Abstract
The choice of reference region in positron emission tomography (PET) human brain imaging of the vesicular monoamine transporter 2 (VMAT2), a marker of striatal dopamine innervation, has been arbitrary, with cerebellar, whole cerebral, frontal, or occipital cortices used. To establish whether levels of VMAT2 are in fact low in these cortical areas, we measured VMAT2 protein distribution by quantitative immunoblotting in autopsied normal human brain (n=6). Four or five species of VMAT2 immunoreactivity (75, 55, 52, 45, 35 kDa) were detected, which were all markedly reduced in intensity in nigrostriatal regions of patients with parkinsonian conditions versus matched controls (n=9 to 10 each). Using the intact VMAT2 immunoreactivity, cerebellar and cerebral neocortices had levels of the transporter >100-fold lower than the VMAT2-rich striatum and with no significant differences among the cortical regions. We conclude that human cerebellar and cerebral cortices contain negligible VMAT2 protein versus the striatum and, in this respect, all satisfy a criterion for a useful reference region for VMAT2 imaging. The slightly lower PET signal for VMAT2 binding in occipital (the currently preferred reference region) versus cerebellar cortex might not therefore be explained by differences in VMAT2 protein itself but possibly by other imaging variables, for example, partial volume effects.
Keywords: cerebellum, occipital cortex, positron emission tomography, reference region, substantia nigra, vesicular monoamine transporter 2
Introduction
The vesicular monoamine transporter 2 (VMAT2), a member of the solute carrier family 18 (SLC18A2) with 12 transmembrane domains, is the protein responsible for transporting monoamine neurotransmitters (dopamine, noradrenaline, serotonin) into synaptic vesicles for subsequent storage and release (Erickson et al, 1996). Tetrabenazine or its metabolite dihydrotetrabenazine (DTBZ) is a specific inhibitor of the transporter used in radioligand-binding studies to measure the in vitro concentration of VMAT2 in mammalian brain (Scherman et al, 1988; Darchen et al, 1989; Vander Borght et al, 1995; Wilson et al, 1996). Positron emission tomography (PET) imaging of [11C]DTBZ binding has now been developed as a noninvasive tool to estimate brain levels of VMAT2 in vivo (Kilbourn et al, 1993; Frey et al, 1996; Chan et al, 1999; Koeppe et al, 1999), in particular in the striatum (caudate and putamen) in which most of VMAT2 (90% to 95%) is considered to be localized to the dopamine nerve terminals (cf. Boileau et al, 2008). The advantage of VMAT2 imaging over other presynaptic dopamine terminal markers including the decarboxylase (by 18F-DOPA) and the dopamine transporter (e.g., by 11C-methylphenidate) is the insensitivity of VMAT2 protein to several dopaminergic modulations (cf. Boileau et al, 2008; Tong et al, 2008) although it was recently demonstrated that in vivo [11C]DTBZ uptake could be influenced by presumably large changes in vesicular dopamine levels (see Boileau et al, 2010; Kilbourn et al, 2010 for references and discussion). Positron emission tomography VMAT2 imaging has been commonly used to assess striatal dopamine terminal integrity in a variety of human neuropsychiatric conditions, for example, in Parkinson's disease (PD) (Frey et al, 1996; Brooks et al, 2003; Okamura et al, 2010).
A practical issue with PET VMAT2 imaging is selection of a reference region for the measurement of free and nonspecific binding (Chan et al, 1999). In principle, an ideal reference region should have identical free and nonspecific properties as compared with regions of interest with specific binding, a reasonably high uptake of the radiotracer, and a negligible contribution of specific binding to the tracer uptake in this region, that is, very low levels of the protein target. Among the PET VMAT2 studies, the selection of reference region has been arbitrary, with cerebellar (Chan et al, 1999), whole cerebral (Gilman et al, 1998, 1999), frontal (Frey et al, 1996; Gilman et al, 1996), parieto-occipital (Troiano et al, 2010), or occipital cortices (Chan et al, 1999; Koeppe et al, 1999; Boileau et al, 2008; Okamura et al, 2010) having been used. Currently, the occipital cortex appears to be a preferred reference region in human brain imaging studies apparently because the data obtained are less variable and of consistently higher values as compared with those using cerebellar cortex as the reference region (Chan et al, 1999). However, animal studies have repeatedly showed that the cerebellar cortex contains less DTBZ-binding sites than cerebral cortices (Scherman et al, 1988; Darchen et al, 1989; Vander Borght et al, 1995) and in the development of new DTBZ-based PET tracers the cerebellum, for practical reasons, continues to be used as the reference region in preclinical studies (Kilbourn et al, 2007).
To address the question of the most appropriate reference region for VMAT2 PET imaging, we reasoned that it would be helpful to know the relative concentrations of VMAT2 protein in different regions of the human brain. The current study therefore used quantitative VMAT2 immunoblotting in autopsied human brain. Given that several VMAT2-immunoreactive species of uncertain identity have been reported in the literature (Wang et al, 1997; Jassen et al, 2005; Cruz-Muros et al, 2008), we also made efforts to confirm these VMAT2 Western blot bands.
Materials and methods
Subjects
This study was approved by the Research Ethics Board of the Centre for Addiction and Mental Health at Toronto. A total of six (4M/2F) autopsied brains from neurologically normal subjects (age: 48±0.3 years; postmortem interval: 16±3 hours; mean±s.e.m.) were used in the VMAT2 regional distribution study. To help characterize the VMAT2-immunoreactive protein bands (which should be reduced in intensity in degenerative nigrostriatal dopamine deficiency disorders) in the Western blots, autopsied brains were also obtained from a total of 10 patients with PD (5M/5F), 10 patients with progressive supranuclear palsy (PSP; 9M/1F), 9 patients with multiple system atrophy (MSA; 5M/4F), and 10 normal subjects (4M/6F). No significant difference (one-way analysis of variance) was found among the four groups in postmortem interval (control: 12±1; PD: 14±2; PSP: 11±2; MSA: 14±2 hours) or in age (control: 70±3; PD: 75±2, PSP: 73±4; MSA: 64±4 years). One-half brain was used for neuropathological examination, whereas the other half was frozen for neurochemical analyses. The characteristics and pathological findings of the patients have been previously published (Tong et al, 2010). The causes of death for the neuropathologically normal control subjects were cardiovascular illnesses (n=12), bronchopneumonia (n=2), pulmonary edema (n=1), and trauma (n=1).
Tissue Sample Preparation, Sodium Dodecyl Sulfate Polyacrylamide Gel Electrophoresis, and Western Blotting
Cerebral cortical subdivisions were excised according to Brodmann classification. Dissection of the subcortical areas from the ∼3 mm thick coronal sections followed published procedures (Kish et al, 2005; Tong et al, 2010). Brain tissue homogenates were used throughout this study. Sample preparation, sodium dodecyl sulfate polyacrylamide gel electrophoresis, and Western blot followed published procedures (Kish et al, 2005). Protein concentration was determined using the Bio-Rad Protein Assay Kit (Bio-Rad, Hercules, CA, USA) with bovine plasma albumin as the standard. Five concentrations of tissue standard (0.25 to 3 μg of protein), consisting of a pooled human striatal samples, were run on each blot together with the samples (2 to 40 μg of protein, depending on regional levels of VMAT2; see Figure 4). As VMAT2 has a strong propensity to aggregate during heating or long-term storage in sodium dodecyl sulfate polyacrylamide gel electrophoresis sample buffer in the freezer, the samples were prepared by incubation at room temperature for 30 minutes immediately before use. The antibody used for quantitative determination of VMAT2 levels was a well-characterized (Miller et al, 1999; Haycock et al, 2003) rabbit polyclonal antibody raised against the 19-amino-acid C-terminus peptide of human VMAT2 (AB1767; Chemicon International, Temecula, CA, USA). For measurement of the level of the ∼35 kDa VMAT2 band IV (see Figures 1, 2, 3 and 4), blots were cut between the two colored molecular weight (MW) markers (47.5 and 32.5 kDa), with the upper and lower half probed with the primary antibody at a dilution of 1:10,000 and 1:3,000, respectively. The same dilution of 1:10,000 was used for the goat anti-rabbit IgG (H+L) horseradish peroxidase secondary antibody (cat# 4050-05; SouthernBiotech, Birmingham, AL, USA). Another recently available anti-human VMAT2 monoclonal antibody (clone 9E11, cat# TA500506, OriGene Technologies, Rockville, MD, USA) raised against the recombinant, full-length VMAT2 protein was also used to help characterize the VMAT2-immunoreactive protein bands. Western blot of the ‘control' protein neuron specific enolase followed published procedures (Kish et al, 2005).
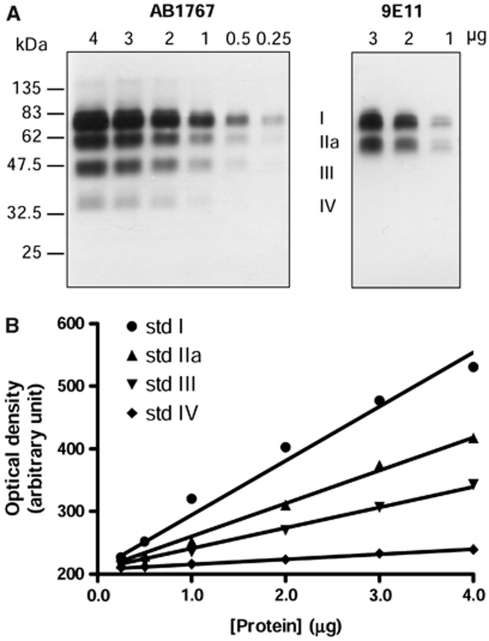
Figure 1.
Western blotting of vesicular monoamine transporter 2 (VMAT2) in the striatum of autopsied human brain. (A) Representative immunoblots of the tissue standard comprised of pooled human putamen and caudate samples probed with the rabbit polyclonal antibody raised against a C-terminus 19-amino-acid peptide of human VMAT2 (AB1767 from Chemicon) and the mouse monoclonal antibody (clone 9E11, subtype IgG1) raised against recombinant, full-length human VMAT2. The four VMAT2 bands detected by AB1767 in the striatum were assigned as bands I, IIa, III, and IV, respectively. Note the absence of bands III and IV by 9E11. (B) Curve fitting of the four standard curves. The mean ratios of levels of VMAT2 bands IIa, III, and IV versus I in the tissue standard from five determinations were 0.529±0.033, 0.358±0.023, and 0.115±0.009, respectively.
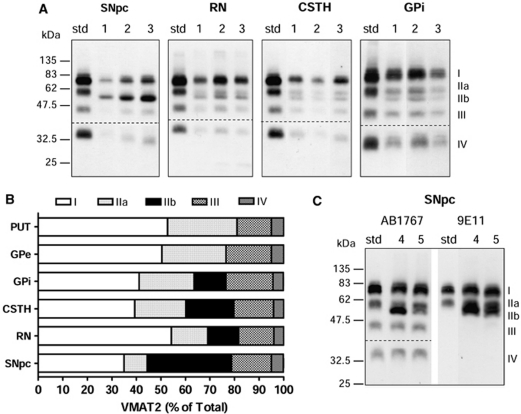
Figure 2.
Vesicular monoamine transporter 2 (VMAT2) band IIb in the substantia nigra and neighboring regions of autopsied human brain. (A) Representative immunoblots with AB1767 in the substantia nigra pars compacta (SNpc; 10 μg), red nucleus (RN; 10 μg), subthalamic nucleus (CSTH; 20 μg), and globus pallidus internal (GPi; 20 μg) of three normal subjects (#1–3). Note the presence of a protein band (IIb) of slightly smaller molecular weight in these brain regions as compared with band IIa in the tissue standard of striatum (std; 2 μg). (B) Composition of the VMAT2 protein bands (percent of total) in SNpc (n=16), RN (n=6), CSTH (n=6), and GPi (n=6) as compared with that of putamen (PUT; n=10) and globus pallidus external (GPe; n=6). Note similar levels of bands I and IIb in SNpc and the difference between GPi and GPe. (C) Band IIb in SNpc was also detected by the monoclonal antibody 9E11. Shown are representative immunoblots of SNpc (10 μg) from another two normal subjects (#4 and #5) and the tissue standard of striatum (2 μg) probed with the two antibodies AB1767 and 9E11, respectively. The dotted lines in (A, C) show the cut of the blots for separate probing of the upper (1:10,000) and lower (1:3,000) halves with different dilution of the primary antibody.
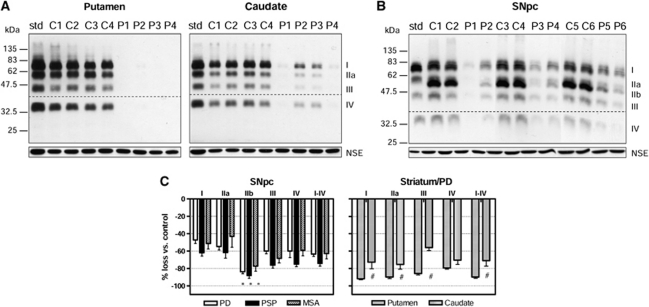
Figure 3.
Vesicular monoamine transporter 2 (VMAT2) in the brain of patients with Parkinson's disease (PD), progressive supranuclear palsy (PSP), and multiple system atrophy (MSA). (A) Immunoblots (AB1767) of putamen and caudate (2 μg) of PD patients (P1–P4) as compared with that of the controls (C1–C4). Note more severe loss of VMAT2 in putamen versus caudate in PD. (B) Immunoblots of substantia nigra pars compacta (SNpc; 10 μg) of PD patients (P1–P6) as compared with that of the controls (C1–C6). Note the preferential loss of band IIb versus other bands in SNpc of PD. Also shown in (A, B) are the tissue standard (3 μg) and blots of the control protein neuron specific enolase (NSE). The dotted lines in (A, B) show the cut of the blots for separate probing of the upper (1:10,000) and lower (1:3,000) halves with different dilution of the primary antibody. (C) Marked loss (mean±s.e.m.) of all VMAT2 proteins (I–IV) in the striatum (putamen and caudate) of patients with PD (n=4) and in the SNpc of patients with PD (n=10), PSP (n=10), and MSA (n=9). #P<0.05, caudate versus putamen in percentage loss of VMAT2 proteins in PD (paired Student's t-tests); *P<0.05, percentage loss of VMAT2 band IIb versus other bands in SNpc of PD, PSP, and MSA except versus bands III and IV in MSA (repeated measures analysis of variance followed by post hoc Bonferroni adjustments).
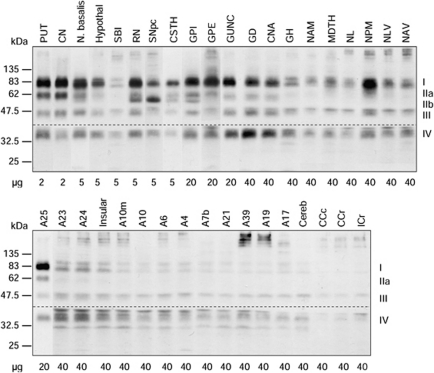
Figure 4.
Representative immunoblots of vesicular monoamine transporter 2 (VMAT2) in 37 brain regions of a normal control subject. ‘A' refers to Brodmann cerebral cortical areas; A23, cingulate gyrus posterior; A24, cingulate gyrus anterior; A25, parolfactory gyrus; CCc, corpus callosum caudal; CCr, corpus callosum rostral; Cereb, cerebellar cortex; CN, caudate; CNA, hippocampal Ammon's horn; CSTH, subthalamic nucleus; GD, dentate gyrus; GH, hippocampal gyrus; GPe, globus pallidus external; GPi, globus pallidus internal; GUNC, gyrus of uncus; Hypothal, hypothalamus; ICr, internal capsule rostral; Insular, insular cortex; MDTH, mediodorsal thalamus; N. basalis, nucleus basalis; NAM, amygdala; NAV, anterior ventral nucleus of thalamus; NL, nucleus lateralis of thalamus; NLV, lateral ventral nucleus of thalamus; NPM, medial pulvinar of thalamus; PUT, putamen; RN, red nucleus; SBI, substantia innominata; SNpc, substantia nigra pars compacta.
Data Analyses
Four linear standard curves were constructed for each of the VMAT2 protein bands (I, IIa, III, and IV; see Figure 1B for an example) on each blots. The relative levels of the four VMAT2 protein bands in the tissue standard were determined by ratios of the slopes of the standard curves of band IIa, III, and IV to that of band I, that is, RIIa/I, RIII/I, and RIV/I, which were measured in blots probed with one concentration of the primary antibody (Figures 1A and 1B). The concentration of each protein band in a sample was determined by interpolation from the respective standard curves, with levels of band IIa/b, III, and IV then standardized against band I by multiplying the ratio R, and the results were expressed as μg tissue standard (band I)/μg sample protein. The within and between blots coefficient of variation for each VMAT2 protein bands were 4.9% and 14.2% for band I, 7.7% and 11.7% for band IIa/b, 9.6% and 14.4% for band III, and 8.5% and 10.7% for band IV, respectively. For simplicity only, VMAT2 ‘immunoreactivity' will be referred to as ‘levels.'
Differences in VMAT2 levels among control and parkinsonian patients were assessed by one-way analysis of variance followed by post hoc Bonferroni adjustments. Differences in percentage loss versus control among the five VMAT2 bands in the substantia nigra of parkinsonian patients were assessed by repeated measures analysis of variance. Vesicular monoamine transporter 2 regional distribution was examined by one-way repeated measures analysis of variance followed by post hoc Bonferroni adjustments. No significant correlation was observed between the levels of VMAT2 proteins and postmortem interval of the subjects examined.
Results
Characterization of Vesicular Monoamine Transporter 2-Immunoreactive Protein Bands in Western Blots of Normal Autopsied Human Striatum
The polyclonal antibody raised against a 19-amino-acid C-terminus peptide of human VMAT2 (AB1767 from Chemicon) has been widely used to examine VMAT2 protein by Western blot in striatum of human brain (Miller et al, 1999; Haycock et al, 2003; Little et al, 2003) and in the brain of rodents under a variety of conditions (Wang et al, 1997; Gainetdinov et al, 1998; Cruz-Muros et al, 2008). As expected (Haycock et al, 2003), the antibody detected in human striatal samples a total of four broad protein bands with an MW of ∼75 (I), 55 (IIa), 45 (III), and 35 kDa (IV), respectively (Figure 1A). The ∼75 kDa VMAT2 I was the predominant band, accounting for 53%±2% (n=10) and 56%±3% (n=10) of the total VMAT2 immunoreactivity in putamen and caudate, respectively. The intensity of the smaller bands decreased along with their sizes in the putamen (28%±1%, 14%±1%, and 5%±1% of total, respectively; see Figure 2B) and caudate (26%±2%, 14%±1%, and 5%±1% of total, respectively). The ∼35 kDa band IV was either ignored (Haycock et al, 2003; Jassen et al, 2005) or not reported (Miller et al, 1999) in previous studies due to its weak staining.
To address the literature controversy with respect to the identity of the multiple VMAT2-immunoreactive bands detected in the striatum (Wang et al, 1997; Gainetdinov et al, 1998; Miller et al, 1999; Jassen et al, 2005; Cruz-Muros et al, 2008), we performed immunoblotting with another recently available monoclonal antibody (9E11) raised against recombinant, full-length human VMAT2. As shown in Figure 1A, the monoclonal antibody detected in human striatal samples only the two larger forms (I and IIa) of VMAT2, with the relative intensity of the two bands also similar to that observed with the polyclonal antibody. Therefore, our observations with two antibodies against different epitopes of VMAT2 provide some support for the notion that the minor VMAT2-immunoreactive species at 45 and 35 kDa might be N-terminally truncated forms (Wang et al, 1997; Gainetdinov et al, 1998; Miller et al, 1999; Jassen et al, 2005).
Distinct Vesicular Monoamine Transporter 2 Immunoreactivity in the Normal Human Substantia Nigra
The four-band pattern (I, IIa, III, and IV) of VMAT2 detected with the polyclonal antibody was generally observed throughout the human brain regions examined (see Figure 4). However, we found that another protein band (∼52 kDa, IIb) of slightly smaller MW than the 55-kDa band could be detected by the polyclonal antibodies in substantia nigra pars compacta, the neighboring midbrain regions red nucleus and subthalamic nucleus, and in the internal portion of the globus pallidus (GPi) (Figure 2A, see also Figures 3B and 4). Moreover, in the substantia nigra, the 52-kDa band IIb was of (at least) comparable intensity as that of the mature 75 kDa band I (35%±1% versus 35%±2% of total levels of VMAT2, respectively; Figure 2B), with levels of the 55-kDa band IIa low in the dopamine cell body region (9%±1%). The 75-kDa band I nevertheless was predominant in other brain regions. The percentage levels of band I, IIa, and IIb were 54%±2%, 15%±1%, and 13%±2% in the red nucleus, 39%±3%, 21%±2%, and 20%±1% in the subthalamic nucleus, and 41%±2%, 22%±1%, and 13%±1% in the GPi. We also confirmed that the 52-kDa band IIb in the substantia nigra was immunoreactive to the monoclonal antibody (Figure 2C).
Vesicular Monoamine Transporter 2 Immunoreactivity Is Decreased in Substantia Nigra and Striatum of Patients with Degenerative Parkinsonian Conditions
To provide further evidence that the protein bands (I, IIa, IIb, III, and IV) detected by the antibodies were in fact derived from VMAT2, we examined brain of patients with PD, the classic degenerative dopamine, and VMAT2 deficiency condition (Frey et al, 1996; Wilson et al, 1996). As expected, in the striatum (putamen and caudate) of patients with PD, levels of all four VMAT2 bands I, IIa, III, and IV were markedly and evenly decreased, with the loss significantly more severe in putamen (−92%±1%, −90%±2%, −86%±2%, and −79%±2% for bands I to IV, respectively) than in caudate (−73%±8%, −75%±6%, −56%±3%, and −70%±5%, respectively; Figures 3A and 3C). The overall loss of VMAT2 immunoreactivity was −90%±2% and −71%±6% for putamen and caudate, respectively, consistent with the literature showing that the putamen has more severe dopamine marker loss in PD than the caudate (Frey et al, 1996; Wilson et al, 1996). Similarly in the substantia nigra, levels of all five VMAT2-immunoreactive bands were significantly reduced (−47%±4%, −55%±4%, −84%±2, −60%±3, and −60%±8%, respectively) in PD although to a less overall extent (−63%±3%) than those in the striatal terminal regions (Figures 3B and 3C). Again, this is in line with the pathological finding that the loss of dopamine cell body is less severe than the loss of dopamine terminals in the striatum in PD (cf. Hornykiewicz, 1998). Interestingly, we found that the 52-kDa band IIb was preferentially lost as compared with the other bands (−84% versus −47% to −60% P<0.05) in the substantia nigra of PD, with the extent of loss similar to that observed in the striatum (Figures 3B and 3C). Similar findings were observed in the substantia nigra of patients with PSP (−62%±4%, −62%±6%, −88%±3%, −76%±3%, and −75%±3% for bands I to IV, respectively) and MSA (−51%±6%, −43%±12%, −77%±6%, −68%±5%, and −59%±7%, respectively), two atypical parkinsonian conditions characterized, in part, by degeneration of nigrostriatal dopamine neurones. The overall loss of nigral VMAT2 protein was slightly higher in PSP (−74%±3%) than in MSA (−63%±6%) or in PD (−63%±3%).
Levels of neuron specific enolase, a control protein, were normal in the putamen, caudate, and substantia nigra of patients with PD (Figures 3A and 3B). However, neuron specific enolase levels in the substantia nigra were significantly decreased in patients with PSP (−47%) and MSA (−33%).
Regional Distribution of Vesicular Monoamine Transporter 2 in Normal Human Brain
A total of 37 brain regions covering the entire human forebrain and including both primarily dopaminergic and nondopaminergic areas were examined (see Figure 4 for representative immunoblots). It should be mentioned that with higher loading of protein, additional protein bands including some high MW bands and two sharp bands immediately above and below the 35-kDa VMAT2 band IV could be observed with the polyclonal antibody, particularly in thalamus and cerebral cortices. However, these protein bands appeared to be nonspecific reaction products as, unlike VMAT2 proteins, they were enriched in the soluble fraction. All four or five VMAT2 protein bands were measured quantitatively and normalized against putamen, the brain region with highest levels of VMAT2. As shown in Figure 5, the regionally normalized data for levels of mature VMAT2 (band I), intact VMAT2 (bands I+II, including both IIa and IIb), and total VMAT2 were not significantly different (region × measure: F72, 540=0.095, P=1.0), suggesting that the regional difference (F36, 540=90.3, P<0.0001) in the levels of the different VMAT2 bands were proportional (except for band IIb in substantia nigra; see above). Therefore, in the following description of the results, the regional pattern of total VMAT2 versus that of the individual species was highly similar.
Figure 5.
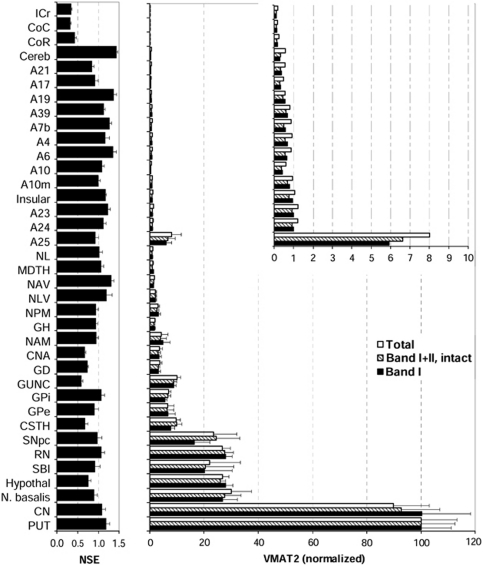
Regional brain distribution of vesicular monoamine transporter 2 (VMAT2) in normal human brain (n=6). Shown are normalized levels (mean±s.e.m.) of total (bands I–IV), intact (bands I–IIa/b), and mature (band I only) VMAT2 against the respective levels in putamen, the brain region with the highest levels of VMAT2 proteins. The inset shows enlarged graph of the cerebral and cerebellar cortical regions and white matters. Also shown are regional levels (in μg std/μg sample) of the control protein neuron specific enolase (NSE). See Figure 4 for abbreviations. Note that levels of VMAT2 in frontal (A10, A10m, A6, and A4), temporal (A21), parietal (A7b and A39), and occipital (A17 and A19) cerebral cortical areas and in cerebellar cortex are only a small fraction of those in the VMAT2-rich caudate and putamen.
The brain gray matter regions could be separated into four groups according to VMAT2 protein levels. The highest levels of VMAT2 were in the striatum and were at least threefold higher than that of any other brain region. Concentrations of VMAT2 in putamen were somewhat higher than in caudate although the difference was not statistically significant. The basal forebrain regions including nucleus basalis, hypothalamus, and substantia innominata and the midbrain regions substantia nigra pars compacta and red nucleus had moderate concentrations of VMAT2 that were 20% to 30% of that of putamen. The other subcortical brain structures together with a few allocortical/archicortical areas, including the basal ganglia output regions subthalamic nucleus, globus pallidus external (GPe) and GPi, the medial temporal lobe regions gyrus of uncus, dentate gyrus, Ammon's horn, amygdala, and hippocampal gyrus, the thalamic subregions medial pulvinar, lateral ventral, anterior ventral, mediodorsal, and lateralis, and the parolfactory cortical area A25 contained low levels of VMAT2 (1% to 10% of putamen).
All of the cerebral neocortical areas and cerebellar cortex had a level of mature or intact VMAT2 that was <1% of that of the striatum (Figure 5, inset). No statistically significant difference was observed among these brain regions, in particular between cerebellar and occipital cortex, although there were trends for the cingulate (A24 and A23) and insular cortices to have relatively higher levels of VMAT2 than other neocortical regions. The cerebral white matters, including the corpus callosum and internal capsule, had the lowest levels of VMAT2 (<0.25% of putamen) that were barely detectable.
In contrast to the above (Figure 5), the house-keeping protein neuron specific enolase had a different brain distribution, with levels quite homogenous across the gray matter regions but, as expected, low in white matter, a finding consistent with our previous reports (Kish et al, 2005).
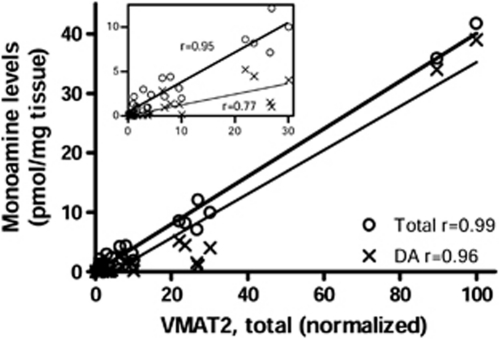
Given that VMAT2 is localized to dopamine, noradrenaline, and serotonin neurons, we reasoned that VMAT2 protein levels in the different brain areas (having variable dopamine–noradrenaline–serotonin ratios) might show a good correlation with the sum of the concentration of the three neurotransmitters. Using regional brain monoamine neurotransmitter data reported in our previous studies (a total of 27 brain regions; Tong et al, 2007), we found a positive correlation (Pearson) between protein levels of VMAT2 and tissue concentrations of total monoamines neurotransmitters (dopamine, noradrenaline, and serotonin; r=0.99 or 0.95, with or without the two striatal outlier points, respectively; P<0.0001). This correlation was not as strong using dopamine concentrations alone (r=0.96 or 0.77, with or without the two striatal outlier points, respectively; Figure 6).
Figure 6.
Correlation (Pearson) between levels of vesicular monoamine transporter 2 (VMAT2) protein and tissue concentrations of total monoamines (dopamine, noradrenaline, and serotonin) or dopamine (DA) alone among brain regions examined (n=27). The inset shows correlations after excluding the two striatal outlier points (putamen and caudate).
In our samples of normal subjects (n=10 to 16), we did not observe any significant correlation (Pearson) between the levels of VMAT2 protein and the age of the subjects (47 to 80 years) in the putamen (r=−0.32, P=0.36), caudate (r=−0.14, P=0.71), or substantia nigra (r=0.21, P=0.44).
Discussion
To our knowledge, this is the first detailed quantitative examination of VMAT2-immunoreactive species in different human brain regions. The major finding from a practical neuroimaging perspective is that the levels of VMAT2 protein in the cerebellar cortex and the widespread cerebral neocortical areas were all at least 100-fold lower than those in striatum. This supports the usefulness of these areas as potential reference regions for PET imaging of VMAT2 in the human. We also found that the human substantia nigra was different in VMAT2 immunoreactivity from the dopamine terminal regions by containing a distinct dominant 52 kDa band IIb that was preferentially lost in parkinsonian conditions.
The multiple bands (I, IIa, IIb, III, IV) of VMAT2 detected by the commonly used C-terminus antibodies (AB1767 used in this study and another similar antibody AB1598P from Chemicon, initially developed by Erickson et al (1996) and Edwards and colleagues (Peter et al, 1995), respectively) and their uncertain identity have been a major challenge for quantitative studies of VMAT2 protein in the brain. Many studies thus chose to examine only the major band detected (Sandoval et al, 2002; Haycock et al, 2003; Little et al, 2003; Gonzalez-Hernandez et al, 2004; Fortune and Lurie, 2009), which in most cases has been the mature band I (Wang et al, 1997; Miller et al, 1999; Haycock et al, 2003; Little et al, 2003; Gonzalez-Hernandez et al, 2004; Jassen et al, 2005; Cruz-Muros et al, 2008) although in a few instances a 55-kDa band was found to be the major band in rodent brains (Holtje et al, 2003; Duchemin et al, 2009; Fortune and Lurie, 2009). The reason for the inconsistencies is not clear but could arise from differential sample preparation, particular Western blot conditions and antibody used, and/or the batch-to-batch variance of the antibodies. We have used four batches of the same version (AB1767) of the rabbit polyclonal antibodies against the C-terminus 19 amino acids of human VMAT2 from different commercial sources and have obtained the same results as observed by most of the studies, that is, the 75-kDa band I was dominant but accounted for only ∼50% of total VMAT2 immunoreactivity in the striatum.
In the present study, by using a newly available monoclonal antibody, by examining multiple brain regions, and by including brain of patients with degenerative parkinsonism, we established that (1) the minor smaller bands III and IV are most likely N-terminal truncated forms of VMAT2; (2) the banding pattern described above in the striatum (I, IIa, III, IV) also applies to most of the monoamine terminal fields except in the nigral dopamine cell body region (and its close proximity regions including red nucleus, subthalamic nucleus, and GPi), in which a band (IIb) of slightly smaller MW than that of band IIa could be detected; and (3) the overall extent of loss of VMAT2 immunoreactivity in the brain regions of patients with PD was correlated with the expected degree of dopaminergic neuronal damage (putamen>caudate>substantia nigra). An exception is band IIb in substantia nigra, with the extent of loss similar to that observed in the terminal regions caudate and putamen. This interesting finding, together with the limited distribution of band IIb in human brain that might be related to the distance from the dopamine cell body (e.g., GPi versus GPe), suggest that VMAT2 IIb might be the precursor to IIa and I being posttranslationally modified and transported to the terminals. However, we cannot exclude the possibility that bands I/IIa/b might represent the transporter associated with different monoaminergic neurons or different subcellular structures, for example, small synaptic vesicles versus large dense core vesicles (Nirenberg et al, 1996). The lack of an aging change of VMAT2 protein in the nigrostriatal system in our limited sample size and within a limited age span is nonetheless consistent with a previous Western blotting study (Haycock et al, 2003) and a recent PET VMAT2 imaging report (Troiano et al, 2010) although the question of the influence of aging on striatal VMAT2 in the human is still controversial (cf. Troiano et al, 2010).
Notwithstanding the generic limitation of Western blot investigations that bands with different MW could well be differentially transferred and detected, we attempted to measure the levels of total, intact, and mature VMAT2 proteins. As the tetrabenazine-binding site on VMAT2 has been mapped to the juxtaposed N- and C-terminals (Sievert and Ruoho, 1997), in particular a conserved phenylalanine (F135 of hVMAT2) in the second transmembrane domain (Gros and Schuldiner, 2010) and glycosylation does not appear to influence the transporter's activity and binding of tetrabenazine (Yelin et al, 1998), the VMAT2 proteins that are capable of binding DTBZ might include all the intact species, that is, I, IIa, and IIb, but not the N-terminally truncated III and IV. However, since the distribution patterns of total, intact, and mature VMAT2 immunoreactivity were all similar, possibly differential DTBZ-binding capacity of the different VMAT2 species should reasonably not affect the regional pattern of DTBZ binding (except, possibly, in the substantia nigra due to the unique abundance of VMAT2 IIb). Indeed, the overall brain distribution of VMAT2 proteins was generally consistent with in vivo PET imaging data with (+)-[11C]DTBZ (Koeppe et al, 1999) and with findings of the in vitro radioligand-binding study of autopsied human brain by [3H]DTBZ (Scherman et al, 1988), showing striatum≫substantia nigra (midbrain), hypothalamus>globus pallidus, hippocampus, thalamus>cerebral cortices (occipital and cerebellar cortices were not measured in the Scherman study).
However, one notable difference between the results of our in vitro measurement of VMAT2 protein and the in vivo PET imaging of [11C]DTBZ binding was in cerebellum. Whereas we did not observe any difference in VMAT2 protein levels between the cerebellar cortex and the cerebral neocortices, PET imaging detects small but consistently higher binding in the cerebellum or cerebellar cortex (Kilbourn et al, 1993; Chan et al, 1999; Koeppe et al, 1999). Therefore, the cerebral cortical regions, in particular the occipital cortex rather than cerebellar cortex, have been the preferred reference region for PET VMAT2 imaging. One possible explanation for the in vitro versus in vivo ‘discrepancy' is that VMAT2 protein levels determined in autopsied brains might not correspond to that available for binding of the radioligand in vivo due to a variety of uncertain factors including differential subcellular localization and posttranslational modification status of the transporter, for example, phosphorylation. Possible differences in nonspecific binding profiles of the tracer among different brain regions could also be a contributing factor. Alternatively, differences in VMAT2 binding between cerebellar and cerebral cortices as assessed by PET imaging might be explained by other imaging variables, for example, partial volume effects. The cerebral and cerebellar cortices are quite different in anatomical structure, with the former having a much smaller surface area/volume ratio and lower percentage of surface area hidden within fissures than the latter (Henery and Mayhew, 1989), which could cause more marked partial volume effects and artificially lower binding in cerebral versus cerebellar cortices. In this regard, previous PET studies of [11C]flumazenil (Aston et al, 2002) and [11C]-arachidonic acid (Giovacchini et al, 2004) brain uptake have disclosed a larger effect of partial volume correction in most regions of the cerebral cortices than in the cerebellar cortex.
In conclusion, although no cerebral gray matter region in the human brain is free of VMAT2 protein, levels of the transporter in the cerebral and cerebellar cortex were sufficiently low to satisfy this criterion as a reference region in PET VMAT2 imaging. We also found that the cerebellar cortex and the (now preferred reference region) occipital cortex were not different in VMAT2 protein concentrations. Differences in PET VMAT2 binding between the two regions might in this respect provide an example of bias caused by imaging variables such as partial volume effects in the reference region.
The authors declare no conflict of interest.
Footnotes
This study was supported by USA NIH NIDA DA025096, the Friedman MSA Fund, the William S Storey and Al Silverberg PSP Funds, the CAMH PSP Fund, and the Abe Memorial Fund.
References
- Aston JA, Cunningham VJ, Asselin MC, Hammers A, Evans AC, Gunn RN. Positron emission tomography partial volume correction: estimation and algorithms. J Cereb Blood Flow Metab. 2002;22:1019–1034. doi: 10.1097/00004647-200208000-00014. [DOI] [PubMed] [Google Scholar]
- Boileau I, Houle S, Rusjan PM, Furukawa Y, Wilkins D, Tong J, Selby P, Wilson AA, Kish SJ. Influence of a low dose of amphetamine on vesicular monoamine transporter binding: a PET (+)[11C]DTBZ study in humans. Synapse. 2010;64:417–420. doi: 10.1002/syn.20743. [DOI] [PubMed] [Google Scholar]
- Boileau I, Rusjan P, Houle S, Wilkins D, Tong J, Selby P, Guttman M, Saint-Cyr JA, Wilson AA, Kish SJ. Increased vesicular monoamine transporter binding during early abstinence in human methamphetamine users: is VMAT2 a stable dopamine neuron biomarker. J Neurosci. 2008;28:9850–9856. doi: 10.1523/JNEUROSCI.3008-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks DJ, Frey KA, Marek KL, Oakes D, Paty D, Prentice R, Shults CW, Stoessl AJ. Assessment of neuroimaging techniques as biomarkers of the progression of Parkinson's disease. Exp Neurol. 2003;184 (Suppl 1:S68–S79. doi: 10.1016/j.expneurol.2003.08.008. [DOI] [PubMed] [Google Scholar]
- Chan GL, Holden JE, Stoessl AJ, Samii A, Doudet DJ, Dobko T, Morrison KS, Adam M, Schulzer M, Calne DB, Ruth TJ. Reproducibility studies with 11C-DTBZ, a monoamine vesicular transporter inhibitor in healthy human subjects. J Nucl Med. 1999;40:283–289. [PubMed] [Google Scholar]
- Cruz-Muros I, Afonso-Oramas D, Abreu P, Rodriguez M, Gonzalez MC, Gonzalez-Hernandez T. Deglycosylation and subcellular redistribution of VMAT2 in the mesostriatal system during normal aging. Neurobiol Aging. 2008;29:1702–1711. doi: 10.1016/j.neurobiolaging.2007.04.003. [DOI] [PubMed] [Google Scholar]
- Darchen F, Masuo Y, Vial M, Rostene W, Scherman D. Quantitative autoradiography of the rat brain vesicular monoamine transporter using the binding of [3H]dihydrotetrabenazine and 7-amino-8-[125I]iodoketanserin. Neuroscience. 1989;33:341–349. doi: 10.1016/0306-4522(89)90214-5. [DOI] [PubMed] [Google Scholar]
- Duchemin AM, Zhang H, Neff NH, Hadjiconstantinou M. Increased expression of VMAT2 in dopaminergic neurons during nicotine withdrawal. Neurosci Lett. 2009;467:182–186. doi: 10.1016/j.neulet.2009.10.038. [DOI] [PubMed] [Google Scholar]
- Erickson JD, Schafer MK, Bonner TI, Eiden LE, Weihe E. Distinct pharmacological properties and distribution in neurons and endocrine cells of two isoforms of the human vesicular monoamine transporter. Proc Natl Acad Sci USA. 1996;93:5166–5171. doi: 10.1073/pnas.93.10.5166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortune T, Lurie DI. Chronic low-level lead exposure affects the monoaminergic system in the mouse superior olivary complex. J Comp Neurol. 2009;513:542–558. doi: 10.1002/cne.21978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey KA, Koeppe RA, Kilbourn MR, Vander Borght TM, Albin RL, Gilman S, Kuhl DE. Presynaptic monoaminergic vesicles in Parkinson's disease and normal aging. Ann Neurol. 1996;40:873–884. doi: 10.1002/ana.410400609. [DOI] [PubMed] [Google Scholar]
- Gainetdinov RR, Fumagalli F, Wang YM, Jones SR, Levey AI, Miller GW, Caron MG. Increased MPTP neurotoxicity in vesicular monoamine transporter 2 heterozygote knockout mice. J Neurochem. 1998;70:1973–1978. doi: 10.1046/j.1471-4159.1998.70051973.x. [DOI] [PubMed] [Google Scholar]
- Gilman S, Frey KA, Koeppe RA, Junck L, Little R, Vander Borght TM, Lohman M, Martorello S, Lee LC, Jewett DM, Kilbourn MR. Decreased striatal monoaminergic terminals in olivopontocerebellar atrophy and multiple system atrophy demonstrated with positron emission tomography. Ann Neurol. 1996;40:885–892. doi: 10.1002/ana.410400610. [DOI] [PubMed] [Google Scholar]
- Gilman S, Koeppe RA, Adams KM, Junck L, Kluin KJ, Johnson-Greene D, Martorello S, Heumann M, Bandekar R. Decreased striatal monoaminergic terminals in severe chronic alcoholism demonstrated with (+)[11C]dihydrotetrabenazine and positron emission tomography. Ann Neurol. 1998;44:326–333. doi: 10.1002/ana.410440307. [DOI] [PubMed] [Google Scholar]
- Gilman S, Koeppe RA, Junck L, Little R, Kluin KJ, Heumann M, Martorello S, Johanns J. Decreased striatal monoaminergic terminals in multiple system atrophy detected with positron emission tomography. Ann Neurol. 1999;45:769–777. doi: 10.1002/1531-8249(199906)45:6<769::aid-ana11>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Giovacchini G, Lerner A, Toczek MT, Fraser C, Ma K, DeMar JC, Herscovitch P, Eckelman WC, Rapoport SI, Carson RE. Brain incorporation of 11C-arachidonic acid, blood volume, and blood flow in healthy aging: a study with partial-volume correction. J Nucl Med. 2004;45:1471–1479. [PubMed] [Google Scholar]
- Gonzalez-Hernandez T, Barroso-Chinea P, De La Cruz Muros I, Del Mar Perez-Delgado M, Rodriguez M. Expression of dopamine and vesicular monoamine transporters and differential vulnerability of mesostriatal dopaminergic neurons. J Comp Neurol. 2004;479:198–215. doi: 10.1002/cne.20323. [DOI] [PubMed] [Google Scholar]
- Gros Y, Schuldiner S. Directed evolution reveals hidden properties of VMAT, a neurotransmitter transporter. J Biol Chem. 2010;285:5076–5084. doi: 10.1074/jbc.M109.081216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haycock JW, Becker L, Ang L, Furukawa Y, Hornykiewicz O, Kish SJ. Marked disparity between age-related changes in dopamine and other presynaptic dopaminergic markers in human striatum. J Neurochem. 2003;87:574–585. doi: 10.1046/j.1471-4159.2003.02017.x. [DOI] [PubMed] [Google Scholar]
- Henery CC, Mayhew TM. The cerebrum and cerebellum of the fixed human brain: efficient and unbiased estimates of volumes and cortical surface areas. J Anat. 1989;167:167–180. [PMC free article] [PubMed] [Google Scholar]
- Holtje M, Winter S, Walther D, Pahner I, Hortnagl H, Ottersen OP, Bader M, Ahnert-Hilger G. The vesicular monoamine content regulates VMAT2 activity through Galphaq in mouse platelets. Evidence for autoregulation of vesicular transmitter uptake. J Biol Chem. 2003;278:15850–15858. doi: 10.1074/jbc.M212816200. [DOI] [PubMed] [Google Scholar]
- Hornykiewicz O. Biochemical aspects of Parkinson's disease. Neurology. 1998;51:S2–S9. doi: 10.1212/wnl.51.2_suppl_2.s2. [DOI] [PubMed] [Google Scholar]
- Jassen AK, Brown JM, Panas HN, Miller GM, Xiao D, Madras BK. Variants of the primate vesicular monoamine transporter-2. Brain Res Mol Brain Res. 2005;139:251–257. doi: 10.1016/j.molbrainres.2005.05.028. [DOI] [PubMed] [Google Scholar]
- Kilbourn MR, Butch ER, Desmond T, Sherman P, Harris PE, Frey KA. In vivo [11C]dihydrotetrabenazine binding in rat striatum: sensitivity to dopamine concentrations. Nucl Med Biol. 2010;37:3–8. doi: 10.1016/j.nucmedbio.2009.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilbourn MR, DaSilva JN, Frey KA, Koeppe RA, Kuhl DE. In vivo imaging of vesicular monoamine transporters in human brain using [11C]tetrabenazine and positron emission tomography. J Neurochem. 1993;60:2315–2318. doi: 10.1111/j.1471-4159.1993.tb03521.x. [DOI] [PubMed] [Google Scholar]
- Kilbourn MR, Hockley B, Lee L, Hou C, Goswami R, Ponde DE, Kung MP, Kung HF. Pharmacokinetics of [(18)F]fluoroalkyl derivatives of dihydrotetrabenazine in rat and monkey brain. Nucl Med Biol. 2007;34:233–237. doi: 10.1016/j.nucmedbio.2007.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kish SJ, Furukawa Y, Chang LJ, Tong J, Ginovart N, Wilson A, Houle S, Meyer JH. Regional distribution of serotonin transporter protein in postmortem human brain: is the cerebellum a SERT-free brain region. Nucl Med Biol. 2005;32:123–128. doi: 10.1016/j.nucmedbio.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Koeppe RA, Frey KA, Kuhl DE, Kilbourn MR. Assessment of extrastriatal vesicular monoamine transporter binding site density using stereoisomers of [11C]dihydrotetrabenazine. J Cereb Blood Flow Metab. 1999;19:1376–1384. doi: 10.1097/00004647-199912000-00011. [DOI] [PubMed] [Google Scholar]
- Little KY, Krolewski DM, Zhang L, Cassin BJ. Loss of striatal vesicular monoamine transporter protein (VMAT2) in human cocaine users. Am J Psychiatry. 2003;160:47–55. doi: 10.1176/appi.ajp.160.1.47. [DOI] [PubMed] [Google Scholar]
- Miller GW, Erickson JD, Perez JT, Penland SN, Mash DC, Rye DB, Levey AI. Immunochemical analysis of vesicular monoamine transporter (VMAT2) protein in Parkinson's disease. Exp Neurol. 1999;156:138–148. doi: 10.1006/exnr.1998.7008. [DOI] [PubMed] [Google Scholar]
- Nirenberg MJ, Chan J, Liu Y, Edwards RH, Pickel VM. Ultrastructural localization of the vesicular monoamine transporter-2 in midbrain dopaminergic neurons: potential sites for somatodendritic storage and release of dopamine. J Neurosci. 1996;16:4135–4145. doi: 10.1523/JNEUROSCI.16-13-04135.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamura N, Villemagne VL, Drago J, Pejoska S, Dhamija RK, Mulligan RS, Ellis JR, Ackermann U, O'Keefe G, Jones G, Kung HF, Pontecorvo MJ, Skovronsky D, Rowe CC. In vivo measurement of vesicular monoamine transporter type 2 density in Parkinson disease with (18)F-AV-133. J Nucl Med. 2010;51:223–228. doi: 10.2967/jnumed.109.070094. [DOI] [PubMed] [Google Scholar]
- Peter D, Liu Y, Sternini C, de Giorgio R, Brecha N, Edwards RH. Differential expression of two vesicular monoamine transporters. J Neurosci. 1995;15:6179–6188. doi: 10.1523/JNEUROSCI.15-09-06179.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandoval V, Riddle EL, Hanson GR, Fleckenstein AE. Methylphenidate redistributes vesicular monoamine transporter-2: role of dopamine receptors. J Neurosci. 2002;22:8705–8710. doi: 10.1523/JNEUROSCI.22-19-08705.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherman D, Raisman R, Ploska A, Agid Y. [3H]dihydrotetrabenazine, a new in vitro monoaminergic probe for human brain. J Neurochem. 1988;50:1131–1136. doi: 10.1111/j.1471-4159.1988.tb10583.x. [DOI] [PubMed] [Google Scholar]
- Sievert MK, Ruoho AE. Peptide mapping of the [125I]Iodoazidoketanserin and [125I]2-N-[(3′-iodo-4′-azidophenyl)propionyl]tetrabenazine binding sites for the synaptic vesicle monoamine transporter. J Biol Chem. 1997;272:26049–26055. doi: 10.1074/jbc.272.41.26049. [DOI] [PubMed] [Google Scholar]
- Tong J, Hornykiewicz O, Furukawa Y, Kish SJ. Marked dissociation between high noradrenaline versus low noradrenaline transporter levels in human nucleus accumbens. J Neurochem. 2007;102:1691–1702. doi: 10.1111/j.1471-4159.2007.04636.x. [DOI] [PubMed] [Google Scholar]
- Tong J, Wilson AA, Boileau I, Houle S, Kish SJ. Dopamine modulating drugs influence striatal (+)-[11C]DTBZ binding in rats: VMAT2 binding is sensitive to changes in vesicular dopamine concentration. Synapse. 2008;62:873–876. doi: 10.1002/syn.20573. [DOI] [PubMed] [Google Scholar]
- Tong J, Wong H, Guttman M, Ang LC, Forno LS, Shimadzu M, Rajput AH, Muenter MD, Kish SJ, Hornykiewicz O, Furukawa Y. Brain alpha-synuclein accumulation in multiple system atrophy, Parkinson's disease and progressive supranuclear palsy: a comparative investigation. Brain. 2010;133:172–188. doi: 10.1093/brain/awp282. [DOI] [PubMed] [Google Scholar]
- Troiano AR, Schulzer M, de la Fuente-Fernandez R, Mak E, McKenzie J, Sossi V, McCormick S, Ruth TJ, Stoessl AJ. Dopamine transporter PET in normal aging: dopamine transporter decline and its possible role in preservation of motor function. Synapse. 2010;64:146–151. doi: 10.1002/syn.20708. [DOI] [PubMed] [Google Scholar]
- Vander Borght TM, Sima AA, Kilbourn MR, Desmond TJ, Kuhl DE, Frey KA. [3H]methoxytetrabenazine: a high specific activity ligand for estimating monoaminergic neuronal integrity. Neuroscience. 1995;68:955–962. doi: 10.1016/0306-4522(95)00167-h. [DOI] [PubMed] [Google Scholar]
- Wang YM, Gainetdinov RR, Fumagalli F, Xu F, Jones SR, Bock CB, Miller GW, Wightman RM, Caron MG. Knockout of the vesicular monoamine transporter 2 gene results in neonatal death and supersensitivity to cocaine and amphetamine. Neuron. 1997;19:1285–1296. doi: 10.1016/s0896-6273(00)80419-5. [DOI] [PubMed] [Google Scholar]
- Wilson JM, Levey AI, Rajput A, Ang L, Guttman M, Shannak K, Niznik HB, Hornykiewicz O, Pifl C, Kish SJ. Differential changes in neurochemical markers of striatal dopamine nerve terminals in idiopathic Parkinson's disease. Neurology. 1996;47:718–726. doi: 10.1212/wnl.47.3.718. [DOI] [PubMed] [Google Scholar]
- Yelin R, Steiner-Mordoch S, Aroeti B, Schuldiner S. Glycosylation of a vesicular monoamine transporter: a mutation in a conserved proline residue affects the activity, glycosylation, and localization of the transporter. J Neurochem. 1998;71:2518–2527. doi: 10.1046/j.1471-4159.1998.71062518.x. [DOI] [PubMed] [Google Scholar]