Abstract
Transcranial direct current stimulation (tDCS) is a noninvasive brain stimulation technique that induces changes in cortical excitability: anodal stimulation increases while cathodal stimulation reduces excitability. Imaging studies performed after unilateral stimulation have shown conflicting results regarding the effects of tDCS on surrogate markers of neuronal activity. The aim of this study was to directly measure these effects on activation-induced changes in regional cerebral blood flow (ΔrCBF) using positron emission tomography (PET) during bilateral tDCS. Nine healthy subjects underwent repeated rCBF measurements with 15O-water and PET during a simple motor task while receiving tDCS or sham stimulation over the primary motor cortex (M1). Motor evoked potentials (MEPs) were also assessed before and after real and sham stimulation. During tDCS with active movement, ΔrCBF in M1 was significantly lower on the cathodal than the anodal side when compared with sham stimulation. This decrease in ΔrCBF was accompanied by a decrease in MEP amplitude on the cathodal side. No effect was observed on resting or activated rCBF relative to sham stimulation. We thus conclude that it is the interaction of cathodal tDCS with activation-induced ΔrCBF rather than the effect on resting or activated rCBF itself which constitutes the physiological imaging correlate of tDCS.
Keywords: finger movements, human motor cortex, motor evoked potentials, positron emission tomography, transcranial direct current stimulation
Introduction
The electrophysiological modulation of brain excitability induced by transcranial direct current stimulation (tDCS) has been extensively studied in the past decade. There is a general consensus that after anodal stimulation, the amplitude of motor evoked potentials (MEPs) is increased (Furubayashi et al, 2008; Jeffery et al, 2007; Nitsche and Paulus 2000, 2001; Nitsche et al, 2007; Power et al, 2006) while it is decreased with cathodal stimulation (Ardolino et al, 2005; Furubayashi et al, 2008; Nitsche and Paulus, 2000; Nitsche et al, 2003c, 2007; Power et al, 2006; Priori, 2003). This modulation of cortical excitability is possibly caused by hyperpolarization/depolarization of neuronal membranes, resulting in a shift of resting membrane potential (Bindman et al, 1964). In fact, DC stimulation modulates neurons either by inhibition or by excitation depending on the polarity of the applied current (Lefaucheur, 2008).
A limited number of studies have investigated the after-effect of tDCS on brain activation using surrogate markers such as blood oxygen level-dependent (BOLD) contrast or changes in regional cerebral blood flow (rCBF), yielding to some extent conflicting results. Three studies examined how brain activation is modulated after tDCS (Baudewig et al, 2001; Jang et al, 2009; Lang et al, 2005). These activation-induced changes in rCBF and BOLD during motor tasks were found in widespread cortical networks and it was cathodal tDCS that induced the largest changes (Baudewig et al, 2001; Lang et al, 2005). Anodal tDCS, however, seems to only generate modest changes after stimulation periods of varying duration (5, 10, or 20 minutes; Baudewig et al, 2001; Jang et al, 2009; Lang et al, 2005). These changes in rCBF and BOLD signals after tDCS are more complex than the electrophysiological findings where the increase in MEPs after anodal stimulation shows the strongest effect while the decreasing effects of cathodal stimulation are usually less. In addition, the changes in rCBF and BOLD signals are observed on a global scale although the polarizing effects of tDCS are generally thought to be restricted to the area under the electrodes (Nitsche et al, 2003b, 2004). It thus cannot be ruled out that the widespread after-effects, observed on a network level, might be secondary adaptations of the network to the previous stimulation rather than the direct effect of stimulation itself.
Only one study has assessed the effect of anodal stimulation on resting BOLD signal with magnetic resonance imaging (MRI) during tDCS (Kwon et al, 2008). In this study, no BOLD-contrast changes were detected during anodal tDCS except in the last 21 seconds of the total 84 seconds of stimulation. Nevertheless, those changes observed in the left M1, left supplementary motor area, and right posterior parietal cortex were rather small. These minor effects on resting BOLD signal are expected, because tDCS is thought to modulate neuronal excitability rather than inducing action potentials (Zaghi et al, 2010). An effect might thus only be expected during induced neuronal activity (i.e., the generation of action potentials with behavioral tasks).
The objective of this study was to determine the immediate effects of tDCS on resting and activation-induced rCBF during stimulation rather than tDCS after-effects, using a bilateral electrode mount in young healthy subjects. The use of bilateral tDCS allows for the simultaneous assessment of anodal and cathodal effects during a single positron emission tomography (PET) session comprising 12 measurements of rCBF, while still respecting dose limits for radiation exposure. It has recently been shown that in contrast to unilateral stimulation, simultaneous anodal and cathodal tDCS over motor cortex in both hemispheres may provide an additive effect that facilitates motor task performance in young healthy subjects at the behavioral level (Vines et al, 2008) and might thus translate into more pronounced rCBF changes. We hypothesize that bilateral tDCS will affect brain activation during movement-related activity but not during rest. Specifically, using 15O-water PET we expect to show an increase in rCBF change under the anode and a decrease in rCBF change under the cathode.
Materials and methods
Subjects
Nine healthy subjects (mean age: 28 years (range: 18 to 35); three males) without history of neurologic disease participated in this study. Eight subjects were right handed (mean score +85) as assessed by the Edinburgh Handedness Inventory (left handed subject' score=−100). All subjects gave their informed consent in accordance to the McGill Faculty of Medicine Institutional Review Board regulations for human subjects' studies before their participation in the study.
Protocol
All subjects first underwent structural and functional MRI scans to determine optimal tDCS electrode placement. The effect of bilateral tDCS on rCBF and on MEPs was then tested in two separate sessions, at least 1 week apart, to avoid any possible after-effect of tDCS on brain activity.
Magnetic Resonance Imaging
A structural brain scan was first obtained in all patients using a whole body 1.5 T Siemens Sonata Magnetic Resonance Imaging (MRI) scanner (Siemens AG, Erlangen, Germany) with an 8-channel coil. T1-weighted images were acquired as isotropic data sets of 1 mm3 voxel size (echo time=9.2 ms; repetition time=22 ms; flip angle=30°). This was followed by two sessions of functional MRI to locate the first dorsal interosseous muscle (FDI) representation area for accurate positioning of the tDCS electrodes. Twenty-four parallel axial slices (thickness=4 mm) were obtained across the entire brain volume using an echo-planar imaging sequence (64 × 64 matrix, field of view=256 × 256 mm2, echo time=50 ms, repetition time=2.03 seconds, flip angle=90°).
Functional MRI was conducted while subjects performed an acoustically paced motor task with (1) their dominant index finger and (2) their nondominant index finger. During the functional task, subjects executed finger flexions using a custom-made MRI-compatible pneumatic pressure piston that produces a constant resistance to the index finger. During the entire duration of the task, computer-generated tones (2.03 Hz), serving as acoustical pacing signals (duration of tone=10 ms), were relayed to the subject's headphones. Subjects received a visual ‘stop' and ‘go' signal over a back-projected mirror on top of the head coil, prompting them to start or stop finger movements. Each active and rest epoch lasted 20.3 seconds and was repeated 14 times each. Presentation Software from Neurobehavioral Systems was used to synchronize data collection onset and offset as well as stimuli appearance (auditory and visual cues). Subjects practiced the task before they entered the MRI to ensure it was fully understood.
Images obtained from the functional scan were processed to determine FDI muscle cortical representation using the FMRI Expert Analysis Tool (FEAT, version 5.98) of the FMRIB Software Library (FSL, version 4.1.4; http://www.fmrib.ox.ac.uk/fsl, Functional Magnetic Resonance lmaging of the Brain Center, University of Oxford, Oxford, UK) using standard parameter settings: 6 mm full width at half maximum Gaussian filter for spatial smoothing, intensity normalization across images, high-pass temporal filter cutoff 40.3 seconds, multiple regression model, P value 0.05 corrected. Functional images were superimposed and coregistered with the high resolution structural brain scan using the Multi-Modality Matching (v1.8) registration plug-in of the Volume Imaging in Neurological Research, coregistration and regions of interest included (VINCI 2.57) software (Cizek et al, 2004).
Electrode Placement
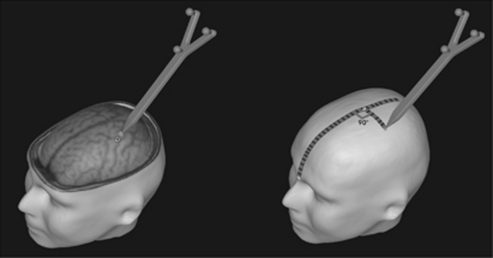
3D surface renderings of structural and functional magnetic resonance data sets were created with the Nexstim Navigated Brain Stimulation TMS system using the software supplied by the manufacturer. The exact location of the FDI M1 representation was obtained with the navigation system and its position relative to the midsagittal line was measured on the subject's skull using surface distance measurements for later electrode positioning in the PET scanner, where no navigation system is available (Figure 1). We have previously demonstrated that the precision of this localization method is ∼8 mm (Weiduschat et al, 2009), which is appropriate for an electrode size of 5 cm × 5 cm.
Figure 1.
Illustration of the measurement used for transcranial direct current stimulation (tDCS) electrode placement over the first dorsal interosseous muscle (FDI) hotspot, as determined by functional magnetic resonance imaging (fMRI). The antero-posterior distance of the M1 representation from the nasion was first measured after the subject's skull on the midline. The lateral component of the electrodes was then measured from the midline on each side. These measures were used to position the tDCS electrodes on the subject.
Transcranial Direct Current Stimulation
For both rCBF (PET) and MEPs (transcranial magnetic stimulation (TMS)) assessment sessions, bilateral tDCS was applied using the Eldith plus stimulator (Magstim Company Ltd., Whitland, UK). The cathodal electrode was positioned over the nondominant FDI M1 and the anodal electrode over the dominant FDI M1. Current intensity was 2 mA (current density of 0.08 mA/cm2; 5 seconds ramp on and 5 seconds ramp off) and applied for 4 minutes per trial. This stimulus duration was selected based on Nitsche and Paulus (2000) who showed that the effect of 4 minutes of tDCS completely faded away after 4 to 5 minutes. Thus, for both PET and TMS procedures, inter-tDCS trial intervals of at least 8 minutes were chosen to minimize the possibility of recording tDCS after-effects. During preliminary testing of the tDCS device, some subjects reported an itching sensation throughout the entire stimulation period and not only at the onset and offset of stimulation. For this reason, we decided to use a sham stimulation consisting of small current pulses occurring every 550 ms (0.11 mA over 15 ms with peak current lasting for 3 ms, as supplied by the manufacturer). The average current over time was <0.002 mA, which has no therapeutic effect (Nitsche and Paulus, 2000) but yields a sensation on the skin similar to real tDCS. None of the subjects could distinguish between sham and real stimulation, when debriefed after the scanning session.
Positron Emission Tomography
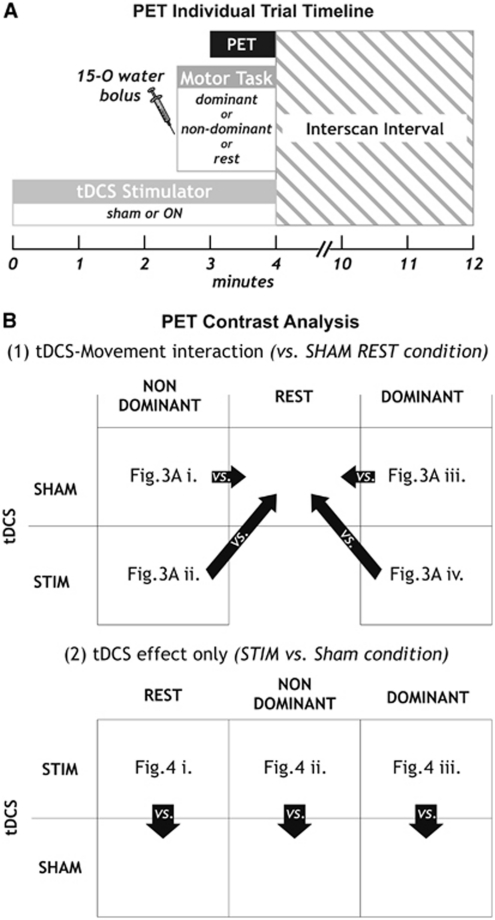
Twelve PET imaging scans were acquired using a Siemens ECAT HR+scanner (Siemens AG, Erlangen, Germany) in 3D mode. Subjects lay supine in the scanner with their head resting on foam pads. A VELCRO® brand (Velcro Industries B.V., Manchester, NH, USA) strap was tightly fixed across their forehead to maintain head position. Scans were reconstructed to 63 contiguous slices of 2.425 mm thickness and 2.059 mm voxel size, using the manufacturer-provided 3D FBP (Hanning Filtered with a 6 mm kernel) equivalent approach which includes a proprietary scatter projection technique. A 10-minute transmission scan was performed before the emission scans for attenuation correction. During each scan, the subjects either performed an externally paced sequential finger opposition task at 1.16 Hz with the dominant or nondominant hand or were at rest. Each experimental condition was replicated twice (six experimental conditions: tDCS or sham X dominant hand, nondominant hand or no movement, see Figure 2). Subjects started finger tapping with the intravenous bolus injection of 370 MBq of 15O-water 2.5 minutes after the start of real or sham tDCS (as illustrated in Figure 2A). Data acquisition started automatically, when the number of registered true counts exceeded the baseline level by 40% and lasted for 60 seconds. Injections were given at least 12 minutes apart (corresponding to six physical half-lives) to ensure adequate radioactive tracer decay and to minimize background noise and tDCS after-effects. Data from two out of nine subjects were discarded because subject motion during acquisition.
Figure 2.
Positron emission tomography (PET) experimental set-up. Individual trial timeline is shown in (A). The contrasts tested are shown in (B) for (1) transcranial direct current stimulation (tDCS)–movement interaction, contrasting conditions with tDCS SHAM during rest condition and (2) for stimulation effects only, where tDCS STIM was contrasted with tDCS SHAM for each of the three motor tasks. Each contrast is shown as Z-transformed difference images averaged across subjects in Figures 3 and 4, as indicated in the respective boxes. STIM, stimulation.
Transcranial Magnetic Stimulation
Single-pulse TMS was delivered with a bifocal figure-of-eight-shaped coil (50 mm mean winding diameter) using a Nexstim eXimia NBS system with Navigated Brain Stimulation, TMS, and electromyography (Nexstim Ltd, Helsinki, Finland). Coil positioning was in posterolateral orientation, perpendicular to the central sulcus thus inducing a current in an anterior direction. Disposable surface electrodes (Ag/AgCl, Ambu Neuroline, 95 mm2 area) were placed in a monopolar configuration over the bilateral FDI muscles. Electromyography signals were amplified, band-pass filtered (10 to 500 Hz) and sampled at 3,000 Hz. Motor evoked potentials were recorded in eight out of nine subjects (no consistent response could be obtained in one subject). The coil position producing the largest MEP was used for recording and determination of resting motor threshold (minimal stimulus intensity eliciting MEPs>50 μV in 6 out of 10 trials). Four trials of tDCS were acquired: two with tDCS and two during sham stimulation. After registration of the MRI to the subject's head, 10 baseline MEPs at 110% resting motor threshold were acquired. Transcranial direct current stimulation electrodes were positioned on the subject's head and stimulation was applied for 4 minutes. Transcranial direct current stimulation electrodes were then removed and MEPs were recorded over 4 minutes using randomized interpulse intervals. Transcranial direct current stimulation trials were given at least 8 minutes apart.
Data Analysis
Positron emission tomography activation
Both emission (for activation) and transmission (for electrode positioning) images were coregistered with the structural MRI (T1-weighted) using the VINCI software (Cizek et al, 2004). The two PET scans of each condition were averaged and ratio normalized. Activation images were calculated as difference images of rCBF changes after smoothing with a Gaussian filter of 12 mm full width at half maximum and Z-transformation using a global variance estimate as previously described (Thiel et al, 2001a, 2001b). The following contrasts were analyzed, as shown in Figure 2B: (1) stimulation–movement interaction by subtracting sham tDCS during rest from tDCS with dominant or nondominant finger movements (including movement effect only sham tDCS with finger movements versus sham tDCS during rest) or (2) stimulation effect only by subtracting sham tDCS during rest from tDCS during rest or by subtracting sham tDCS with finger movements from tDCS with the respective side finger movements.
For analysis, PET activation images were rendered onto each subject's 3D reconstructed structural image and the tDCS anode and cathode were reconstructed from the transmission scan. Regional cerebral blood flow change was measured on the surface renderings under the center of the respective electrodes using a cylindrical probe volume of interest (VOI) measuring 1 cm in depth and 5 cm diameter as described previously (Thiel et al, 2006; Von Stockhausen et al, 1998). Differences in rCBF change were assessed using paired t-tests. Although quantitative VOI-based analysis was performed on individual data sets, voxel-based average images for each contrast were generated from the individual images after stereotaxic normalization (statistical parametric mapping 8) to assess possible changes outside the VOI and for illustration purposes (Figures 3 and 4).
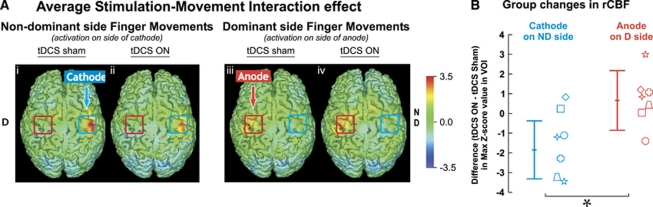
Figure 3.
Regional cerebral blood flow (rCBF) changes with bilateral transcranial direct current stimulation (tDCS) during finger movements. Z-transformed difference images averaged across seven subjects are shown in (A). Activations are shown during finger movements on the nondominant (i and ii) and dominant (iii and iv) hand. Differences are obtained from contrast with the sham rest condition. The red (anode) and blue (cathode) squares drawn on each image correspond to the tDCS electrodes. (B) Mean (±1s.d.) group changes in rCBF during cathodal and anodal stimulation are shown. Individual subjects are represented by the seven different symbols. *P<0.05, one-tailed paired t-test.
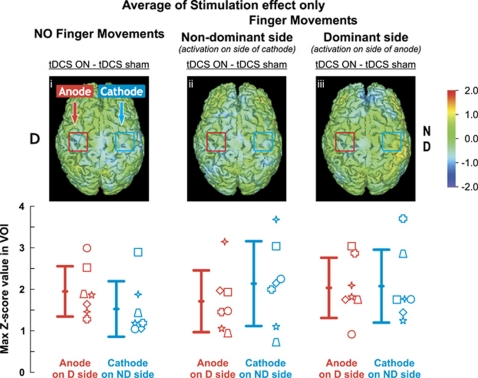
Figure 4.
Regional cerebral blood flow (rCBF) changes with bilateral transcranial direct current stimulation (tDCS) only. Z-transformed differences images averaged across seven subjects are shown above and group means (±1s.d.) are shown in the bottom row. Activations are shown during rest (versus sham rest) on the left panel (i), during movements of the nondominant hand (versus sham nondominant movement) in center panel (ii) and for the dominant hand (versus sham dominant movement) in the right panel (iii). No change was observed in ΔrCBF when contrasting tDCS stimulation versus sham with nondominant (center panel) or dominant (right panel) finger movements. The red (anode) and blue (cathode) squares drawn on each image correspond to the tDCS electrodes. Individual subjects are represented by the seven different symbols.
Motor evoked potential assessment
Peak-to-peak amplitude values were automatically detected using the software supplied by the manufacturer and visually inspected. Trials in which the FDI muscle was not relaxed before TMS pulse and MEP amplitudes below 50 μV as well as outliers (values>2s.d., <20 for all subjects) were removed. No differences were measured in the baseline MEPs between trials, thus confirming the absence of after-effects. Changes in MEP due to tDCS were calculated as ratio of MEP amplitudes during stimulation divided by each subjects' baseline. Thus, a value of one represents no change from baseline, a value higher than one reflects increased MEPs relative to baseline and a value smaller than one reflects that MEPs are smaller than baseline.
Results
Figure 3A, left panel, shows average changes in activation-induced rCBF relative to the sham rest condition when subjects executed nondominant side finger movements during sham (Figure 3Ai) or real (Figure 3Aii) tDCS. As expected, the sequential finger movement task alone significantly increased rCBF in M1 (task effect); as shown in Figure 3Ai, during sham tDCS with finger movements on the nondominant side, rCBF was significantly increased over the M1 representation of the nondominant hemisphere (increase in max Z-score value in VOI on nondominant hemisphere: 5.85 s.d.2.47) as well as on the dominant hemisphere with dominant finger movements (Figure 3Aiii; 4.56 s.d.1.15).
Effects of Bilateral Transcranial Direct Current Stimulation During an Active Motor Task (Task and Stimulation Effect)
During movement of the nondominant hand and activation of the nondominant motor cortex under the cathode, bilateral tDCS caused a ΔrCBF decrease when compared with the resting condition under sham tDCS stimulation (decrease of 1.84 in Z-score, P=0.02, paired t-test, one-tailed; Figure 3Aii). The effect of bilateral tDCS under the anode during movement was similar to that of sham tDCS during movement (increase of 0.67 in Z-score, P=0.31). Yet, changes under the anode and cathode during bilateral tDCS were significantly different and in opposite directions. The regional mean (±1s.d.) difference in peak Z-score value for ΔrCBF between active and sham tDCS stimulation for the VOI region under both cathode and anode is shown in Figure 3B.
Effects of Bilateral Transcranial Direct Current Stimulation Only (Stimulation Effect)
Bilateral tDCS had no significant effect on ΔrCBF under the cathode or the anode during the resting condition (mean increase in Z-score <2.0) or finger movement, as shown in Figure 4.
Immediate After-Effects of Bilateral Transcranial Direct Current Stimulation on Motor Evoked Potentials
After-effects measured by MEPs in 4 minutes after bilateral tDCS were similar to the effects observed on rCBF during stimulation. As shown in Figure 5, MEPs measured on the cathode side were significantly reduced as compared with baseline. On the side of the anode, however, no difference could be observed between the sham and real stimulation. Changes in MEP amplitude were not correlated with ΔrCBF during stimulation (cathode: r=−0.18, P=0.73 and anode: r=−0.56, P=0.25).
Figure 5.
Changes in motor evoked potentials (MEPs) after bilateral transcranial direct current stimulation (tDCS). Values for sham on the cathodal and anodal side were pooled together.
Discussion
This is the first study quantifying online changes in brain activation during bilateral tDCS. Our results suggest that activation-induced rCBF changes measured during tDCS differ from more widespread brain activity-related changes measured 5 to 60 minutes after tDCS, as described in previous studies (Baudewig et al, 2001; Jang et al, 2009; Lang et al, 2005). We found a significant focal decrease in ΔrCBF under the cathode positioned over the M1 region during a finger-tapping task. As in Kwon et al (2008), we did not find significant changes in rCBF during tDCS when subjects were either at rest or active. It seems to be the interaction of tDCS with a task-induced change in activity that modulates the ΔrCBF. Stimulation alone seems to have little effect if neuronal activity does not change between two activation states. In addition, we showed that a bilateral tDCS electrode mount can be used to induce changes in brain activation during movement and that it is the cathode that induces stronger effects.
After-Effects Versus Immediate Effects
Previous imaging studies focused on tDCS-induced after-effects and only reported changes in neuronal activation patterns on a more global scale, i.e., outside the stimulated region (Baudewig et al, 2001; Jang et al, 2009; Lang et al, 2005). These network-wide effects reported after stimulation might thus reflect a different mechanism between online and after-effects where these after-effects may represent more the adaptation of the network to the stimulation effect rather than the effect itself. It has been suggested that immediate effects (in the range of several milliseconds after tDCS onset) are secondary to changes in membrane potentials and that after-effects of tDCS are mediated by long-term depression (LTD) and long-term potentiation (LTP)-like synaptic mechanisms (Furubayashi et al, 2008). Thus, the effects of tDCS on activation-induced rCBF changes in the brain region primarily affected by the behavioral task may only be seen if imaging is performed online. We did not observe significant modulation of the ΔrCBF in other cortical regions.
The online effects of tDCS have been assessed in one previous study during rest and no convincing evidence for change in BOLD signal was observed (Kwon et al, 2008). The online effects of tDCS, as we have shown here with active movements, seem to be localized under the tDCS electrodes. Because the tDCS currents used in the present study are well below threshold to induce action potentials, our results imply that the immediate effects of tDCS are detected when neurons are active and support the hypothesis that low current tDCS mainly modulates resting membrane potential thresholds (e.g., Liebetanz et al, 2002; Nitsche et al, 2003a; Zaghi et al, 2010). According to this theory, tDCS would thus modulate neuronal activation by inhibiting or facilitating the generation of action potentials.
Imaging Effects Versus Electrophysiological Effects
The imaging findings in our study were consistent with the electrophysiological effects of bilateral tDCS observed in the same subjects using identical stimulation parameters. The cathodal stimulation resulted in a decrease of MEP amplitude relative to sham while no significant effect was observed for anodal stimulation. Similar findings were reported by Williams et al (2010) who applied cathodal stimulation over the dominant and anodal over the nondominant M1 for 40 minutes. They observed a 20% reduction of MEP amplitudes on the cathodal side 3 hours after end of stimulation but only a 13% increase in MEP amplitude immediately after the end of stimulation on the anodal side. In contrast to our study, they only used 1 mA currents and stimulation was combined with a motor learning task.
The modulation of MEP amplitude after tDCS was not correlated with the change in rCBF. Results from studies on neurovascular coupling suggest that there is a strong relationship between ΔrCBF and the postsynaptic activity in the activated area measured as the sum of local field potentials but not with the spike rate of the efferent neurons (Lauritzen and Gold, 2003). Motor evoked potential amplitude, however, is mainly determined by action potentials elicited in pyramidal tract neurons by the TMS pulse either indirectly via interneurons (I-waves) or—at higher stimulation intensities—directly at the first node of Ranvier (D-waves; Rothwell et al, 1991). Although both processes are related to the excitability of neuronal membranes, they measure different aspects of neuronal excitability in so far as ΔrCBF is related to intrinsic, evoked postsynaptic activity and MEP amplitude to passively induced action potentials in pyramidal cells. A simple relationship between both might thus not be expected.
The anodal effect of bilateral tDCS on MEPs thus seems to be less pronounced than in unilateral stimulation (Furubayashi et al, 2008; Jeffery et al, 2007; Nitsche and Paulus, 2000, 2001; Nitsche et al, 2007; Power et al, 2006), while the cathodal effects appear to be similar (Ardolino et al, 2005; Furubayashi et al, 2008; Nitsche and Paulus, 2000; Nitsche et al, 2003c, 2007; Power et al, 2006; Priori, 2003).
Study Limitations
Differences in stimulation intensity or duration might be one explanation for the absence of an anodal effect. It has been shown that larger current densities result in stronger effects of tDCS (Iyer et al, 2005; Nitsche and Paulus, 2000). In the current protocol, we used a current density of 0.08 mA/cm2 which is larger than what has been used in most tDCS studies (∼0.02 mA/cm2). Only two other studies have safely used stronger current intensities (0.141 mA/cm2) but with shorter stimulation duration (Furubayashi et al, 2008; Kwon et al, 2008). Using these higher intensities, Kwon et al were unable to show a convincing effect of tDCS. Stimulation intensity is thus not likely to caused the effects observed in the present study. Lang et al (2005), as in our study, showed that only cathodal tDCS had a lasting effect on movement-related activity and suggested that cathodal tDCS might be more effective at interfering with motor execution than anodal tDCS. With a longer tDCS stimulation protocol (20 minutes; more than three times longer than our current protocol), Jang et al (2009) have observed a small increase in brain activation under the anode after stimulation. Although the differences in stimulation intensities and duration cannot be entirely excluded as cause for the absence of an anodal effect, the asymmetry of transcallosal inhibition between dominant and nondominant hemisphere should be considered (Netz et al, 1995). Inhibition from the nondominant to the dominant side is weaker than in the opposite direction. Since we applied cathodal stimulation to the nondominant side, the reduction in transcallosal inhibition might not have been sufficient to yield an increase in MEP amplitudes on the anodal side. This effect of hemispheric dominance could only have been tested by inversing stimulus polarity (anode on nondominant and cathode on dominant side), which was not possible in our PET study due to radiation dose limitations. Previous studies testing the effect of cathode and anode stimulation on the same dominant hemisphere (Baudewig et al, 2001; Lang et al, 2005) have yielded results similar to ours: cathodal stimulation had the largest effect on modulating brain activity. The effect of tDCS polarity, therefore, seems to outweigh a potential effect of hemispheric dominance.
Another possible explanation for the absence of an anodal effect might have been caused by tDCS spillover effects during the PET study. Although we did allow sufficient time to elapse between consecutive trials (10 minutes), it cannot entirely be ruled out that tDCS effects might have cumulated and caused spillover effects (i.e., tDCS ON given before tDCS sham condition). Such a potential effect, however, could be regarded as minor because our results did show a clear difference between conditions (movement with tDCS versus tDCS alone) and would not have been expected only with anodal stimulation.
Finally, the bilateral electrode mount might have been responsible for the asymmetric effect of anodal and cathodal stimulation, because the direction of polarization depends strictly on the orientation of axons and dendrites in the induced electrical field (Zaghi et al, 2010). Stronger left–right asymmetries in the orientation of axons and dendrites within M1 in some subjects might thus also have contributed to the missing overall anodal effect at the group level. Indeed, we did observe interindividual variations in the response to stimulation across subjects. Some subjects showed increased ΔrCBF under the anode while others did not (an increase in Z-score between 0.43 and 3.01, and two subjects who did not change (0.04) or decreased (−1.40)), thus rendering the overall anodal effect nonsignificant.
Study Implications
The effects of tDCS on motor cortex excitability can actually translate into improved movement performance in healthy controls as well as in patients with chronic stroke (Boggio et al, 2006, 2007; Hummel and Cohen, 2005; Tanaka et al, 2009; Vines et al, 2006). In the current study, we show that it is the interaction of tDCS with neuronal activity induced by active movement, which may constitute the physiological correlate of tDCS on rCBF modulation. This finding may imply that future studies investigating the effect of tDCS on motor stroke rehabilitation should aim at combining tDCS stimulation with task-specific training during the stimulation period, rather than using tDCS after-effects. Our findings may thus have implications for the potential therapeutic use of tDCS in other nonmotor diseases such as chronic pain (Fregni et al, 2006a, 2006b), depression (Brunoni et al, 2011) and Parkinson's disease (Benninger et al, 2010) in so far as tDCS may be especially effective when combined with behavioral therapies targeting relevant cortical areas.
It has also been suggested that bilateral tDCS stimulation might bear some advantage for stroke rehabilitation over unilateral application, since the anodal electrode placed over the affected hemisphere would facilitate recruitment of the affected motor cortex directly while the cathode placed over the unaffected hemisphere would reduce transcallosal inhibition to the affected hemisphere and facilitate movement in the affected limb, indirectly (Fregni and Pascual-Leone, 2007; Schlaug and Renga, 2008). The results from our study do not directly support this hypothesis because we only found cathodal stimulation to be effective in terms of MEP and rCBF changes, thus favoring the reduction of transcallosal inhibition as the stronger pathophysiological principle (Traversa et al, 1998).
In conclusion, we showed that tDCS stimulation can modulate rCBF change directly under the cathode. These modulating effects of tDCS on rCBF change are only measured when the stimulated cortical region is recruited by voluntary movement. Transcranial direct current stimulation thus seems to have little to no effect on the resting state. The interaction of tDCS with activation-induced rCBF change thus may constitute the physiological imaging correlate of tDCS.
Acknowledgments
The authors thank the Cyclotron Unit of the McConnell Brain Imaging Center for their skillful assistance.
The authors declare no conflict of interest.
Footnotes
This study was supported by grants from the Canadian Institutes for Health Research (CIHR) and Canada Foundation for Innovation leader opportunity fund to A Thiel who is a research scholar supported by the Fonds de la recherche en santé du Québec. C Paquette is supported by a Fellowship in the Area of Mobility in Aging from the CIHR. This study was presented in part at the 16th Annual Meeting of the Organization for Human Brain Mapping.
References
- Ardolino G, Bossi B, Barbieri S, Priori A. Non-synaptic mechanisms underlie the after-effects of cathodal transcutaneous direct current stimulation of the human brain. J Physiol. 2005;568:653–663. doi: 10.1113/jphysiol.2005.088310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baudewig J, Nitsche MA, Paulus W, Frahm J. Regional modulation of BOLD MRI responses to human sensorimotor activation by transcranial direct current stimulation. Magn Reson Med. 2001;45:196–201. doi: 10.1002/1522-2594(200102)45:2<196::aid-mrm1026>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Benninger DH, Lomarev M, Lopez G, Wassermann EM, Li X, Considine E, Hallett M. Transcranial direct current stimulation for the treatment of Parkinson's disease. J Neurol Neurosurg Psychiatry. 2010;81:1105–1111. doi: 10.1136/jnnp.2009.202556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bindman LJ, Lippold OC, Redfearn JW. The action of brief polarizing currents on the cerebral cortex of the rat (1) during current flow and (2) in the production of long-lasting after-effects. J Physiol. 1964;172:369–382. doi: 10.1113/jphysiol.1964.sp007425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boggio PS, Castro LO, Savagim EA, Braite R, Cruz VC, Rocha RR, Rigonatti SP, Silva MT, Fregni F. Enhancement of non-dominant hand motor function by anodal transcranial direct current stimulation. Neurosci Lett. 2006;404:232–236. doi: 10.1016/j.neulet.2006.05.051. [DOI] [PubMed] [Google Scholar]
- Boggio PS, Nunes A, Rigonatti SP, Nitsche MA, Pascual-Leone A, Fregni F. Repeated sessions of noninvasive brain DC stimulation is associated with motor function improvement in stroke patients. Restor Neurol Neurosci. 2007;25:123–129. [PubMed] [Google Scholar]
- Brunoni AR, Ferrucci R, Bortolomasi M, Vergari M, Tadini L, Boggio PS, Giacopuzzi M, Barbieri S, Priori A. Transcranial direct current stimulation (tDCS) in unipolar vs. bipolar depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:96–101. doi: 10.1016/j.pnpbp.2010.09.010. [DOI] [PubMed] [Google Scholar]
- Cizek J, Herholz K, Vollmar S, Schrader R, Klein J, Heiss WD. Fast and robust registration of PET and MR images of human brain. Neuroimage. 2004;22:434–442. doi: 10.1016/j.neuroimage.2004.01.016. [DOI] [PubMed] [Google Scholar]
- Fregni F, Boggio PS, Lima MC, Ferreira MJ, Wagner T, Rigonatti SP, Castro AW, Souza DR, Riberto M, Freedman SD, Nitsche MA, Pascual-Leone A. A sham-controlled, phase II trial of transcranial direct current stimulation for the treatment of central pain in traumatic spinal cord injury. Pain. 2006a;122:197–209. doi: 10.1016/j.pain.2006.02.023. [DOI] [PubMed] [Google Scholar]
- Fregni F, Gimenes R, Valle AC, Ferreira MJ, Rocha RR, Natalle L, Bravo R, Rigonatti SP, Freedman SD, Nitsche MA, Pascual-Leone A, Boggio PS. A randomized, sham-controlled, proof of principle study of transcranial direct current stimulation for the treatment of pain in fibromyalgia. Arthritis Rheum. 2006b;54:3988–3998. doi: 10.1002/art.22195. [DOI] [PubMed] [Google Scholar]
- Fregni F, Pascual-Leone A. Technology insight: noninvasive brain stimulation in neurology-perspectives on the therapeutic potential of rTMS and tDCS. Nat Clin Pract Neurol. 2007;3:383–393. doi: 10.1038/ncpneuro0530. [DOI] [PubMed] [Google Scholar]
- Furubayashi T, Terao Y, Arai N, Okabe S, Mochizuki H, Hanajima R, Hamada M, Yugeta A, Inomata-Terada S, Ugawa Y. Short and long duration transcranial direct current stimulation (tDCS) over the human hand motor area. Exp Brain Res. 2008;185:279–286. doi: 10.1007/s00221-007-1149-z. [DOI] [PubMed] [Google Scholar]
- Hummel F, Cohen LG. Improvement of motor function with noninvasive cortical stimulation in a patient with chronic stroke. Neurorehabil Neural Repair. 2005;19:14–19. doi: 10.1177/1545968304272698. [DOI] [PubMed] [Google Scholar]
- Iyer MB, Mattu U, Grafman J, Lomarev M, Sato S, Wassermann EM. Safety and cognitive effect of frontal DC brain polarization in healthy individuals. Neurology. 2005;64:872–875. doi: 10.1212/01.WNL.0000152986.07469.E9. [DOI] [PubMed] [Google Scholar]
- Jang SH, Ahn SH, Byun WM, Kim CS, Lee MY, Kwon YH. The effect of transcranial direct current stimulation on the cortical activation by motor task in the human brain: an fMRI study. Neurosci Lett. 2009;460:117–120. doi: 10.1016/j.neulet.2009.05.037. [DOI] [PubMed] [Google Scholar]
- Jeffery DT, Norton JA, Roy FD, Gorassini MA. Effects of transcranial direct current stimulation on the excitability of the leg motor cortex. Exp Brain Res. 2007;182:281–287. doi: 10.1007/s00221-007-1093-y. [DOI] [PubMed] [Google Scholar]
- Kwon YH, Ko MH, Ahn SH, Kim YH, Song JC, Lee CH, Chang MC, Jang SH. Primary motor cortex activation by transcranial direct current stimulation in the human brain. Neurosci Lett. 2008;435:56–59. doi: 10.1016/j.neulet.2008.02.012. [DOI] [PubMed] [Google Scholar]
- Lang N, Siebner HR, Ward NS, Lee L, Nitsche MA, Paulus W, Rothwell JC, Lemon RN, Frackowiak RS. How does transcranial DC stimulation of the primary motor cortex alter regional neuronal activity in the human brain. Eur J Neurosci. 2005;22:495–504. doi: 10.1111/j.1460-9568.2005.04233.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauritzen M, Gold L. Brain function and neurophysiological correlates of signals used in functional neuroimaging. J Neurosci. 2003;23:3972–3980. doi: 10.1523/JNEUROSCI.23-10-03972.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefaucheur JP. Principles of therapeutic use of transcranial and epidural cortical stimulation. Clin Neurophysiol. 2008;119:2179–2184. doi: 10.1016/j.clinph.2008.07.007. [DOI] [PubMed] [Google Scholar]
- Liebetanz D, Nitsche MA, Tergau F, Paulus W. Pharmacological approach to the mechanisms of transcranial DC-stimulation-induced after-effects of human motor cortex excitability. Brain. 2002;125:2238–2247. doi: 10.1093/brain/awf238. [DOI] [PubMed] [Google Scholar]
- Netz J, Ziemann U, Homberg V. Hemispheric asymmetry of transcallosal inhibition in man. Exp Brain Res. 1995;104:527–533. doi: 10.1007/BF00231987. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Paulus W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J Physiol. 2000;527 (Part 3:633–639. doi: 10.1111/j.1469-7793.2000.t01-1-00633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitsche MA, Paulus W. Sustained excitability elevations induced by transcranial DC motor cortex stimulation in humans. Neurology. 2001;57:1899–1901. doi: 10.1212/wnl.57.10.1899. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Fricke K, Henschke U, Schlitterlau A, Liebetanz D, Lang N, Henning S, Tergau F, Paulus W. Pharmacological modulation of cortical excitability shifts induced by transcranial direct current stimulation in humans. J Physiol. 2003a;553:293–301. doi: 10.1113/jphysiol.2003.049916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitsche MA, Liebetanz D, Lang N, Antal A, Tergau F, Paulus W. Safety criteria for transcranial direct current stimulation (tDCS) in humans. Clin Neurophysiol. 2003b;114:2220–2222. doi: 10.1016/s1388-2457(03)00235-9. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Niehaus L, Hoffmann KT, Hengst S, Liebetanz D, Paulus W, Meyer BU. MRI study of human brain exposed to weak direct current stimulation of the frontal cortex. Clin Neurophysiol. 2004;115:2419–2423. doi: 10.1016/j.clinph.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Nitsche MS, Klein CC, Tergau F, Rothwell JC, Paulus W. Level of action of cathodal DC polarisation induced inhibition of the human motor cortex. Clin Neurophysiol. 2003c;114:600–604. doi: 10.1016/s1388-2457(02)00412-1. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Roth A, Kuo MF, Fischer AK, Liebetanz D, Lang N, Tergau F, Paulus W. Timing-dependent modulation of associative plasticity by general network excitability in the human motor cortex. J Neurosci. 2007;27:3807–3812. doi: 10.1523/JNEUROSCI.5348-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power HA, Norton JA, Porter CL, Doyle Z, Hui I, Chan KM. Transcranial direct current stimulation of the primary motor cortex affects cortical drive to human musculature as assessed by intermuscular coherence. J Physiol. 2006;577:795–803. doi: 10.1113/jphysiol.2006.116939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priori A. Brain polarization in humans: a reappraisal of an old tool for prolonged non-invasive modulation of brain excitability. Clin Neurophysiol. 2003;114:589–595. doi: 10.1016/s1388-2457(02)00437-6. [DOI] [PubMed] [Google Scholar]
- Rothwell JC, Thompson PD, Day BL, Boyd S, Marsden CD. Stimulation of the human motor cortex through the scalp. Exp Physiol. 1991;76:159–200. doi: 10.1113/expphysiol.1991.sp003485. [DOI] [PubMed] [Google Scholar]
- Schlaug G, Renga V. Transcranial direct current stimulation: a noninvasive tool to facilitate stroke recovery. Expert Rev Med Devices. 2008;5:759–768. doi: 10.1586/17434440.5.6.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka S, Hanakawa T, Honda M, Watanabe K. Enhancement of pinch force in the lower leg by anodal transcranial direct current stimulation. Exp Brain Res. 2009;196:459–465. doi: 10.1007/s00221-009-1863-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiel A, Herholz K, Koyuncu A, Ghaemi M, Kracht LW, Habedank B, Heiss WD. Plasticity of language networks in patients with brain tumors: a positron emission tomography activation study. Ann Neurol. 2001a;50:620–629. doi: 10.1002/ana.1253. [DOI] [PubMed] [Google Scholar]
- Thiel A, Lottgen J, Grond M, Pietrzyk U, Heiss WD. Estimation of regional cerebral blood flow levels in ischemia using [(15)O]water of [(11)C]flumazenil PET without arterial input function. J Comput Assist Tomogr. 2001b;25:446–451. doi: 10.1097/00004728-200105000-00019. [DOI] [PubMed] [Google Scholar]
- Thiel A, Schumacher B, Wienhard K, Gairing S, Kracht LW, Wagner R, Haupt WF, Heiss WD. Direct demonstration of transcallosal disinhibition in language networks. J Cereb Blood Flow Metab. 2006;26:1122–1127. doi: 10.1038/sj.jcbfm.9600350. [DOI] [PubMed] [Google Scholar]
- Traversa R, Cicinelli P, Pasqualetti P, Filippi M, Rossini PM. Follow-up of interhemispheric differences of motor evoked potentials from the ‘affected' and ‘unaffected' hemispheres in human stroke. Brain Res. 1998;803:1–8. doi: 10.1016/s0006-8993(98)00505-8. [DOI] [PubMed] [Google Scholar]
- Vines BW, Cerruti C, Schlaug G. Dual-hemisphere tDCS facilitates greater improvements for healthy subjects' non-dominant hand compared to uni-hemisphere stimulation. BMC Neurosci. 2008;9:103. doi: 10.1186/1471-2202-9-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vines BW, Nair DG, Schlaug G. Contralateral and ipsilateral motor effects after transcranial direct current stimulation. Neuroreport. 2006;17:671–674. doi: 10.1097/00001756-200604240-00023. [DOI] [PubMed] [Google Scholar]
- Von Stockhausen HM, Pietrzyk U, Herholz K, Thiel A, Ilmberger J, Reulen HJ, Heiss WD.1998A method for surface-based quantification of functional data from the human cortex Quantitative functional brain imaging with positron emission tomography(Richard EC, Margaret ED-W, Peter H, eds),San Diego, CA: Academic Press; 139–142. [Google Scholar]
- Weiduschat N, Habedank B, Lampe B, Poggenborg J, Schuster A, Haupt WF, Heiss WD, Thiel A. Localizing Broca's area for transcranial magnetic stimulation: comparison of surface distance measurements and stereotaxic positioning. Brain Stimul. 2009;2:93–102. doi: 10.1016/j.brs.2008.09.005. [DOI] [PubMed] [Google Scholar]
- Williams JA, Pascual-Leone A, Fregni F. Interhemispheric modulation induced by cortical stimulation and motor training. Phys Ther. 2010;90:398–410. doi: 10.2522/ptj.20090075. [DOI] [PubMed] [Google Scholar]
- Zaghi S, Acar M, Hultgren B, Boggio PS, Fregni F. Noninvasive brain stimulation with low-intensity electrical currents: putative mechanisms of action for direct and alternating current stimulation. Neuroscientist. 2010;16:285–307. doi: 10.1177/1073858409336227. [DOI] [PubMed] [Google Scholar]