Abstract
The spatiotemporal dynamics of postischemic hyperperfusion (HP) remains incompletely understood. Diffusion, perfusion, T2, T1, angiographic, dynamic susceptibility-contrast magnetic resonance imaging (MRI) and magnetic resonance angiography were acquired longitudinally at multiple time points up to 7 days after stroke in rats subjected to 30-, 60-, and 90-minutes middle cerebral artery occlusion (MCAO). The spatiotemporal dynamics of postischemic HP was analyzed and compared with T1, T2 and blood–brain barrier (BBB) changes. No early HP within 3 hours after recanalization was observed. Late (⩾12 hours) HP was present in all animals of the 30-minute MCAO group (N=20), half of the animals in the 60-minute MCAO group (N=8), and absent in the 90-minute MCAO group (N=9). Dynamic susceptibility-contrast MRI and magnetic resonance angiography corroborated HP. Hyperperfusion preceded T2 increase in some animals, but HP and T2 changes temporally coincided in others. T2 peaked first at 24 hours whereas HP peaked at 48 hours after occlusion, and HP resolved by day 7 in most animals at which point the arteries became tortuous. Pixel-by-pixel tracking analysis showed that tissue did not infarct (migrated from core or mismatch at 30 minutes to normal at 48 hours) showed normal cerebral blood flow (CBF), whereas infarct tissue (migrated from core or mismatch at 30 minutes to infarct at 48 hours) showed exaggerated CBF, indicating that HP was associated with poor outcome.
Keywords: ADC, arterial spin labeling, CBF, cerebral ischemia, dynamic susceptibility-contrast, relaxation time
Introduction
Postischemic hyperperfusion (HP)—also known as ‘luxury perfusion' or ‘hyperemia' where blood flow exceeds metabolic needs in the brain—has long been documented (Lassen, 1966) to be a frequent, yet poorly understood, phenomenon. Hyperperfusion has been studied using positron emission tomography and magnetic resonance imaging (MRI) techniques in animal stroke models (Heiss et al, 1997; Kastrup et al, 1999) and stroke patients (Kidwell et al, 2001; Marchal et al, 1996a).
Early postischemic HP sometimes observed immediately after recanalization is a hallmark of efficient recanalization after stroke (Sundt et al, 1969; Tasdemiroglu et al, 1992) and it has been reported to be both beneficial (i.e., salvage tissue in and around the ischemic zone or prevent infarct growth) (Marchal et al, 1996b) and harmful (i.e., aggravate edema and hemorrhage, and neuronal damage from reperfusion injury) (Pan et al, 2007; Schaller and Graf, 2004). By contrast, late postischemic HP (48 hours after onset) is often associated with tissue necrosis (Ackerman et al, 1981; Baron et al, 1981, 1983; Tran Dinh et al, 1997). Many studies have investigated the mechanisms underlying HP. Accumulated by-products (such as free radicals) could result in delayed neuronal death as well as production of vasoactive metabolites (such as lactic acid and adenosine) that could induce vasodilation through relaxation of vascular smooth muscle (Berne and Rubio, 1974; Kontos and Wei, 1985). Some of these metabolites are implicated in modulating blood–brain barrier (BBB) permeability (Joo, 1986), potentially enhancing cerebral edema. Others have suggested neurogenic vasodilation (Macfarlane et al, 1991) and passive physiological coupling (Marchal et al, 1996b). Histopathological investigation of stroke cat showed that late HP in the necrotic core could in part reflects neovascularization with increased capillary density and endothelial hypertrophy (Yamaguchi, 1977). However, the underlying spatiotemporal characteristics of postischemic HP and its progression with respect to other imaging markers (such as T1, T2, diffusion and contrast-enhanced MRI) remain incompletely understood. Most published noninvasive longitudinal studies to characterize HP had limited time points and terminal studies at different time points were confounded by intersubject variations. Improved understanding of the HP spatiotemporal characteristics with respect to other imaging markers could lead to better understanding of stroke pathophysiology, which could ultimately improve clinical stroke management.
The goal of this study was to longitudinally investigate the spatiotemporal dynamics of late HP in same animals subjected to 30, 60, and 90 minutes intraluminal middle cerebral artery occlusion (MCAO) in rats. Multiparametric MRI data including diffusion, perfusion, T2, T1, dynamic susceptibility-contrast (DSC) MRI and magnetic resonance angiography (MRA) were acquired longitudinally at multiple time points up to 7 days after stroke. The spatiotemporal progression of HP was compared with T1, T2, diffusion, angiographic, and BBB changes.
Materials and methods
Animal Preparation
All experimental procedures were approved by the Institutional of Animal Care and Utilization Committee, UT Health Science Center at San Antonio. Thirty-seven male Sprague-Dawley rats (250 to 350 g, Taconic Farms, NY, USA) were anesthetized with 2% isoflurane in air during surgery. Transient focal brain ischemia of the right hemisphere was induced by intraluminal MCAO (Shen et al, 2003, 2004a). Rats were secured in a supine position using an magnetic resonance compatible rat stereotaxic headset, anesthesia was reduced to 1.2% to 1.3% isoflurane and maintained throughout MRI. Magnetic resonance imaging was performed during occlusion and animal was slided out on the rail to withdraw the occluder while the animal was in the holder at 30 minutes (n=20), 60 minutes (n=8), or 90 minutes (n=9) after occlusion. The animal was repositioned for additional MRI.
For the 30-minute MCAO group, quantitative cerebral blood flow (CBF) and apparent diffusion coefficient (ADC) were acquired at 30, 40 (immediately after recanalization), 60, 90, 120, and 180 minutes, 12 hours (two rats only for this time point), and 24 hours after occlusion. Quantitative T2 was also acquired at 24 hours after occlusion. In 10 of the 20 rats, T1 map, MRA, and dynamic susceptibility-contrast MRI were also acquired at 1, 2, 3, and 7 days after stroke. T1 maps were obtained before and after intravenous injection of Gd_DTPA via the tail vein. T1 maps were acquired within 15 minutes after intravenous Gd_DTPA injection. Cerebral blood flow functional MRI of hypercapnic challenge was performed on two rats 48 hours after occlusion.
For the 60-minute MCAO group, quantitative CBF and ADC were acquired at 30, 60, 70 (immediately after recanalization), 90, 120, and 180 minutes, and again at 24 hours after occlusion.
For the 90-minute MCAO group, quantitative CBF and ADC were acquired at 30, 60, 90, 100 (immediately after recanalization), 120, and 180 minutes, and again at 24 hours after occlusion.
Rats breathed spontaneously throughout. Rectal temperature was maintained at 37.0±0.5°C. Heart rate and arterial oxygenation saturation (SpO2) were recorded continuously using MouseOx system (STARR Life Science Corp., Oakmont, PA, USA) onto a computer via the Biopac system (Santa Barbara, CA, USA). Respiration rate was derived from chest motion via a force transducer (SA Instruments, Inc., Stony Brook, NY, USA). All recorded physiological parameters were within normal physiological ranges.
Magnetic Resonance Experiments
Magnetic resonance imaging experiments were performed on a 7-T/40-cm magnet, a Biospec Bruker console (Billerica, MA, USA), and a 40-G/cm gradient insert (ID=12 cm, 120 μs rise time). A surface coil (2.3 cm ID) was used for brain imaging and a neck coil (Duong et al, 2000) for perfusion labeling. Coil-to-coil electromagnetic interaction was actively decoupled.
ADC
Diffusion-weighted images were acquired with gradients separately applied along the x, y, or z direction using single-shot, spin-echo echo-planar images with matrix=64 × 64 or 96 × 96 with 5/8 partial Fourier acquisition and reconstructed to 128 × 128, TR (repetition time)=3 seconds (90° flip angle), TE (echo time)=37 ms, b values of 4 and 1,200 s/mm2, Δ (time between two diffusion gradients)=17.53 ms, δ (diffusion gradient duration)=5.6 ms, 2.56 × 2.56 cm2 FOV (field of view), seven 1.5-mm thick slices, and 16 averages.
Basal Cerebral Blood Flow
Basal CBF (air inhalation) was measured using the continuous arterial spin-labeling (cASL) technique with single-shot, gradient-echo, echo-planar image acquisition (Shen et al, 2005). Continuous arterial spin-labeling used a 2.7-s square radiofrequency pulse to the labeling coil. Imaging parameters were matrix=64 × 64 or 96 × 96 with 5/8 partial Fourier acquisition and reconstructed to 128 × 128, FOV=2.56 × 2.56 cm2, TR=3 seconds, TE=14 ms, and seven 1.5-mm thick slices.
T2
T2-weighted images were acquired using fast spin-echo pulse sequence with two effective TEs (50 and 80 ms), TR=2 seconds, FOV=2.56 × 2.56 cm2, matrix=128 × 128, echo train length 8, and 8 signal averages.
Cerebral Blood Flow of Hypercapnic Challenge
Hypercapnic challenge was used to evaluate vascular reactivity at 48 hours after occlusion. Animals breathed 2 minutes of air, 2 minutes of 5% CO2 in air, 4 minutes of air, and 2 minutes of 5% CO2, while cASL CBF were continuously acquired using the parameters for basal CBF above.
T1
T1-weighted images were acquired using single-shot, inversion-recovery gradient-echo echo-planar image sequence with six different inversion delay times (0.025, 0.5, 1, 2, 4, and 8 seconds), matrix=96 × 96 and reconstructed to 128 × 128, TR=12 seconds, and 4 signal averages.
MRA
MRA was acquired using the 3D fast low angle shot (FLASH) sequence with TR/TE/flip angle=15 ms/2.5 ms/20°, FOV=2.56 × 2.56 × 2.56 cm3, matrix=256 × 256 × 256 and zero-fill factor of 2 in the slice direction.
DSC
DSC image was acquired using T2*-weighted single-shot, gradient-echo echo-planar image. Parameters were TR/TE/flip angle=200 ms/15 ms/30°, three central 1.5-mm slices, matrix=64 × 64, and 300 repetitions. A bolus of Gd_DTPA (0.2 mmol/kg) was injected via tail vein around the 60th image (20 seconds).
Data Analysis
Data analysis used codes written in Matlab (MathWorks Inc., Natick, MA, USA) and the STIMULATE software (University of Minnesota). Images among different time points from the same rats were coregistered (Liu et al, 2004). Data were reported as mean±s.d., and all statistical tests used t-test with P<0.05 taken to be statistically significant.
ADC, CBF, and T2 maps were calculated as described previously (Shen et al, 2003, 2004a). A constant T1 of 1.8 seconds was used for CBF calculation in the acute phase, and measured T1 maps were used for CBF calculation in subacute and chronic phase (⩾24 hours). T1 maps were calculated pixel-by-pixel by fitting the data to Si = S0 − 2A e − TIi/T1 where Si is the signal intensity obtained with inversion delay time TIi. T1 map and T1 difference map (T1-diff) between pre- and post-Gd_DTPA were obtained. MRA was derived using maximum intensity projection. Cerebral blood flow was also obtained from DSC data using NordicICE software (NordicNeuroLab, Milwaukee, WI, USA). Blood–brain barrier leakage was evaluated by comparing T1 maps before and after Gd_DTPA injection.
ISODATA automated clustering method (Shen et al, 2004b) was used to classify pixels into normal, mismatch, and core based on the ADC and CBF data before recanalization, and to determine the final lesion volume based on end point T2 MRI data.
Results
Early Hyperperfusion
Recanalization was successful as demonstrated by partial recovery of CBF in all animals. Cerebral blood flow usually improved with time after recanalization but generally did not completely renormalize (Figure 1A). Cerebral blood flow recovery was overall better in the 30-minute groups compared with the 60- and 90-minute MCAO groups. Early postischemic HP immediately (within the first 3 hours) after recanalization was not detected.
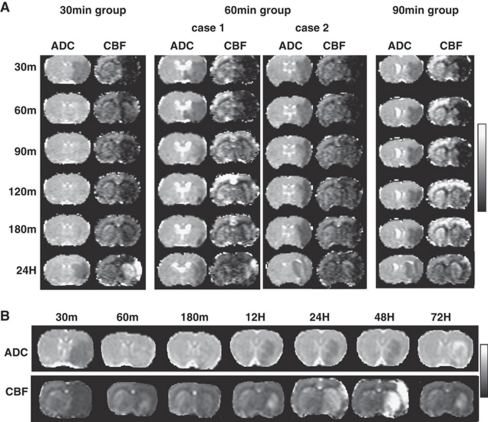
Figure 1.
(A) Spatiotemporal characteristics of ADC and CBF maps of representative rats from the 30-, 60-, and 90-minute middle cerebral artery occlusion (MCAO) groups. For the 60-minute MCAO group, a case with hyperperfusion (HP) and another case without HP are shown. (B) Spatiotemporal characteristics ADC and CBF maps up to 72 hours after occlusion of a representative rat from the 30-minute MCAO group. Display scales are 0 to 1.0 × 10−3 mm2/s for ADC and 0 to 2 mL/g per minute for CBF.
Effects of Occlusion Duration on Late Hyperperfusion
Figure 1A also shows the ADC and CBF maps at different time points from representative animals in the 30-, 60-, and 90-minute MCAO groups. For the 60-minute MCAO group, a case with, and another case without, HP are shown. At 24 hours after occlusion, HP was observed in all rats in the 30-minute MCAO group, four of eight rats in the 60-minute MCAO group, and none in the 90-minute MCAO group. The 90-minute MCAO group was not analyzed further because HP was not detected. In the 60-minute MCAO group, there was no correlation between the incidence of HP and lesion volume (P=0.15, two-side t-test). Subsequent detailed spatiotemporal dynamic analysis was only performed on the 30-minute MCAO group in which HP was observed in all rats.
Temporal Evolution of Late Hyperperfusion
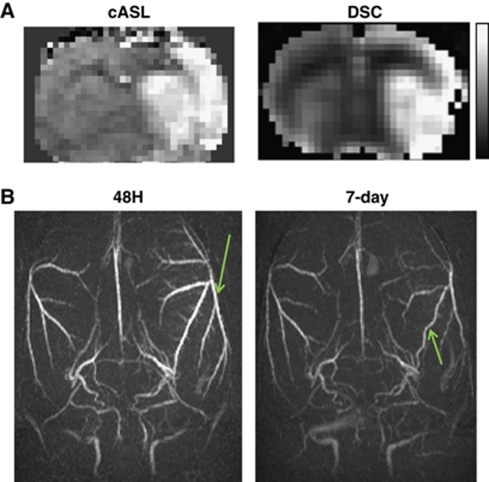
In the 30-minute MCAO group, HP volume was small at 12 hours after occlusion, but grew larger 24 hours after occlusion. The magnitude of HP peaked at 48 hours after occlusion and lasted about a week (Figure 1B). Two additional measurements in the same animals by DSC CBF and MRA independently corroborated late HP. DSC and cASL showed essentially identical HP territories at 48 hours after occlusion (Figure 2A), although quantitative comparison was not performed. MRA at 48 hours after occlusion of the same rat showed thicker and brighter blood vessels with many smaller branches becoming more apparent in the ipsilateral hemisphere compared with the contralateral hemisphere (Figure 2B). MRA on day 7 showed that the ipsilateral arteries had similar size and brightness as the contralateral hemisphere (HP had subsided) but they were tortuous. The vessels were not yet tortuous at 48 or 72 hours (data not shown).
Figure 2.
(A) Cerebral blood flow (CBF) maps obtained by continuous arterial spin-labeling (cASL) and DSC 48 hours after occlusion from the same animal. Display CBF scale is 0 to 2 mL/g per minute. (B) MRA at 48 hours and 7-day after occlusion from the same animal.
Hyperperfusion Correlation with T2, T1, and Blood–Brain Barrier Change
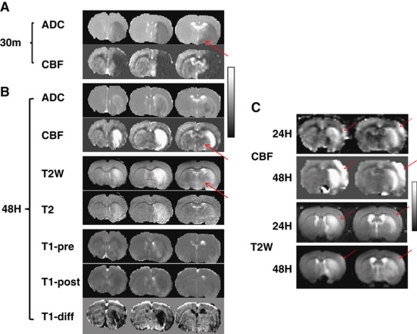
Hyperperfusion regions usually showed increased T2, increased T1 before Gd_DTPA and decreased T1 after Gd_DTPA compared with homologous regions in the contralateral hemisphere (Figure 3A). At 30 minutes after occlusion, there were large areas of abnormal CBF and ADC. Increased T2 and T1 were indicative of edema. T1 changes after Gd_DPTA, as reflected in the T1 difference map, were indicative of BBB leakage. ADC lesion volume at 48 hours was smaller than HP and T2 lesion volumes (Figure 3B) likely because ADC pseudo-normalized. Hyperperfusion region matched well with areas of T2 increase. Hyperperfusion area was larger than the area of BBB leakage at 48 hours, suggesting that the cause of late HP is not exclusively due to BBB leakage. Moreover, HP tissue at 48 hours was mostly localized to ADC lesion defined at 30 minutes after MCAO before recanalization. Tissue that did not infract (no increased T2) due to recanalization did not show HP (arrow in Figure 3A and 3B).
Figure 3.
(A) ADC and CBF maps at 30 minutes, and (B) ADC, CBF, T2-weighted, T2 map, T1 map before and after Gd_DTPA injection (T1-pre and T1-post), and the difference image (T1-diff) 48 hours after occlusion. The animal was subjected to a 30-minutes middle cerebral artery occlusion (MCAO). (C) CBF and T2-W images at 24 and 48 hours after occlusion, showing hyperperfusion (HP) occurring before T2-W hyperintensity. Display scales are 0 to 1 × 10−3 mm2/s for ADC, 0 to 2 mL/g per minute for CBF, 30 to 80 ms for T2, 1,000 to 2,500 ms for T1 and 0 to 100 ms for T1 subtract image. Arrows show tissue did not infract due to recanalization.
In 3 out of 20 rats, HP occurred before T2 increase (Figure 3C). In these three rats, lesion started from striatum and then expanded to cortex, consistent with our previously finding that striatum lesion appeared earlier than cortical lesion in this MCAO model. Reperfusion delayed the cortical infarction but not striatum. At 24 hours after occlusion, HP was clearly visible in the cortex and the striatum, whereas hyperintensive T2-weighted signal was not visible in the cortex until 48 hours after occlusion. Cortical HP region expanded from 24 to 48 hours after occlusion. The remaining 17 rats showed HP temporally coincided with T2 increase.
Normal ADC was (0.72±0.02) × 10−3 mm2/s, CBF was 0.91 × 0.11 mL/g per minute, T2 was 50.5±1.7 ms, and T1 was 1,786±13 ms from the whole contralateral hemisphere. Group-averaged changes in ADC, CBF, T1, and T2 data of the HP pixels at 30 minutes and 48 hours with respect to normal values are summarized in Table 1. Hyperperfusion pixels were defined as pixels with CBF > mean+2 s.d. of normal at 48 hours. At 30 minutes after occlusion, ADC and CBF were abnormally low. At 48 hours after occlusion, ADC pseudo-normalized, T2 was markedly elevated, T1 was significantly elevated precontrast (likely due to mild edema) but significantly decreased postcontrast (likely due to trapped Gd_DTPA).
Table 1. Group-averaged changes in ADC, CBF, T2, and T1 of pixels with CBF (at 48 hours after occlusion) greater than (mean+2 s.d.) of normal (i.e., hyperperfusion pixels).
| % of normal at 30 minutes after MCAO | % of normal at 48 hours after MCAO | ||||||
|---|---|---|---|---|---|---|---|
| ADC | CBF | ADC | CBF | T2 | T1_pre | T1_post | T1_diff |
| 72±4* | 5±3* | 101±8 | 263±22* | 125±3* | 108±6* | 92±4* | 16±5* |
Abbreviations: ADC, apparent diffusion coefficient; CBF, cerebral blood flow; MCAO, middle cerebral artery occlusion.
T1_pre, T1_post, and T1_diff indicate T1 precontrast, postcontrast, and the difference, respectively.
*P<0.05 compared with normal. All except for 48 hours ADC were significantly different from normal values.
Hyperperfusion Correlation with Tissue Fates
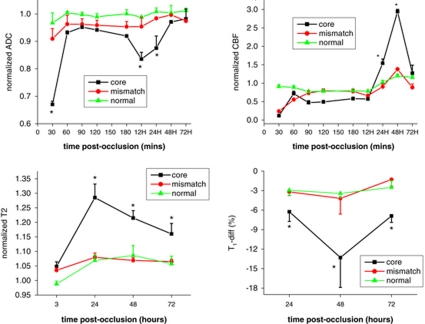
To evaluate the effects of HP on tissue outcome, ‘normal,' ‘mismatch,' and ‘ischemic core' at 30 minutes after occlusion were classified using ISODATA and their ADC, CBF, T2, and T1-diff (T1 difference between pre- and post-Gd_DTPA) values were tracked over time (Figure 4). ADC, CBF, and T2 were normalized to their own normal values in the contralateral left hemisphere. For normal pixels, ADC, CBF, and T2 values were normal with no significant time-dependent changes. Gd-DTPA caused slightly T1 decrease (∼3%) in the normal tissue as expected. For core pixels, ADC decreased at 30 minutes, recovered after recanalization, decreased again and reached a minimum at 12 hours, and pseudo-normalized at 48 to 72 hours. Hyperperfusion peaked at 48 hours after occlusion. T2 contrast peaked strongly at 24 hours after occlusion. T1-difference between precontrast and postcontrast peaked at 48 hours after occlusion. For mismatch pixels, which mostly did not infract, the trends of ADC, CBF, T2, and T1-diff values were similar to normal tissue.
Figure 4.
Temporal evolution of the group-averaged normalized ADC, CBF, T2, and T1-diff values of the normal, mismatch, and core tissue classified at 30 minutes after occlusion. The ADC, CBF, and T2 values were normalized to their own values in the contralateral normal hemisphere. T1-diff values were percentage difference between precontrast and postcontrast. *Indicates statistically significant difference from normal value (P<0.05). All CBF values of core and mismatch tissue between 30 minutes and 48 hours after stroke were statistically different from each other.
Cerebral blood flow values of tissue that did not infract (from core or mismatch at 30 minutes to normal at 48 hours after occlusion) and tissue that infract (from core or mismatch at 30 minutes to infarct at 48 hours after occlusion) were tracked pixel-by-pixel in Figure 5. Tissue that did not infract due to reperfusion did not show late HP and infracted tissue showed late HP.
Figure 5.
Pixel-by-pixel tracking of CBF values of tissue that did not infract (from core or mismatch at 30 minutes to normal at 48 hours after occlusion) and tissue that infracted (from core or mismatch at 30 minutes to infarct at 48 hours after occlusion). *Indicates statistically significant difference from normal value (P<0.05).
Association with Impaired Vascular Reactivity
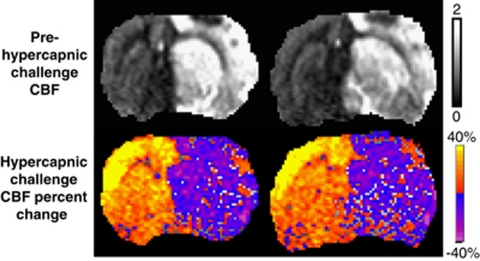
Basal CBF (prehypercapnic challenge) and hypercapnia-induced CBF change of a representative rat 48 hours after stroke are shown in Figure 6. In normal tissue, hypercapnia induced significant CBF increase (P<0.05), indicative of vasodilation. In contrast, in HP tissue, hypercapnia induced a small CBF decrease, suggesting autoregulatory dysfunction.
Figure 6.
Hypercapnic challenge CBF percent-change map and the corresponding prehypercapnic challenge CBF map of a 30-minute middle cerebral artery occlusion (MCAO) rat at 48 hours after occlusion. Display CBF scale is 0 to 2 mL/g per minute.
Discussion
The main findings of this study are (1) early HP within 3 hours of recanalization was not detected in all three MCAO groups; (2) late (⩾12 hours) HP was present consistently in the 30-minute MCAO group, present in half of the animals in the 60-minute MCAO group, and absent in the 90-minute MCAO group; (3) DSC CBF MRI and MRA independently corroborated HP detected by cASL; (4) HP preceded T2 increase in some animals, and HP and T2 changes coincided in others; (5) T2 peaked first at 24 hours whereas HP peaked at 48 hours after occlusion, and HP resolved by day 7 in most animals at which point the arteries also became tortuous; and (6) HP was exclusively associated with poor outcome whereas tissue that was not infracted did not show HP.
Potential Issues with Cerebral Blood Flow Measurement in Ischemia
Stroke could affect tissue T1, arterial transit time, and BBB permeability and thus could affect cASL CBF quantification. Cerebral blood flow maps were calculated using T1 map obtained at each time point, and thus ischemia-induced T1 effect per se on CBF is likely negligible. Under normal conditions, water permeability is limited by BBB (Schwarzbauer et al, 1997). With a disrupted BBB in stroke, water in the blood can extravasate more freely, which could cause overestimation of cASL CBF in stroke and exaggerate HP. The effects of physiological perturbations, stroke and measurements parameters on late HP as measured by cASL and DSC techniques have been evaluated in embolic stroke rats (Tanaka et al, 2010) and they found both cASL and DSC CBF quantification in postischemic HP were affected differently by ischemia-induced changes in T1 and BBB. Cerebral blood flow by cASL in HP tissue was likely overestimated slightly. However, it is clear that the observed postischemic HP herein was not an artifact, as independently corroborated by DSC and MRA.
Early Hyperperfusion
Cerebral blood flow increased significantly after recanalization compared with during occlusion (P<0.05) but did not completely renormalize. No early postischemic HP (i.e., within 3 hours after recanalization) was observed, consistent with some studies (Bardutzky et al, 2007; Jokivarsi et al, 2010). In other studies, early HP was observed (Heiss et al, 1997; Tasdemiroglu et al, 1992). The discrepancy could be due to differences of animal models, duration and severity of ischemia, duration the animal under anesthesia, and recanalization efficiency across different laboratories.
Incidence of Late Hyperperfusion
Late HP was detected in all animals in the 30-minute MCAO group and half of the animals in the 60-minute MCAO group, and none in the 90-minute MCAO group. A likely explanation is that shorter MCAO is less likely to completely damage blood vessels whereas longer MCAO is more likely to irreversibly damage blood vessels. Numerous stroke studies have previously reported late HP using different CBF measurement techniques across different species. In an anesthetized cat study of various MCAO durations, 30-minute MCAO group showed a transient HP (detected by positron emission tomography) after recanalization with fast normalization of CBF, but no or small infarcts in the deep nuclei were found in histological analysis (Heiss et al, 1997). In the 60 and 120 minutes of MCAO group, the degree of HP was related to the severity of prior ischemia (reaching up to 300% of preocclusion values) and 50% of animals survived (Heiss et al, 1997). Although it was not statistically significant, animals that eventually died from brain swelling exhibited higher HP than those that survived. Because the degree of HP was related to the severity of prior ischemia, it could not be concluded that the more severe HP per se was responsible for the worse outcome. It is possible that severe brain swelling was a consequence of recanalization in a severely damaged vascular bed, not of the degree of HP (Heiss et al, 1997). In our study, we found no correlation between the incidence of HP and lesion volume in the 60-minute MCAO group, although there was a weak trend of HP was correlated with a smaller lesion volume.
Spatial Characteristics of Late Hyperperfusion
The spatial and temporal characteristics of late HP were analyzed and compared with other imaging modalities. Spatially, HP correlated well with T2 lesion and BBB permeability increase overall, although there were some notable differences. Most of the HP was localized to the initial ischemic core (i.e., initial ADC lesion before recanalization). In contrast, mismatch tissue that did not infract showed no major HP at all time points. The slight changes in these parameters in the mismatch tissue were likely due to partial-volume effect with the core pixels or some mismatch and normal tissue might indeed infarct at later time points. Tissue that was not infracted (no increased T2) due to recanalization did not show HP (arrow in Figure 3A and 3B). Consistent with our findings, others have also reported the outcome of HP areas to be almost universally poor, exhibiting necrosis on the late structural imaging procedures (Ackerman et al, 1981; Baron et al, 1981, 1983; Tran Dinh et al, 1997). To our knowledge, late HP has not been reported to be associated with positive stroke outcome.
Other studies compared HP with metabolism and pH. Hakim et al (1989) analyzed changes in hemodynamic, oxygen, glucose metabolism, and pH in the severely hypometabolic (presumably infracted) cortex in patients. They found that this class of tissue exhibited HP in one third of their patients studied >23 hours after clinical onset. In these regions, anaerobic glycolysis was enhanced without persistent regional acidosis. By defining the probable core of the developing infarct as the most severely affected region on CBF or glucose consumption images, Fink et al found regions with significant HP surrounding such core in ∼40% of the patients and such HP regions were often mixed with hypoperfused areas. Hyperperfusion regions had a glucose extraction fraction lower than corresponding contralateral regions but relatively preserved glucose consumption, and they did not become completely necrotic (Fink et al, 1993).
Temporal Characteristics of Late Hyperperfusion
Temporally, HP appeared ∼12 hours after occlusion, peaked at 48 hours and disappeared ∼1 week after occlusion, although some rats with large lesions still showed some residual HP at 1 week after occlusion. Hyperperfusion correlated with BBB permeability increase but lagged T2 changes in peak times. While it is not surprising that increased BBB permeability leads to edema, the different peak times suggest that at least some of the factors responsible for BBB permeability increase differ from those responsible for edema (Macfarlane et al, 1991). It is interesting to note that HP disappeared by day 7 and vessels became tortuous but BBB permeability and edema (T2) remained slightly elevated. Vessels were not tortuous at 48 or 72 hours after stroke. These results suggest that vasoactive elements at least contributed to HP at some of the time points and it was not a passive effect of BBB permeability increase (Berne and Rubio, 1974; Kontos and Wei, 1985).
Several positron emission tomography studies reported that the occurrence of late HP increased in frequency until ∼10 to 15 days after stroke in patients (Ackerman et al, 1981; Baron et al, 1981), and that it was consistently associated with low oxygen extraction fraction and markedly reduced CMRO2, indicative of luxury perfusion (Baron et al, 1983). Durukan et al (2009) reported BBB leakage peaked at 1 week, but was not significant different from other time points.
The temporal profiles of T1 and T2 changes in our study peaked at 24 hours after stroke, consistent with previous reports that T1 and T2 reached a maximum at 24 to 48 hours (Kavec et al, 2004; Kettunen et al, 2000) and decreased slightly thereafter, and stabilized or increased again slightly (when cysts are formed) over the next 2 weeks (Ishii et al, 1998; Lin et al, 2002).
In some studies, BBB disruption has been reported to be biphasic, with an early first opening (during the first half hour of recanalization) followed by partial closing, and then a delayed, but progressive, opening between 22 and 46 hours after recanalization (Belayev et al, 1996; Huang et al, 1999). Other works showed a continuous BBB leakage for days (Nagel et al, 2004) or weeks (Durukan et al, 2009). This study showed that BBB leakage was apparent 24 hours after stroke and peaked at 48 hours, but did not investigate BBB integrity at the acute phase.
Tissue Fate and Hyperperfusion
Tracking of tissue fate showed that pixels that did not infract (ischemic core or mismatch at 30 minutes but became normal at 48 hours) showed CBF returning to normal. By contrast, pixels that did infract (ischemic core or mismatch at 30 minutes but became ischemic core at 48 hours) showed HP. These results further supported the notion that HP was associated with poor outcome whereas tissue that was not infracted (no T2 lesion) did not show HP. It is worth noting that CBF of the pixels transitioning from core to normal was higher than those transitioning from mismatch to normal. This is because the CBF threshold for the initially defined core pixels must be higher than that of the mismatch in order for them not to infract given they had experienced a more severe insult.
Impaired Vascular Reactivity
The hypercapnic challenge experiment showed that tissue with late HP showed decreased CBF responses to CO2 inhalation, in contrast to significant increased CBF in normal tissue. These results indicated that vessels in the HP territories had impaired vasodilatory functions. A ‘blood stealing' effect (Paksoy et al, 2003) diverging blood to the contralateral hemisphere and elsewhere in the body was observed because vessels in normal tissue vasodilated but those in ischemic tissue could not. Vascular dysfunction is further corroborated by the tortuous vessels in the HP territories. Future studies will probe CO2 reactivity at earlier time points postischemia.
Conclusion
This study longitudinally imaged the spatiotemporal dynamics of postischemic HP in the same stroke animals up to 7 days after stroke. The spatiotemporal progression of late HP was mostly correlated with T1, T2, and BBB changes, although in some animals HP preceded T2 increase, and T2 peaked first at 24 hours whereas HP peaked at 48 hours after occlusion. Pixel-by-pixel tracking of tissue fates showed that late HP was associated with tissue infarction whereas tissue that was not infarcted did not show HP. Future studies will compare immunochemical markers (e.g., NF-κB and Nitric oxide synthases) with spatiotemporal dynamic profiles of postischemic HP and investigate hypercapnic responses at earlier time points after recanalization.
The authors declare no conflict of interest.
Footnotes
This work was supported by the NIH (R01-NS45879) and the American Heart Association (EIA 0940104N, SDG-0430020N, and SDG-0830293N).
References
- Ackerman RH, Correia JA, Alpert NM, Baron JC, Gouliamos A, Grotta JC, Brownell GL, Taveras JM. Positron imaging in ischemic stroke disease using compounds labeled with oxygen 15. Initial results of clinicophysiologic correlations. Arch Neurol. 1981;38:537–543. doi: 10.1001/archneur.1981.00510090031002. [DOI] [PubMed] [Google Scholar]
- Bardutzky J, Shen Q, Henninger N, Schwab S, Duong TQ, Fisher M. Characterizing tissue fate after transient cerebral ischemia of varying duration using quantitative diffusion and perfusion imaging. Stroke. 2007;38:1336–1344. doi: 10.1161/01.STR.0000259636.26950.3b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron JC, Bousser MG, Comar D, Soussaline F, Castaigne P. Noninvasive tomographic study of cerebral blood flow and oxygen metabolism in vivo. Potentials, limitations, and clinical applications in cerebral ischemic disorders. Eur Neurol. 1981;20:273–284. doi: 10.1159/000115247. [DOI] [PubMed] [Google Scholar]
- Baron JC, Delattre JY, Bories J, Chiras J, Cabanis EA, Blas C, Bousser MG, Comar D. Comparison study of CT and positron emission tomographic data in recent cerebral infarction. AJNR Am J Neuroradiol. 1983;4:536–540. [PMC free article] [PubMed] [Google Scholar]
- Belayev L, Busto R, Zhao W, Ginsberg MD. Quantitative evaluation of blood-brain barrier permeability following middle cerebral artery occlusion in rats. Brain Res. 1996;739:88–96. doi: 10.1016/s0006-8993(96)00815-3. [DOI] [PubMed] [Google Scholar]
- Berne RM, Rubio R. Regulation of coronary blood flow. Adv Cardiol. 1974;12:303–317. doi: 10.1159/000395474. [DOI] [PubMed] [Google Scholar]
- Duong TQ, Silva AC, Lee SP, Kim SG. Functional MRI of calcium-dependent synaptic activity: cross correlation with CBF and BOLD measurements. Magn Reson Med. 2000;43:383–392. doi: 10.1002/(sici)1522-2594(200003)43:3<383::aid-mrm10>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Durukan A, Marinkovic I, Strbian D, Pitkonen M, Pedrono E, Soinne L, Abo-Ramadan U, Tatlisumak T. Post-ischemic blood-brain barrier leakage in rats: one-week follow-up by MRI. Brain Res. 2009;1280:158–165. doi: 10.1016/j.brainres.2009.05.025. [DOI] [PubMed] [Google Scholar]
- Fink GR, Herholz K, Pietrzyk U, Huber M, Heiss W-D. Peri-infarct perfusion in human ischemia: its relation to tissue metabolism, morphology, and clinical outcome. J Stroke Cerebrovasc Dis. 1993;3:123–131. doi: 10.1016/S1052-3057(10)80239-3. [DOI] [PubMed] [Google Scholar]
- Hakim AM, Evans AC, Berger L, Kuwabara H, Worsley K, Marchal G, Biel C, Pokrupa R, Diksic M, Meyer E, Gjedde A, Marrett S. The effect of nimodipine on the evolution of human cerebral infarction studied by PET. J Cereb Blood Flow Metab. 1989;9:523–534. doi: 10.1038/jcbfm.1989.76. [DOI] [PubMed] [Google Scholar]
- Heiss W-D, Graf R, Lottgen J, Ohta K, Fujita T, Wagner R, Grond M, Weinhard K. Repeat positron emission tomographic studies in transient middle cerebral artery occlusion in cats. J Cereb Blood Flow Metab. 1997;17:388–400. doi: 10.1097/00004647-199704000-00004. [DOI] [PubMed] [Google Scholar]
- Huang ZG, Xue D, Preston E, Karbalai H, Buchan AM. Biphasic opening of the blood-brain barrier following transient focal ischemia: effects of hypothermia. Can J Neurol Sci. 1999;26:298–304. doi: 10.1017/s0317167100000421. [DOI] [PubMed] [Google Scholar]
- Ishii H, Arai T, Morikawa S, Inubushi T, Tooyama I, Kimura H, Mori K. Evaluation of focal cerebral ischemia in rats by magnetic resonance imaging and immunohistochemical analyses. J Cereb Blood Flow Metab. 1998;18:931–934. doi: 10.1097/00004647-199809000-00001. [DOI] [PubMed] [Google Scholar]
- Jokivarsi KT, Hiltunen Y, Tuunanen PI, Kauppinen RA, Grohn OH. Correlating tissue outcome with quantitative multiparametric MRI of acute cerebral ischemia in rats. J Cereb Blood Flow Metab. 2010;30:415–427. doi: 10.1038/jcbfm.2009.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joo F. The blood-brain barrier. New aspects to the function of the cerebral endothelium. Nature. 1986;321:197–198. doi: 10.1038/321197a0. [DOI] [PubMed] [Google Scholar]
- Kastrup A, Engelhorn T, Beaulieu C, de Crespigny A, Moseley ME. Dynamics of cerebral injury, perfusion, and blood-brain barrier changes after temporary and permanent middle cerebral artery occlusion in the rat. J Neurol Sci. 1999;166:91–99. doi: 10.1016/s0022-510x(99)00121-5. [DOI] [PubMed] [Google Scholar]
- Kavec M, Grohn OH, Kettunen MI, Silvennoinen MJ, Garwood M, Kauppinen RA. Acute cerebral ischemia in rats studied by Carr-Purcell spin-echo magnetic resonance imaging: assessment of blood oxygenation level-dependent and tissue effects on the transverse relaxation. Magn Reson Med. 2004;51:1138–1146. doi: 10.1002/mrm.20089. [DOI] [PubMed] [Google Scholar]
- Kettunen MI, Grohn OH, Lukkarinen JA, Vainio P, Silvennoinen MJ, Kauppinen RA. Interrelations of T(1) and diffusion of water in acute cerebral ischemia of the rat. Magn Reson Med. 2000;44:833–839. doi: 10.1002/1522-2594(200012)44:6<833::aid-mrm3>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Kidwell CS, Saver JL, Mattiello J, Starkman S, Vinuela F, Duckwiller G, Gobin YP, Jahan R, Vespa JP, Villablanca JP, Liebeskind DS, Woods RP, Alger JR. Diffusion-perfusion MRI characterization of post-recanalization hyperperfusion in humans. Neurology. 2001;57:2015–2021. doi: 10.1212/wnl.57.11.2015. [DOI] [PubMed] [Google Scholar]
- Kontos HA, Wei EP. Oxygen-dependent mechanisms in cerebral autoregulation. Ann Biomed Eng. 1985;13:329–334. doi: 10.1007/BF02584251. [DOI] [PubMed] [Google Scholar]
- Lassen NA. The luxury-perfusion syndrome and its possible relation to acute metabolic acidosis localised within the brain. Lancet. 1966;2:1113–1115. doi: 10.1016/s0140-6736(66)92199-4. [DOI] [PubMed] [Google Scholar]
- Lin SP, Schmidt RE, McKinstry RC, Ackerman JJ, Neil JJ. Investigation of mechanisms underlying transient T2 normalization in longitudinal studies of ischemic stroke. J Magn Reson Imaging. 2002;15:130–136. doi: 10.1002/jmri.10052. [DOI] [PubMed] [Google Scholar]
- Liu ZM, Schmidt KF, Sicard KM, Duong TQ. Imaging oxygen consumption in forepaw somatosensory stimulation in rats under isoflurane anesthesia. Magn Reson Med. 2004;52:277–285. doi: 10.1002/mrm.20148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macfarlane R, Moskowitz MA, Sakas DE, Tasdemiroglu E, Wei EP, Kontos HA. The role of neuroeffector mechanisms in cerebral hyperperfusion syndromes. J Neurosurg. 1991;75:845–855. doi: 10.3171/jns.1991.75.6.0845. [DOI] [PubMed] [Google Scholar]
- Marchal G, Beaudouin V, Rioux P, de la Sayette V, Le Doze F, Viader F, Derlon JM, Baron JC. Prolonged persistence of substantial volumes of potentially viable brain tissue after stroke: a correlative PET-CT study with voxel-based data analysis. Stroke. 1996a;27:599–606. doi: 10.1161/01.str.27.4.599. [DOI] [PubMed] [Google Scholar]
- Marchal G, Furlan M, Beaudouin V, Rioux P, Hauttement JL, Serrati C, de la Sayette V, Le Doze F, Viader F, Derlon JM, Baron JC. Early spontaneous hyperperfusion after stroke. A marker of favourable tissue outcome. Brain. 1996b;119 (Part 2:409–419. doi: 10.1093/brain/119.2.409. [DOI] [PubMed] [Google Scholar]
- Nagel S, Wagner S, Koziol J, Kluge B, Heiland S. Volumetric evaluation of the ischemic lesion size with serial MRI in a transient MCAO model of the rat: comparison of DWI and T1WI. Brain Res Brain Res Protoc. 2004;12:172–179. doi: 10.1016/j.brainresprot.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Paksoy Y, Genc BO, Genc E. Retrograde flow in the left inferior petrosal sinus and blood steal of the cavernous sinus associated with central vein stenosis: MR angiographic findings. AJNR Am J Neuroradiol. 2003;24:1364–1368. [PMC free article] [PubMed] [Google Scholar]
- Pan J, Konstas AA, Bateman B, Ortolano GA, Pile-Spellman J. Reperfusion injury following cerebral ischemia: pathophysiology, MR imaging, and potential therapies. Neuroradiology. 2007;49:93–102. doi: 10.1007/s00234-006-0183-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaller B, Graf R. Cerebral ischemia and reperfusion: the pathophysiologic concept as a basis for clinical therapy. J Cereb Blood Flow Metab. 2004;24:351–371. doi: 10.1097/00004647-200404000-00001. [DOI] [PubMed] [Google Scholar]
- Schwarzbauer C, Morrissey SP, Deichmann R, Hillenbrand C, Syha J, Adolf H, Noth U, Haase A. Quantitative magnetic resonance imaging of capillary water permeability and regional blood volume with an intravascular MR contrast agent. Magn Reson Med. 1997;37:769–777. doi: 10.1002/mrm.1910370521. [DOI] [PubMed] [Google Scholar]
- Shen Q, Fisher M, Sotak CH, Duong TQ. Effects of reperfusion on ADC and CBF pixel-by-pixel dynamics in stroke: characterizing tissue fates using quantitative diffusion and perfusion imaging. J Cereb Blood Flow Metab. 2004a;24:280–290. doi: 10.1097/01.WCB.0000110048.43905.E5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Q, Meng X, Fisher M, Sotak CH, Duong TQ. Pixel-by-pixel spatiotemporal progression of focal ischemia derived using quantitative perfusion and diffusion imaging. J Cereb Blood Flow Metab. 2003;23:1479–1488. doi: 10.1097/01.WCB.0000100064.36077.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Q, Ren H, Bouley J, Fisher M, Duong TQ. Dynamic tracking of acute ischemic tissue fates using improved unsupervised ISODATA analysis of high-resolution quantitative perfusion and diffusion data. J Cereb Blood Flow Metab. 2004b;24:887–897. doi: 10.1097/01.WCB.0000124321.60992.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Q, Ren H, Cheng H, Fisher M, Duong TQ. Functional, perfusion and diffusion MRI of acute focal ischemic brain injury. J Cereb Blood Flow Metab. 2005;25:1265–1279. doi: 10.1038/sj.jcbfm.9600132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundt TM, Jr, Grant WC, Garcia JH. Restoration of middle cerebral artery flow in experimental infarction. J Neurosurg. 1969;31:311–321. doi: 10.3171/jns.1969.31.3.0311. [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Nagaoka T, Nair G, Ohno K, Duong TQ.2010Arterial spin labeling and dynamic susceptibility contrast CBF MRI in postischemic hyperperfusion, hypercapnia, and after mannitol injection J Cereb Blood Flow Metabadvance online publication, 22 December 2010; [e-pub ahead of print] PMID: 21179070 [DOI] [PMC free article] [PubMed]
- Tasdemiroglu E, Macfarlane R, Wei EP, Kontos HA, Moskowitz MA. Pial vessel caliber and cerebral blood flow become dissociated during ischemia-reperfusion in cats. Am J Physiol. 1992;263:H533–H536. doi: 10.1152/ajpheart.1992.263.2.H533. [DOI] [PubMed] [Google Scholar]
- Tran Dinh YR, Ille O, Guichard JP, Haguenau M, Seylaz J. Cerebral postischemic hyperperfusion assessed by Xenon-133 SPECT. J Nucl Med. 1997;38:602–607. [PubMed] [Google Scholar]
- Yamaguchi T. Regional cerebral blood flow in experimental cerebral infarction, with special reference to hyperemia in the ischemic cerebral hemisphere. Int J Neurol. 1977;11:162–178. [PubMed] [Google Scholar]