Abstract
Recent animal studies at high field have shown that blood oxygen level-dependent (BOLD) contrast can be specific to the laminar vascular architecture of the cortex, by differences in its temporal dynamics in reference to cortical depth. In this study, we characterize the temporal dynamics of the hemodynamic response (HDR) across cortical depth in the human primary motor and visual cortex, at 7 T and using very short stimuli and with high spatial and temporal resolution. We find that the shape and temporal dynamics of the HDR changed in an orderly manner across cortical depth. Compared with the pial vasculature, HDRs in deeper gray matter are significantly faster in onset time (by ∼0.5 second) and peak time (∼2 seconds), and are narrower (by ∼1 second) and with smaller amplitude, in line with the known vascular organization across cortical depth and the transit of deoxygenated blood through the vasculature. The width of the HDR in deeper gray matter was as short as 2.1 seconds, indicating that neurovascular coupling takes place at a shorter timescale than previously reported in the human brain. These findings open the possibility to probe layer-specific hemodynamics and neurovascular coupling mechanisms in human gray matter.
Keywords: BOLD contrast, brain imaging, cerebral hemodynamics, functional MRI (fMRI), neurovascular coupling
Introduction
Blood oxygen level-dependent (BOLD) functional magnetic resonance imaging (fMRI) is the most widely used technique to study brain function in the human brain. Blood oxygen level-dependent fMRI relies on the coupling between increases in neuronal activity and increases in blood flow and volume that accompany the local increase in oxygen demand. With electrophysiological methods, changes in neuronal activity can be localized to the submillimeter scale of columnar or laminar structures, depending on the brain function studied (Mountcastle, 1997). Even though the ensuing changes in hemodynamics are related to neuronal activity (Logothetis et al, 2001), both the spatial and temporal extent of the hemodynamic response (HDR) is dispersed due to the vascular organization and dynamics, resulting in inaccurate mapping onto neuronal activity.
In principle, the local increase in oxygen demand on neuronal activation is met by increased blood flow from the arterial vasculature into the microvasculature in the deeper cortical layers (capillary bed; diameters <20 μm; Duvernoy et al, 1981) directly serving the active sites. From there on, blood flows into the macrovasculature (intracortical veins) ascending to the cortical surface, and is drained by the larger pial veins at the cortical surface (Duvernoy et al, 1981). The macrovasculature, such as the pial veins (diameters>100 μm and up to several millimeters for the larger vessels; Duvernoy et al, 1981), drain blood from several sites of active tissue (Turner, 2002). In practice, the BOLD signal reflects an aggregate change from the several vessel sizes, due to partial volume effects (imaging resolution) and the nature of the contrast that is sensitive to both intravascular and extravascular signals. At field strengths of 1.5 and 3 T, the intravascular component of the BOLD response dominates signal change in larger vessels, while the extravascular component dominates that in microvasculature (Marques and Bowtell, 2008; Ogawa et al, 1998). The separation of the microvascular and macrovascular signals is not only essential in improving the specificity of BOLD fMRI but also in understanding the mechanisms underlying neurovascular control.
At high field strength (⩾7 T), the intravascular signal is virtually eliminated due to the faster T2* relaxation of blood as compared with lower field strengths (Uludag et al, 2009; Yacoub et al, 2001). Even more, BOLD fMRI signal models predict an increased contrast-to-noise ratio for the microvasculature at 7 T due to increasing extravascular effects (Ogawa et al, 1993; Uludag et al, 2009). Recent animal studies at high field strength (⩾7 T) have shown that BOLD can be specific to the laminar vascular architecture of the cortex, by differences in temporal dynamics of the BOLD signal in reference to cortical depth (Jin and Kim, 2008; Silva and Koretsky, 2002; Tian et al, 2010). The onset times and time-to-peak (TTP) of the BOLD signal were found to be shorter in the deeper gray matter layers as compared with the pial surface. The translation of these findings to the human brain, however, is not direct due to the differences in vascular geometry and dynamics between the species, and experimental conditions such as anesthesia. The human brain has larger veins that drain to longer distances possibly resulting in increased contribution of large vessel effects. The possibility to differentiate between the vascular compartments contributing to the BOLD signal has been suggested in previous studies, also based on the temporal information of the HDR on neural activation. A range of delays of BOLD signal change at 1.5 T was reported to be associated with (visual) proximity to larger draining vessels, suggesting that long delays signify larger vessels (Lee et al, 1995). This finding was supported by a later study at 3 T, where the delay and width of the BOLD response were found increased for the larger venous vasculature as compared with gray matter (de Zwart et al, 2005).
In the present work, we characterize the temporal dynamics of the BOLD response across cortical depth in the human brain. We show that the shape and temporal evolution of the BOLD response vary across the cortical depth, with the faster and narrower responses corresponding to the deeper gray matter, presumably reflecting the source of neural activity, and that these measures increase in a linear manner toward the cortical surface. This was achieved by capitalizing on the advantages of 7 T in terms of contrast-to-noise ratio and resolution (Pfeuffer et al, 2002), minimal intravascular contribution (Uludag et al, 2009; Yacoub et al, 2001), and fast sampling of the BOLD response. We focused on the primary visual cortex (V1) because it can be stimulated in a highly controlled manner (Boynton et al, 1996), and quantified parameters of the BOLD HDR evolution expressed as onset time, TTP, full-width-at-half-maximum (FWHM), and maximum percent signal change (PSC). In addition, the same method was applied to the primary motor cortex (M1), using fist clenching as stimulus, to assess the generalizability of findings to cortical areas that do not primarily serve sensory input.
Materials and methods
Seven healthy subjects participated in the study after giving written informed consent in accordance to the Institutional Review Board of the Utrecht University Medical Center. fMRI data were acquired using an event-related fMRI paradigm for either the visual or the motor cortex. Each event-related fMRI scan contained a functional localizer part consisting of an off/on block design. Significantly activated voxels were selected for analysis according to the localizer and divided into a ‘large vessel' compartment and a ‘gray matter' compartment based on high resolution T2*-weighted anatomical images. Hemodynamic responses were then estimated for each voxel in both compartments and the TTP, FWHM, and PSC were computed from these HDRs. By registering the functional data to the high resolution anatomical images, we associated the measured HDR parameters with their corresponding anatomical location. Additionally, we obtained estimates of the onset time for the average HDRs for each anatomical location. The methods are described in detail below.
Data Acquisition
The subjects were scanned at a Philips 7 T (Philips Healthcare, Cleveland, OH, USA) system with a 16-channel SENSE head coil (Nova Medical, MA, USA); five subjects participated in the visual cortex study and two in the motor cortex study.
Visual cortex: Functional data were obtained using a multislice single-shot gradient-echo echo planar imaging acquisition with repetition time (TR)/echo time=440/27 ms, flip angle=60°, SENSE factor=2.2, an isotropic voxel size of 1.5 mm3, field-of-view=150 × 120 mm2, and seven slices with a 1-mm slice gap covering visual areas V1 and V2. Third order image-based shimming was performed on the field-of-view of the functional scans after brain extraction (Smith, 2002), using in-house developed software in IDL (v6.3 RSI, Boulder, CO, USA). A high resolution whole brain T2*-weighted scan was acquired as an anatomical reference, and to visualize the larger vessels, as follows: 3D multishot gradient-echo echo planar imaging, TR/echo time=76.6/27 ms, flip angle=20°, SENSE factor=2 in both phase- and slice-encoding direction, an isotropic voxel size of 0.6 mm3 and field-of-view=188 × 188 × 160 mm3. An echo planar imaging readout was used for the anatomical scan to have similar geometric distortions as in the functional images.
Motor cortex: For the functional data, the same scan was used as for the visual cortex except 13 oblique axial-sagittal slices covering the primary motor cortex were acquired (without slice gap), with a TR of 880 ms and flip angle of 65°. The same high resolution T2*-weighted anatomical scan was also acquired except with a field-of-view of 188 × 188 × 75 mm3. Additionally, hand movements were recorded from both subjects using a DataGlove 5 Ultra MRI (5DT, Irvine, CA, USA, sampling rate 20 ms) to measure the onsets of the fist clenching. Cardiac and respiratory rate data were recorded during all scans for all subjects for subsequent correction of physiological noise.
Functional Paradigm
Visual cortex: Each functional scan consisted of four parts: (1) 31 seconds baseline period, (2) 437 seconds event-related part, (3) 31 seconds baseline period, and (4) 79 seconds block design (localizer) part with off/on periods of 15.8/15.8 seconds (uniform gray screen/8 Hz reversing checkerboard). The event train for the event-related part was generated with interstimulus intervals (ISIs) taken from a uniform permuted distribution between 6 × TR=2.64 seconds and 26 × TR=11.44 seconds in 1 × TR steps (Burock et al, 1998; Hagberg et al, 2001). In total, 61 stimuli were presented with a stimulus duration of 250 ms (two 125 ms opposing checkerboard frames) and a mean ISI of 7.04 seconds. Short stimuli together with a minimal ISI of 2.64 seconds will yield a narrow HDR with minimal hemodynamic nonlinearities (Miezin et al, 2000; Pfeuffer et al, 2003; Zhang et al, 2008). All conditions included a central red fixation point.
Motor cortex: The same paradigm structure was used except the event-related part was 463 seconds long with ISIs taken from an exponential distribution (Hagberg et al, 2001). In total, 54 stimuli were presented as a color changing dot from red to green, indicating a short fist clench action. Minimal, maximal, and mean ISI were 3.52, 18.84, and 7.77 seconds, respectively.
Data Processing
Visual cortex: The functional scans were corrected for motion and linear drift, and then corrected for cardiac and respiratory fluctuations using RETROICOR (Glover et al, 2000). The localizer part was processed using FMRI Expert Analysis Tool (FEAT) including high pass filtering (cutoff at 1/31.6 Hz), Z threshold=3.5, slice-timing correction and no spatial smoothing (FSL, FMRIB Software Library, Oxford). The largest significant cluster (cluster P threshold=0.05, corrected for multiple comparisons) was selected and used as a region of interest for the event-related fMRI analysis. A mask depicting the larger vasculature was created on the high resolution T2*-weighted scan, where low signal intensity spots indicate the larger vasculature (e.g., see Figure 1A). To account for the extravascular extent of the ‘large vessel' signals, voxels adjacent to ‘large vessels' were also included in the mask. Next, a manually delineated mask containing visual area V1 was created using the high resolution T2*-weighted scan as anatomical reference. Estimation of the HDR was performed by means of conjugate gradients for deconvolution (Ari and Yen, 2001) after normalization by the baseline signal (mean of the two baseline periods) and 7-fold Fourier interpolation (yielding an effective sampling time of 440/7=68 ms). This level of interpolation was equal to the number of slices for the purpose of slice-timing correction; slice-timing correction was performed simultaneously with the HDR estimation by shifting the stimuli model for each slice according to the slice acquisition time. The estimated HDRs were temporally smoothed (robust LOESS filter function in Matlab with span 0.15, The Mathworks Inc., Natick, MA, USA) to reduce high frequency noise (>1 Hz). For every HDR, we computed TTP, FWHM, and PSC.
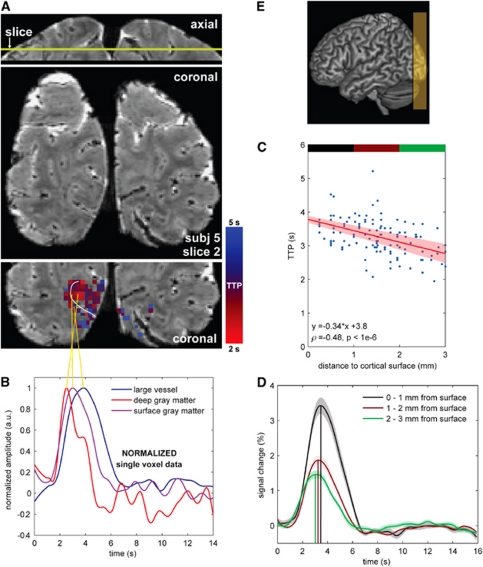
Figure 1.
(A) T2*-weighted anatomical image with the time-to-peak (TTP) map ranging from short TTP (red) to long TTP (blue). Top image shows the axial view indicating the slice location of the middle and bottom images. Middle and bottom images contain the same coronal slice, where the middle image shows the underlying anatomical structures and the bottom image the TTP map. The map encompasses the voxels identified as active according to the functional localizer. The white line represents the cortical surface boundary (only for that slice). The larger vasculature (diameter >∼0.3 mm) is recognized on the high resolution T2*-weighted anatomy as the lowest intensity spots compared with the surrounding tissue (cerebrospinal fluid (CSF), white and gray matter). (B) Three normalized hemodynamic response (HDR) curves (single voxel) selected according to their distance from the cortical surface and for a large vessel (orange lines). The amplitude of the single voxel HDR curves is normalized to clearly illustrate the timing differences associated with their anatomical location. (C) TTP as a function of the distance to the cortical surface; the red line shows the linear fit (shaded area reflects the standard error of the mean of the fit parameters, s.e.m.). Spearman correlation coefficient is also given. (D) Average HDR curves per distance section; black: 0 to 1 mm, brown: 1 to 2 mm, and green: 2 to 3 mm. Shaded area reflects the s.e.m. (E) Position of the field-of-view.
Motor cortex: For the motor cortex data, the same processing was performed except 10-fold Fourier interpolation was applied on the time series to match the sampling rate of the DataGlove (yielding an effective sampling time of 880/10=88 ms).
Spatiotemporal Analysis
The dependency of TTP, FWHM, and percent signal change as a function of distance to the cortical surface was estimated as follows.
Visual cortex: First, the functional and high resolution T2*-weighted images were both interpolated to a common grid of 0.5 mm3 isotropic voxel size. An affine registration was performed to co-register the high resolution T2*-weighted image to the functional image using Analysis of Functional NeuroImages (AFNI). A weighting mask incorporating the slice gaps was used in the registration algorithm to account for the slice gap in the functional images. This weighting mask guarantees that the slice gaps are ignored in the registration algorithm and not regarded as zero signal between the slices which would result in artificial contrast. For slices where the calcarine sulcus was clearly visible, the cortical surface was delineated manually on the high resolution T2*-weighted image.
Motor cortex: The same approach was performed as above except the incorporation of the slice gaps was omitted in the registration part, and the central sulcus within the motor cortex was delineated.
Distance to cortical surface estimation: The shortest distance from each voxel to the cortical surface was computed in the high resolution (0.5 mm3 isotropic) grid, which was used for the co-registration. In this grid, each voxel from the functional scan was represented by nine smaller voxels in-plane. The shortest distance was defined as the in-plane Euclidean distance from the center of each 9-voxel group to the cortical surface delineation thus giving the distance to the voxel originally sampled. Next, from each of the originally sampled voxels the HDR TTP was identified as a function of distance to the cortical surface. To further explore the HDR heterogeneity as a function of distance to the cortical surface, we divided the distance in three sections: 0 to 1, 1 to 2, and 2 to 3 mm (3 mm is approximately the cortical thickness; Duvernoy, 1999; Fischl and Dale, 2000) and computed the average HDR for every section. The onset time was estimated for the average HDR of each gray matter section. The onset time was defined by fitting a line to the slope between 20% and 80% of the peak of the HDR and computing the intercept with the baseline (Tian et al, 2010). Note that voxels including or adjacent to large vessels were excluded from this analysis.
Results
Hemodynamic Response Properties Reflecting the Underlying Vascular Anatomy
Significantly activated tissue in human primary visual cortex (V1) and motor cortex (M1), identified based on the block design functional localizer, is shown as the color overlay in Figure 1A and Supplementary Figure S1A for the visual cortex and in Figure 2A for the motor cortex. For each of the voxels identified as active, the HDR curve and its temporal properties as the TTP and FWHM were estimated from the subsequent event-related paradigm. A range of values for TTP, FWHM, and PSC was found (Figures 3B, 3C, and 3D) and they were distributed in an orderly manner across the cortex, apparently in accordance to the underlying vascular anatomy.
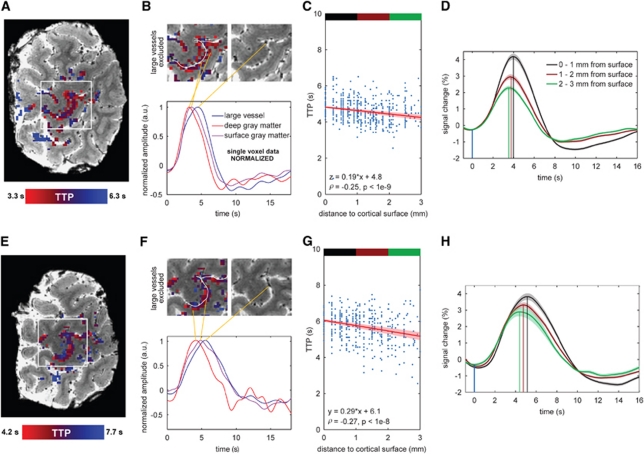
Figure 2.
(A, E) T2*-weighted anatomical image of the motor cortex for subjects 6 and 7, respectively, with the time-to-peak (TTP) map ranging from short TTP (red) to long TTP (blue). (B, F) Three normalized hemodynamic response (HDR) curves (single voxel) selected according to their distance from the cortical surface and for a large vessel (orange lines). The amplitude of the single voxel HDR curves is normalized to clearly illustrate the timing differences associated with their anatomical location. The two images above the plot show on the left the TTP map (excluding the identified large vessels) with the cortical surface boundary (white line, only for that slice) and on the right the underlying anatomy. (C, G) TTP as a function of the distance to the cortical surface with in red the linear fit (shaded area reflects the s.e.m. of the fit parameters). Spearman correlation coefficient is also given. (D, H) Average HDR curves per distance section; black: 0 to 1 mm, brown: 1 to 2 mm, and green: 2 to 3 mm. The blue line indicates the hand movement onset time as recorded from the DataGlove. Shaded area reflects the s.e.m.
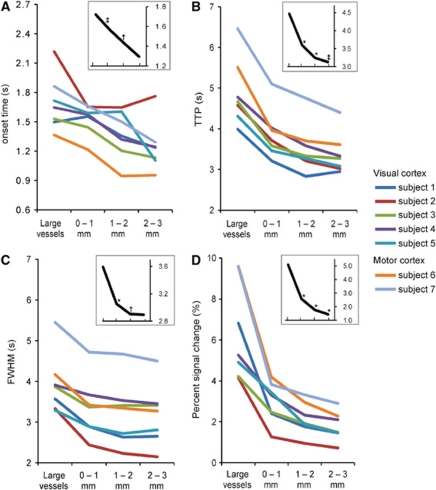
Figure 3.
(A–D) Onset time, time-to-peak (TTP), full-width-at-half-maximum (FWHM) and percent signal change (PSC) values, respectively, for all subjects for different anatomical locations (large vessels and distance to cortical surface: 0 to 1, 1 to 2, and 2 to 3 mm). The onset time was defined by fitting a line to the slope between 20% and 80% of the peak of the HDR and computing the intercept with the baseline. The inset curves (black) show the mean values for the visual cortex. * Denotes significant difference with the anatomical location directly on the left (paired one-sided Student's t-test for P<0.01, † for P<0.05, and ‡ for P<0.09).
The spatiotemporal pattern in the TTP with respect to the underlying vascular distribution is visible in Figure 1A and Supplementary Figure S1A for the visual cortex and in Figures 2A and 2E for the motor cortex. The long TTP HDRs (blue, TTP ≈5 seconds) are associated with larger vessels and the pial veins on the cortical surface (white line), while the short TTP HDRs (red, TTP ≈2.5 seconds) are more confined to the deeper gray matter. Note that in Figure 1A and Supplementary Figure S1A active gray matter voxels adjacent to cortical surfaces, which were not delineated can also show prolonged TTP values (see for instance the ‘blue' pixels along the outer surface perpendicular to the white line in Figure 1A). Individual, single voxel curves from shorter to longer measured TTP are illustrated in Figure 1B, Supplementary Figure S1B, and Figures 2B and 2F. These correspond to a voxel in deeper gray matter (>1.5 mm from the cortical surface, in red) presumably including the capillary bed, a voxel adjacent to the sulcus (in purple) presumably including intracortical and pial vessels, and one in the extravascular space of a larger vein within the sulcus (in blue). The delay in TTP was correlated with the FWHM of the HDR for all subjects (see Table 1), indicating a similar spatial distribution for the FWHM measures. In other words, short TTP HDRs were also narrower and they appeared confined to the deeper gray matter. Single voxel HDR TTP and FWHM differences were on the order of 2 and 1 second, respectively, across subjects, for the different anatomical locations described above. Figure 3 shows the HDR onset time, TTP, FWHM, and PSC in V1 and M1 for all subjects, separate for different voxel locations with respect to the cortical surface. All measures decreased significantly across the cortical depth (P value <0.08), with the exception of onset time and FWHM for the deeper gray matter (2 to 3 mm from the cortical surface). The HDR TTP, FWHM, and PSC were significantly larger in the extravascular space of large vessels as compared with gray matter activation (Figure 3), in agreement with previous studies performed at lower fields (de Zwart et al, 2005; Lee et al, 1995).
Table 1. Correlations between HDR TTP, FWHM, and PSC parameters (visual cortex).
| Subject | TTP versus FWHM | TTP versus PSC | FWHM versus PSC | # voxels |
|---|---|---|---|---|
| 1 | 0.36* | 0.05 | 0.33* | 698 |
| 2 | 0.47* | 0.28* | 0.57* | 397 |
| 3 | 0.38* | −0.04 | 0.12* | 528 |
| 4 | 0.36* | 0.11* | 0.18* | 772 |
| 5 | 0.38* | 0.10 | 0.13† | 168 |
TTP, time-to-peak; HDR, hemodynamic response; FWHM, full-width-at-half-maximum; PSC, percent signal change.
Visual cortex (excluding large vessels): TTP versus FWHM, TTP versus PSC and FWHM versus PSC. * Denotes a significant correlation (Pearson), two-sided Student's t-test for P<0.01, † for P<0.1.
Hemodynamic Response Properties Within Cortical Gray Matter
Further quantitative assessment of the measured HDR parameters with respect to cortical depth was conducted on voxels within cortical gray matter, excluding the voxels on or adjacent to larger vessels. The relationship of the HDR TTP with the distance from the cortical surface (Figure 1A; Supplementary Figure S1A; Figures 2B and 2F) is shown in Figure 1C and Supplementary Figure S1C for the visual cortex and in Figures 2C and 2G for the motor cortex, for all voxels found active. A significant trend was observed between TTP and distance from the cortical surface in every subject (P values indicated on the graphs for each subject). For the visual cortex, the decrease in TTP with cortical depth was 0.22±0.08 s/mm (mean±std across subjects), computed from the linear fit slopes (Figure 1C; Supplementary Figure S1C). For the motor cortex (Figures 2C and 2G), a decrease in TTP with cortical depth of 0.24±0.07 s/mm (mean±std across subjects) was found. Analyses on the FWHM data did not yield consistent results across cortical depth.
The average nonnormalized HDR curves across the cortical depth compartments (0 to 1, 1 to 2, and 2 to 3 mm from the cortical surface; see color bars in Figure 1C, Supplementary Figure S1C and Figures 2C and 2E) are shown in Figure 1D and Supplementary Figure S1D (visual cortex) and Figures 2D and 2H (motor cortex) and reveal significant differences in HDR parameters for different distances from the cortical surface (see also Figure 3). Differences between adjacent depth compartments were most pronounced in TTP and amplitude of signal change. Furthermore, for the visual cortex differences in onset time were found between the surface gray matter voxels and the middle and deeper gray matter voxels (0.14 and 0.27 seconds earlier, respectively, P values <0.04; see inset Figure 3A). Differences in onset time between the middle and deeper gray matter voxels were not significant (P value=0.13). Strong correlations were found between TTP and FWHM across voxels in cortical gray matter (voxels around large vessels were excluded), for every single subject. Correlations were surprisingly similar for all subjects, illustrating the consistency of the relationship across individuals. Correlations were also prominent between FWHM and PSC, but less so for TTP versus PSC (see Table 1).
Discussion
Our results show that the shape and temporal dynamics of the BOLD response vary across cortical depth in the human brain, with faster and narrower responses corresponding to the deeper gray matter, and these measures increase in an orderly manner toward the cortical surface. This spatiotemporal heterogeneity of the BOLD response matched the known vascular organization across cortical depth (Duvernoy et al, 1981), which is expected to be closely related to functional organization in the human cerebral cortex (Friston et al, 1994; Gati et al, 1997; Turner, 2002). Furthermore, these relationships were similar for the visual (sensory) and motor cortex (nonsensory), suggesting that we probed basic and similar neurovascular mechanisms between cortices.
Traversing from deeper gray matter to the cortical surface, the maximum differences in HDR shape as observed in onset time, TTP, FWHM, and PSC were on the order of 0.27, 0.47, 0.17 seconds, 76% and 0.32, 0.53, 0.19 seconds, 56%, on average for the visual and motor cortex, respectively (Figure 3). The evolution of the HDR shape when ascending toward the cortical surface showed an increase in TTP and FWHM consistent with the blood pooling time and dispersion of oxyhemoglobin across the cortical depth (visual cortex: Figure 1D and Supplementary Figure S1D; motor cortex: Figures 2D and 2H). The feeding arterioles enter the cortex perpendicularly from the pial surface and are spatially matched to a cortical unit (whisker barrel, Woolsey et al, 1996). From there they feed into capillaries, the highest density of which is located in layer IV (Bell and Ball, 1985; Weber et al, 2008). Next, blood drains to postcapillary venules oriented toward the surface. Postcapillary venules, however, drain more than a single cortical unit as shown in rat whisker barrels (Woolsey et al, 1996). Given the relatively slow transit of blood in capillaries (Stefanovic et al, 2008), the fact that most capillaries are located in layer IV, and the increased distance traveled from adjacent cortical units, the duration of oxygen-rich blood passing through is increased in postcapillary venules yielding an increase in TTP and FWHM when ascending to the cortical surface. Taking the average slope of 0.22±0.08 s/mm for the TTP versus distance to the cortical surface (visual cortex) and assuming a cortical thickness of ∼3 mm, we can estimate a blood pooling time of oxygenated blood contributing to the BOLD response from the capillary bed to the pial surface of ∼0.7 seconds (range ∼0.4 to ∼1.0 second). A similar estimate for the motor cortex yields a blood pooling time of ∼0.6 and ∼0.9 seconds for subjects 6 and 7, respectively. This is in the same range as the transit time of blood across the cortical vasculature of around 1 second as recorded in rat brain (Stefanovic et al, 2008). However, estimating the blood pooling time from the TTP values could be contaminated by spatial blurring caused by the extravascular effects, particularly around the pial vessels. This could also result in longer TTP at the surface gray matter. Most pial vessels were identified on the high resolution T2*-weighted anatomical images and removed from the analysis (together with adjacent voxels, 1.5 mm). Any remaining extravascular contamination from the pial veins would disappear rapidly with distance to the vessel; in the worst case scenario it drops off by a factor of four at a distance of half the vessel diameter (Ogawa et al, 1993). Hence, extravascular effects decline rapidly with distance from the vessel.
The FWHM of the HDRs in the deeper gray matter was on the order of 2.2 to 3.5 seconds for the visual cortex and 3.3 to 4.5 seconds for the motor cortex (Figure 3C). For the visual cortex data, where a short stimulus was used, we anticipated a narrow HDR (Miezin et al, 2000; Pfeuffer et al, 2003; Zhang et al, 2008). The values measured indicate a fast temporal resolution for the neurovascular coupling mechanism, in line with reports in the rat brain (Silva et al, 2007).
Additionally, we observed a significant increase in PSC (Figure 3D) from the deeper gray matter toward the surface gray matter. Multiple factors may contribute to this, including across cortical depth differences in blood volume, blood pooling, flow and vessel diameter (Duvernoy et al, 1981; Hutchinson et al, 2006; van Zijl et al, 1998; Zhao et al, 2006). The HDRs for the larger draining veins, as identified on the high resolution anatomy scan, exhibited larger onset time, TTP, FWHM, and PSC than those corresponding to gray matter (Figure 1; Supplementary Figure S1; Figures 2 and 3). This confirms the predictions for the larger draining vasculature from early reports (de Zwart et al, 2005; Lee et al, 1995).
Given the direction of drainage toward the sulcus (de Zwart et al, 2005; Hutchinson et al, 2006), and the velocity of capillary blood in rats of an estimated 0.7 mm/s during activation (Stefanovic et al, 2008), one might expect a delay in the onset of the BOLD response ascending toward the cortical surface, as has been shown in rats (Jin and Kim, 2008; Silva and Koretsky, 2002). Differences in the BOLD onset could also be due to the varying delays in microvascular (arterial) dilation across the cortical depth (Tian et al, 2010). Our results for the visual cortex showed a significant increase in onset time for the upper gray matter (0 to 1 mm) as compared with the deeper gray matter compartments (1 to 2 and 2 to 3 mm). We found on average a difference of ∼0.27 and ∼0.32 seconds in onset time between the deeper and upper gray matter for the visual and motor cortex, respectively. These values are faster than the reported transit time of blood across the cortical vasculature in rats (∼1 second) (Stefanovic et al, 2008). This implies a cortical depth-dependent local vascular response in humans, in addition to the blood pooling along the cortical vasculature, as was recently suggested for the rat brain (Silva and Koretsky, 2002; Tian et al, 2010). However, we did not observe a significant difference in onset time between the two deeper gray matter compartments (1 to 2 and 2 to 3 mm, respectively). Onset differences in deeper gray matter have been reported in the rat brain, specifically between layers V-IV and VI (Silva and Koretsky, 2002). It is likely that at our imaging resolution may not be high enough to sample directly from specific layers; at 1.5 mm voxel size we most likely averaged across layers, thereby reducing any potential onset time variation across layers. However, Tian et al (2010) did not find any significant onset time differences in the deeper gray matter (cortical layers IV to VI), although they used a lower spatial and temporal resolution than Silva and Koretsky (2002). Furthermore, the shape of the BOLD response has been shown to change with stimulus duration due to nonlinearities in the seconds range (Boynton et al, 1996; Wager et al, 2005; Figure 2 displays onsets) and in the subsecond range (Birn et al, 2001). It may be that onset differences across layers are more pronounced at longer stimulus duration (⩾2 seconds) (Silva and Koretsky, 2002) due to variability of nonlinearity effects across layers given differences in vascular architecture. Differences in the cerebrovascular organization across cortex and species (rats versus humans) may also lead to differences in these observations.
For mapping the spatiotemporal heterogeneity of the HDRs with high fidelity, high spatial and temporal resolution is critical. In addition, to generate very narrow and fast evolving HDRs a very short stimulus duration (250 ms for the visual cortex) is needed. Both were feasible in the present study due to the benefits of 7 T for BOLD fMRI imaging in terms of high BOLD contrast-to-noise ratio and high signal-to-noise ratio together with more efficient parallel imaging at very high field strength (Wiesinger et al, 2006). Increasing the spatial resolution even further would reduce partial volume effects and could open up the possibility to probe cortical layer-dependent HDRs as suggested by animal work (Jin and Kim, 2008; Silva and Koretsky, 2002; Tian et al, 2010). In this study, the temporal resolution was chosen as high as possible given the spatial resolution and needed cortical coverage. Increasing the temporal resolution further by using a shorter repetition time would increase the statistical power in estimating the HDR function as more samples are recorded per unit of time, but at a cost in coverage and spatial resolution.
Implications
The findings presented reveal that temporal HDR parameters mark the position of voxels relative to the pial surface. Although further investigation is required for other primary and notably for associative cortices, it seems reasonable to expect a similar relationship between the HDR response and voxel position. Separation of voxels at the pial surface from deeper-located voxels in cortical gray matter improves accuracy of localizing activated parenchyma, as has also been suggested by Zhang et al (2009). The shape of the BOLD response is determined by both voxel position in cortical gray matter and stimulus characteristics including duration. Nonlinearity of the BOLD response has a role in longer stimulus duration (than the one we used) (Birn et al, 2001) and in short ISIs (Wager et al, 2005). Nonlinearity of the BOLD response may well be much less prominent in deeper voxels devoid of larger vessels (Zhang et al, 2009). Reassessment of nonlinearity features seems warranted, and may lead to new insights in neurovascular coupling in humans.
Acknowledgments
The authors thank Patrik Andersson and Dora Hermes for their contribution to the data processing routines.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Journal of Cerebral Blood Flow & Metabolism website (http://www.nature.com/jcbfm)
Supplementary Material
References
- Ari N, Yen Y.2001Extraction of the hemodynamic response in randomized event-related functional MRIIn: Proceedings of the 23rd Annual Conference. IEEE/EMBS, 25–28 October 2001, Istanbul, Turkey (2001
- Bell MA, Ball MJ. Laminar variation in the microvascular architecture of normal human visual cortex (area 17) Brain Res. 1985;335:139–143. doi: 10.1016/0006-8993(85)90284-7. [DOI] [PubMed] [Google Scholar]
- Birn RM, Saad ZS, Bandettini PA. Spatial heterogeneity of the nonlinear dynamics in the FMRI BOLD response. Neuroimage. 2001;14:817–826. doi: 10.1006/nimg.2001.0873. [DOI] [PubMed] [Google Scholar]
- Boynton GM, Engel SA, Glover GH, Heeger DJ. Linear systems analysis of functional magnetic resonance imaging in human V1. J Neurosci. 1996;16:4207–4221. doi: 10.1523/JNEUROSCI.16-13-04207.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burock MA, Buckner RL, Woldorff MG, Rosen BR, Dale AM. Randomized event-related experimental designs allow for extremely rapid presentation rates using functional MRI. Neuroreport. 1998;9:3735–3739. doi: 10.1097/00001756-199811160-00030. [DOI] [PubMed] [Google Scholar]
- de Zwart JA, Silva AC, van Gelderen P, Kellman P, Fukunaga M, Chu R, Koretsky AP, Frank JA, Duyn JH. Temporal dynamics of the BOLD fMRI impulse response. Neuroimage. 2005;24:667–677. doi: 10.1016/j.neuroimage.2004.09.013. [DOI] [PubMed] [Google Scholar]
- Duvernoy H. The Human Brain. New York: Springer; 1999. [Google Scholar]
- Duvernoy HM, Delon S, Vannson JL. Cortical blood vessels of the human brain. Brain Res Bull. 1981;7:519–579. doi: 10.1016/0361-9230(81)90007-1. [DOI] [PubMed] [Google Scholar]
- Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci USA. 2000;97:11050–11055. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Jezzard P, Turner R. Analysis of functional MRI time-series. Hum Brain Mapp. 1994;1:153–171. [Google Scholar]
- Gati JS, Menon RS, Ugurbil K, Rutt BK. Experimental determination of the BOLD field strength dependence in vessels and tissue. Magn Reson Med. 1997;38:296–302. doi: 10.1002/mrm.1910380220. [DOI] [PubMed] [Google Scholar]
- Glover GH, Li TQ, Ress D. Image-based method for retrospective correction of physiological motion effects in fMRI: RETROICOR. Magn Reson Med. 2000;44:162–167. doi: 10.1002/1522-2594(200007)44:1<162::aid-mrm23>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Hagberg GE, Zito G, Patria F, Sanes JN. Improved detection of event-related functional MRI signals using probability functions. Neuroimage. 2001;14:1193–1205. doi: 10.1006/nimg.2001.0880. [DOI] [PubMed] [Google Scholar]
- Hutchinson EB, Stefanovic B, Koretsky AP, Silva AC. Spatial flow-volume dissociation of the cerebral microcirculatory response to mild hypercapnia. Neuroimage. 2006;32:520–530. doi: 10.1016/j.neuroimage.2006.03.033. [DOI] [PubMed] [Google Scholar]
- Jin T, Kim SG. Cortical layer-dependent dynamic blood oxygenation, cerebral blood flow and cerebral blood volume responses during visual stimulation. Neuroimage. 2008;43:1–9. doi: 10.1016/j.neuroimage.2008.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee AT, Glover GH, Meyer CH. Discrimination of large venous vessels in time-course spiral blood-oxygen-level-dependent magnetic-resonance functional neuroimaging. Magn Reson Med. 1995;33:745–754. doi: 10.1002/mrm.1910330602. [DOI] [PubMed] [Google Scholar]
- Logothetis NK, Pauls J, Augath M, Trinath T, Oeltermann A. Neurophysiological investigation of the basis of the fMRI signal. Nature. 2001;412:150–157. doi: 10.1038/35084005. [DOI] [PubMed] [Google Scholar]
- Marques JP, Bowtell RW. Using forward calculations of the magnetic field perturbation due to a realistic vascular model to explore the BOLD effect. NMR Biomed. 2008;21:553–565. doi: 10.1002/nbm.1224. [DOI] [PubMed] [Google Scholar]
- Miezin FM, Maccotta L, Ollinger JM, Petersen SE, Buckner RL. Characterizing the hemodynamic response: effects of presentation rate, sampling procedure, and the possibility of ordering brain activity based on relative timing. Neuroimage. 2000;11:735–759. doi: 10.1006/nimg.2000.0568. [DOI] [PubMed] [Google Scholar]
- Mountcastle VB. The columnar organization of the neocortex. Brain. 1997;120 (Part 4:701–722. doi: 10.1093/brain/120.4.701. [DOI] [PubMed] [Google Scholar]
- Ogawa S, Menon RS, Tank DW, Kim SG, Merkle H, Ellermann JM, Ugurbil K. Functional brain mapping by blood oxygenation level-dependent contrast magnetic resonance imaging. A comparison of signal characteristics with a biophysical model. Biophys J. 1993;64:803–812. doi: 10.1016/S0006-3495(93)81441-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa S, Menon RS, Kim SG, Ugurbil K. On the characteristics of functional magnetic resonance imaging of the brain. Annu Rev Biophys Biomol Struct. 1998;27:447–474. doi: 10.1146/annurev.biophys.27.1.447. [DOI] [PubMed] [Google Scholar]
- Pfeuffer J, McCullough JC, de Moortele PFV, Ugurbil K, Hu X. Spatial dependence of the nonlinear BOLD response at short stimulus duration. Neuroimage. 2003;18:990–1000. doi: 10.1016/s1053-8119(03)00035-1. [DOI] [PubMed] [Google Scholar]
- Pfeuffer J, van de Moortele P-F, Yacoub E, Shmuel A, Adriany G, Andersen P, Merkle H, Garwood M, Ugurbil K, Hu X. Zoomed functional imaging in the human brain at 7 Tesla with simultaneous high spatial and high temporal resolution. Neuroimage. 2002;17:272–286. doi: 10.1006/nimg.2002.1103. [DOI] [PubMed] [Google Scholar]
- Silva AC, Koretsky AP. Laminar specificity of functional MRI onset times during somatosensory stimulation in rat. Proc Natl Acad Sci USA. 2002;99:15182–15187. doi: 10.1073/pnas.222561899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva AC, Koretsky AP, Duyn JH. Functional MRI impulse response for BOLD and CBV contrast in rat somatosensory cortex. Magn Reson Med. 2007;57:1110–1118. doi: 10.1002/mrm.21246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanovic B, Hutchinson E, Yakovleva V, Schram V, Russell JT, Belluscio L, Koretsky AP, Silva AC. Functional reactivity of cerebral capillaries. J Cereb Blood Flow Metab. 2008;28:961–972. doi: 10.1038/sj.jcbfm.9600590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian P, Teng IC, May LD, Kurz R, Lu K, Scadeng M, Hillman EM, De Crespigny AJ, D'Arceuil HE, Mandeville JB, Marota JJ, Rosen BR, Liu TT, Boas DA, Buxton RB, Dale AM, Devor A. Cortical depth-specific microvascular dilation underlies laminar differences in blood oxygenation level-dependent functional MRI signal. Proc Natl Acad Sci USA. 2010;107:15246–15251. doi: 10.1073/pnas.1006735107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner R. How much cortex can a vein drain? Downstream dilution of activation-related cerebral blood oxygenation changes. Neuroimage. 2002;16:1062–1067. doi: 10.1006/nimg.2002.1082. [DOI] [PubMed] [Google Scholar]
- Uludag K, Müller-Bierl B, Ugurbil K. An integrative model for neuronal activity-induced signal changes for gradient and spin echo functional imaging. Neuroimage. 2009;48:150–165. doi: 10.1016/j.neuroimage.2009.05.051. [DOI] [PubMed] [Google Scholar]
- van Zijl PC, Eleff SM, Ulatowski JA, Oja JM, Ulug AM, Traystman RJ, Kauppinen RA. Quantitative assessment of blood flow, blood volume and blood oxygenation effects in functional magnetic resonance imaging. Nat Med. 1998;4:159–167. doi: 10.1038/nm0298-159. [DOI] [PubMed] [Google Scholar]
- Wager TD, Vazquez A, Hernandez L, Noll DC. Accounting for nonlinear BOLD effects in fMRI: parameter estimates and a model for prediction in rapid event-related studies. Neuroimage. 2005;25:206–218. doi: 10.1016/j.neuroimage.2004.11.008. [DOI] [PubMed] [Google Scholar]
- Weber B, Keller AL, Reichold J, Logothetis NK. The microvascular system of the striate and extrastriate visual cortex of the macaque. Cereb Cortex. 2008;18:2318–2330. doi: 10.1093/cercor/bhm259. [DOI] [PubMed] [Google Scholar]
- Wiesinger F, de Moortele P-FV, Adriany G, Zanche ND, Ugurbil K, Pruessmann KP. Potential and feasibility of parallel MRI at high field. NMR Biomed. 2006;19:368–378. doi: 10.1002/nbm.1050. [DOI] [PubMed] [Google Scholar]
- Woolsey TA, Rovainen CM, Cox SB, Henegar MH, Liang GE, Liu D, Moskalenko YE, Sui J, Wei L. Neuronal units linked to microvascular modules in cerebral cortex: response elements for imaging the brain. Cereb Cortex. 1996;6:647–660. doi: 10.1093/cercor/6.5.647. [DOI] [PubMed] [Google Scholar]
- Yacoub E, Shmuel A, Pfeuffer J, Moortele PFVD, Adriany G, Andersen P, Vaughan JT, Merkle H, Ugurbil K, Hu X. Imaging brain function in humans at 7 Tesla. Magn Reson Med. 2001;45:588–594. doi: 10.1002/mrm.1080. [DOI] [PubMed] [Google Scholar]
- Zhang N, Yacoub E, Zhu X-H, Ugurbil K, Chen W. Linearity of blood-oxygenation-level dependent signal at microvasculature. Neuroimage. 2009;48:313–318. doi: 10.1016/j.neuroimage.2009.06.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N, Zhu X-H, Chen W. Investigating the source of BOLD nonlinearity in human visual cortex in response to paired visual stimuli. Neuroimage. 2008;43:204–212. doi: 10.1016/j.neuroimage.2008.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao F, Wang P, Hendrich K, Ugurbil K, Kim SG. Cortical layer-dependent BOLD and CBV responses measured by spin-echo and gradient-echo fMRI: insights into hemodynamic regulation. Neuroimage. 2006;30:1149–1160. doi: 10.1016/j.neuroimage.2005.11.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.