme systems in the lens. ALDH1 is highly abundant in the adult lens and was reported to be a component of crystallin complexes in old human lenses.46,47 Importantly, the senofilcon A class 1 UV-blocking contact lenses protected against the loss of ALDH1 from the lens epithelium/cortex (Table 4, Fig. 5). It should be noted that our study was limited by the fact that each spot analyzed contained multiple proteins. Spectrum counts shown in the tables suggest that the proteins with the highest spectral counts changed the most, but this is not guaranteed. If one protein stood out above all others (e.g., spot 2614 in Table 1, in which 41 of the assigned spectra are for lamin A/C), we can surmise that this protein was likely to be changed the most by UVB radiation. In addition, we did not separate the central lens epithelium from the cortical fibers in our experiments; thus, we cannot be certain of the localization of the human lens proteins that were affected by UVB radiation. However, most of the UVB radiation that impinges on the lens was absorbed by the central lens epithelial and cortical fiber cell proteins, which we analyzed together. Therefore, the reported UVB-induced effects are important and relevant to the human lens.
UVB and UVA radiation from sunlight can reach the eye.48,49 Although the cornea absorbs most of the UVB radiation, a small amount of UVB penetrates the lens and is absorbed by lens epithelium and cortical fiber cells. This penetrating UVB radiation causes cataracts in animal models. The fluence used in this study is roughly equivalent to 85 hours of sunlight exposure by the human lens.1 However, this is an estimate only, and, because absorption by the lens chromophores is greater at 302 nm than at 305 or 310 nm, 1568 mJ/cm2 of 302 nm radiation will be more damaging than longer wavelengths of UVB radiation. The aggregation of lens proteins, the formation of tryptophan photoproducts, and the loss of visible light transmission have been extensively studied in animal models of human cataracts and lens aging.6,10,50–53 UV-absorbing chromophores, which include tryptophan residues, tryptophan photoproducts such as N-FK, and lens glycation products, enhance light absorption and photodamage in the lens with age. Epidemiologic studies suggest a correlation between increased UVB exposure and risk for cortical cataracts15; however, estimates for UVB exposure do not include cumulative childhood exposure. Determining childhood UV exposure and including such an estimation in epidemiologic studies would further assist in understanding the mechanisms of human cataract formation and the role of UVB radiation in this process.16
In this study, UVB exposure of human lens induced the loss of αA-, αB-, βB1-, βB2-, βA3-, and βS-crystallins, and this decrease was partially prevented by UV-blocking contact lenses. Thus, these results support the hypothesis that wearing class 1 UV-blocking contact lenses provides some protection to the lens compared with non–UV-blocking contact lenses. These findings are supported by previous reports that UV-blocking contact lenses minimize UV-induced ocular damage.20,54 In agreement with this, the lotrafilcon A non–UV-blocking lenses did not prevent UVB exposure-induced changes in human lens proteins. Thus, UV-blocking senofilcon A contact lenses may be a useful way to protect the lens from solar UVB damage. In conclusion, senofilcon A class 1 UV-blocking contact lenses are beneficial for protecting against UVB-induced changes in protein abundance in lens epithelial cells and human lenses.
Supplementary Material
Footnotes
Supported by Vistakon, Johnson & Johnson Vision Care, Inc.; National Institutes of Health Grant R01EY05681 (UPA), Core Grant EY02687, CTSA Grant UL1 RR024992, Neuroscience Blueprint Core Grant P30NS057105 (Washington University), and National Centers of Research Resources Grant P41-RR00954; and Research to Prevent Blindness (Departmental of Ophthalmology and Visual Sciences).
Disclosure: U.P. Andley, Vistakon, Johnson & Johnson Vision Care, Inc. (F) ; J.P. Malone, None; R.R. Townsend, None
References
- 1. Zigman S. Environmental near-UV radiation and cataracts. Optom Vis Sci. 1995;72:899–901 [DOI] [PubMed] [Google Scholar]
- 2. Zigman S. Lens UVA photobiology. J Ocul Pharmacol Ther. 2000;16:161–165 [DOI] [PubMed] [Google Scholar]
- 3. Andley UP. Photodamage to the eye. Photochem Photobiol. 1987;46:1057–1066 [DOI] [PubMed] [Google Scholar]
- 4. Andley UP, Lewis RM, Reddan JR, Kochevar IE. Action spectrum for cytotoxicity in the UVA- and UVB-wavelength region in cultured lens epithelial cells. Invest Ophthalmol Vis Sci. 1994;35:367–373 [PubMed] [Google Scholar]
- 5. Roberts JE. Ocular phototoxicity. J Photochem Photobiol B. 2001;64:136–143 [DOI] [PubMed] [Google Scholar]
- 6. Andley UP. Ocular lens photobiology. In: Photobiology for the 21st Century. Overland Park, KS: Valdenmar Publishing Company; 2001:135–141 [Google Scholar]
- 7. Andley UP, Song Z, Mitchell DL. DNA repair and survival in human lens epithelial cells with extended lifespan. Curr Eye Res. 1999;18:224–230 [DOI] [PubMed] [Google Scholar]
- 8. Long AC, Colitz CM, Bomser JA. Apoptotic and necrotic mechanisms of stress-induced human lens epithelial cell death. Exp Biol Med. 2004;229:1072–1080 [DOI] [PubMed] [Google Scholar]
- 9. Andley UP, Walsh A, Kochevar IE, Reddan JR. Effect of ultraviolet-B radiation on protein synthesis in cultured lens epithelial cells. Curr Eye Res. 1990;9:1099–1106 [DOI] [PubMed] [Google Scholar]
- 10. Andley UP, Clark BA. Generation of oxidants in the near-UV photooxidation of human lens alpha-crystallin. Invest Ophthalmol Vis Sci. 1989;30:706–713 [PubMed] [Google Scholar]
- 11. Andley UP, Sutherland P, Liang JN, Chakrabarti B. Changes in tertiary structure of calf-lens alpha-crystallin by near-UV irradiation: role of hydrogen peroxide. Photochem Photobiol. 1984;40:343–349 [DOI] [PubMed] [Google Scholar]
- 12. Dillon J, Wang RH, Atherton SJ. Photochemical and photophysical studies on human lens constituents. Photochem Photobiol. 1990;52:849–854 [DOI] [PubMed] [Google Scholar]
- 13. Taylor LM, Aquilina J Andrew, Jamie JF, Truscott RJ. UV filter instability: consequences for the human lens. Exp Eye Res. 2002;75:165–175 [DOI] [PubMed] [Google Scholar]
- 14. Taylor LM, Aquilina JA, Willis RH, Jamie JF, Truscott RJ. Identification of a new human lens UV filter compound. FEBS Lett. 2001;509:6–10 [DOI] [PubMed] [Google Scholar]
- 15. Taylor HR, West SK, Rosenthal FS, et al. Effect of ultraviolet radiation on cataract formation. N Engl J Med. 1988;319:1429–1433 [DOI] [PubMed] [Google Scholar]
- 16. West S. Ocular ultraviolet B exposure and lens opacities: a review. J Epidemiol. 1999;9:S97–S101 [DOI] [PubMed] [Google Scholar]
- 17. Ayala MN, Michael R, Soderberg PG. Influence of exposure time for UV radiation-induced cataract. Invest Ophthalmol Vis Sci. 2000;41:3539–3543 [PubMed] [Google Scholar]
- 18. Andley UP, Fritz C, Morrison AR, Becker B. The role of prostaglandins E2 and F2 alpha in ultraviolet radiation-induced cortical cataracts in vivo. Invest Ophthalmol Vis Sci. 1996;37:1539–1548 [PubMed] [Google Scholar]
- 19. Dillon J, Zheng L, Merriam JC, Gaillard ER. The optical properties of the anterior segment of the eye: implications for cortical cataract. Exp Eye Res. 1999;68:785–795 [DOI] [PubMed] [Google Scholar]
- 20. Chandler HL, Reuter KS, Sinnott LT, Nichols JJ. Prevention of UV-induced damage to the anterior segment using class I UV-absorbing hydrogel contact lenses. Invest Ophthalmol Vis Sci. 2010;51:172–178 [DOI] [PubMed] [Google Scholar]
- 21. Sliney DH. Photoprotection of the eye: UV radiation and sunglasses. J Photochem Photobiol B. 2001;64:166–175 [DOI] [PubMed] [Google Scholar]
- 22. Sliney DH. How light reaches the eye and its components. Int J Toxicol. 2002;21:501–509 [DOI] [PubMed] [Google Scholar]
- 23. Kwok LS, Daszynski DC, Kuznetsov VA, Pham T, Ho A, Coroneo MT. Peripheral light focusing as a potential mechanism for phakic dysphotopsia and lens phototoxicity. Ophthalmic Physiol Opt. 2004;24:119–129 [DOI] [PubMed] [Google Scholar]
- 24. Andley UP, Patel HC, Xi JH, Bai F. Identification of genes responsive to UV-A radiation in human lens epithelial cells using complementary DNA microarrays. Photochem Photobiol. 2004;80:61–71 [DOI] [PubMed] [Google Scholar]
- 25. Moore L, Ferreira JT. Ultraviolet (UV) transmittance characteristics of daily disposable and silicone hydrogel contact lenses. Contact Lens Anterior Eye. 2006;29:115–122 [DOI] [PubMed] [Google Scholar]
- 26. Andley UP, Rhim JS, Chylack LT, Jr, Fleming TP. Propagation and immortalization of human lens epithelial cells in culture. Invest Ophthalmol Vis Sci. 1994;35:3094–3102 [PubMed] [Google Scholar]
- 27. Fleming TP, Song Z, Andley UP. Expression of growth control and differentiation genes in human lens epithelial cells with extended life span. Invest Ophthalmol Vis Sci. 1998;39:1387–1398 [PubMed] [Google Scholar]
- 28. Andley UP, Weber JG. Ultraviolet action spectra for photobiological effects in cultured human lens epithelial cells. Photochem Photobiol. 1995;62:840–846 [DOI] [PubMed] [Google Scholar]
- 29. Andley UP, Sawardekar MA, Burris JL. Action spectrum for photocross-linking of human lens proteins. Photochem Photobiol. 1997;65:556–559 [DOI] [PubMed] [Google Scholar]
- 30. Andley UP, Song Z, Wawrousek EF, Bassnett S. The molecular chaperone αA-crystallin enhances lens epithelial cell growth and resistance to UVA stress. J Biol Chem. 1998;273:31252–31261 [DOI] [PubMed] [Google Scholar]
- 31. Bai F, Xi JH, Wawrousek EF, Fleming TP, Andley UP. Hyperproliferation and p53 status of lens epithelial cells derived from αB-crystallin knockout mice. J Biol Chem. 2003;278:36876–36886 [DOI] [PubMed] [Google Scholar]
- 32. Mendelsohn BA, Malone JP, Townsend RR, Gitlin JD. Proteomic analysis of anoxia tolerance in the developing zebrafish embryo. Comp Biochem Physiol Part D Genomics Proteomics. 2009;4:21–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hu Y, Malone JP, Fagan AM, Townsend RR, Holtzman DM. Comparative proteomic analysis of intra- and interindividual variation in human cerebrospinal fluid. Mol Cell Proteomics. 2005;4:2000–2009 [DOI] [PubMed] [Google Scholar]
- 34. Amacher DE, Adler R, Herath A, Townsend RR. Use of proteomic methods to identify serum biomarkers associated with rat liver toxicity or hypertrophy. Clin Chem. 2005;51:1796–1803 [DOI] [PubMed] [Google Scholar]
- 35. Marionneau C, LeDuc RD, Rohrs HW, Link AJ, Townsend RR, Nerbonne JM. Proteomic analyses of native brain K(V)4.2 channel complexes. Channels (Austin). 2009;3:284–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Keller A, Nesvizhskii AI, Kolker E, Aebersold R. Empirical statistical model to estimate the accuracy of peptide identifications made by MS/MS and database search. Anal Chem. 2002;74:5383–5392 [DOI] [PubMed] [Google Scholar]
- 37. Nesvizhskii AI, Keller A, Kolker E, Aebersold R. A statistical model for identifying proteins by tandem mass spectrometry. Anal Chem. 2003;75:4646–4658 [DOI] [PubMed] [Google Scholar]
- 38. Wang-Su ST, McCormack AL, Yang S, et al. Proteome analysis of lens epithelia, fibers, and the HLE B-3 cell line. Invest Ophthalmol Vis Sci. 2003;44:4829–4836 [DOI] [PubMed] [Google Scholar]
- 39. Andley UP. Crystallins in the eye: function and pathology. Prog Retin Eye Res. 2007;26:78–98 [DOI] [PubMed] [Google Scholar]
- 40. Andley UP. The lens epithelium: focus on the expression and function of the alpha-crystallin chaperones. Int J Biochem Cell Biol. 2008;40:317–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bloemendal H, de Jong W, Jaenicke R, Lubsen NH, Slingsby C, Tardieu A. Ageing and vision: structure, stability and function of lens crystallins. Prog Biophys Mol Biol. 2004;86:407–485 [DOI] [PubMed] [Google Scholar]
- 42. Horwitz J. Alpha-crystallin can function as a molecular chaperone. Proc Natl Acad Sci U S A. 1992;89:10449–10453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Liang JN, Andley UP, Chylack LT., Jr Spectroscopic studies on human lens crystallins. Biochim Biophys Acta. 1985;832:197–203 [DOI] [PubMed] [Google Scholar]
- 44. Liang JN, Chakrabarti B. Spectroscopic investigations of bovine lens crystallins, 1: circular dichroism and intrinsic fluorescence. Biochemistry. 1982;21:1847–1852 [DOI] [PubMed] [Google Scholar]
- 45. Jedziniak J, Arredondo M, Andley U. Oxidative damage to human lens enzymes. Curr Eye Res. 1987;6:345–350 [DOI] [PubMed] [Google Scholar]
- 46. Graham C, Hodin J, Wistow G. A retinaldehyde dehydrogenase as a structural protein in a mammalian eye lens: gene recruitment of eta-crystallin. J Biol Chem. 1996;271:15623–15628 [DOI] [PubMed] [Google Scholar]
- 47. Srivastava K, Chaves JM, Srivastava OP, Kirk M. Multi-crystallin complexes exist in the water-soluble high molecular weight protein fractions of aging normal and cataractous human lenses. Exp Eye Res. 2008;87:356–366 [DOI] [PubMed] [Google Scholar]
- 48. Taylor HR. Ultraviolet radiation and the eye: an epidemiologic study. Trans Am Ophthalmol Soc. 1989;87:802–853 [PMC free article] [PubMed] [Google Scholar]
- 49. Taylor HR. Ocular effects of UV-B exposure. Doc Ophthalmol. 1994;88:285–293 [DOI] [PubMed] [Google Scholar]
- 50. Andley UP, Clark BA. The effects of near-UV radiation on human lens beta-crystallins: protein structural changes and the production of O2- and H2O2. Photochem Photobiol. 1989;50:97–105 [DOI] [PubMed] [Google Scholar]
- 51. Giblin FJ, Leverenz VR, Padgaonkar VA, et al. UVA light in vivo reaches the nucleus of the guinea pig lens and produces deleterious, oxidative effects. Exp Eye Res. 2002;75:445–458 [PMC free article] [PubMed] [Google Scholar]
- 52. Liang JN, Rossi MR, Andley UP. Fluorescence studies on the age related changes in bovine and human lens membrane structure. Curr Eye Res. 1989;8:293–298 [DOI] [PubMed] [Google Scholar]
- 53. Andley UP, Becker B, Hebert JS, Reddan JR, Morrison AR, Pentland AP. Enhanced prostaglandin synthesis after ultraviolet-B exposure modulates DNA synthesis of lens epithelial cells and lowers intraocular pressure in vivo. Invest Ophthalmol Vis Sci. 1996;37:142–153 [PubMed] [Google Scholar]
- 54. Giblin FJ, Lin LR, Leverenz VR, Dang L. A class I (senofilcon A) soft contact lens prevents UVB-induced ocular effects, including cataract, in the rabbit in vivo. Invest Ophthalmol Vis Sci. 2011;52:3667–3675 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.