Abstract
The hypothesis tested by these studies states that in addition to interendothelial cell tight junction proteins, matrix adhesion by β1-integrin receptors expressed by endothelial cells have an important role in maintaining the cerebral microvessel permeability barrier. Primary brain endothelial cells from C57 BL/6 mice were incubated with β1-integrin function-blocking antibody (Ha2/5) or isotype control and the impacts on claudin-5 expression and microvessel permeability were quantified. Both flow cytometry and immunofluorescence studies demonstrated that the interendothelial claudin-5 expression by confluent endothelial cells was significantly decreased in a time-dependent manner by Ha2/5 exposure relative to isotype. Furthermore, to assess the barrier properties, transendothelial electrical resistance and permeability measurements of the monolayer, and stereotaxic injection into the striatum of mice were performed. Ha2/5 incubation reduced the resistance of endothelial cell monolayers significantly, and significantly increased permeability to 40 and 150 kDa dextrans. Ha2/5 injection into mouse striatum produced significantly greater IgG extravasation than the isotype or the control injections. This study demonstrates that blockade of β1-integrin function changes interendothelial claudin-5 expression and increases microvessel permeability. Hence, endothelial cell–matrix interactions via β1-integrin directly affect interendothelial cell tight junction claudin-5 expression and brain microvascular permeability.
Keywords: β1-integrins, cerebral endothelial cells, claudin-5, extracellular matrix, matrix adhesion receptors, microvessel permeability
Introduction
Very early after ischemic stroke, within hours, the blood–brain barrier of cerebral microvessels becomes permeable. The leakiness of the cerebral microvasculature during focal ischemia contributes to brain edema and hemorrhagic transformation that can complicate the evolution of the tissue injury. A two-part permeability barrier ordinarily prevents the plasma and blood cells from entering the perivascular neural tissue. The barrier properties of cerebral microvessels depend on the cohesive and resistance attributes of the endothelial cells, that involve the interendothelial tight junction proteins (e.g., zonula occludens (ZO-1), occludin, claudin-5), and on the integrity of the extracellular matrix (ECM) of the basal lamina. We have suggested that the adhesive interactions (1) between endothelial cells and the basal lamina matrix components, on the abluminal aspect of cerebral microvessels and (2) between astrocytes and the basal lamina are necessary for the barrier phenotype (del Zoppo and Milner, 2006; Milner et al, 2008a, 2008b). These interactions, mediated by select and characteristic matrix adhesion receptors, also appear necessary for the juxtaposition and cooperation between the endothelium and astrocytes.
Adhesion of both endothelial cells and cells of epithelial origin to their subjacent ECM is required for cell viability. Endothelial cells require interaction via their β1-integrins to matrix ligands, as shown by cell demise when contact to a matrix sublayer is prevented (Meredith et al, 1993). In adult brain, endothelial cells express β1-integrins in all microvessel diameter classes (Haring et al, 1996). And, β1-integrins interact with the ECM proteins laminin, collagen type IV, and perlecan, found in the basal lamina of all central nervous system microvessels (Haring et al, 1996; Tagaya et al, 2001).
While interendothelial cell tight junction integrity (‘horizontal' cohesion) has been the focus of considerable research, the roles of matrix adhesion have not been addressed. Recent studies imply that β1-integrins expressed by endothelial cells, among other matrix adhesion receptors, could have important roles in maintaining the permeability barrier (‘vertical' adhesion) (del Zoppo and Milner, 2006). The rapidity of the loss of β1-integrin immunoreactivity in the ischemic core following middle cerebral artery occlusion (Tagaya et al, 2001) and its temporal relation to edema suggest that β1-integrin-dependent adhesion could be relevant to permeability barrier integrity. However, during focal ischemia endothelial cell demise is infrequent, suggesting the stability of endothelial cell–matrix adhesion (Tagaya et al, 1997). In contrast, focal ischemia induces detachment of astrocyte end-feet from the basal lamina (Garcia et al, 1971; Milner et al, 2008b; Kwon et al, 2009).
The tight junction (TJ) protein claudin-5, found at the endothelial cell–cell borders of all brain blood vessel segments (Morita et al, 1999) appears to have a major role in the blood–brain permeability barrier. Claudin-5 expression relates inversely to brain microvessel permeability both in vivo and in vitro. Tracer experiments have shown that small molecules (<800 Da) extravasate into the perivascular cerebral tissue in claudin-5-deficient mice (Nitta et al, 2003). After 7 days hypoxia, retinal vascular claudin-5 expression in the rat decreases and extravasation of an injected 534 Da tracer increases compared with normoxic retina (Koto et al, 2007). Complementary work in culture has demonstrated that after transfection of claudin-5 gene into rat brain capillary endothelial cells, the inulin paracellular permeability coefficient of the endothelial cell monolayer decreases (Ohtsuki et al, 2007). Separately, increased claudin-5 protein expression paralleled an increase in the transendothelial electrical resistance (TEER) of bEnd.3 monolayers during the growth phase. However, when claudin-5 expression was inhibited by transfection of RNAi, TEER decreased (Koto et al, 2007).
Basal lamina matrix composition can determine integrin receptor expression by cerebral endothelial cells (Milner et al, 2008a). Recent work has suggested that tight junction expression and function may also be related to the composition of the matrix that endothelial cells are exposed to. Wolburg and colleagues have shown that expression of occludin by endothelial cells in glioblastoma multiforme depends on the presence of agrin in the basal lamina matrix (Liebner et al, 2000; Rascher et al, 2002). Rat brain endothelial cells (RBE4.B) grown on collagen type IV-coated plates appear to express occludin better than when grown on collagen type I or on laminin (Savettieri et al, 2000). The basement membrane proteins collagen IV, laminin, and fibronectin increase the transcellular electrical resistance of porcine brain capillary endothelial cell monolayers (Tilling et al, 1998).
Based on those observations and the premise that vertical adhesion could be a central determinant of the endothelial portion of the intact blood–brain barrier, the hypothesis tested by these experiments states that blockade of the interaction of β1-integrins on confluent cerebral endothelial cells with the matrix (1) directly affects tight junction protein expression (e.g., claudin-5) and (2) increases microvessel permeability in the central nervous system. The studies reported here demonstrate that the endothelial cell–matrix interactions via the adhesion receptor β1-integrins can directly affect the interendothelial cell TJ expression and alter brain microvascular permeability.
Materials and methods
Animals
These experiments were reviewed and approved by the University of Washington Institutional Animal Care and Use Committee and were performed following an institutionally approved protocol in accordance with the National Institute of Health's Guide for the Care and Use of Laboratory Animals. All animals were maintained under pathogen-free conditions in a closed breeding colony before and during the studies.
Antibodies and Reagents
Immunochemicals
For immunohistochemistry, monoclonal antibodies (MoAbs) to mouse IgG (Jackson ImmunoResearch, West Grove, PA, USA) and polyclonal antibodies to the TJ protein claudin-5 (Zymed/Invitrogen, Carlsbad, CA, USA) were employed. The immunofluorescence studies used MoAb to the mouse β1-integrin clone 9EG7 (BD Pharmingen, San Diego, CA, USA) and the same polyclonal antibodies to claudin-5 (Zymed/Invitrogen) as for immunohistochemistry. For secondary antibodies to the primary antibodies, DyLight 488-conjugated goat anti-rabbit IgG and Texas Red dye-conjugated goat anti-rat IgG were obtained from Jackson ImmunoResearch. Cell nuclei were stained with 4′,6′-diamidino-2-phenylindole ((DAPI); Sigma, St Louis, MO, USA).
For flow cytometry, a PE-conjugated mouse MoAb was used to identify mouse β1-integrin (clone HMβ1-1; Biolegend, San Diego, CA, USA) throughout. Rabbit polyclonal antibodies identified claudin-5 with fluorescein isothiocyanate (FITC)-conjugated secondary antibodies against rabbit IgG (Vector Laboratories, Inc., Burlingame, CA, USA).
Functional blockade of the integrin β1 subunit was achieved with a well-characterized purified hamster IgM MoAb against mouse β1-integrin (at 10 μg/mL, clone Ha2/5; BD Pharmingen) and was paired with the matched isotype IgM that does not bind β1-integrins or any known epitopes (BD Pharmingen). Ha2/5 specifically binds the β1-integrin subunit, and has function-blocking properties (Mendrick and Kelly, 1993).
Reagents
Purified matrix proteins served as growth substrates for the primary murine endothelial cells. These included collagen I from calf skin for colony expansion, and collagen IV from Engelbreth–Holm–Swarm murine sarcoma basement membrane or laminin from Engelbreth–Holm–Swarm murine sarcoma basement membrane for growth (all Sigma).
Endothelial Cell Culture
Primary brain microvessel endothelial cells from 2- to 3-month-old C57 BL/6 mice (Jackson Laboratory, Bar Harbor, ME, USA) were prepared as previously described (Sapatino et al, 1993; Milner et al, 2008a). Brains were removed, cleaned of meninges and external blood vessels, then finely minced, and dissociated in a solution containing 20 U/mL papain, and 250 U/mL, DNase I type IV (Worthington Biochemicals, Lakewood, NJ, USA) in 2 mL Minimum Essential Medium 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid modification (MEM-HEPES) (Milner and ffrench-Constant, 1994). After 1 hour incubation, the disrupted brain tissue was minced further, added to a 15-mL tube containing 22% bovine serum albumin, and centrifuged at 1000 g for 20 minutes. The isolated tubes and cells were then washed and centrifuged in MEM-HEPES before resuspension in endothelial cell growth media (ECGM). Endothelial cell growth media consists of Hams F12, supplemented with 10% fetal bovine serum, heparin, ascorbic acid, -glutamine (all from Sigma) and endothelial cell growth supplement (Millipore, Billerica, MA, USA). The endothelial cells in ECGM were then added to matrix protein-treated six-well plates (Nunc, Rochester, NY, USA), and incubated at 37 °C under 5% CO2. The wells were previously coated with solutions containing collagen I (200 μg/mL in phosphate-buffered saline (PBS)) for 2 hours at 37 °C before use.
Endothelial cell growth media was changed the next day and the cultures were treated with puromycin (3 μg/mL; Sigma) for a total of 3 days (Perriere et al, 2005). The ECGM culture media was then replaced every 3 days thereafter. Assessed by live immunostaining for the endothelial cell-specific proteins CD31 platelet endothelial cell adhesion molecule-1 (PECAM-1) and by von Willebrand factor antigen, the confluent endothelial cell cultures were >99% pure.
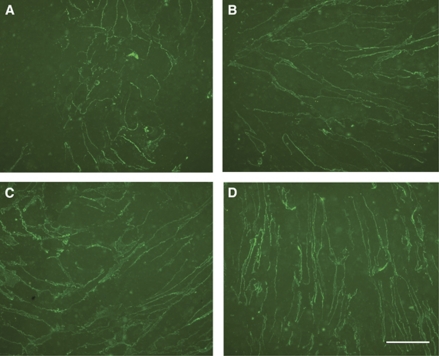
Endothelial cells were grown in subconfluent or confluent format depending on the experiment purpose. In the subconfluent format, murine brain endothelial cells (passage 2) were grown 3 days short of confluence (4 days after splitting) on either collagen IV- or laminin-coated six-well plates (Nunc). The ECM coatings were applied at 10 μg/mL for 2 hours at 37 °C. The cells were 40% to 60% confluent and formed small colonies. In the confluent format, brain endothelial cells (passage 2) were grown on collagen IV- or laminin-coated inserts (six-well; Greiner bio-one, Frickenhausen, Germany) until the insert surface area was completely covered with cells in monolayer and claudin-5 expression appeared maximal. This was usually achieved by 7 days after seeding at a density of 2.0 × 105 cells/insert (Figure 1).
Figure 1.
Progressive expression of immunoreactive claudin-5 with time by primary cerebral endothelial cells grown on collagen IV (insert). Panel (A) day 1; (B) day 3; (C) day 4; and (D) day 7. Magnification bar=50 μm.
Tight Junction Expression
Cells grown to subconfluence on matrix-coated wells or to confluence on inserts were incubated with either 10 μg/mL Ha2/5 for β1-integrin function blockade or the matched isotype IgM for specific times. These experiments were performed in the presence of serum supplementation. To determine the effect on claudin-5 expression (1) cells from both formats were assayed by flow cytometry and (2) only cells grown to confluence on inserts were assayed by dual immunofluorescence.
Flow cytometry
The endothelial cells were removed from the culture plates or inserts using a cell scraper, suspended in 5% goat serum in PBS, incubated with PE-conjugated anti-β1-integrin MoAb for 1 hour on ice, and then washed twice with PBS. The cells were fixed and permeabilized for 20 minutes in Cytofix/Cytoperm (BD Pharmingen). The permeabilized cells were then incubated for 1 hour on ice with the polyclonal antibody against claudin-5, washed twice in the BD Perm/Wash PBS (BD Pharmingen), labeled with the FITC-conjugated secondary antibody for 1 hour on ice, and washed twice. The PE- and FITC-fluorescence intensities of labeled cells and fragments (‘events') were then analyzed on a Becton Dickinson FACScan machine (San Diego, CA, USA). For each experiment, mean fluorescent intensity was calculated by FlowJo (Tree Star, Inc., Ashland, OR, USA).
Western blots
For whole-cell extracts, cells were harvested in cold radio-immunoprecipitation assay buffer (Thermo Scientific, Rockford, IL, USA) and protease inhibitors (Sigma). After centrifugation at 14 000 r.p.m. for 5 minutes (4 °C), the supernatants were collected. Proteins (10 μg) were subjected to Tris-glycine sodium dodecyl sulfate polyacrylamide gel electrophoresis. They were probed with primary rat anti-mouse β1-integrin antibody (1:500; Millipore), followed by a secondary horseradish peroxidase-conjugated antibody (1:20,000; Jackson ImmunoResearch). Signals were detected by enhanced chemiluminescence and semiquantified by densitometry. The membranes were stripped and probed with an anti-β-actin antibody to confirm even protein loading.
Immunohistochemistry of confluent cells
Claudin-5 expression by confluent endothelial cells was demonstrated by immunoperoxidase or by immunofluorescence methods as required (Abumiya et al, 1999).
The impact of β1-integrin function blockade on interendothelial claudin-5 expression was determined with the aid of quantitative video-imaging microscopy. After treatment, the cells on the inserts were washed with PBS and fixed with methanol for 5 minutes. While on the membranes, the cells were permeabilized and blocked with 0.3% Triton-X and 5% goat serum in PBS for 20 minutes (at room temperature), then incubated with the primary rabbit anti-claudin-5 polyclonal antibody (4 μg/mL) and rat anti-mouse anti-β1-integrin MoAb (1:100) overnight at 4 °C. The cells were rinsed with PBS and incubated with the appropriate secondary antibodies (e.g., DyLight 488-conjugated goat anti-rabbit IgG (5 μg/mL) and Texas Red dye-conjugated goat anti-rat IgG (5 μg/mL)) for 1 hour, washed with PBS, and then stained with DAPI (1 μg/mL) for 5 minutes. The washed inserts and processed cells were mounted on slides with Vectashield (Vector Laboratories) and sealed. The fluorescence signals were captured by quantitative video-imaging microscopy using a modified Zeiss S100 Invert equipped with computer-assisted staging and AxioVision and KS software (Zeiss, Oberkochen, Germany).
Quantitative Video-Imaging Microscopy
Each glass slide-mounted insert was evaluated by capturing 10 1.80 mm2 noncontiguous regions of interest randomly chosen across the insert. Full-field photographic images were obtained, coded for interendothelial expression of claudin-5 of interest, and actively traced with resident software, rendering the distances in μm. Cell numbers were identified by DAPI stain, so that the length of TJ expression per cell was measured as (total TJ distance in μm)/(number of DAPI nuclei) for each region of interest. Assessments were made by an observer blinded to the coded assignments.
Stereotaxic Injections
C57 Bl/6 mice (male, 2 to 3 months old) were employed for cerebral injection experiments to determine the effect of β1-integrin function blockade on vascular permeability in vivo. Mice were anesthetized with isoflurane (1.0% to 2.5%) and placed in a stereotaxic frame. A midline scalp incision was made, and a skull window opened using a dental tool. A 33-gauge beveled needle (World Precision Instruments, Inc., Sarasota, FL, USA) was lowered into the center of the right striatum (from bregma: 1 mm anterior, 2 mm lateral, 3.5 mm depth), and 2 μL of either Ha2/5 (1 mg/mL) or matched IgM isotype antibody was infused into the right striatum (at 1 μL/min). The needle was left in place for 10 minutes after completion of the infusion, to prevent leakage of either agent from the brain, and then removed slowly (1 mm/min). The skull window was sealed with bone wax, the scalp closed, and the mice allowed to recover.
At 24 hours after injection, the subjects underwent transcardiac pressure perfusion with buffer containing bovine serum albumin (50 g/L), Na+ nitroprusside (6.7 mmol/L; MP Biomedicals, LLC, Solon, OH, USA), and heparin (2000 IU/L; American Pharmaceutical Partners, Inc., East Schaumburg, IL, USA) in Plasmalyte (Baxter Healthcare Corporation, Deerfield, IL, USA) under CO2 narcosis. The brains were then removed, placed on ice, divided in the coronal plane at the level of the injection site into anterior and posterior segments, placed in OCT compound (Tissue-Tek, Sakura Finetek USA, Inc., Torrance, CA, USA), and quick frozen. Samples were stored at −80 °C until sectioning and study.
Serial 10 μm frozen sections of perfused brain tissues from the stereotaxically injected mice were developed for the antigens of interest by immunoperoxidase methods (Tagaya et al, 2001; Fukuda et al, 2004). The sections were fixed with methanol, and incubated with the primary antibody overnight at 4 °C, then developed using the appropriate secondary antibodies (Vectastain ABC Kit, Vector Laboratories), according to the manufacturer's instructions, and finally stained with the DAB peroxidase substrate.
Cell Viability Assays
The survival of cells in culture, exposed to Ha2/5 or isotype antibodies, in 24-well plates was determined using the PI (propidium iodide) method in which 33 μg/mL PI solution (Sigma) was added to each well and incubated for 5 minutes. Stained cells were counted and viability was calculated as the percentage PI-stained cells per total cells. A second method made use of the conversion of MTS tetrazolium to formazan (CellTiter 96 Aqueous One Solution Cell Proliferation Assay, Promega, Madison, WI, USA) by endothelial cells in a 96-well plate assay. A third assay determined lactate dehydrogenase (LDH release into the culture supernatant via kit assay (Roche Diagnostics GmbH, Penzberg Basel, Switzerland) according to the manufacturer's instructions.
For tissue samples, cells displaying evidence of DNA scission were detected by incorporation of dUTP (digoxigenin-deoxyuridine triphosphate) with DNA polymerase I into nuclear DNA as previously described (Tagaya et al, 1997).
BrdU uptake into cell nuclei was measured via kit assay (5-Bromo-2-deoxy-uridine Labeling and Detection Kit III; Roche Diagnostics GmbH, Mannheim Germany), according to the manufacturer's instructions.
Permeability Measurements
Endothelial cell monolayer integrity was assessed by TEER measurements and dextran permeability. Mouse primary brain microvessel endothelial cells (2.0 × 104 cells/100 μL of ECGM) were seeded on 6.5 mm Transwell inserts (24-well polystyrene, 0.4 μm pore; Corning Incorporated, Lowell, MA, USA) coated with collagen IV. The ECGM (0.6 mL) was then added to the lower compartments. One day after endothelial cell seeding, the culture media was changed to media containing Ha2/5 or isotype antibody (at 10 μg/mL each). Resistance of the endothelial cell monolayer was measured using a calibrated Endohm-6 electrode chamber and an EVOM2 epithelial voltohmmeter (World Precision Instruments) at 0, 18, 24, 42, and 48 hours after applying the antibody (Wessells et al, 2009). Before each measurement, the chamber was filled with 0.7 mL fresh ECGM and incubated 20 minutes under 5% CO2 at 37 °C. The inserts with endothelial cells were transferred to the chamber and measured sequentially. Transendothelial electrical resistance was calculated by subtracting background resistance of the insert without endothelial cells.
In parallel experiments, after incubation of monolayers with Ha2/5 or isotype antibodies, fluoresein, 40 kDa-FITC-dextran, or 150 kDa-FITC-dextran (all Sigma) were added in the upper chamber (at 1 mg/mL) and 50 μL aliquots were collected from the lower chamber at 0, 5, 10, 20, 40, and 60 minutes. The volume sampled was replaced with fresh media at each time point. The apparent permeability coefficient (Papp, cm/s) of each group was calculated using the equation: Papp=(dM/dt)/(A × C), where dM/dt is the cumulative measured fluorescence intensity in the lower chamber per unit time (RFU/s) corrected for dilution due to sampling, A is the surface area of the insert membrane (0.33 cm2), and C is the initial concentration (RFU/mL) in the upper chamber (Hsuchou et al, 2010).
Statistical Analyses
The data are expressed as mean±s.d. of replicate experiments (with parallel duplicate or triplicate measures) on separate days. The number of measurements is indicated.
Analyses were performed using nonparametric tests for comparisons of twin data sets, two-way analysis of variance with corrections for multiple comparisons (e.g., TEER data), or loglinear model analyses whether the interventions Ha2/5 or isotype differentially affected cell expression (e.g., likelihood ratio χ2 for flow cytometry) where appropriate. Loglinear analyses were undertaken in Systat 12, and the other analyses with Prism software (GraphPad Software, La Jolla, CA, USA).
Significance was set at 2P<0.05 or P<0.05 where appropriate.
Results
Tight Junction Expression by Endothelial Cells
Confluent and subconfluent cells expressed claudin-5 at the interendothelial cell junctions. Over 7 days, confluent endothelial cells grown on collagen IV on inserts expressed interendothelial claudin-5 that increased successively with time from day 1 (Figure 1).
β1-Integrin Expression by Endothelial Cells
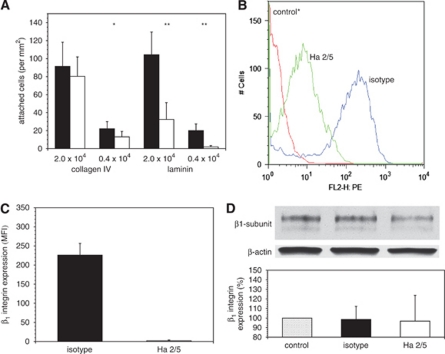
Incubation of primary endothelial cells with Ha2/5 prevented cell adherence and growth, decreasing adherence significantly by 41.1%±27.1% on collagen IV at the low cell concentration (n=15, 2P=0.0022), and decreasing adherence to laminin by 90.7%±6.8% at low cell concentrations (n=15, 2P<0.0001) compared with the isotype antibody (Figure 2A). Hence, adherence of vascular endothelial cells to collagen IV and laminin could be significantly prevented by blockade of β1-integrin function.
Figure 2.
Expression of immunoreactive β1-integrin by murine primary cerebral endothelial cells. (A) Decreased β1-integrin-dependent endothelial cell adherence to collagen IV and laminin after incubation with Ha2/5 (white bars) compared with isotype antibody (black bars) at two different cell concentrations (n=15 images each; *2P=0.0022, **2P<0.0001). (B) β1-Integrin expression by subconfluent endothelium on collagen IV after exposure to the control* antibody, Ha2/5, or isotype antibodies (flow cytometry). (C) β1-Integrin expression by subconfluent endothelium on collagen IV (flow cytometry). Ha2/5 (white bar) significantly decreased β1-integrin expression compared with the isotype antibody (black bar) (n=21 each, 2P<0.0001). (D) No changes in β1-integrin expression were observed on confluent endothelium on collagen IV by Western immunoblot (percentage total β1-integrin expression compared with control) (n=3 each). Control* is the PE-conjugated IgG antibody for β1-integrin (flow cytometry). Please see text.
β1-Integrin expression on primary cerebral microvessel endothelial cells cultured on collagen IV is readily identified by flow cytometry (Figure 2B). To test the ability of basal β1-integrins to accomplish matrix adhesion, subconfluent endothelial cell cultures on collagen IV were exposed to Ha2/5 or to the isotype MoAb in parallel for 18 hours, harvested, and then assayed by flow cytometry. Ha2/5 significantly reduced β1-integrin subunit immunoreactivity to 1.02%±1.05% of that by adherent endothelial cells exposed to the isotype IgM (Figure 2C).
β1-Integrin subunit is identified as ∼140 kDa doublet bands by Western immunoblot (Yu et al, 2005). Total β1-integrin expression by confluent endothelium did not change after the treatment with isotype or Ha2/5, compared with control (no intervention) (Figure 2D).
β1-Integrin Function Blockade and Claudin-5 Expression by Endothelial Cells
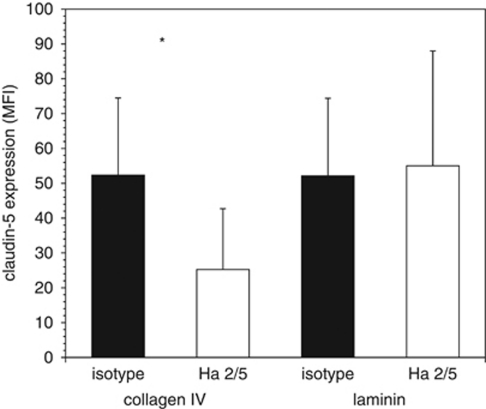
Next, the impact of ECM exposure on claudin-5 expression by inhibition of β1-integrin function was quantitated. Primary endothelial cells grown to target subconfluence in wells either on collagen type IV or on laminin developed well-formed colonies, and were then exposed to either Ha2/5 or isotype MoAb for 18 hours. A significant reduction in claudin-5 expression was observed by flow cytometry when the endothelium was grown on collagen IV, but not laminin (Figure 3). Hence, all subsequent experiments examined cells grown on collagen IV.
Figure 3.
Altered claudin-5 expression by cerebral endothelial cells grown to 60% target confluence in wells (single label flow cytometry). The relative effect of Ha2/5 (white bars) compared with isotype antibody (black bars) on claudin-5 expression when the cells were grown on collagen IV (n=18) or on laminin (n=11). *2P=0.001.
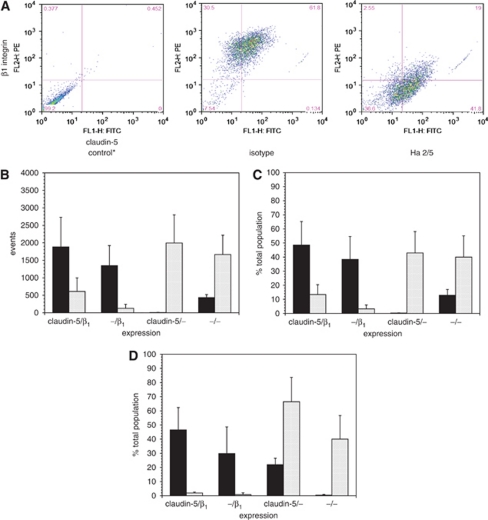
To determine whether β1-integrin–matrix adhesion is required for claudin-5 expression, claudin-5 immunoreactivity was analyzed by dual label flow cytometry following functional β1-integrin blockade (Figure 4A). Ha2/5 highly significantly decreased claudin-5 expression (likelihood ratio χ2=41808, df=3, n=24, P<10−12). After 18 hours Ha2/5 exposure, subconfluent endothelial cells expressing both β1-integrin and claudin-5 decreased from 48.59%±16.65% to 13.38%±7.01% (2P=0.0078), matched by a significant reciprocal increase in the proportion of cells lacking both claudin-5 and β1-integrin (12.83%±4.18% to 39.97%±15.17% 2P=0.0004) (Figures 4B and 4C). Overall, β1-integrin immunoreactivity decreased from 86.91%±4.14% to 16.60%±7.74% (2P=0.0078) in these studies. Hence, β1-integrin blockade was associated with an increase in the cell population lacking claudin-5. This could be explained either by (1) a decrease in claudin-5 expression because of disruption of β1-integrin–matrix adhesion, or (2) a shift in the claudin-5-nonimmunoreactive/β1-integrin-immunoreactive (claudin-5−/β1+) population to β1-integrin nonimmunoreactive. Also, total claudin-5 expression and event number measured by flow cytometry increased in the Ha2/5-exposed group compared with isotype (data not shown). This implies the exposure of hidden claudin-5 epitopes by β1-integrin blockade and/or the disruption of clusters of endothelial cells thereby increasing claudin-5 exposure. By Western immunoblot, however, no change in claudin-5 content was observed even up to 42 hours exposure to Ha2/5 or isotype (data not shown).
Figure 4.
Effect of functional inhibition of β1-integrin on claudin-5 expression by primary cerebral endothelial cells (dual label flow cytometry). (A) Examples of distributions of β1-integrin/claudin-5 expression following incubation of cells for 18 hours without antibody or with the isotype or Ha2/5 antibodies. Relative fluorescence: ordinate, claudin-5; abscissa, β1-integrin (A–C, cells grown on collagen IV on wells to 60% target confluence). (B) Distributions of β1-integrin and claudin-5 on subconfluent cerebral endothelial cells incubated with isotype antibody (black bars) or Ha2/5 (hatched bars) for 18 hours (data from panel (A), n=24 each). Four β1-integrin/claudin-5 expression types could be determined. The individual cell population distributions between the two interventions were highly significant (P<10−10). (C) Percent distribution of β1-integrins and claudin-5 (data from panel (B), n=24 each). (D) Percent distribution of β1-integrin and claudin-5 on confluent cerebral endothelial cells incubated with isotype antibody (black bars) or Ha2/5 (hatched bars) for 18 hours (on collagen IV-coated inserts grown to 100% target confluence, n=9 each). Please see text.
To determine whether functional blockade of endothelial β1-integrins altered claudin-5 expression in claudin-5+ cells, primary endothelial cells were grown to confluence on collagen IV-coated inserts, so that the ‘basal surface' could be directly exposed to either antibody. The effects of Ha2/5 versus isotype antibody exposure for 18 hours on β1-integrin and claudin-5 expression by confluent endothelium (using flow cytometry) were very similar to those observed with the subconfluent preparation (Figure 4D).
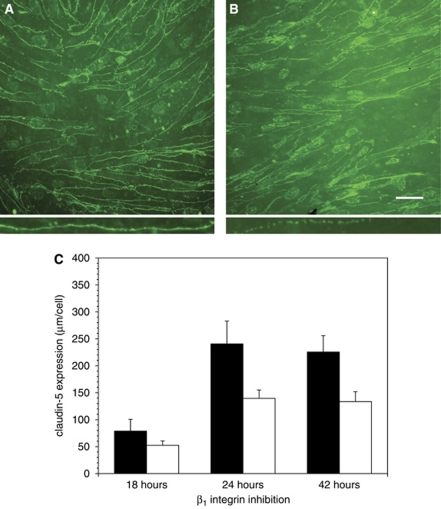
To determine how claudin-5 expression changed with β1-integrin blockade, endothelial cells grown to confluence on inserts were incubated with either Ha2/5 or isotype IgM antibodies for 18, 24, or 42 hours, and the length of the endothelial cell circumference displaying residual claudin-5 immunoreactivity was quantified in situ for each intervention with the aid of video-imaging microscopy (Figure 5). The effect of β1-integrin blockade on claudin-5 expression was significantly time dependent (2P<0.0001) (Figure 5C). Incubation for 18 hours with Ha2/5 produced a 33.7%±10.5% decrease in per cell claudin-5 circumference compared with isotype antibody, which became significant by 24 hours (a 42.0%±6.5% reduction in interendothelial claudin-5 immunoreactivity was seen in the Ha2/5 group (2P<0.05)). Per cell claudin-5 circumference in the Ha2/5 group remained significantly reduced (by 40.7%±8.1%) at 42 hours compared with the isotype group (Figure 5C). Notable was the increase in claudin-5 expression during this exposure time in the isotype cohorts, which corresponded to the observed increase in claudin-5 expression with culture maturation (see Figure 1). The interendothelial claudin-5 expression clearly changed from the continuous to a discontinuous morphology when exposed to Ha2/5 (Figures 5A and 5B, insets). The number of cells per field increased between 24 and 42 hours in both groups, although the change was not significant.
Figure 5.
Effect of functional inhibition of β1-integrins on claudin-5 expression by confluent primary cerebral endothelial cells. (A, B) Claudin-5 immunoreactivity with isotype antibody and with Ha2/5, respectively, at 24 hours. Note, disruption of continuous expression of claudin-5 (‘beading') among cells treated with Ha2/5 compared with isotype antibody (inset panel (B) compared with inset panel (A), interfaces in insets are ∼100 μm in length). (C) Time course of expression after isotype antibody (black bars) or Ha2/5 (white bars) exposure. Ha2/5 significantly reduced claudin-5 expression throughout (n=10 images each, 2P<0.0001). Cells grown on collagen IV on inserts, circumference of immunoreactive interendothelial claudin-5 expression (μm per cell). Magnification bar=50 μm.
Cell Viability and Proliferation
Prevention of β1-integrin–matrix adhesion promotes demise of unattached endothelial cells (Meredith et al, 1993). Although downregulation of already attached β1-integrins might induce cell demise, functional β1-integrin blockade had little impact on endothelial cell survival in these studies (Supplementary Table). After continuous exposure to Ha2/5 or the isotype antibody, endothelial cell viability remained 95% to 100% compared with untreated cells or internal controls throughout the study period as measured by PI uptake, LDH release, and MTS assay.
There was also no difference among the treatment cohorts with respect to BrdU incorporation, which remained constant at baseline levels, compared with much greater uptake by bEnd.3 cells plated in parallel at the same cell concentrations under identical conditions (data not shown). Hence, there was no effect on proliferative potential by either Ha2/5 or the isotype antibody.
Effect of β1-Integrin Blockade on Permeability In Vitro
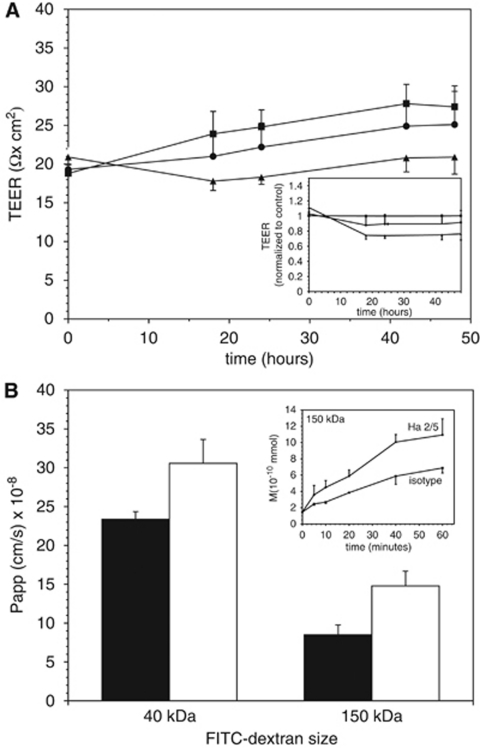
The impermeability of confluent endothelial cell cultures was measured by two methods. By 18 hours after treatment, endothelial cell monolayers continuously exposed to Ha2/5 showed lower electrical resistance than to isotype exposure (17.8±1.2 versus 21.0±2.6 Ω × cm2, respectively). Significantly decreased resistance with Ha2/5 was seen at 24, 42, and 48 hours (two-way analysis of variance, 2P<0.0001; comparison between Ha2/5 and isotype at 48 hours, 2P=0.0313; Figure 6A). Diffusion experiments were then performed with fluorescein (376 Da), 40 kDa-FITC-dextran, and 150 kDa-FITC-dextran. By 24 hours, Ha2/5 significantly increased dextran transit at both 40 and 150 kDa molecular masses (Figure 6B). Ha2/5 caused a 73.6%±22.6% increase in 150 kDa-FITC-dextran transit compared with isotype (Papp=8.5±1.2 × 10−8 versus 14.8±1.9 × 10−8 cm/s, P<0.05) and a 30.7%±13.3% increase in 40 kDa-FITC-dextran transit (23.3±1.0 × 10−8 versus 30.6±3.1 × 10−8 cm/s, P<0.05). A trend in favor of increased fluorescein transit with Ha2/5 was also observed (data not shown).
Figure 6.
Effect of β1-integrin blockade on permeability of confluent endothelial cell monolayers on collagen IV-coated inserts. (A) Serial transendothelial electrical resistance (TEER) measurements for the Ha2/5 and isotype antibody-exposed cultures were lower than untreated controls (squares). Ha2/5 (triangles) significantly decreased the resistance by 16.8%±6.2% (n=6 cultures for each time point; at 48 hours, 2P=0.0313) compared with isotype antibody exposure (circles). Inset replots the data taking the measurements of the untreated control as 1.0. (B) Apparent permeability coefficients (Papp, cm/s) for 40 and 150 kDa dextrans in separate experiments (n=3 each point). Papp increased significantly when exposed to Ha2/5 (white bars) compared with isotype antibody (black bars) for 24 hours (P<0.05 for each set). Inset demonstrates transit measurements for the 150-kDa-FITC-dextran as an example.
Effect of β1-Integrin Blockade on Permeability Barrier Integrity In Vivo
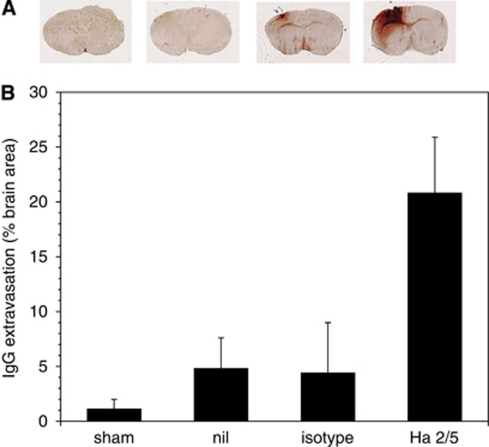
Focal cerebral ischemia causes a significant and rapid decrease in microvessel-associated β1-integrin immunoreactivity in concert with increased barrier permeability (Haring et al, 1996; Tagaya et al, 1997, 2001). To test whether the blockade of β1-integrin function alters blood–brain barrier integrity in situ, the impact at 24 hours of Ha2/5 or isotype stereotaxically delivered antibodies into the striatum of mice on normal cerebral vascular impermeability (i.e., no IgG extravasation) was quantified. While injection of the isotype antibody produced little additional IgG compared with the sham and needle-only cohorts, significantly greater leakage of IgG was noted in the Ha2/5 cohort (4.44%±4.58% versus 20.80%±5.08% of the total coronal surface area, respectively; 2P=0.0006; Figure 7).
Figure 7.
Change in permeability (IgG extravasation) by functional inhibition of β1-integrin 24 hours after stereotaxic injection of Ha2/5 or isotype antibody into the murine striatum (A). The surface areas (in mm2) of IgG extravasation into the surrounding tissue in the coronal plane of the striatal injection in animals undergoing sham procedure (n=6), needle insertion without injection (nil, n=6), isotype antibody injection (n=8), and Ha2/5 injection (n=8) were normalized to the total area of the coronal section (B). The areas of extravasation of the sham, needle-only, and isotype were not different. Ha2/5 significantly increased IgG extravasation compared with the isotype antibody (2P=0.0006).
Injections of either Ha2/5 or its control antibody were occasionally associated with the appearance of cells that incorporated dUTP along the insertion site. There was no difference among the treatment cohorts in the number of these cells (which did not differ from the needle-only group, data not shown), and no relationship of dUTP+ cells to the degree of permeability. Histochemical stains suggested that the dUTP+ cells were extracerebral in origin, compatible with leukocytes.
Discussion
Cells adhere to their ECM protein ligands by structural transmembrane receptors (e.g., integrins, dystroglycan) that, together with growth factor receptors, organize and coordinate cell responses to changes in the local environment. Certain integrin receptors can initiate internal signaling processes when ligated to specific matrix proteins (Hynes and Bader, 1997). β1-Integrins associate with collagen IV, laminin, and perlecan. The nature of integrin structure and function implies that, for the cerebral microvascular endothelium, matrix adhesion receptor-mediated binding/signaling could be required to develope and maintain the permeability barrier (del Zoppo and Milner, 2006). This notion implies that, in cerebral microvessels, endothelial cell β1-integrin–matrix adhesion is required for TJ integrity and function. However, it is not yet known whether coordinated basal lamina-endothelial cell-TJ communication participates in permeability barrier integrity.
The integrity of the microvessel portion of the blood–brain permeability barrier has been attributed to the interendothelial tight junctions, which involve the cohesion of ZO-1, occludin, and claudins (claudin-3, claudin-5, and claudin-12), junctional adhesion molecule-1, E-cadherin, and other cytoskeleton components (Wolburg et al, 2009). These molecules and their intracellular scaffolding provide the lateral or ‘horizontal' vector of barrier integrity (del Zoppo and Milner, 2006). Largely ignored have been forces and receptors that are required for positioning of the endothelium on the vascular basal lamina to permit the horizontal interactions to take place. Although other interactions are possible, the prominent position of β1-integrins on the endothelial abluminal surface suggests their involvement in ‘vertical' matrix adhesion.
These studies are the first to demonstrate that abrogation of microvascular endothelial cell β1-integrin function or expression (Figures 4 and 5) substantially changes the expression of a TJ protein, here claudin-5, known to be important for endothelial permeability barrier integrity (Ohtsuki et al, 2007). Blockade of endothelial cell β1-integrin function by the MoAb Ha2/5 (1) significantly reduced β1-integrin expression; (2) significantly decreased interendothelial claudin-5 expression by confluent or subconfluent endothelial cells in culture; and (3) consequently, significantly increased cerebral microvessel permeability in vitro and in vivo over a period of 18 to 24 hours. These observations cannot be explained by endothelial cell demise or disruption. The findings support the necessity for β1-integrin function to maintain the intact permeability barrier.
In previous studies with the nonhuman primate, we demonstrated that β1-integrin immunoreactivity on microvessel endothelium is lost in the ischemic core within 2 hours of middle cerebral artery occlusion. This loss is sustained and is not recovered after restoration of flow. But, in the regions peripheral to the ischemic cores microvessel β1-integrin gene expression is increased (Tagaya et al, 2001).
Claudin-5 was chosen for its known roles in microvascular permeability barrier integrity. It resides within cerebral and pulmonary endothelial cells at the cell–cell border, but not in epithelial cells, including astrocyte end-feet (Nitta et al, 2003). Claudin-5 is predominantly expressed in brain capillaries, and appears to have a key role in the barrier to egress of small molecules by brain capillary endothelial cells (Morita et al, 1999; Ohtsuki et al, 2007). In one study, the normalized claudin-5 mRNA level in the rat brain capillary fraction was 35.6-fold greater than that in the brain (Ohtsuki et al, 2007).
Mice lacking claudin-5 display no apparent changes in cerebral microvessel structure compared with wild type, and do not exhibit edema or hemorrhage (Nitta et al, 2003). Importantly, tracer experiments have shown that small molecules (<800 Da) extravasate into the perivascular tissue in the claudin-5-deficient brain, whereas larger molecules (>1,900 Da) do not (Nitta et al, 2003). In concert with those findings, Koto et al (2007) showed that after 7 days hypoxia, the microvessel permeability barrier is disrupted in the rat retina, a condition accompanied by decreased endothelial cell claudin-5 expression and the extravasation of small molecules. Claudin-5 expression decreased and extravasation of an injected small molecule (534 Da) tracer increased compared with the normoxic retina, while 10 kDa dextran remained inside the vessels under both conditions. Hence, claudin-5 appears to have a major role in selective exclusion of small molecules in the blood–brain barrier permeability phenotype (Koto et al, 2007). In contrast, Bake et al (2009) recently demonstrated, in aging rodents, that extravasation of IgG into the hippocampus is inversely related to interendothelial claudin-5 expression.
The binding of Ha2/5 to β1-integrins significantly decreased claudin-5 expression in both confluent and subconfluent endothelium in vitro. The generation of claudin-5−/β1− events seen by flow cytometry could be interpreted as either a consequence of the blockade of the β1-integrin on claudin-5–/β1+-expressing cells or the conversion of claudin-5+/β1+ to claudin-5− events, or both, as flow cytometry cannot distinguish the baseline antigen state after Ha2/5 exposure. It seems unlikely that the generation of claudin-5−/β1− events was an artifact of β1-integrin binding by Ha2/5. The in vitro immunohistochemistry experiments demonstrated clearly that exposure to Ha2/5 produces a significant decrease in interendothelial claudin-5 expression, which is compatible with the conversion of claudin-5+/β1+ cells to claudin-5−/β1− cells (Figure 5). The increase in event number observed with Ha2/5 by flow cytometry could reflect decreased cell–matrix adherence or reduced cell–cell cohesion when β1-integrin function was blocked.
An interesting aspect of the decrease in interendothelial claudin-5 expression is its time dependence. We and others have shown that the expression of claudin-5 at the interendothelial cell interface from cytoplasmic pools in in vitro culture depends on cell density and the time from plating (Koto et al, 2007). Hence, at the time of Ha2/5 and isotype antibody exposure, maturation of cell–cell contacts and claudin-5 expression are still proceeding (Figure 1). It is possible that at 42 hours, the change in β1-integrin expression, and perhaps the decrease in claudin-5 expression, could also have limited cell growth in the insert environment.
While functional blockade of the β1-integrin subunit prevents firm adhesion of endothelial cells, and is known to promote cell demise in some cultures (Meredith et al, 1993), the endothelial cell viability was not different from control here. Parallel experiments indicated that Ha2/5 did not affect cell viability up to 42 hours exposure. Matrix binding established before Ha2/5 exposure is likely to have employed other matrix adhesion receptors not affected by β1-integrin blockade. The overall results, then, are consistent with the notion that matrix contact and contact to adjacent cells is required for endothelial cell viability.
Furthermore, Ha2/5 exposure for extended periods decreased TEER by 15% to 17% compared with the isotype antibody, indicating that interruption of β1-integrin function increases monolayer permeability. Dextran-based permeability studies demonstrated that β1-integrin blockade allowed ready passage of 40 and 150 kDa particles (the latter compatible with IgG) (Figure 6B). Koto et al (2007) demonstrated that the TEER of bEnd.3 cell monolayers under normoxia decreased when subject to hypoxia, which paralleled changes in claudin-5 expression. The TEER of porcine brain endothelial cell monolayers was also reduced by hypoxia over 1.5 to 24.0 hours, which paralleled increased sucrose and inulin flux across the monolayers (Deli et al, 2005). It is quite possible here that the passage of 40 and 150 kDa particles through confluent endothelium reflects an impact of β1-integrin dysfunction on ZO-1 and occludin, or other components of the adherens complex, in addition to claudin-5.
The in vitro findings are supported by the appearance of IgG extravasation following stereotaxic Ha2/5 injection to the striatum and β1-integrin blockade compared with the isotype antibody (Figure 7). The effect of Ha2/5 action in vivo is most likely due to alterations in the vascular β1-integrin–matrix interactions, with impact on permeability barrier integrity (as shown in vitro), because the isotype antibody has no effect on the barrier, there was no evidence of vascular cell, glial, or neuron demise, and neurologic function was grossly intact. However, the action of Ha2/5 in vivo could be more complex than targeting of the barrier. Blockade of β1-integrin has been shown to increase tissue edema in other settings (Reed et al, 1992). In addition, IgG transit increases in microvessels in which claudin-5 expression is decreased (Bake et al, 2009). Those experiments and ours can be explained if the adhesive functions of cerebral microvessel endothelial cells are also altered by β1-integrin blockade.
Stereotaxic injection itself causes local injury not unlike a stab wound. Tracer studies have demonstrated leakage of Evans blue albumin within 24 hours after stab wounds in rat brain, and glial uptake of Evans blue albumin 8 to 24 hours after the injury (Persson et al, 1976). Stab wounds are also associated with an inflammatory response. In the mouse, increases in monocyte chemoattractant protein-1 (MCP-1 mRNA, MCP-1 protein, and monocytes and polymorphonuclear (PMN leukocytes were detected within 24 hours after injury (Glabinski et al, 1996; Matsumoto et al, 2007). Occasionally, cells compatible with inflammatory cells were observed along the injection track here, but their presence was unrelated to the inhibitor choice or control. Despite the known local impact of activated PMN leukocytes on microvascular integrity, there is so far no evidence that inhibition of β1-integrin enhances PMN leukocyte invasion in this setting.
The mechanism(s) by which functional blockade of β1-integrins on confluent cerebral endothelial cells can decrease TJ expression (e.g., claudin-5) are yet to be worked out. It is possible that communication could proceed via (1) the actin cytoskeleton, (2) the β1-integrin cytoplasmic tail, (3) tight junction-related kinases, (4) cytoplasmic Ca+2 pools, or (5) a combination of these or other events. Evidence to support the involvement of the cytoskeleton derives from observations of barrier permeability and actin integrity. Signaling from β1-integrin involves activation of the actin cytoskeleton (Wolburg et al, 2009). In addition, TJ proteins including ZO-1, occludin, and claudin-5 bind to the cytoskeleton actin. Hypoxia can change endothelial cell permeability in association with changes in the structural organization of actin (Huber et al, 2001). During hypoxia and reoxygenation, the expression of actin protein in bovine brain microvessel endothelial cells increased compared with normoxic monolayers, which paralleled the reduction of the monolayer barrier properties (Mark and Davis, 2002). After 48 hours hypoxia, an increase in the permeability of brain capillary endothelial cell monolayers was observed that was associated with rearrangements of F-actin filaments and decreased ATP levels (Plateel et al, 1995). Changes in blood–brain barrier permeability are associated with protein phosphorylations that are regulated by protein kinase C isotypes, tyrosine phosphatase, or kinase activity (Staddon et al, 1995; Tsukamoto and Nigam, 1999; Fleegal et al, 2005). It is likely that cytoskeletal reorganization has an important role in the relation between β1-integrin adhesion and tight junction integrity.
Calcium channel inhibitors (nifedipine and SKF-96365) prevented increased permeability of bovine brain microvessel endothelial cells monolayer induced by hypoxia–aglycemia (Abbruscato and Davis, 1999). Mark and Davis (2002) showed that hypoxia increases endothelial monolayer permeability, but expression of the TJ proteins ZO-1, ZO-2, and occludin changed little. The localization of these TJ proteins correlated with the permeability change. Here, total claudin-5 expression did not change, but claudin-5 morphology was altered by Ha2/5 (Figures 5A and 5B, insets). The findings with Ha2/5 here are reminiscent of changes in epithelial barrier cohesion wherein β1-integrin blockade induced albumin leakage and edema in rat skin in vivo (Reed et al, 1992).
Interference with the interactions between β1-integrins on confluent cerebrovascular endothelial cells and the matrix ligand collagen IV decreases interendothelial tight junction (e.g., claudin-5) expression and decreases permeability barrier integrity, allowing IgG transit. Typically, loss of claudin-5 expression is associated with small molecule leakage, hence IgG transit may reflect β1-integrin-dependent changes in other TJ proteins. We have not shown that the decline of claudin-5 causes the increase in 40 and 150 kDa (IgG) leakage. The results here imply that (1) β1-integrin-mediated adherence to the basal lamina is central to the integrity of the blood–brain permeability barrier, (2) leakiness may reflect β1-integrin density, (3) disorders (e.g., focal ischemia) that interfere with β1-integrin–matrix interactions could significantly alter blood–brain barrier integrity, and (4) the timing and location of leakiness may be determined by the vulnerability of or density of β1-integrin expression. Also implied is that pharmacological manipulation to locally increase blood–brain barrier permeability must take account of endothelial cell adhesion to the basal lamina. These findings remain limited, as the impact of the interruption of β1-integrin–matrix interactions on occludin or ZO-1 expression, and the mechanisms of these changes must be defined. Nonetheless, it is now quite clear that changes in ‘vertical' adhesion can modulate ‘horizontal' cohesion processes among cerebral microvascular endothelial cells.
Acknowledgments
The authors thank Dr JA Koziol for input on specific statistical matters, and Drs Rothwell, Stewart, and Tejima for their involvement during early discussions of this project. Technical assistance by H Frankowski is appreciated. The authors specially thank G Berg for manuscript preparation.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Journal of Cerebral Blood Flow & Metabolism website (http://www.nature.com/jcbfm)
This study was supported in part by NIH Grants NS 053716, NS 038710, and NS 036945 to Dr del Zoppo. Dr Osada also received funding from the Kanae Foundation for the Promotion of Medical Science.
Supplementary Material
References
- Abbruscato TJ, Davis TP. Combination of hypoxia/aglycemia compromises in vitro blood-brain barrier integrity. J Pharmacol Exp Ther. 1999;289:668–675. [PubMed] [Google Scholar]
- Abumiya T, Lucero J, Heo JH, Tagaya M, Koziol JA, Copeland BR, del Zoppo GJ. Activated microvessels express vascular endothelial growth factor and integrin alpha(v)beta3 during focal cerebral ischemia. J Cereb Blood Flow Metab. 1999;19:1038–1050. doi: 10.1097/00004647-199909000-00012. [DOI] [PubMed] [Google Scholar]
- Bake S, Friedman JA, Sohrabji F. Reproductive age-related changes in the blood brain barrier: expression of IgG and tight junction proteins. Microvasc Res. 2009;78:413–424. doi: 10.1016/j.mvr.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Zoppo GJ, Milner R. Integrin-matrix interactions in the cerebral microvasculature. Arterioscler Thromb Vasc Biol. 2006;26:1966–1975. doi: 10.1161/01.ATV.0000232525.65682.a2. [DOI] [PubMed] [Google Scholar]
- Deli MA, Abraham CS, Kataoka Y, Niwa M. Permeability studies on in vitro blood-brain barrier models: physiology, pathology, and pharmacology. Cell Mol Neurobiol. 2005;25:59–127. doi: 10.1007/s10571-004-1377-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleegal MA, Hom S, Borg LK, Davis TP. Activation of PKC modulates blood-brain barrier endothelial cell permeability changes induced by hypoxia and posthypoxic reoxygenation. Am J Physiol Heart Circ Physiol. 2005;289:H2012–H2019. doi: 10.1152/ajpheart.00495.2005. [DOI] [PubMed] [Google Scholar]
- Fukuda S, Fini CA, Mabuchi T, Koziol JA, Eggleston LL, del Zoppo GJ. Focal cerebral ischemia induces active proteases that degrade microvascular matrix. Stroke. 2004;35:998–1004. doi: 10.1161/01.STR.0000119383.76447.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia JH, Cox JV, Hudgins WR. Ultrastructure of the microvasculature in experimental cerebral infarction. Arch Neuropathol (Berlin) 1971;18:273–285. doi: 10.1007/BF00688441. [DOI] [PubMed] [Google Scholar]
- Glabinski AR, Balasingam V, Tani M, Kunkel SL, Strieter RM, Yong VW, Ransohoff RM. Chemokine monocyte chemoattractant protein-1 is expressed by astrocytes after mechanical injury to the brain. J Immunol. 1996;156:4363–4368. [PubMed] [Google Scholar]
- Haring H-P, Akamine P, Habermann R, Koziol JA, del Zoppo GJ. Distribution of integrin-like immunoreactivity on primate brain microvasculature. J Neuropathol Exp Neurol. 1996;55:236–245. doi: 10.1097/00005072-199602000-00012. [DOI] [PubMed] [Google Scholar]
- Hsuchou H, Kastin AJ, Tu H, Joan AN, Couraud PO, Pan W. Role of astrocytic leptin receptor subtypes on leptin permeation across hCMEC/D3 human brain endothelial cells. J Neurochem. 2010;115:1288–1298. doi: 10.1111/j.1471-4159.2010.07028.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber JD, Egleton RD, Davis TP. Molecular physiology and pathophysiology of tight junctions in the blood-brain barrier. Trends Neurosci. 2001;24:719–725. doi: 10.1016/s0166-2236(00)02004-x. [DOI] [PubMed] [Google Scholar]
- Hynes RO, Bader BL. Targeted mutations in integrins and their ligands: their implications for vascular biology. Thromb Haemost. 1997;78:83–87. [PubMed] [Google Scholar]
- Koto T, Takubo K, Ishida S, Shinoda H, Inoue M, Tsubota K, Okada Y, Ikeda E. Hypoxia disrupts the barrier function of neural blood vessels through changes in the expression of claudin-5 in endothelial cells. Am J Pathol. 2007;170:1389–1397. doi: 10.2353/ajpath.2007.060693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon I, Kim EH, del Zoppo GJ, Heo JH. Ultrastructural and temporal changes of the microvascular basement membrane and astrocyte interface following focal cerebral ischemia. J Neurosci Res. 2009;87:668–676. doi: 10.1002/jnr.21877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebner S, Fischmann A, Rascher G, Duffner F, Grote EH, Kalbacher H, Wolburg H. Claudin-1 and claudin-5 expression and tight junction morphology are altered in blood vessels of human glioblastoma multiforme. Acta Neuropathol. 2000;100:323–331. doi: 10.1007/s004010000180. [DOI] [PubMed] [Google Scholar]
- Mark KS, Davis TP. Cerebral microvascular changes in permeability and tight junctions induced by hypoxia-reoxygenation. Am J Physiol Heart Circ Physiol. 2002;282:1485–1494. doi: 10.1152/ajpheart.00645.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto H, Kumon Y, Watanabe H, Ohnishi T, Shudou M C, II, Takahashi H, Imai Y, Tanaka J. Antibodies to CD11b, CD68, and lectin label neutrophils rather than microglia in traumatic and ischemic brain lesions. J Neurosci Res. 2007;85:994–1009. doi: 10.1002/jnr.21198. [DOI] [PubMed] [Google Scholar]
- Mendrick DL, Kelly DM. Temporal expression of VLA-2 and modulation of its ligand specificity by rat glomerular epithelial cells in vitro. Lab Invest. 1993;69:690–702. [PubMed] [Google Scholar]
- Meredith JE, Jr, Fazeli B, Schwartz MA. The extracellular matrix as a cell survival factor. Mol Biol Cell. 1993;4:953–961. doi: 10.1091/mbc.4.9.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner R, ffrench-Constant C. A developmental analysis of oligodendroglial integrins in primary cells: changes in αv-associated β subunits during differentiation. Development. 1994;120:3497–3506. doi: 10.1242/dev.120.12.3497. [DOI] [PubMed] [Google Scholar]
- Milner R, Hung S, Wang X, Berg GI, Spatz M, del Zoppo GJ. Responses of endothelial cell and astrocyte matrix-integrin receptors to ischemia mimic those observed in the neurovascular unit. Stroke. 2008a;39:191–197. doi: 10.1161/STROKEAHA.107.486134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner R, Hung S, Wang X, Spatz M, del Zoppo GJ. The rapid decrease in astrocyte-associated dystroglycan expression by focal cerebral ischemia is protease-dependent. J Cereb Blood Flow Metab. 2008b;28:812–823. doi: 10.1038/sj.jcbfm.9600585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita K, Sasaki H, Furuse M, Tsukita S. Endothelial claudin: claudin-5/TMVCF constitutes tight junction strands in endothelial cells. J Cell Biol. 1999;147:185–194. doi: 10.1083/jcb.147.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitta T, Hata M, Gotoh S, Seo Y, Sasaki H, Hashimoto N, Furuse M, Tsukita S. Size-selective loosening of the blood-brain barrier in claudin-5-deficient mice. J Cell Biol. 2003;161:653–660. doi: 10.1083/jcb.200302070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtsuki S, Sato S, Yamaguchi H, Kamoi M, Asashima T, Terasaki T. Exogenous expression of claudin-5 induces barrier properties in cultured rat brain capillary endothelial cells. J Cell Physiol. 2007;210:81–86. doi: 10.1002/jcp.20823. [DOI] [PubMed] [Google Scholar]
- Perriere N, Demeuse P, Garcia E, Regina A, Debray M, Andreux JP, Couvreur P, Scherrmann JM, Temsamani J, Couraud PO, Deli MA, Roux F. Puromycin-based purification of rat brain capillary endothelial cell cultures. Effect on the expression of blood-brain barrier-specific properties. J Neurochem. 2005;93:279–289. doi: 10.1111/j.1471-4159.2004.03020.x. [DOI] [PubMed] [Google Scholar]
- Persson L, Hansson HA, Sourander P. Extravasation, spread and cellular uptake of Evans blue-labelled albumin around a reproducible small stab-wound in the rat brain. Acta Neuropathol. 1976;34:125–136. doi: 10.1007/BF00684663. [DOI] [PubMed] [Google Scholar]
- Plateel M, Dehouck MP, Torpier G, Cecchelli R, Teissier E. Hypoxia increases the susceptibility to oxidant stress and the permeability of the blood-brain barrier endothelial cell monolayer. J Neurochem. 1995;65:2138–2145. doi: 10.1046/j.1471-4159.1995.65052138.x. [DOI] [PubMed] [Google Scholar]
- Rascher G, Fischmann A, Kroger S, Duffner F, Grote EH, Wolburg H. Extracellular matrix and the blood-brain barrier in glioblastoma multiforme: spatial segregation of tenascin and agrin. Acta Neuropathol. 2002;104:85–91. doi: 10.1007/s00401-002-0524-x. [DOI] [PubMed] [Google Scholar]
- Reed RK, Rubin K, Wiig H, Rodt S. Blockade of β1-integrins in skin causes edema through lowering of interstitial fluid pressure. Circ Res. 1992;71:978–983. doi: 10.1161/01.res.71.4.978. [DOI] [PubMed] [Google Scholar]
- Sapatino BV, Welsh CJ, Smith CA, Bebo BF, Linthicum DS. Cloned mouse cerebrovascular endothelial cells that maintain their differentiation markers for factor VIII, low density lipoprotein, and angiotensin-converting enzyme. In Vitro Cell Dev Biol Anim. 1993;29A:923–928. doi: 10.1007/BF02634230. [DOI] [PubMed] [Google Scholar]
- Savettieri G, Di Liegro I, Catania C, Licata L, Pitarresi GL, D′Agostino S, Schiera G, De Caro V, Giandalia G, Giannola LI, Cestelli A. Neurons and ECM regulate occludin localization in brain endothelial cells. Neuroreport. 2000;11:1081–1084. doi: 10.1097/00001756-200004070-00035. [DOI] [PubMed] [Google Scholar]
- Staddon JM, Herrenknecht K, Smales C, Rubin LL. Evidence that tyrosine phosphorylation may increase tight junction permeability. J Cell Sci. 1995;108 (part 2:609–619. doi: 10.1242/jcs.108.2.609. [DOI] [PubMed] [Google Scholar]
- Tagaya M, Haring H-P, Stuiver I, Wagner S, Abumiya T, Lucero J, Lee P, Copeland BR, Seiffert D, del Zoppo GJ. Rapid loss of microvascular integrin expression during focal brain ischemia reflects neuron injury. J Cereb Blood Flow Metab. 2001;21:835–846. doi: 10.1097/00004647-200107000-00009. [DOI] [PubMed] [Google Scholar]
- Tagaya M, Liu K-F, Copeland B, Seiffert D, Engler R, Garcia JH, del Zoppo GJ. DNA scission after focal brain ischemia. Temporal differences in two species. Stroke. 1997;28:1245–1254. doi: 10.1161/01.str.28.6.1245. [DOI] [PubMed] [Google Scholar]
- Tilling T, Korte D, Hoheisel D, Galla HJ. Basement membrane proteins influence brain capillary endothelial barrier function in vitro. J Neurochem. 1998;71:1151–1157. doi: 10.1046/j.1471-4159.1998.71031151.x. [DOI] [PubMed] [Google Scholar]
- Tsukamoto T, Nigam SK. Role of tyrosine phosphorylation in the reassembly of occludin and other tight junction proteins. Am J Physiol. 1999;276:F737–F750. doi: 10.1152/ajprenal.1999.276.5.F737. [DOI] [PubMed] [Google Scholar]
- Wessells H, Sullivan CJ, Tsubota Y, Engel KL, Kim B, Olson NE, Thorner D, Chitaley K. Transcriptional profiling of human cavernosal endothelial cells reveals distinctive cell adhesion phenotype and role for claudin 11 in vascular barrier function. Physiol Genomics. 2009;39:100–108. doi: 10.1152/physiolgenomics.90354.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolburg H, Noell S, Mack A, Wolburg-Buchholz K, Fallier-Becker P. Brain endothelial cells and the glio-vascular complex. Cell Tissue Res. 2009;335:75–96. doi: 10.1007/s00441-008-0658-9. [DOI] [PubMed] [Google Scholar]
- Yu W, Datta A, Leroy P, O′Brien LE, Mak G, Jou TS, Matlin KS, Mostov KE, Zegers MM. Beta1-integrin orients epithelial polarity via Rac1 and laminin. Mol Biol Cell. 2005;16:433–445. doi: 10.1091/mbc.E04-05-0435. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.