Abstract
Elevation of intraluminal pressure increases vasomotor tone, which thought to have a substantial role in regulation of cerebral blood flow (CBF). Interestingly, responses of cerebral vessels to increases in flow varied and have not been studied in human cerebral arteries. We hypothesized that increases in flow elicit constrictions of isolated human and rat cerebral arteries and aimed to elucidate the underlying mechanisms. Human cerebral arteries and rat middle cerebral arteries constricted to increases in flow (P<0.05). Simultaneous increase in intraluminal flow+pressure further reduced the diameter compared with pressure-induced changes (P<0.05), leading to constant estimated CBF. Flow-induced constrictions were abolished by HET0016 (inhibitor of synthesis of 20-hydroxyeicosatetraenoic acid (20-HETE) or inhibition of COXs or blocking TP (thromboxane A2/prostaglandin H2, receptors and attenuated by scavenging reactive oxygen species (ROS). Flow-enhanced ROS formation was significantly reduced by HET0016. In conclusion, in human and rat cerebral arteries (1) increases in flow elicit constrictions, (2) signaling mechanism of flow-induced constriction of cerebral arteries involves enhanced production of ROS, COX activity, and mediated by 20-HETE via TP receptors, and (3) we propose that simultaneous operation of pressure- and flow-induced constrictions is necessary to provide an effective autoregulation of CBF.
Keywords: autoregulation, COX, cytochrome P450 4A, ROS, TP receptors
Introduction
Regulation of cerebral blood flow (CBF) is the result of complex, multilevel, interrelated mechanisms to ensure many roles, such as nutritional supply of brain tissue, appropriate gas exchange between blood and tissue, maintenance of intracranial volume and pressure. The metabolic and neural regulation have been well delineated and described by Kontos and others, such as the role of pCO2, adenosine, intrinsic and extrinsic innervations, role of surrounding astrocytes, etc. (Harder et al, 1998; Kontos, 1981; van Beek et al, 2008). In addition, several endo-thelium-derived factors were shown to contribute to the regulation of CBF (Andresen et al, 2006; Shafi et al, 2008). Moreover, propagated vasodilation was also found to modulate cerebral circulation (Iadecola et al, 1997). Changes in hemodynamic forces are also known to affect CBF. Previous studies showed that increases in pressure elicit increases in resistance of cerebral vessels (Cipolla et al, 2002; Davis and Hill, 1999; Faraci et al, 1989; Heistad and Baumbach, 1992; Kontos, 1981; Osol et al, 2002; Wallis et al, 1996). However, vasomotor responses to increases in flow in isolated cerebral vessels varied: dilation (Drouin et al, 2011; Drouin and Thorin, 2009; Gaw and Bevan, 1993), constriction (Bryan et al, 2001a; Madden and Christman, 1999), and biphasic responses (Garcia-Roldan and Bevan, 1990, 1991; Ngai and Winn, 1995; Shimoda et al, 1996; Shimoda et al, 1998; Thorin-Trescases and Bevan, 1998) were observed. Importantly, no data are available regarding human cerebral vessels preventing the translation of knowledge from vertebrates to humans. In theory, flow-induced dilation would reduce the magnitude of myogenic constriction of cerebral vessels, which would reduce the gain of autoregulation of CBF, whereas if flow elicited constriction, it could contribute to a more efficient autoregulation of CBF.
In the present study, we hypothesized that increases in flow elicit constriction of isolated human and rat middle cerebral arteries and assess the potential contribution of flow-induced response to the autoregulation of CBF and aimed to elucidate the underlying molecular mechanisms.
Materials and methods
Isolation of Intracerebral Arteries of Human and Rat Middle Cerebral Arteries
All procedures were approved by the institutional animal care and use committe of New York Medical College, Valhalla, New York, USA and University of Pecs, Medical School, Pecs, Hungary. Studies of human samples were carried out under the approvement of the Regional Ethic and Review Commeette of the University of Pecs.
Brain samples were obtained from discarded tissues of patients undergoing neurosurgic treatment of epileptic disorder or cerebral tumors (n=6, age: 32±10 years; Wallis et al, 1996). They did not have any comorbidity. Preoperative contrast enhanced magnetic resonance imaging (e.g., magnetization prepared rapid acquisition gradient echo (MP-RAGE) sequences) was carefully performed to visualize areas with increased (pathologic) blood brain barrier permeability. Vessels for the study were selected to be removed from normal, non-enhancing areas that had to be removed because of operative technical reasons to be able to approach deep-seated tumors. In epilepsy patients, areas to be removed were defined by means of MR/CT fusion-based neuronavigation and neither preoperative nor operative cortical electrodes were used. Patients were on only ordinary anticonvulsive medications that directly do not influence microvascular functions. After removal of the brain tissue from the frontotemporal cortex, it was placed in 0°C–4°C physiologic salt solution (PSS) composed of (in mmol/L) 110.0 NaCl, 5.0 KCl, 2.5 CaCl2, 1.0 MgSO4, 1.0 KH2PO4, 5.5 glucose, and 24.0 NaHCO3 equilibrated with a gas mixture of 20% O2 and 5% CO2, balanced with nitrogen at pH ∼7.3. Under an operating microscope, with microsurgical instruments small human cerebral arteries (HCAs, 200 to 300 μm active diameters) were isolated from the brain tissue. Male Wistar–Kyoto rats (250–350 g), fad standard rat chow and free acces to tap water, were anesthetized (intraperitoneal pentobarbital sodium) and decapitated. The brains were immediately removed and placed in PSS. Middle cerebral arteries (MCAs) were isolated from both sides of brain of each animal (n=61) (Ungvari et al, 1999).
Flow-, Pressure-, and Simultaneous Flow- and Pressure-Induced Responses of Isolated Cerebral Arteries
After isolation, cerebral arteries were transferred into a custom-made pressure-flow chamber. Inflow and outflow pressures were controlled and measured by a pressure servo-control system (Living Systems Instrumentation, Burlington, VE, USA). Perfusate flow was measured by a ball flow meter (Omega, Omega Engineering Inc., Stamford, CT, USA). The internal diameter was measured by videomicroscopy with a microangiometer (Texas A&M University System). Changes in arterial diameter were continuously recorded digitally by PowerLab system (AD Instruments, Sydney, Australia) connected to a computer for later analysis. The size of glass pipettes used in this study was matched to both each other and the diameter of the vessels to achieve equal resistance (see our previous publications (Racz et al, 2010; Ungvari et al, 1999). In addition, inflow and outflow reservoirs and position of the chamber were built in a symmetrical manner providing equal pressures or generating flow in the presence of constant pressure in the midsection of vessels. By the end of the 60 minutes incubation, the vessels developed a spontaneous myogenic tone in response to 80 mm Hg of intraluminal pressure. First, changes in diameter of cerebral arteries were obtained to stepwise increases in flow elicited by pressure differences (ΔP; established by changing the inflow and outflow pressure to an equal degree, but opposite direction; ΔP=5, 10, 20, 30, 40 corresponding to 3 to 32 μL/minute intraluminal flow (Racz et al, 2010). The following general protocol was used: (1) vessels were exposed to 3 minutes at each flow rate to reach a steady-state diameter. (2) Next, changes in diameter of cerebral arteries were measured to stepwise increase in intraluminal pressure (0–140 mm Hg) in the absence of intraluminal flow by elevating simultaneously the inflow and outflow reservoir to the same level (10 minutes at each pressure step). (3) Then, changes in diameter were measured to stepwise simultaneous increase in pressure and flow. Inflow reservoir was raised from 0 to 140 mm Hg and the outflow reservoir was set at 0 mm Hg. In a series of experiments the vessels were incubated at flow Δ20 mm Hg for 60 minutes, then flow was decreased to Δ10 mm Hg, and then increased to Δ40 mm Hg. At the end of each experiment, the passive diameters were measured at each intraluminal pressure step in the presence of Ca2+-free PSS containing nifedipine 10−5 mol/L.
Calculations
We have estimated the change in CBF (in arbitrary units) by using diameter values induced by only changes in pressure and then simultaneous changes in pressure and flow using the Hagen–Poiseuille equation (Q=r4ΔPπ/L8 η, where Q, flow; r, radius; ΔP, pressure difference; L, length; η, viscosity). We have also calculated a ‘gain factor (G)' indicating the strength or efficacy of the autoregulation of blood flow used in previous studies (Osol and Halpern, 1985). Accordingly, G=1-{[(P2d24/P1d14)-1]/[(P2-P1)/P1]}, where P, intraluminal pressure; d, diameter; 1 and 2, initial and final pressure values of a pressure step. Thus, G=1 indicates perfect autoregulation, whereas G<1 means inefficient autoregulation, when cerebral blood flow increases as a function of intraluminal pressure.
Administration of Vasoactive Agents and Inhibitors
At 80 mm Hg intraluminal pressure, flow-induced diameter changes of HCA and MCA were repeated in the presence of 20-hydroxyeicosatetraenoic acid (20-HETE) synthesis inhibitor HET0016 (10−6 mol/L; Gebremedhin et al, 2000) for 30 minutes. Then, flow-induced diameter responses of MCA were repeated in the presence of cyclooxygenase inhibitor indomethacin 2 × 10−6 mol/L for 30 minutes; thromboxane A2 (TXA2)/prostaglandin H2 (PGH2) receptor (TP receptor) blocker SQ 29,548 2 × 10−6 mol/L for 30 minutes; free-radical scavenger superoxide dismutase, 200 U/ml for 30 minutes; catalase, 130 U/ml for 30 minutes; and TXA2-synthase inhibitor ozagrel 10−5 mol/L for 30 minutes. Afterward, 20-HETE (10−7 mol/L) was directly administered into the vessel chamber and diameter changes were recorded. In a series of experiments in the presence of Δ40 mm Hg adenosine (10−5 mol/L) was added into the chamber. The intact vasomotor function of endothelium and smooth muscle was verified by dilation to administration of adenosine triphosphate (10−5 mol/L). To test the specificity of HET0016, acetylcholine-induced (10−5 mol/L, n=5) responses were obtained before and after incubation of vessels with HET0016. All drugs were purchased from Sigma Aldrich (St Louis, MO, USA), except SQ 29,548 and HET0016 (Cayman Chemical Company, Ann Arbor, MI, USA).
Expression of CYP450 4A Proteins in Cerebral Vessels
Western blot analysis of vessels was performed as previously described. In brief, samples of MCAs and gracilis muscle arterioles, as control, isolated from rats were loaded onto a 10% acrylamide sodium dodecyl sulfate gel and electrophoresed at 100 V for 3 hours before being transferred to an Immobilon-P nylon membrane (Millipore, Billerica, MA, USA). The membrane was blocked in 5% milk/TBS/0.5% Tween for 2 hours at room temperature before the antibody was added and the incubation continued overnight at 4°C. The blots were washed and the secondary antibody was added for 2 hours at room temperature. The primary antibodies included anti-cytochrome P450 (1:4000 dilution, #ab22615, Abcam, Cambridge, MA, USA). The blots were washed and a 1:20,000 dilution of the anti-rabbit horseradish peroxidase secondary antibody (Amersham, Buckinghamshire, UK) was used. Bands were visualized using a Thermo SuperSignal West Pico kit (Thermo Scientific Pierce Protein Research Products, Rockford, IL, USA). Care was taken to ensure that band density remained within the linear range of the film and did not saturate the film, by performing exposures of different times. Band density was quantified using AlphaEaseFC software (AlphaInnotech, San Leando, CA, USA).
Detection of Superoxide Formation
Superoxide production was assessed in MCA of rat by the dihydroethidium fluorescence method Ethidium bromide (EB) thought to be specific primarily for superoxide (Fink et al, 2004). Middle cerebral arteries isolated from rat brain were placed in a vessels chamber, cannulated, and incubated in the presence of intraluminal flow generated by Δ40 mm Hg pressure difference in PSS at 37°C for 30 minutes. Some of these experiments were repeated in the presence of HET0016 10−5 mol/L for 60 minutes. Control MCAs were incubated in the same conditions in the absence of intraluminal flow. Then, dihydroethidium (3 × 10−5 mol/L) was added to the vials and incubated for 15 minutes. Afterward, MCAs were washed out with PSS and immersed in an embedding medium. Frozen sections of MCAs were visualized by a digital camera attached to a fluorescence microscope (Olympus BX61WI, Olympus Corporation, Center Valley, PA, USA). Intensity of EB fluorescence of the arterial wall was measured and quantified by Image J software (Image Processing and Analysis in Java, freely available from the National Institutes of Health). Relative EB fluorescence intensity was counted by extracting the intensity of the background. Measurement was repeated five times, and EB fluorescence was presented as intensity/total area.
Statistical Analysis
Statistical analysis was performed by two-way ANOVA followed by a Tukey's post hoc test or Student's t-test. P-values <0.05 were considered to be significant. Data are expressed as either micrometer or percentage of passive diameter at corresponding intraluminal pressure and are presented as mean±s.e.m.
Results
Flow-Induced Responses of Cerebral Arteries and Calculations of CBF
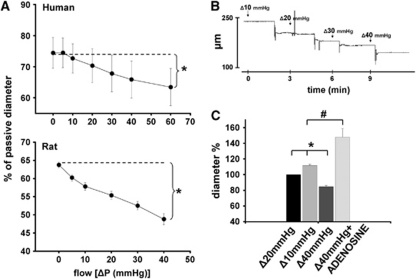
Original records show that increases in flow in the presence of constant pressure (80 mm Hg), elicited by increases in pressure differences caused substantial constrictions of a HCA (from 246 to 160 μm; Figure 1), as a function of time. Summary data (Figure 1) show that increases in flow elicited significant constrictions of vessels (human: from 74±4.9% to 63±5%, rat: from 63.8±0.8% to 48.8±1.5% of passive diameter at 80 mm Hg, P<0.05). Figure 1 also shows that diameter of MCAs incubated in the presence of flow Δ20 mm Hg increased when flow was decreased to Δ10 mm Hg (to 111±1.7% of diameter at flow Δ20 mm Hg), and decreased when flow was increased to Δ40 mm Hg (to 84±1.5% of diameter at flow Δ20 mm Hg). Also, adenosine (10−5 mol/L) increased the diameter of MCAs perfused by flow Δ40 mm Hg significantly above the baseline diameter (to 148±10% of diameter at flow Δ20 mm Hg).
Figure 1.
(A) Summary data of diameter changes (as % of passive diameter at 80 mm Hg) of human cerebral arteries (n=6) and rat middle cerebral arteries (n=12) as a function of intraluminal flow indicated as [ΔP(mm Hg)]. (B) Original record of diameter changes (μm) of a human cerebral artery to increases in intraluminal flow (ΔP=10, 20, 30, and 40 mm Hg) as a function of time (minutes). (C) Diameter changes (as diameter %) of rat middle cerebral arteries (n=6) perfused by flow Δ20 mm Hg to decreased flow (Δ10 mm Hg) and increased flow (Δ40 mm Hg), and to administration of adenosine (10−5 mol/L) in the presence of flow Δ40 mm Hg. Data are mean±s.e.m. (*,#P<0.05).
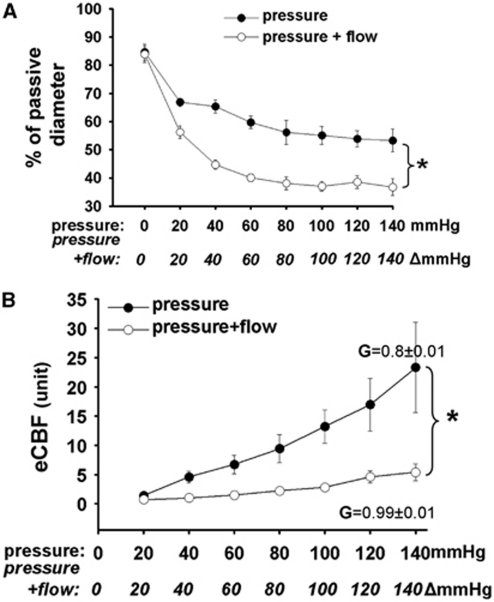
We found that increases in intraluminal pressure decreased normalized diameter of MCA (from 84±3% to 53±4%, n=6), whereas simultaneous increase of pressure+flow enhanced the only pressure-induced decrease in diameter (from 83.8±3% to 36±3%, P<0.05; Figure 2A).
Figure 2.
(A) Summary data of diameter changes (as % of passive diameter) of rat middle cerebral arteries (n=6) as a function of intraluminal pressure (mm Hg) and pressure+flow (Δmm Hg). (B) Changes of estimated CBF (eCBF) in arbitrary units (unit) as a function of pressure (mm Hg) and pressure+flow (Δmm Hg). Calculated gain (G) of CBF was 0.8±0.01 when pressure was raised, and increased to 0.99±0.01 when pressure+flow were raised. Data are mean±s.e.m. (*P<0.05).
When only pressure was increased estimated CBF (eCBF) showed a linear increase from 1.4±0.1 to 23.3±7.6 in arbitrary units. In contrast, when pressure+flow increased simultaneously first the eCBF decreased significantly to 0.7±0.1 and then increased only to 5.4±1.4 in arbitrary unit (Figure 2B). The gain of autoregulation (G) calculated using diameters induced by pressure alone was 0.8±0.01, whereas G was 0.99±0.01 when pressure+flow were increased simultaneously.
Mechanism of Flow-Induced Response of Cerebral Arteries
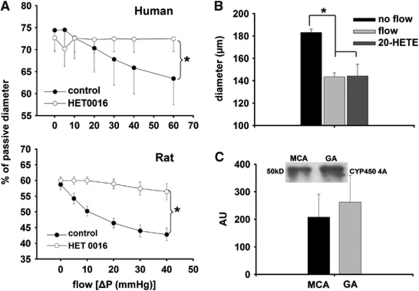
Summary data show that incubation of the vessels with HET0016 abolished the decrease in diameter of both HCA and MCA elicited by increases in flow (Figure 3A). Direct administration of 20-HETE (10−7 mol/L) decreased the diameter of MCA similarly to flow (at ΔP=40 mm Hg, flow:42±3, 20-HETE: 34±9.8 Δμm; Figure 3B).
Figure 3.
(A) Diameter changes (as % of passive diameter) of human intracerebral arteries (n=6) and rat middle cerebral arteries (n=6) as a function of intraluminal flow [ΔP(mm Hg)] in the presence of 20-hydroxyeicosatetraenoic acid (20-HETE) synthesis inhibitor HET0016 (10−6 mol/L). (B) Summary data of diameter changes (μm) of rat middle cerebral arteries (n=4) to increased intraluminal flow (ΔP=40 mm Hg), and to direct administration of 20-HETE (10−7 mol/L). (C) Expression of cytochrome P450 4A protein (CYP450 4A) in middle cerebral arteries (MCAs) and gracilis arterioles (GA; as control; n=4). Integrated density value is expressed as arbitrary unit (AU). Data are mean±s.e.m. (*P<0.05).
Dilations of MCA in response to acetylcholine (ACh) were not affected significantly by HET0016 (before: 53±4.6%, after: 46±5.4% of passive diameter). Figure 3C shows that cytochrome P450 4A enzymes are present in the MCA of rat.
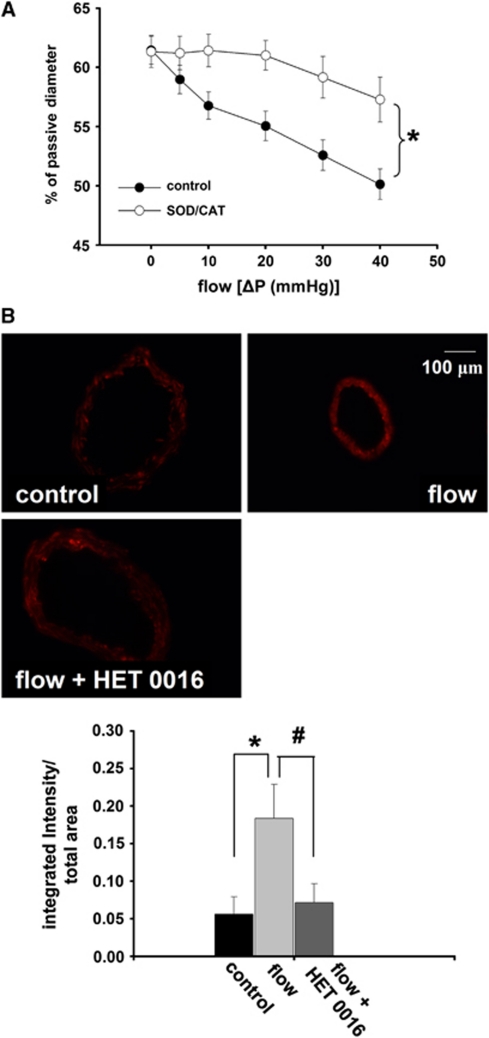
Incubation of the vessels in superoxide dismutase/catalase significantly decreased the reduction in diameter of rat cerebral arteries elicited by increases in flow. Representative EB fluorescent images of sections of MCA and summary data show an enhanced EB fluorescence in the vessels exposed to flow compared with control (absence of flow). Enhanced EB fluorescence was reduced to the control level by HET0016 (10−6 m/L; control: 0.05±0.02, flow: 0.18±0.04, flow+HET0016: 0.07±0.02 integrated intensity/total area, respectively; P<0.05; Figures 4A and B).
Figure 4.
(A) Diameter changes of rat middle cerebral arteries (n=6) as a function of intraluminal flow in the presence of superoxide scavenger superoxide dismutase plus catalase (SOD/CAT, 200/130 U/mL, respectively). (B) Representative pictures and summary data (as integrated intensity/total area) of ethidium bromide (EB) fluorescence of sections of middle cerebral arteries of rat in the absence (control) and presence of flow (flow), generated by Δ40 mm Hg pressure difference, and in the presence flow and 20-hydroxyeicosatetraenoic acid synthesis (cytochrome P450 4A) inhibitor HET0016 (10−6 mol/L) (flow+HET0016). Data are mean±s.e.m. (*,#P<0.05).
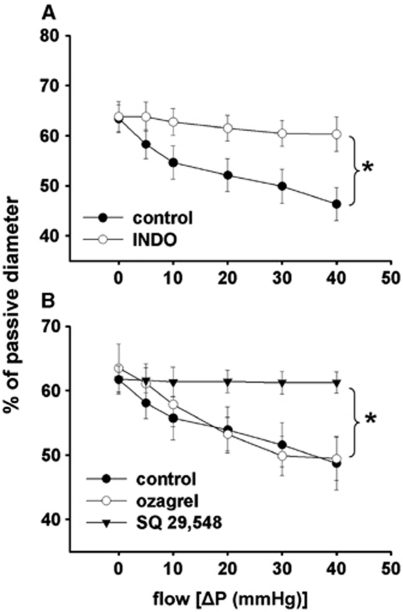
Summary data show that incubation of the vessels with indomethacin or SQ 29,548 inhibited the constriction of MCA to increases in flow, whereas ozagrel did not have an effect (Figure 5A and B).
Figure 5.
(A) Summary data of diameter changes (as % of passive diameter) of rat middle cerebral arteries (n=6) as a function of intraluminal flow [ΔP(mm Hg)] in the presence of cyclooxygenase inhibitor indomethacin (2 × 10−6 mol/L) and (B) in the presence of thromboxane synthase inhibitor ozagrel (10−5 mol/L; n=6) and thromboxane A2 receptor (TP) antagonist SQ 29,548 (2 × 10−6 mol/L; n=6). Data are mean±s.e.m. (*P<0.05).
Discussion
The novel findings of the present study are: (1) increases in flow elicit constrictions in isolated human intracerebral arteries and rat middle cerebral arteries, (2) simultaneous increases of pressure and flow elicited significantly greater constriction than pressure alone, (3) the underlying mechanism of flow-induced constriction of cerebral arteries involves increased production of reactive oxygen species (ROS), activity of COX, and mediated by 20-HETE acting via TP receptors.
Flow-Induced Constriction of Isolated Cerebral Arteries and its Contribution to an Effective Autoregulation of CBF
At present, the myogenic vasomotor mechanism is used primarily to explain the presence of autoregulation of CBF, which aims to protect the brain from high volume and pressure (Faraci et al, 1989; Harper et al, 1984; Mellander, 1989; Mueller et al, 1977). Much less is known regarding the role of flow-dependent regulation of CBF, although in vivo during changes in pressure, flow changes, as well.
In peripheral vessels, the vascular response to increases in flow is dilation (Koller et al, 1993). In previous studies, flow-induced responses of isolated cerebral vessels varied: depending on the prevailing pressure, conditions and species dilations, constrictions and biphasic responses were observed (Bryan et al, 2001a; Bryan et al, 2001b; Fujii et al, 1991; Garcia-Roldan and Bevan, 1990, ; Gaw and Bevan, 1993; Madden and Christman, 1999; Ngai and Winn, 1995; Paravicini et al, 2006; Shimoda et al, 1996). Importantly, no studies have been conducted to elucidate the nature of responses of isolated human cerebral arteries to increases in flow.
In theory, if cerebral arteries dilated to flow it would reduce the magnitude of myogenic constriction. Also, it was found that in isolated large cerebral arteries, which are responsible for significant part of total cerebrovascular resistance (in contrast to peripheral vascular beds) (Faraci et al, 1988; Kontos et al, 1978; Stromberg and Fox, 1972), in the physiologic range of perfusion pressure (∼40–140 mm Hg) increases in pressure do not elicit substantial reduction in diameter (Osol et al, 2002; Wallis et al, 1996), which would allow an increase in CBF. However, in vivo measurements of CBF by various magnetic resonance imaging and ultrasound techniques showed that autoregulation of flow does occur in the brain (Bellapart and Fraser, 2009; Zaharchuk et al, 1999). Because during increases in pressure flow increases as well, we hypothesized that increases in flow elicit constrictions of isolated cerebral arteries, which contribute to the maintenance of constant CBF, i.e., autoregulation of CBF.
Here, we showed for the first time that isolated HCAs constrict to increases in flow and confirmed similar findings of Bryan et al, (2001a) in rat MCA (Figure 1). Also, pressure+flow together caused a significantly greater constriction of cerebral arteries than pressure alone (Figure 2). By estimation (e) of CBF and the gain of autoregulation we aimed to extrapolate these in vitro findings to in vivo conditions (Osol and Halpern, 1985) and found that when only pressure increased, eCBF increased linearly. However, when pressure+flow increased simultaneously, eCBF remained constant, i.e., autoregulation did occur (Figure 2). The gain of the regulation of eCBF, when only pressure increased was below 1, indicating inefficient autoregulation. However, when pressure+flow increased simultaneously, the gain of autoregulation was close to 1, indicating an efficient autoregulation (Figure 2). Thus, we propose that flow-induced constriction contributes to the autoregulation of CBF and thus intracranial volume and pressure (Figure 2), as put forward by the Kelly–Monroe doctrine. Of course, there are serious limitation of such extrapolation, because flow through a small vessel and responses of single vessels may not represent those of vascular network. For example, Fujii and others found that in vivo basilar arteries of rats dilate to increases in flow elicited by bilateral carotid artery occlusion (Fujii et al, 1991; Fujii et al, 1992; Paravicini et al, 2006). Thus, on the basis of previous and present studies we propose that flow-induced responses show regional differences in the cerebrovascular tree.
Whereas flow-induced constriction (together with the pressure sensitive myogenic response) may have an important role in the regulation and maintenance of cerebral volume and intracranial pressure, increased local neural activity/function (and consequent cellular metabolic changes) can increase the diameter of cerebral vessels adjacent to the neural cells and thus increase local CBF (Ingvar, 1976; Kontos, 1981; Sokoloff, 1977; van Beek et al, 2008). This idea is in line with previous findings showing that metabolic effects (such as hypercapnia, acidosis, hypoxia, and adenosine) can override the vasoconstrictor effects of pressure (Raisis et al, 1979) and with the finding of the present study demonstrating that flow-induced constriction could be converted to dilation by adenosine, a key molecule of metabolic regulation (Figure 1). In addition, as shown by Iadecola et al, (1997) increased synaptic activity elicited local functional hyperemia of cerebral arterioles, which is then propagated to upstream vessels via intravascular mechanisms. Thus, in vivo it is difficult to study or prove the presence and role of a single vasomotor mechanism.
The present study aimed to investigate one vaso-motor mechanism intrinsic to the vascular wall, thus the result should be carefully related directly to in vivo complex conditions.
In conclusion, we propose that during increases in systemic pressure the pressure- and flow-sensitive constrictor mechanisms ‘set' the vasomotor tone, which can be modulated or overridden by other factors sensitive to the needs of neural tissues. Thus CBF is regulated by multilevel and redundant mechanisms to adapt to the lack of one or two mechanisms.
Mechanisms Mediating Flow-Induced Constriction of Cerebral Arteries
The mechanisms of flow-induced constriction of cerebral vessels are still not clarified. Arachidonic acid and its metabolites (such as CYP450 4A-derived 20-HETE, COX-derived TXA2,) have important roles in the regulation of cerebrovascular resistance (Dunn et al, 2008; Ellis et al, 1977; Gebremedhin et al, 2000). Harder et al, (1994) and Gebremedhin et al, (1998) showed that AA is metabolized by cytochrome P450 ω-hydroxylases (CYP450 4A) into 20-HETE and it has an important role in the regulation of cerebrovascular tone, by mediating agonists- and pressure-induced constrictions of vascular smooth muscle of cerebral vessels (Gebremedhin et al, 2000; Yu et al, 2004). Thus, it seemed to be logical to hypothesize that 20-HETE could be the constrictor factor mediating the flow-induced constriction of cerebral arteries as well. Indeed, we found that flow-induced constrictions of HCAs and MCAs of rat were abolished by administration of HET0016 (Figure 3), an inhibitor of 20-HETE production. Consistently to these functional findings we found that CYP450 4A enzymes are present in the MCA of the rat, a finding similar to that of Dunn et al, (2008) and Gebremedhin et al, (2000).
It is also known that direct administration and production of 20-HETE by cytochrome P450 can produce ROS (Terashvili et al, 2006). Thus, we hypothesized that in response to flow ROS are produced as well, and scavenging ROS would affect the constrictor response. We found that administration of ROS scavengers significantly reduced the flow-induced constriction of cerebral arteries (Figure 4). In addition, our findings showed increased EB fluorescence in MCA after exposing the vessels to flow, suggesting flow-induced increased ROS production. The enhanced production of ROS was reversed by inhibition of 20-HETE production (Figure 4), suggesting ROS is generated during synthesis of 20-HETE, which is elicited by increases in flow. This finding also confirms that ROS are generated during activation of CYP450 4A (Terashvili et al, 2006). Although HET0016 abolished flow-induced constriction, ROS likely have direct vasomotor effect, as well.
Previous studies (Harder et al, 1997; Zou et al, 1996) have proposed that 20-HETE constricts cerebral arteries by various pathways. For example, 20-HETE activates protein kinase C, depolarizes smooth muscle cells by inhibition of large-conductance KCa channel, and increases Ca2+ influx via L-type Ca2+ channels.
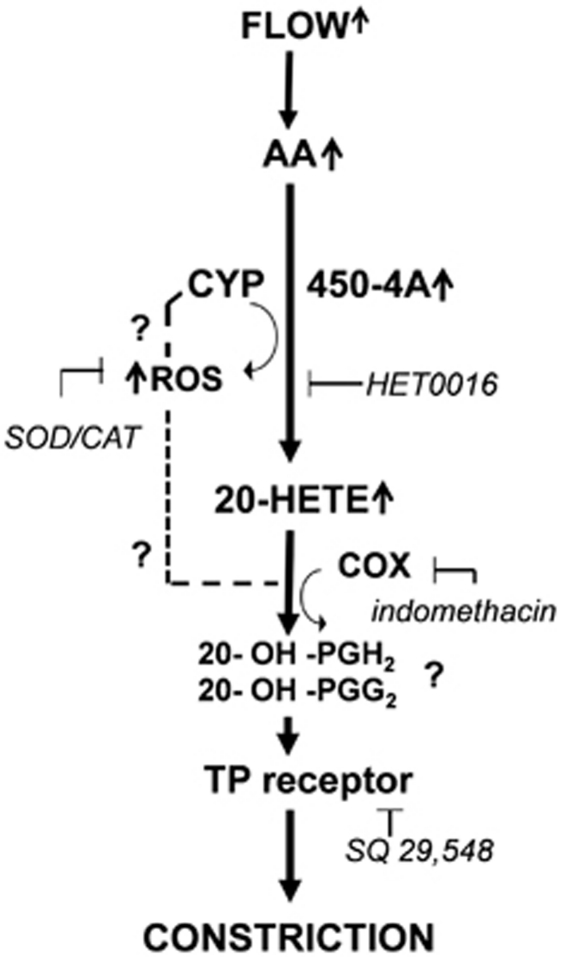
In addition, thromboxane A2 is another constrictor metabolite of AA and known to have a profound effect on cerebrovascular tone (Ellis et al, 1977). Thus, we evaluated the effect of TXA2 synthase inhibitor ozagrel, and TP receptor blocker SQ 29,548. Importantly, SQ 29548 abolished the flow-induced constriction, but ozagrel did not have any effects (Figure 5). Our results showing that both inhibition of 20-HETE production and antagonizing TP receptor abolished flow-induced constriction and suggest that 20-HETE may act on TP receptor. Consistently with this hypothesis, previous studies by Escalante et al, (1989) and Schwartzman et al, (1989) proposed that 20-HETE caused constriction of arteries via TP receptor after 20-HETE was metabolized by COX into 20-endoperoxides (20-OH-PGH2, 20-OH-PGG2). This finding is supported by our finding that indomethacin also blocked flow-induced constrictions of MCA. The proposed molecular mechanisms mediating flow-induced constriction of human and rat cerebral arteries are summarized in Figure 6.
Figure 6.
Proposed mechanisms of flow-induced constrictions of cerebral arteries. Increases in flow activate arachidonate production, which is further metabolized by cytochrome P450 4A enzymes (CYP450 4A) into 20-hydroxyeicosatetraenoic acid (20-HETE). CYP450 4A also produces reactive oxygen species (ROS), which contribute to the constriction. The flow-induced constriction is mediated via thromboxane A2/prostaglandin H2 (TP) receptors and requires COX activity.
Interestingly, in vivo application of ROS caused dilation of pial arterioles and systemic administration of scavengers of ROS or inhibition of COXs reduced or did not affect CBF (Didion and Faraci, 2002; Lacza et al, 2009; Zhang and Ellis, 1991). These findings could be because of multiple sources of ROS and COXs are present in the cerebral vessels and in the surrounding neural tissues and astrocytes (Harder et al, 1998), releasing both constrictor and dilator factors (Cosentino et al, 1994; Wei et al, 1996). Thus, in vivo administration of drugs generating or inhibiting ROS production could activate several opposing vasomotor mechanisms. Also, to ensure appropriate blood flow to cerebral tissues several multilevel, confounding mechanisms are present. Thus, lack or inhibition of one mechanism activates compensatory mechanisms to maintain CBF.
In conclusion, the novel findings of the present study are: (1) increases in flow elicit constrictions in isolated human intracerebral arteries and rat middle cerebral arteries, (2) simultaneous increases of pressure and flow elicited significantly greater constriction than pressure alone, (3) the underlying mechanism of flow-induced constriction of cerebral arteries involves increased production of ROS, activity of COX, and mediated by 20-HETE acting via TP receptors.
Acknowledgments
We thank Dr John G. Edwards, and Jamie Mathew for performing the western blot measurements.
The authors declare no conflict of interest.
Footnotes
Sources of Support: American Heart Association, Founders Affiliate, 0855910D, NIH PO-1 HL-43023, Hungarian National Science Research Fund (OTKA) K71591 and K67984.
References
- Andresen J, Shafi NI, Bryan RM., Jr Endothelial influences on cerebrovascular tone. J Appl Physiol. 2006;100:318–327. doi: 10.1152/japplphysiol.00937.2005. [DOI] [PubMed] [Google Scholar]
- Bellapart J, Fraser JF. Transcranial Doppler assessment of cerebral autoregulation. Ultrasound Med Biol. 2009;35:883–893. doi: 10.1016/j.ultrasmedbio.2009.01.005. [DOI] [PubMed] [Google Scholar]
- Bryan RM, Jr, Marrelli SP, Steenberg ML, Schildmeyer LA, Johnson TD. Effects of luminal shear stress on cerebral arteries and arterioles. Am J Physiol Heart Circ Physiol. 2001a;280:H2011–H2022. doi: 10.1152/ajpheart.2001.280.5.H2011. [DOI] [PubMed] [Google Scholar]
- Bryan RM, Jr, Steenberg ML, Marrelli SP. Role of endothelium in shear stress-induced constrictions in rat middle cerebral artery. Stroke. 2001b;32:1394–1400. doi: 10.1161/01.str.32.6.1394. [DOI] [PubMed] [Google Scholar]
- Cipolla MJ, Gokina NI, Osol G. Pressure-induced actin polymerization in vascular smooth muscle as a mechanism underlying myogenic behavior. FASEB J. 2002;16:72–76. doi: 10.1096/cj.01-0104hyp. [DOI] [PubMed] [Google Scholar]
- Cosentino F, Sill JC, Katusic ZS. Role of superoxide anions in the mediation of endothelium-dependent contractions. Hypertension. 1994;23:229–235. doi: 10.1161/01.hyp.23.2.229. [DOI] [PubMed] [Google Scholar]
- Davis MJ, Hill MA. Signaling mechanisms underlying the vascular myogenic response. Physiol Rev. 1999;79:387–423. doi: 10.1152/physrev.1999.79.2.387. [DOI] [PubMed] [Google Scholar]
- Didion SP, Faraci FM. Effects of NADH and NADPH on superoxide levels and cerebral vascular tone. Am J Physiol Heart Circ Physiol. 2002;282:H688–H695. doi: 10.1152/ajpheart.00576.2001. [DOI] [PubMed] [Google Scholar]
- Drouin A, Bolduc V, Thorin-Trescases N, Belanger E, Fernandes P, Baraghis E, Lesage F, Gillis MA, Villeneuve L, Hamel E, Ferland G, Thorin E. Catechin treatment improves cerebrovascular flow-mediated dilation and learning abilities in atherosclerotic mice. Am J Physiol Heart Circ Physiol. 2011;300:H1032–H1043. doi: 10.1152/ajpheart.00410.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drouin A, Thorin E. Flow-induced dilation is mediated by Akt-dependent activation of endothelial nitric oxide synthase-derived hydrogen peroxide in mouse cerebral arteries. Stroke. 2009;40:1827–1833. doi: 10.1161/STROKEAHA.108.536805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn KM, Renic M, Flasch AK, Harder DR, Falck J, Roman RJ. Elevated production of 20-HETE in the cerebral vasculature contributes to severity of ischemic stroke and oxidative stress in spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol. 2008;295:H2455–H2465. doi: 10.1152/ajpheart.00512.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis EF, Nies AS, Oates JA. Cerebral arterial smooth muscle contraction by thromboxane A2. Stroke. 1977;8:480–483. doi: 10.1161/01.str.8.4.480. [DOI] [PubMed] [Google Scholar]
- Escalante B, Sessa WC, Falck JR, Yadagiri P, Schwartzman ML. Vasoactivity of 20-hydroxyeicosatetraenoic acid is dependent on metabolism by cyclooxygenase. J Pharmacol Exp Ther. 1989;248:229–232. [PubMed] [Google Scholar]
- Faraci FM, Mayhan WG, Schmid PG, Heistad DD. Effects of arginine vasopressin on cerebral microvascular pressure. Am J Physiol. 1988;255:H70–H76. doi: 10.1152/ajpheart.1988.255.1.H70. [DOI] [PubMed] [Google Scholar]
- Faraci FM, Baumbach GL, Heistad DD.1989Myogenic mechanisms in the cerebral circulation J Hypertens Suppl 7S61–S64.discussion S5 [PubMed] [Google Scholar]
- Fink B, Laude K, McCann L, Doughan A, Harrison DG, Dikalov S. Detection of intracellular superoxide formation in endothelial cells and intact tissues using dihydroethidium and an HPLC-based assay. Am J Physiol Cell Physiol. 2004;287:C895–C902. doi: 10.1152/ajpcell.00028.2004. [DOI] [PubMed] [Google Scholar]
- Fujii K, Heistad DD, Faraci FM. Flow-mediated dilatation of the basilar artery in vivo. Circ Res. 1991;69:697–705. doi: 10.1161/01.res.69.3.697. [DOI] [PubMed] [Google Scholar]
- Fujii K, Heistad DD, Faraci FM. Effect of diabetes mellitus on flow-mediated and endothelium-dependent dilatation of the rat basilar artery. Stroke. 1992;23:1494–1498. doi: 10.1161/01.str.23.10.1494. [DOI] [PubMed] [Google Scholar]
- Garcia-Roldan JL, Bevan JA. Flow-induced constriction and dilation of cerebral resistance arteries. Circ Res. 1990;66:1445–1448. doi: 10.1161/01.res.66.5.1445. [DOI] [PubMed] [Google Scholar]
- Garcia-Roldan JL, Bevan JA. Augmentation of endothelium-independent flow constriction in pial arteries at high intravascular pressures. Hypertension. 1991;17:870–874. doi: 10.1161/01.hyp.17.6.870. [DOI] [PubMed] [Google Scholar]
- Gaw AJ, Bevan JA.1993Flow-induced relaxation of the rabbit middle cerebral artery is composed of both endothelium-dependent and -independent components Stroke 24105–109.discussion 9–10 [DOI] [PubMed] [Google Scholar]
- Gebremedhin D, Lange AR, Narayanan J, Aebly MR, Jacobs ER, Harder DR. Cat cerebral arterial smooth muscle cells express cytochrome P450 4A2 enzyme and produce the vasoconstrictor 20-HETE which enhances L-type Ca2+ current. J Physiol. 1998;507 (Part 3:771–781. doi: 10.1111/j.1469-7793.1998.771bs.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebremedhin D, Lange AR, Lowry TF, Taheri MR, Birks EK, Hudetz AG, Narayanan J, Falck JR, Okamoto H, Roman RJ, Nithipatikom K, Campbell WB, Harder DR. Production of 20-HETE and its role in autoregulation of cerebral blood flow. Circ Res. 2000;87:60–65. doi: 10.1161/01.res.87.1.60. [DOI] [PubMed] [Google Scholar]
- Harder DR, Gebremedhin D, Narayanan J, Jefcoat C, Falck JR, Campbell WB, Roman R. Formation and action of a P-450 4A metabolite of arachidonic acid in cat cerebral microvessels. Am J Physiol. 1994;266:H2098–H2107. doi: 10.1152/ajpheart.1994.266.5.H2098. [DOI] [PubMed] [Google Scholar]
- Harder DR, Lange AR, Gebremedhin D, Birks EK, Roman RJ. Cytochrome P450 metabolites of arachidonic acid asintracellular signaling molecules in vascular tissue. J Vasc Res. 1997;34:237–243. doi: 10.1159/000159228. [DOI] [PubMed] [Google Scholar]
- Harder DR, Alkayed NJ, Lange AR, Gebremedhin D, Roman RJ. Functional hyperemia in the brain: hypothesis for astrocyte-derived vasodilator metabolites. Stroke. 1998;29:229–234. doi: 10.1161/01.str.29.1.229. [DOI] [PubMed] [Google Scholar]
- Harper SL, Bohlen HG, Rubin MJ. Arterial and microvascular contributions to cerebral cortical autoregulation in rats. Am J Physiol. 1984;246:H17–H24. doi: 10.1152/ajpheart.1984.246.1.H17. [DOI] [PubMed] [Google Scholar]
- Heistad DD, Baumbach GL. Cerebral vascular changes during chronic hypertension: good guys and bad guys. J Hypertens Suppl. 1992;10:S71–S75. [PubMed] [Google Scholar]
- Iadecola C, Yang G, Ebner TJ, Chen G. Local and propagated vascular responses evoked by focal synaptic activity in cerebellar cortex. J Neurophysiol. 1997;78:651–659. doi: 10.1152/jn.1997.78.2.651. [DOI] [PubMed] [Google Scholar]
- Ingvar DH. Functional landscapes of the dominant hemisphere. Brain Res. 1976;107:181–197. doi: 10.1016/0006-8993(76)90109-8. [DOI] [PubMed] [Google Scholar]
- Koller A, Sun D, Kaley G. Role of shear stress and endothelial prostaglandins in flow- and viscosity-induced dilation of arterioles in vitro. Circ Res. 1993;72:1276–1284. doi: 10.1161/01.res.72.6.1276. [DOI] [PubMed] [Google Scholar]
- Kontos HA, Wei EP, Navari RM, Levasseur JE, Rosenblum WI, Patterson JL., Jr Responses of cerebral arteries and arterioles to acute hypotension and hypertension. Am J Physiol. 1978;234:H371–H383. doi: 10.1152/ajpheart.1978.234.4.H371. [DOI] [PubMed] [Google Scholar]
- Kontos HA. Regulation of the cerebral circulation. Annu Rev Physiol. 1981;43:397–407. doi: 10.1146/annurev.ph.43.030181.002145. [DOI] [PubMed] [Google Scholar]
- Lacza Z, Hortobagyi L, Horvath B, Horvath EM, Sandor P, Benyo Z. Additive effect of cyclooxygenase and nitric oxide synthase blockade on the cerebrocortical microcirculation. Neuroreport. 2009;20:1027–1031. doi: 10.1097/wnr.0b013e32832d6a93. [DOI] [PubMed] [Google Scholar]
- Madden JA, Christman NJ. Integrin signaling, free radicals, and tyrosine kinase mediate flow constriction in isolated cerebral arteries. Am J Physiol. 1999;277:H2264–H2271. doi: 10.1152/ajpheart.1999.277.6.H2264. [DOI] [PubMed] [Google Scholar]
- Mellander S.1989Functional aspects of myogenic vascular control J Hypertens Suppl 7S21–S30.discussion S1 [PubMed] [Google Scholar]
- Mueller SM, Heistad DD, Marcus ML. Total and regional cerebral blood flow during hypotension, hypertension, and hypocapnia. Effect of sympathetic denervation in dogs. Circ Res. 1977;41:350–356. doi: 10.1161/01.res.41.3.350. [DOI] [PubMed] [Google Scholar]
- Ngai AC, Winn HR. Modulation of cerebral arteriolar diameter by intraluminal flow and pressure. Circ Res. 1995;77:832–840. doi: 10.1161/01.res.77.4.832. [DOI] [PubMed] [Google Scholar]
- Osol G, Halpern W. Myogenic properties of cerebral blood vessels from normotensive and hypertensive rats. Am J Physiol. 1985;249:H914–H921. doi: 10.1152/ajpheart.1985.249.5.H914. [DOI] [PubMed] [Google Scholar]
- Osol G, Brekke JF, McElroy-Yaggy K, Gokina NI. Myogenic tone, reactivity, and forced dilatation: a three-phase model of in vitro arterial myogenic behavior. Am J Physiol Heart Circ Physiol. 2002;283:H2260–H2267. doi: 10.1152/ajpheart.00634.2002. [DOI] [PubMed] [Google Scholar]
- Paravicini TM, Miller AA, Drummond GR, Sobey CG. Flow-induced cerebral vasodilatation in vivo involves activation of phosphatidylinositol-3 kinase, NADPH-oxidase, and nitric oxide synthase. J Cereb Blood Flow Metab. 2006;26:836–845. doi: 10.1038/sj.jcbfm.9600235. [DOI] [PubMed] [Google Scholar]
- Racz A, Veresh Z, Lotz G, Bagi Z, Koller A. Cyclooxygenase-2 derived thromboxane A(2) and reactive oxygen species mediate flow-induced constrictions of venules in hyperhomocysteinemia. Atherosclerosis. 2010;208:43–49. doi: 10.1016/j.atherosclerosis.2009.06.014. [DOI] [PubMed] [Google Scholar]
- Raisis JE, Kindt GW, McGillicuddy JE, Giannotta SL. The effects of primary elevation of cerebral venous pressure on cerebral hemodynamics and intracranial pressure. J Surg Res. 1979;26:101–107. doi: 10.1016/0022-4804(79)90085-4. [DOI] [PubMed] [Google Scholar]
- Schwartzman ML, Falck JR, Yadagiri P, Escalante B. Metabolism of 20-hydroxyeicosatetraenoic acid by cyclooxygenase. Formation and identification of novel endothelium-dependent vasoconstrictor metabolites. J Biol Chem. 1989;264:11658–11662. [PubMed] [Google Scholar]
- Shafi NI, Andresen J, Marrelli SP, Bryan RM., Jr Erythropoietin potentiates EDHF-mediated dilations in rat middle cerebral arteries. J Neurotrauma. 2008;25:257–265. doi: 10.1089/neu.2007.0347. [DOI] [PubMed] [Google Scholar]
- Shimoda LA, Norins NA, Jeutter DC, Madden JA. Flow-induced responses in piglet isolated cerebral arteries. Pediatr Res. 1996;39:574–583. doi: 10.1203/00006450-199604000-00002. [DOI] [PubMed] [Google Scholar]
- Shimoda LA, Norins NA, Madden JA. Responses to pulsatile flow in piglet isolated cerebral arteries. Pediatr Res. 1998;43:514–520. doi: 10.1203/00006450-199804000-00013. [DOI] [PubMed] [Google Scholar]
- Sokoloff L. Relation between physiological function and energy metabolism in the central nervous system. J Neurochem. 1977;29:13–26. doi: 10.1111/j.1471-4159.1977.tb03919.x. [DOI] [PubMed] [Google Scholar]
- Stromberg DD, Fox JR. Pressures in the pial arterial microcirculation of the cat during changes in systemic arterial blood pressure. Circ Res. 1972;31:229–239. doi: 10.1161/01.res.31.2.229. [DOI] [PubMed] [Google Scholar]
- Terashvili M, Pratt PF, Gebremedhin D, Narayanan J, Harder DR.2006Reactive oxygen species cerebral autoregulation in health and disease Pediatr Clin North Am 531029–1037.xi [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorin-Trescases N, Bevan JA.1998High levels of myogenic tone antagonize the dilator response to flow of small rabbit cerebral arteries Stroke 291194–1200.discussion 200–1 [DOI] [PubMed] [Google Scholar]
- Ungvari Z, Pacher P, Kecskemeti V, Koller A. Fluoxetine dilates isolated small cerebral arteries of rats and attenuates constrictions to serotonin, norepinephrine, and a voltage-dependent Ca(2+) channel opener. Stroke. 1999;30:1949–1954. doi: 10.1161/01.str.30.9.1949. [DOI] [PubMed] [Google Scholar]
- van Beek AH, Claassen JA, Rikkert MG, Jansen RW. Cerebral autoregulation: an overview of current concepts and methodology with special focus on the elderly. J Cereb Blood Flow Metab. 2008;28:1071–1085. doi: 10.1038/jcbfm.2008.13. [DOI] [PubMed] [Google Scholar]
- Wallis SJ, Firth J, Dunn WR. Pressure-induced myogenic responses in human isolated cerebral resistance arteries. Stroke. 1996;27:2287–2290. doi: 10.1161/01.str.27.12.2287. [DOI] [PubMed] [Google Scholar]
- Wei EP, Kontos HA, Beckman JS. Mechanisms of cerebral vasodilation by superoxide, hydrogen peroxide, and peroxynitrite. Am J Physiol. 1996;271:H1262–H1266. doi: 10.1152/ajpheart.1996.271.3.H1262. [DOI] [PubMed] [Google Scholar]
- Yu M, Cambj-Sapunar L, Kehl F, Maier KG, Takeuchi K, Miyata N, Ishimoto T, Reddy LM, Falck JR, Gebremedhin D, Harder DR, Roman RJ. Effects of a 20-HETE antagonist and agonists on cerebral vascular tone. Eur J Pharmacol. 2004;486:297–306. doi: 10.1016/j.ejphar.2004.01.009. [DOI] [PubMed] [Google Scholar]
- Zaharchuk G, Mandeville JB, Bogdanov AA, Jr, Weissleder R, Rosen BR, Marota JJ.1999Cerebrovascular dynamics of autoregulation and hypoperfusion. An MRI study of CBF and changes in total and microvascular cerebral blood volume during hemorrhagic hypotension Stroke 302197–2204.discussion 204–5 [DOI] [PubMed] [Google Scholar]
- Zhang XM, Ellis EF. Superoxide dismutase decreases mortality, blood pressure, and cerebral blood flow responses induced by acute hypertension in rats. Stroke. 1991;22:489–494. doi: 10.1161/01.str.22.4.489. [DOI] [PubMed] [Google Scholar]
- Zou AP, Fleming JT, Falck JR, Jacobs ER, Gebremedhin D, Harder DR, Roman RJ. 20-HETE is an endogenous inhibitor of the large-conductance Ca(2+)-activated K+ channel in renal arterioles. Am J Physiol. 1996;270:R228–R237. doi: 10.1152/ajpregu.1996.270.1.R228. [DOI] [PubMed] [Google Scholar]