Abstract
Brain microvascular endothelium forms an active permeability barrier, the blood–brain barrier (BBB). In neurologic disorders, barrier properties of the BBB are often lost indicating their dependance on molecular cues of the brain microenvironment. In this issue, Osada et al demonstrate that the endothelial extracellular matrix (ECM) provides one of these cues. Their study shows that β1-integrin-mediated adhesion of brain endothelial cells to the surrounding ECM is critical for stabilizing claudin-5 in BBB tight junctions (TJs) and BBB integrity. These observations point to a novel intracellular signaling pathway from β1-integrin/ECM endothelial adhesions to BBB TJs contributing to BBB integrity.
Keywords: β1-integrin, blood–brain barrier, claudin-5, extracellular matrix, tight junction
Homeostasis of the brain microenvironment is a prerequisite for proper neuronal function and is maintained by the blood–brain barrier (BBB). The BBB is established at the level of brain microvascular endothelial cells that due to lack of fenestrae and an extremely low pinocytotic activity inhibit the transcellular passage of molecules across the brain endothelium. In addition, an elaborate network of continuous and complex tight junction (TJ) strands between the individual endothelial cells restricts the paracellular diffusion of water soluble molecules across the BBB—TJ gate function—and importantly also separates the luminal from the abluminal membrane domains of brain endothelial cells establishing cell polarity—TJ fence function (Wolburg et al, 2003).
The BBB TJ strands are composed of integral membrane proteins such as occludin and the claudins, which are linked to the actin cytoskeleton by TJ-associated cytoplasmic peripheral membrane scaffolding proteins of the MAGUK (membrane-associated guanylyl kinase-like) family, such as zonula occludens (ZO)-1, ZO-2, and ZO-3. Claudins display a nonrandom tissue-specific expression pattern and by establishing homophilic and heterophilic interactions via their extracellular loops determine the permeability properties of individual TJs. At the BBB, the expression of claudin-3, claudin-5, and claudin-12 has been described. Whereas the function of claudin-12 and claudin-3 remains to be investigated, the endothelial cell specific claudin-5 displays nonredundant functions at the BBB by controlling the paracellular permeability of BBB TJs for small molecules up to 800 Da (Nitta et al, 2003; Zhang et al, 2010). Interestingly, claudin-3 expression is regulated by Wnt/β-catenin signaling (Liebner et al, 2008), demonstrating that the AJ (adherens junction) component β-catenin is critically involved in the regulation of TJs. In endothelial AJs, the transmembrane protein VE-cadherin is linked to the actin cytoskeleton via β-catenin and plakoglobin binding to α-actinin and vinculin. The crosstalk between AJ and TJs is further supported by the observation that adhesive interactions of VE-cadherin promote claudin-5 transcription by preventing the nuclear accumulation of the transcriptional regulators, FoxO1 and β-catenin (Liebner et al, 2008).
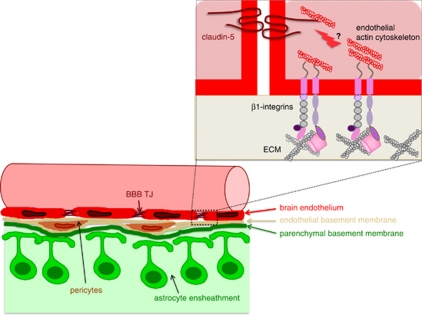
The characteristics of the mature BBB endothelium are well described; however, the cellular and molecular mechanisms that control the maintenance of BBB characteristics in brain endothelial cells remain largely unknown to date. Pathological conditions within the central nervous system, such as ischemia, inflammation, or tumor growth, are typically accompanied by BBB dysfunction, emphasizing that the barrier properties are not simply ‘switched on' in brain endothelium during development, but rather that continuous signals are provided by the tissue microenvironment maintaining BBB features in brain endothelium. In this context, specifically pericytes and astrocytes, which are intimately associated with brain microvascular endothelial cells, have been suggested to form the functional neurovascular unit maintaining the BBB. Morphologically, brain microvessels can be distinguished from microvessels elsewhere in the body by the high number of pericytes embedded in the endothelial cell basement membrane and a second basement membrane layed down by astrocytes, which ensheath the abluminal aspect of brain microvessels with their foot processes (Figure 1).
Figure 1.
Schematic view of the blood–brain barrier (BBB) and the β1-integrin/matrix interactions proposed to support blood–brain barrier integrity. (Bottom left) Brain endothelial cells are surrounded by the endothelial basement membrane, in which a high number of pericytes is embedded. A second—the parenchymal—basement membrane is layed down by astrocytes, which ensheath the abluminal aspect of brain microvessels with their endfeet. The crosstalk between the cellular and acellular elements of brain microvessels establishes a functional neurovascular unit. (Top right) The study by Osada et al demonstrates that β1-integrin engagement of brain endothelial cells to the endothelial basement membrane maintains intracellular signaling events that stabilize claudin-5 localization in BBB tight junctions (TJs) and BBB integrity.
Molecular signals from astrocytes (Abbott et al, 2006) and also from pericytes (Armulik et al, 2010) have been shown to regulate the full BBB phenotype in brain endothelium. In contrast, the role of the extracellular matrix (ECM) in maintaining BBB characteristics in brain endothelium has been largely neglected so far, which might in part be due to the enormous molecular heterogeneity of the ECM being composed of different laminins, collagens, fibronectin, nidogens, etc. In this issue of JCBFM, Osada et al (2011) provide evidence that β1-integrin mediated anchoring of brain endothelial cells to the ECM and thus likely also endothelial polarity is critically involved in the regulation of BBB TJ integrity and permeability. In their present study, the authors show that abrogating β1-integrin-mediated adhesion of brain endothelial cells to collagen IV, a basement membrane component, with an adhesion-blocking antibody in vitro, induced the loss of junctional localization of claudin-5. This alteration in brain endothelial TJ architecture was accompanied by reduced transelectrical resistance and increased permeability of the brain endothelial monolayers to 40 and 100 kDa dextrans, suggesting that β1-integrin-mediated ECM attachment of brain endothelial cells maintains barrier characteristics of brain endothelial cells by stabilizing the molecular integrity of BBB TJs. This notion is further supported by their findings that intrastriatal application of the anti-β1-integrin antibody induced BBB leakiness to circulating IgGs in vivo. While loss of claudin-5 expression on BBB TJs causes leakage of molecules smaller than 800 Da in the mouse and the zebrafish, the present observations suggest that blocking β1-integrin-mediated adhesion of brain endothelium to ECM components might induce even more profound alterations in the molecular architecture of BBB TJs, which have not yet been addressed in the present study.
The observations made by Osada et al direct our attention to the critical role of brain endothelial/ECM interactions in maintaining BBB properties in the microvascular endothelial cells. Although data on the basement membrane composition at the level of brain microvessels is rudimentary, there is some evidence that specific basement membrane components contribute to BBB integrity and function. Agrin, a heparan sulfate proteoglycan, found in the basement membrane of brain microvessels has been proposed to be involved in the development of the BBB by establishing astrocyte polarity, a process in which agrin in the basement membrane initiates a link of the dystroglycan/dystrophin-glycoprotein complex to the astrocyte actin cytoskeleton. A role for agrin in BBB integrity is further supported by the observation that leaky blood vessels in malignant human brain tumors are devoid of agrin and have lost astrocyte polarity (Rascher et al, 2002). Furthermore, mutations in the COL4A1 gene coding for the α1 chain of the most common collagen type IV isoform was shown to cause intracerebral hemorrhage at the level of brain microvessels in mouse and humans (Gould et al, 2005; Vahedi et al, 2007). The latter data suggest that collagen type IV isoforms are important for the structural integrity of small blood vessels, a role that is consistent with the present observations of Osada et al, demonstrating an important role for brain endothelial cell β1-integrin/collagen IV interaction in maintaining junctional localization of claudin-5 and BBB integrity. Interestingly, blocking β1-integrin-mediated adhesion of brain endothelial cells to laminin in the present study did not interfere with junctional localization of claudin-5 in brain endothelial cells in vitro, suggesting that only a select subset of ECM receptors of the β1-integrin family elicits intracellular signals targeting the BBB TJs. The β1-integrin subunit can associate with multiple α-integrin chains to form heterodimeric ECM receptors with different ligand binding properties. Brain endothelial cells have been reported to express the β1-integrins α1β1, α3β1, α6β1, and αvβ1; however, the precise ECM ligands of these receptors and their localization in endothelial and/or parenchymal basement membranes of brain microvessels awaits to be correlated with the endothelial receptor expression patterns.
Further studies will be required to understand the intracellular signals elicited by β1-integrin-mediated endothelial cell adhesion to the ECM leading to BBB TJ stabilization. β1-Integrins have been shown to regulate a number of biological processes including migration, survival, and proliferation of cells. The present study establishes a new layer of crosstalk between β1-integrin-mediated endothelial cell adhesion and intracellular signals maintaining BBB integrity. Binding of the extracellular domain of β1-integrins to ECM proteins will connect the cytoplasmic integrin tail to the endothelial actin cytoskeleton. Since integrins lack an actin-binding domain, all downstream signaling events are mediated by integrin-associated molecules, which function as binding platforms for additional cytoskeletal and signaling molecules. The molecules linking β1-integrin/ECM adhesion and claudin-5 localization to BBB TJs, therefore, remain to be studied.
Interestingly, in nascent endothelium, genetic deletion of β1-integrin was found to lead to the loss of endothelial cell polarity accompanied by decreased expression of the polarity gene partitioning-defective protein (PAR)-3 (Zovein et al, 2010). Par3 has been shown to associate with TJs and to regulate the development of TJs and cell polarity. It is, therefore, tempting to speculate that disruption of β1-integrin-mediated brain endothelial cell adhesion at the fully differentiated BBB may lead to distinct downstream signaling events that affect the endothelial cell polarity complex and consequently TJ integrity thus leading to BBB leakiness.
Acknowledgments
I thank Dr Urban Deutsch for his critical review of this commentary.
The author declares no conflict of interest.
References
- Abbott NJ, Ronnback L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci. 2006;7:41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- Armulik A, Genove G, Mae M, Nisancioglu MH, Wallgard E, Niaudet C, He L, Norlin J, Lindblom P, Strittmatter K, Johansson BR, Betsholtz C. Pericytes regulate the blood-brain barrier. Nature. 2010;468:557–561. doi: 10.1038/nature09522. [DOI] [PubMed] [Google Scholar]
- Gould DB, Phalan FC, Breedveld GJ, van Mil SE, Smith RS, Schimenti JC, Aguglia U, van der Knaap MS, Heutink P, John SW. Mutations in Col4a1 cause perinatal cerebral hemorrhage and porencephaly. Science. 2005;308:1167–1171. doi: 10.1126/science.1109418. [DOI] [PubMed] [Google Scholar]
- Liebner S, Corada M, Bangsow T, Babbage J, Taddei A, Czupalla CJ, Reis M, Felici A, Wolburg H, Fruttiger M, Taketo MM, von Melchner H, Plate KH, Gerhardt H, Dejana E. Wnt/beta-catenin signaling controls development of the blood-brain barrier. J Cell Biol. 2008;183:409–417. doi: 10.1083/jcb.200806024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitta T, Hata M, Gotoh S, Seo Y, Sasaki H, Hashimoto N, Furuse M, Tsukita S. Size-selective loosening of the blood-brain barrier in claudin-5-deficient mice. J Cell Biol. 2003;161:653–660. doi: 10.1083/jcb.200302070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osada T, Gu Y-T, Kanazawa M, Tsubota Y, Hawkins BT, Spatz M, Milner R, del Zoppo GJ.2011Inter-endothelial claudin-5 expression depends upon cerebral endothelial cell matrix adhesion by β1-integrins J Cereb Blood Flow Metabthis issue) [DOI] [PMC free article] [PubMed]
- Rascher G, Fischmann A, Kröger S, Duffner F, Grote E-H, Wolburg H. Extracellular matrix and the blood-brain barrier in glioblastoma multiforme: spatial segregation of tenascin and agrin. Acta Neuropathol. 2002;104:85–91. doi: 10.1007/s00401-002-0524-x. [DOI] [PubMed] [Google Scholar]
- Vahedi K, Kubis N, Boukobza M, Arnoult M, Massin P, Tournier-Lasserve E, Bousser MG. COL4A1 mutation in a patient with sporadic, recurrent intracerebral hemorrhage. Stroke. 2007;38:1461–1464. doi: 10.1161/STROKEAHA.106.475194. [DOI] [PubMed] [Google Scholar]
- Wolburg H, Wolburg-Buchholz K, Kraus J, Rascher-Eggstein G, Liebner S, Hamm S, Duffner F, Grote EH, Risau W, Engelhardt B. Localization of claudin-3 in tight junctions of the blood-brain barrier is selectively lost during experimental autoimmune encephalomyelitis and human glioblastoma multiforme. Acta Neuropathol (Berl) 2003;105:586–592. doi: 10.1007/s00401-003-0688-z. [DOI] [PubMed] [Google Scholar]
- Zhang J, Piontek J, Wolburg H, Piehl C, Liss M, Otten C, Christ A, Willnow TE, Blasig IE, Abdelilah-Seyfried S. Establishment of a neuroepithelial barrier by Claudin5a is essential for zebrafish brain ventricular lumen expansion. Proc Natl Acad Sci USA. 2010;107:1425–1430. doi: 10.1073/pnas.0911996107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zovein AC, Luque A, Turlo KA, Hofmann JJ, Yee KM, Becker MS, Fassler R, Mellman I, Lane TF, Iruela-Arispe ML. Beta1 integrin establishes endothelial cell polarity and arteriolar lumen formation via a Par3-dependent mechanism. Dev Cell. 2010;18:39–51. doi: 10.1016/j.devcel.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]