Abstract
Background
Mediated by reactive oxygen species, the damaging effects of high-intensity ionizing irradiation on tissues are dose, frequency, oxygen concentration and tissue property dependent. Intense ionizing irradiation exposure may cause rapid cellular necrosis by peroxidation of membrane lipids leading to membrane disruption. This leads to a loss of the transmembrane ionic gradients and a subsequent depletion of the cellular ATP store, followed by cellular generation of reactive oxygen species. When membrane disruption is extensive, acute cellular necrosis follows. Triblock copolymer surfactants, such as Poloxamer 188 (P188), are able to seal damaged rhabdomyocyte membranes, increasing post-irradiation viability.
Methods
Separated rat rhabdomyocytes were exposed to 40 Gy (60Co 1.5 Gy min−1) irradiation and treated at 20 minutes with combination permutations of P188, N-acetylcysteine (NAC) and Mg-ATP. Cell viability at 18 and 48 hours was determined using Calcein-AM and Ethidium Homodimer-1 staining.
Results
At 18 hours after irradiation, the combined administration of P188, ATP, and NAC restored cell viability rates to near sham-exposed levels of 60%. At 48 hours post-irradiation, cell viability dropped substantially to the 7%–20% range, regardless of attempted intervention. Nevertheless, the combination of P188, ATP and NAC more than doubled cell viability at the 48-hour time point. Neither 8 kDa polyethylene glycol nor 10 kDa neutral dextran were as effective in enhancing cell viability.
Conclusions
These results indicate that antioxidants and cellular energy substrates improve the efficacy of membrane-sealing copolymer surfactants in prolonging cellular viability following massive radiation exposure.
Classifications: ionizing radiation, membrane disruption, Poloxamer, MgATP, N-acetylcysteine
Introduction
A major need exists to develop therapeutics which effectively inhibit or reverse the harmful effects of ionizing radiation (IR) exposure on humans. Life threatening consequences are a real possibility from military or industrial exposure. In both cases, public health protection strategies are needed to protect against large-scale casualties (Mettler and Voelz 2002; Coleman et al. 2004).
Radiation produces a spectrum of clinical manifestations that correlate with the level of exposure (Anno et al. 1989). Doses of radiation less than 5 Gy create protein and DNA crosslinks which destroy the body’s most rapidly dividing cell lines. Severe immune compromise due to neutropenia and bleeding secondary to thrombocytopenia are seen over a period of 3–5 weeks. At higher levels of exposure (30–100 Gy), even terminally differentiated cells of the body are destroyed. Furthermore, their death occurs rapidly over a period of hours. Radiation induces several additional effects that can be expressed at the cellular and tissue levels including a number of epigenetic phenomena (other than DNA structural changes), alterations in gene transcription, changes in signal transduction and the generation of oxidative stress (Mothersill and Seymour 1998; Yang et al. 2000; Kamiya et al. 2005). The latter consitutes a major source of molecular alteration of the cells (Freeman and Crapo 1982; Stark 1991; Leach et al. 2001).
Oxidative cellular stress is created by an increase in the generation rate of reactive oxygen intermediates (ROIs) that cannot be balanced by the intracellular antioxidant defenses. Therefore, the administration of antioxidants, e.g. superoxide dismutase (Cu/Zn/Mn-containing SOD), nitroxides (tempol) and aminothiols (amifostine), represents a standard approach and a first-line treatment in clinical therapies for ROI-mediated injury. They scavenge free radicals, protecting cellular components against oxidative damage (Delanian et al. 1994; Grdina et al. 2000; Mitchell and Krishna 2002). N-acetylcysteine (NAC) is another thiol-containing antioxidant which restores cellular levels of reduced glutathione, an endogenous antioxidant, in addition to its ability to scavenge aqueous phase free radicals. These mechanisms allow NAC to serve as a radioprotectant against the oxidative effects of gamma rays and other forms of IR (Neal et al. 2003).
At high radiation doses, it is very difficult to completely block the effects of ROI production throughout the intracellular water (Freeman and Crapo 1982; Stark 1991; Leach et al. 2001). Consequently, in parallel with the search for efficient antioxidants, sustained research efforts are currently focused on identifying critical targets of the ROIs at the cellular level and on developing effective treatments for restoring these damaged cellular components (Hannig and Lee 2000; Hannig et al. 2000; Greenebaum et al. 2004).
There is increasing evidence demonstrating that membrane phospholipids are at risk of injury from excessive ROI generation (Hannig and Lee 2000). The ROIs peroxidate the lipids, causing altered bilayer structure and reduced ionic barrier function. Increases in membrane ionic permeability drive up the metabolic energy cost of sustaining transmembrane concentration gradients and deplete cytoplasmic ATP stores. Cellular necrosis secondary to increased membrane permeability is not unique to massive radiation exposure. Other etiologies include mechanical trauma, ischemia-reperfusion injuries, electrical injuries, burns and frostbite (Lee and Astumian 1996). Most cells have several intrinsic methods to repair their membranes. However, when the magnitude of the membrane disruption exceeds the intrinsic repair capability, cell death is inexorable unless a therapeutic intervention is made.
Prior studies have demonstrated that multiblock copolymer surfactants (poloxamers) selectively absorb onto defects in phospholipid membranes and may restore structural integrity by inducing sealing of the defect (Maskarinec et al. 2002, 2005). The addition of Poloxamer188 (P188, Maroon Biotech Corp) to irradiated, terminally differentiated adult rat skeletal muscle cells was reported to prevent acute radiation necrosis that was clearly manifest in the untreated cells over a four hour period of time. Furthermore, despite P188 therapy, there was a substantial decline in cell viability over an 18-hour period after radiation exposure suggesting that other cell death pathways were not arrested by the P188 treatment (Greenbaum et al. 2004).
A complication of membrane disruption is cellular energy depletion, which is followed by accelerated generation of ROIs. Especially under culture conditions of supraphysiological oxygen tension in the media, disrupted cells would generate a large amount of ROIs. This would hamper the ability of poloxamer surfactants to seal the membranes for two reasons. The first is the ongoing lipid peroxidation from radicals generated intracellularly. The second is the vulnerability of poloxamers to oxidative degradation. Thus, it seems reasonable to expect that the prevention of poloxamer degradation by adding ROI scavengers and reducing ROI generation by increasing the intracellular ATP content would increase the efficacy of poloxamers to seal membranes of irradiated cells. Exogenous ATP administration may function to replenish cellular stores and allow for continued structural repair in addition to the restoration of ionic gradients by ATP-fueled pumps. Studies have demonstrated that exogenous ATP provides radioprotective effects in several animal models. The protective mechanisms act by modulating the release of pro-inflammatory cytokines, reducing DNA damage and by other, yet undetermined, processes (Szeinfeld 1990; Swennen et al. 2008).
The purpose of these studies was to determine if the addition of antioxidants and metabolic energy substrates would enhance the efficacy of membrane sealing poloxamers in the preservation of cell viability following massive doses of IR. To be successful, the treatment needs to be administered before the degradative and apoptotic signaling process proceeds beyond reversal. In the following, the intermediate (18hr) and long-term (48hr) viability of irradiated cells was examined, following addition of a membrane-sealing agent (P188), an antioxidant cofactor (NAC) and an exogenous energy substrate (Mg-ATP).
Materials and Methods
A schematic diagram of the experimental protocol used to determine the effect of polymers, antioxidants and high-energy metabolic substrate is presented in Fig. 1.
Figure 1.
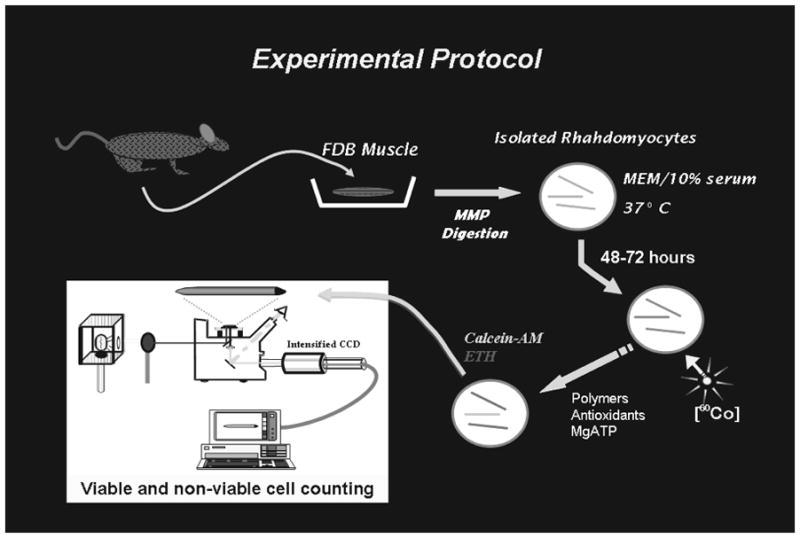
Rhabdomyocyte harvest and culture
Flexor digitorum brevis skeletal muscle cells were harvested from 4-week-old female Sprague-Dawley rats (Harlan-Sprague-Dawley Inc., Indianapolis, IN) through the University of Chicago Carlson Animal Facility. The protocol for using laboratory animals was approved by the Institutional Animal Care and Use Committee. The muscles were extracted within 20 minutes following sacrifice via asphyxiation. They were soaked for 18–20hrs in 0.3% collagenase type III and 0.35% trypsin (Worthington Biochemical Corp., NJ) in phosphate-buffered saline solution containing calcium and the pH buffer N-2-hydroxyethylpiperazine-n-2-ethansulfonic acid. To digest the structures binding individual cells, the samples were incubated for 32 minutes at 37°C. The cells were then washed, separated by gentle trituration and distributed onto tissue culture dishes (Falcon, Cambridge, Mass.), 250–300 per dish. Cells were allowed to recover from trituration, remaining untouched for three days at 37°C and 95% relative humidity in Minimum Essential Medium (Gibco BRL, Grand Island, NY) inside a water jacketed incubator (ThermoForma Scientific model 3326, Marietta, OH). The medium was supplemented with 25mM HEPES, 10% Nu-Serum (Collaborative Biomedical products of Becton Dickinson, Bedford, Ma), 50 U ml−1 penicillin, and 100 μg ml−1 Streptomycin (Gibco BRL).
Small molecule drugs and polymers
DMEM was used to strengthen cytoplasmic buffering of free radicals, and as an aqueous phase antioxidant, reduce the free radical degradation of proteins, lipids and therapeutic polymers. Poloxamer 188 (MW ~8,800 BASF, Parsippany, NJ) was used as the membrane sealing surfactant. N-Acetylcysteine (A9165, Sigma Chemical, St. Louis, Mo) was chosen for its antioxidant and GSH-regenerating properties. Neutral dextran (MW 10,000 Da, Sigma-Aldrich, St. Louis, MO) was used as an osmotic control agent with very little membrane sealing capability. Polyethylene Glycol (MW 8000 Da, Sigma-Aldrich, St. Louis), a hydrophilic polymer, was used as a control for both the osmotic and antioxidant effects of Poloxamer 188 with very little sealing efficacy at the concentrations used. Mg-ATP (Sigma-Aldrich, St. Louis) was used to support cellular metabolism during periods of membrane disruption. All agents were stored in cool and dry conditions and in solid form until sterile filtered in a 10× stock solution of DMEM the day it was used to treat the muscle cells.
Fluorescence viability assay
Baseline pre-irradiation cell viability measurement was performed on the third day after cell harvesting. Ethidium homodimer-1 (EH), dissolved in 1:4 DMSO: water, and calcein-AM (Molecular Probes, Oregon), dissolved in dry DMSO, were added from stock solutions to stain the cells at final concentrations of 10 and 3.3μM, respectively. EH fluoresces red (λex = 528nm; λem = 617nm) and becomes readily visible when it is highly concentrated through binding to DNA within the cell. Entrance of this molecule, Mr = 856.77, into the cell indicates significant cell membrane destabilization and cell death. Calcein-AM fluorescence requires ATP-dependent cleavage by esterases, occurring in the cytosol. Its green fluorescence (λex = 494 nm, λem = 517nm) indicates that the cell maintains both metabolic capabilities as well as a stable membrane. Cells demonstrating any accumulation of EH were deemed non-viable even if green fluorescence was still appreciated. Fifteen minutes after the dye was added to these dishes, their fluorescence was assessed using a Nikon Diophot inverted microscope with fluorescent optics. The excitation wavelength was λex = 480nm. Two dishes from each cell batch were tested and if 70% of the cells were viable, they were considered acceptable for inclusion.
Gamma radiation (IR) exposure protocol
Cells were exposed to 60Co gamma radiation (IR) using a Gammacell® 220 (AECL, Chalk River, Ontario). 40 Gy exposure was selected because surfactant sealing with P188 was previously shown to be most effective in preventing acute necrosis in four hour post-irradiation-cell survival at this dose (Greenebaum et al. 2004). Exposure time was calculated from a dose-rate calibration table (approximately 1.5 Gy min−1) furnished by the Laboratory for Radiation and Oncology Research, University of Chicago. Dishes containing cells to be exposed were placed into the Gammacell® unit and exposed for the time necessary to receive the appropriate dose.
When transported to the IR chamber, the cells were placed on top of a 37°C heating pad, and then inside of an insulated box in order to minimize temperature variation between the samples. The sham-exposed samples were taken to the radiation source along with the cells to be irradiated, in order to be subjected to the same temperature and motion stress caused by handling and transport. The experimental protocol is graphically illustrated in Fig. 1. A decrease in viability of sham-exposed cells by more than 20% was interpreted as defective and subsequently discarded.
Treatment of irradiated or sham exposed muscle cell cultures
Following irradiation or sham exposure, the skeletal muscle culture dishes containing 200–300 rat rhabdomyocytes were returned to the incubator. At 20 minutes post-irradiation or sham exposure, culture dishes were then divided into treatment groups: (1) non-IR sham-exposed controls; (2) IR-exposed untreated controls; (3) IR-exposed and treated with 1mM P188; (4) IR-exposed and treated with 10mM NAC + 0.1mM Mg-ATP; (5) IR-exposed and treated with 10mM NAC + 0.1mM Mg-ATP + 1mM P188; (6) IR-exposed and treated with 1mM neutral dextran (18-hour only); (7) IR-exposed and treated with 2mM P188 (18-hour only) (8) IR-exposed and treated with 1mM PEG (48-hour only); (9) IR-exposed and treated with 10mM NAC (48-hour only); (10) IR-exposed and treated with 1mM P188 + 10mM NAC (48-hour only). The agents were added to the dishes from highly concentrated stock solutions adjusted so that concentrations of other components of the culture medium were not altered. Dishes containing the non-IR sham-exposed cells and IR-exposed, non-treated control cells received additional media culture equivalent to the volume added to the treated dishes. Cells were than returned to the incubator and maintained under culture conditions for either 18 or 48 hours.
Post-irradiation survival
IR-exposed or sham-exposed cells were returned to culture and untouched for 18 or 48 hours at 37°C in Minimum Essential Medium, supplemented with 10% Nu-serum, inside a 37°C, 5% CO2 incubator with 98% relative humidity. At 18 and 48 hours, fluorescent dye was added to cells in order to determine cell survival (using the same method as for initial viability testing). The viability of cells at 18 and 48hrs of testing was given by the percentage of all skeletal muscle cells within the dish exhibiting calcein fluorescence alone.
Data analysis
The results of the fluorescence observations from each sample were tabulated as the number of cells with red EH fluorescence, regardless of concomitant green calcein fluorescence (non-viable), and the number with exclusively green calcein fluorescence (viable). The fraction of the total number of fluorescent cells that were viable was determined (% viable) and used as the primary experimental parameter for statistical analysis.
Our analysis considered the mean percentage viability for the multiple samples conducted at each testing parameter (non-IR sham-exposed, IR-exposed untreated controls, IR-exposed treated with variable permutations of P188, NAC, Mg-ATP, dextran, PEG) at the testing interval (18, 48hrs). Data outside the 95% confidence interval of the mean was excluded. A repeated measure ANOVA analysis was used to test for an effect due to post-IR cofactor treatment with and without P188. If differences existed, Bonferroni’s t-test was used to determine statistical significance. Statistical significance was defined as P values < 0.05. Statistical tests were run using SigmaStat Statistical Analysis Program (SPSS Inc., Chicago, IL).
Results
The rat flexor digitorum brevis skeletal muscle cells harvested for these experiments were typically 1–2mm in length and 25–50μm in diameter. With careful handling to avoid shearing or bending, they were stable in culture.
As previously reported, 40 Gy gamma irradiation delivered at 1.5 Gy min−1 to rat skeletal muscle cells in DMEM culture medium maintained at 21% pO2 resulted in acute necrosis and extensive lysis of greater than 90% of the irradiated skeletal muscle cells within an 18-hour period of time (Greenebaum et al 2004). Sham exposed muscle cell cultures did not manifest significant loss of viability. Fig. 2(A) and (B) are photomicrographs of the rat skeletal muscle cells in a culture dish obtained using an inverted microscope. Fig. 2A is a phase-contrast photomicrograph of many radiation-destroyed cells, one with normal morphology and many contracted, lysed and apparently non-viable cells. Fig. 2B is the corresponding fluorescence photomicrograph. Comparing Fig. 2A and 2B illustrates how the fluorescence vital dye indicator corresponds to cell morphology. The living cells cleaved the calcein-AM and exhibited calcein fluorescence (green) uniformly in the cytoplasm. The non-viable cells exhibited little to no calcein content but high EH fluorescence (red). As noted above, cells exhibiting any EH fluorescent were considered non-viable and counted as such, regardless of whether they also contained calcein fluorescence.
Figure 2.

Skeletal muscle cell survival in culture
The average initial viability of the cultured skeletal muscle cells after the 48-hour recovery in culture was 79.4 ± 5.2% (mean ± SEM). At this point, the viable muscle cells were considered recovered from the harvest and entered into the IR exposure or sham exposure experiments. The viability of the non-IR treated skeletal muscle cells remained stable near 80 ± 6.1% throughout the duration of the experiments. None of the polymers or other therapeutic agents used in these experiments caused a significant change in the viability of non-IR exposed skeletal muscle cultures.
Survival 18 hrs post-irradiation
The effect of various protocols on the survival of skeletal muscle cells 18 hours after 40 Gy IR is illustrated in Fig. 3. Multiple pair-wise comparisons of the cell viability between non-IR sham-exposed control, untreated IR cells and the different post-IR treatments indicated the following. The cells showed greater viability when treated with 1mM P188 than without treatment (20.6 ± 3.3% versus 3.7 ± 1.2%; p < 0.01). The fraction of viable cells, however, was significantly smaller compared to non-IR sham-exposed controls (77.0 ± 2.2%; p < 0.01). The cells treated with 2mM P188 demonstrated similarly improved viability compared to no post-IR treatment (14.0 ± 3.5% versus 3.5 ± 1.1%; p <0.01) and lower viability compared to non-IR sham-exposed controls (78.2 ± 2.1%; p < 0.01). There was no statistically significant difference between the effects of 1 and 2mM P188 (p > 0.05). The treatment with 1mM neutral dextran had essentially no effect on survival compared to untreated cells (5.3 ± 1.1% versus 1.9 ± 0.6%).
Figure 3.

The mean percent survival of cells treated with combined NAC and MgATP is significantly greater than that of the cells that received no treatment (48.2 ± 6% versus 6.9 ± 3%) however is less than that of the sham-exposed cells (78.0 ± 2.3%). The cells treated with the combined cofactors also demonstrated a significantly greater post-radiation viability than those that received P188 alone (48.2 ± 6% versus 20.6 ± 3.3%). Those cells that were treated with both the cofactors and P188 manifested significantly greater viability than the untreated cells (55.2 ± 2.8% versus 6.8 ± 1.7%) however had lower viability than those that received sham-exposure (77.3 ± 1.8%). The addition of P188 to the cofactors did not substantially alter the post-radiation viability (55.2 ± 2.8% versus 48.2 ± 6%).
48hr post-radiation cell viability
The progressive deterioration of survival over time after severe radiation injury can be appreciated in Fig. 4. Regardless of which therapy was used, the slope of the survival curve for the irradiated cells was negative. Adding surfactants to seal, antioxidants to reduce oxidative injury and ATP to improve cell energy economy appear to have an effect on the slope of the survival curve. This curve did not plateau in the 48 hour period observed.
Figure 4.

The comparative effects of different polymers and cofactors on the survival at the 48 hour time point is presented in Table 1 which contains the results of six different treatments on the mean percent viabilities (±SEM) of cells 48hrs after 40Gy IR. ANOVA calculations demonstrated statistically significant effects from the P188 and cofactor treatment permutations but not from that with PEG.
Table 1.
Rhabdomyocyte survival at 48 hours. 48hr mean percent survival (±SEM) of non-IR exposed cells, as well as survival of IR-exposed cells receiving P188 alone, PEG alone, cofactor NAC alone as well as combined with P188 or MgATP and P188 with NAC and MgATP.
| Treatment | Baseline | Sham-exposed | 48hrs Post-Radiation (40Gy) | |
|---|---|---|---|---|
| 0hr Control | 48hr Control | No Treatment | Treatment | |
| 1mM P188 | 89.9% ± 1.5 | 83.3% ± 2.2 | 4.8% ± 0.6 | 8.1% ± 1.5a |
| 1mM PEG | 89.9% ± 1.5 | 87.3% ± 3.1 | 6.8% ± 1.0 | 7.1% ± 1.4 |
| 10mM NAC | 89.9% ± 1.5 | 85.3% ± 3.1 | 6.8% ± 1.0 | 13.4% ± 3.8a |
| 1mM P188, 10mM NAC | 86.9% ± 2.1 | 88.0% ± 1.1 | 7.8% ± 1.9 | 31.2% ± 1.1a |
| 10mM NAC, 0.1mM MgATP | 86.9% ± 2.1 | 82.5% ± 1.2 | 5.5% ± 1.6 | 19.9% ± 1.7a |
| 1mM P188, 10mM NAC, 0.1mM MgATP | 86.9% ± 2.1 | 81.7% ± 1.0 | 7.2% ± 1.4 | 29.5% ± 3.0a |
Results are significantly different than irradiated, untreated samples
Pair-wise comparisons were performed for sham-exposed versus radiated cells with the six different treatment permutations as well as the treated versus untreated cells. Several comparison pairs were also made between the post-radiation viabilities of select permutations. The cells treated with only P188 demonstrated significantly greater viability than those that were untreated (8.1 ± 1.5% versus 4.8 ± 0.6%; p < 0.05) however also demonstrated significantly less viability than the sham-exposed cells (83.3 ± 2.2%; p < 0.0001). The cells treated with PEG did not demonstrate significantly greater viability than those that were untreated (7.1 ± 1.4% versus 6.8 ± 1.0%; p > 0.05) and had significantly less viability than the sham-exposed cells (87.3 ± 3.1%; p < 0.0001). The cells treated with only NAC did not demonstrate greater viability than those that were untreated (13.4 ± 3.8% versus 6.8 ± 1.0%; p > 0.05) and demonstrated viability significantly less than the sham-exposed cells (85.3 ± 3.1%; p < 0.0001). The combination of P188 and NAC, however, did produce greater post-radiation viability compared to those that were untreated (31.2 ± 1.1% versus 7.8 ± 1.9%; p < 0.0001). This permutation also produced significantly less viability than the sham-exposed cells (88.0 ± 1.1%; p < 0.0001). The combination of only the two cofactors produced significantly greater viability than that of the untreated cells (19.9 ± 1.7% versus 5.5 ± 1.6%; p < 0.0005) but again significantly less than the sham-exposed cells (82.5 ± 1.5%; p < 0.0001). The sixth and final permutation of P188 with both cofactors produced significantly greater viability than that of the untreated cells (29.5 ± 3.0% versus 7.2 ± 1.4%; p < 0.0001) and again significantly less than the viability of the sham-exposed cells (81.7 ± 1.0%; p < 0.0001).
Discussion
Following exposure to massive concentrations of IR-generated reactive oxygen species, cell viability is enhanced by sealing of the cellular membrane, scavenging of free radicals and the augmentation of cellular energy metabolism. It was previously reported that under the same IR-exposure and culture conditions, the majority of skeletal muscle cell cultures treated only with fresh media were completely disrupted within four hours of IR exposure. Sealing IR-lysed membranes with sub-critical micelle concentrations of copolymer surfactant P188 in culture media prevented acute necrosis and prolonged survival. At 18 hours the viability of P188-treated radiopermeabilized skeletal muscle cells had decreased to approximately 20% compared to the 3% viability for cells treated with fresh media only (Greenebaum et al. 2004).
In this series of experiments, the addition of NAC and MgATP with P188 to irradiated cells was shown to increase 18-hour viability of radiopermeabilized skeletal muscle fibers comparable to that of non-radiated cells. However, viability of the surfactant, antioxidant and ATP treated cells at 48hrs is much less than what was measured at 18hrs. This finding suggests that multiple cell death pathways are initiated by exposure to massive IR. One possibility is that the prolonged oxidative stress initiates mitochondrial changes that in turn lead to a cascade of irreversible damage and apoptotic cell death (Freeman and Crapo 1982; Stark 1991; Leach et al. 2001). The timeline of the present experiment appears to be consistent with an apoptotic model of cell death. There are multiple mechanisms by which an antioxidant like NAC may augment the efficacy of P188 in massive IR injury. NAC may reduce the ROIs produced by the radiation-injured cells, which would diminish the damage to cellular proteins, organelles or nucleic acids. In addition, reducing the ROIs would also decrease P188 degradation. In theory, this would increase the efficacy of P188 as a membrane sealant. The addition of ATP should also reduce ROI generation in damaged skeletal muscle cells if the source of ROIs is mediated by build up of xanthine oxidase. Adequate ATP supply would prevent utilization of lower affinity AMP for metabolic energy generation.
The present study has two main limitations. Firstly, the therapeutic permutations were administered exclusively at 20 minutes following irradiation. For the therapy to be clinically significant, it would need to be administered in a time frame realistic with that required by an emergency medical team responding to a nuclear accident or detonation of a nuclear weapon. Secondly, although this study demonstrated the therapeutic efficacy in preserving short-term cellular viability as represented by an intact cellular membrane and functioning cellular enzymes (demonstrated by the cleavage of calcein dye), long-term cellular survival was not assessed. Although the treatment as described addresses immediate mechanisms of injury following irradiation, the cellular pathways signaling apoptosis following major irradiation have not yet been elucidated. As these pathways become better understood and therapeutics addressing them are developed, they may be added to the treatment regimen, facilitating analysis of cellular survival weeks after injury.
Radioprotective agents used after exposure to massive doses of IR may be broadly classified into three groups: free radical scavengers, modulators of receptor-coupled pathways, and molecules that stabilize the plasma membrane. Individual therapeutics from each of these classes are slowly being integrated into clinical practice. Amifostine, an antioxidant studied by the US military for soldiers at risk of massive radiation exposure, was shown to provide superior radioprotective properties (Grdina et al. 2000). It is currently approved for the prevention of radiation-induced xerostomia and mucositis in the treatment of head and neck cancers (Brizel et al. 2000). Clinical evidence also suggests that amifostine provides lung and esophageal protection when used in combination with thoracic radiotherapy (Antonadou et al. 2003). Pentoxifylline, a xanthine derivative that decreases blood viscosity, is undergoing studies suggesting that it may provide radioprotective benefits through reduction of inflammatory mediators such as C-reactive protein, TNF-alpha, and Fas or Apo-1 released following radiation exposure (Rube et al. 2002). While cellular studies of these individual modalities have demonstrated success, results from our studies suggest that a multimodal approach to radiation injury may provide additive if not synergistic benefits. The efficacy of these agents in treatment of high–dose irradiation is quite likely to be augmented by the addition of a membrane sealing surfactant to the therapy.
The capability to prolong viability for one to two days following massive IR generation of free radicals has value. IR may trigger cell death processes through multiple pathways. By preventing death due to membrane disruption, a window of opportunity is created to administer inhibitors of ongoing cell death pathways as well as to augment endogenous DNA repair processes. Sealing the cell membrane is one of the critical steps required for cellular viability following exposure to massive doses of IR.
Acknowledgments
This work was fully supported by a grant from the US National Institutes of Health, NIGMS R01-GM53113. Dr. R. Lee is a director of Maroon Biotech, Inc which is developing products based on surfactants and antioxidants
Footnotes
Presented at: Midwest Association of Plastic Surgeons 2010 Conference
Contributor Information
Alexander P. Soneru, Email: soneru@uchicago.edu.
Michael A. Beckett, Email: mbeckett@uchicago.edu.
Ralph R. Weichselbaum, Email: rrw@radonc.bsd.uchicago.edu.
References
- Anno GH, Baum SJ, Withers HR, Young RW. Symptomatology of acute radiation effects in humans after exposure to doses of 0.5–30 Gy. Health Phys. 1989;56:821–838. doi: 10.1097/00004032-198906000-00001. [DOI] [PubMed] [Google Scholar]
- Antonadou D, Petridis A, Synodinou M, Throuvalas N, Bolanos N, Veslemes M, Sagriotis A. Amifostine reduces radiochemotherapy-induced toxicities in patients with locally advanced non-small cell lung cancer. Semin Onc. 2003;30:2–9. doi: 10.1053/j.seminoncol.2003.11.008. [DOI] [PubMed] [Google Scholar]
- Brizel DM, Wasserman TH, Henke M, Strnad V, Rudat V, Monnier A, Eschwege F, Zhang J, Russell L, Oster W, Sauer R. Phase III Randomized Trial of Amifostine as a Radioprotector in Head and Neck Cancer. J Clin Oncol. 2000;18:3339–3345. doi: 10.1200/JCO.2000.18.19.3339. [DOI] [PubMed] [Google Scholar]
- Coleman CN, Stone HB, Moulder JE, Pellmar TC. Modulation of radiation injury. Science. 2004;304:693–694. doi: 10.1126/science.1095956. [DOI] [PubMed] [Google Scholar]
- Delanian S, Baillet F, Huart J, Lefaix JL, Maulard C, Housset Successful treatment of radiation-induced fibrosis using liposomal Cu/Zn superoxide dismutase: clinical trial. Radiother Oncol. 1994;32:12–20. doi: 10.1016/0167-8140(94)90444-8. [DOI] [PubMed] [Google Scholar]
- Freeman BA, Crapo JD. Biology of disease: free radicals and tissue injury. Lab Invest. 1982;47:412–426. [PubMed] [Google Scholar]
- Greenebaum B, Blossfield K, Hannig J, Carrillo CS, Beckett MA, Weichselbaum RR, Lee RC. Poloxamer 188 prevents acute necrosis of adult skeletal muscle cells following high-dose irradiation. Burns. 2004;30:539–547. doi: 10.1016/j.burns.2004.02.009. [DOI] [PubMed] [Google Scholar]
- Grdina DJ, Kataoka Y, Murley JS. Amifostine: Mechanisms of action underlying cytoprotection and chemoprevention. Drug Metabol Drug Interact. 2000;16:237–279. doi: 10.1515/dmdi.2000.16.4.237. [DOI] [PubMed] [Google Scholar]
- Hannig J, Lee RC. Structural changes in cell membranes after ionizing electromagnetic field exposure. IEEE Trans Plasma Sci. 2000;28:97–101. [Google Scholar]
- Hannig J, Zhang D, Canaday DJ, Beckett MA, Astumian RD, Weichselbaum RR, Lee RC. Surfactant sealing of membranes permeabilized by ionizing radiation. Radiat Res. 2000;154:171–177. doi: 10.1667/0033-7587(2000)154[0171:ssompb]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Kamiya K, Yasukawa-Barnes J, Mitchen JM, Gould MN, Clifton KH. Evidence that carcinogenesis involves an imbalance between epigenetic high-frequency initiation and suppression of promotion. Proc Natl Acad Sci USA. 2005;92:1332–1336. doi: 10.1073/pnas.92.5.1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leach JK, Van Tuyle G, Lin PS, Schmidt-Ullrich R, Mikkelsen RB. Ionizing radiation-induced, mitochondria-dependent generation of reactive oxygen/nitrogen. Cancer Res. 2001;61:3894–3901. [PubMed] [Google Scholar]
- Lee RC, Astumian RD. The physicochemical basis for thermal and non-thermal burn injury. Burns. 1996;22:509–519. doi: 10.1016/0305-4179(96)00051-4. [DOI] [PubMed] [Google Scholar]
- Maskarinec SA, Hannig J, Lee RC, Lee KY. Direct obervation of poloxamer 188 insertion into lipid monolayers. Biophys J. 2002;82:1453–1459. doi: 10.1016/S0006-3495(02)75499-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maskarinec SA, Wu G, Lee KY. Membrane sealing by polymers. Ann NY Acad Sci. 2005;1066:310–320. doi: 10.1196/annals.1363.018. [DOI] [PubMed] [Google Scholar]
- Mettler FA, Voelz GL. Major radiation exposure - What to expect and how to respond. N Engl J Med. 2002;346:1554–1561. doi: 10.1056/NEJMra000365. [DOI] [PubMed] [Google Scholar]
- Mitchell JB, Krishna MC. Nitroxides as radiation protectors. Mil Med. 2002;167:49–50. [PubMed] [Google Scholar]
- Mothersill C, Seymour CB. Cell–cell contact during gamma irradiation is not required to induce a bystander effect in normal human keratinocytes: Evidence for release during irradiation of a signal controlling survival into the medium. Radiat Res. 1998;149:256–262. [PubMed] [Google Scholar]
- Neal R, Matthews RH, Lutz P, Ercal N. Antioxidant role of N-acetylcysteine isomers following high dose irradiation. Free Radic Biol Med. 2003;34:689–695. doi: 10.1016/s0891-5849(02)01372-2. [DOI] [PubMed] [Google Scholar]
- Rube CE, Wilfert F, Uthe D, Schmid KW, Knoop R, Willich N, Schuck A, Rube C. Modulation of radiation-induced tumour necrosis factor alpha (TNF-alpha) expression in the lung tissue by pentoxifylline. Radiother Oncol. 2002;64:177–187. doi: 10.1016/s0167-8140(02)00077-4. [DOI] [PubMed] [Google Scholar]
- Stark G. The effect of ionizing radiation on lipid membranes. Biochim Biophys Acta. 1991;1071:103–122. doi: 10.1016/0304-4157(91)90020-w. [DOI] [PubMed] [Google Scholar]
- Swennen EL, Dagnelie PC, Buecken TV, Bast A. Radioprotective effects of ATP in human blood ex vivo. Biochem Biophys Res Commun. 2008;367:383–387. doi: 10.1016/j.bbrc.2007.12.125. [DOI] [PubMed] [Google Scholar]
- Szeinfeld D. Multifactorial role of ATP in repair processes and radioprotection. Med Hypotheses. 1990;32:225–229. doi: 10.1016/0306-9877(90)90127-z. [DOI] [PubMed] [Google Scholar]
- Yang CR, Wilson-Van Patten C, Planchon SM, Wuerzberger-Davis, Davis TW, Cuthill S, Miyamoto S, Boothman DA. Coordinate modulation of Sp1, NF-kappa B, and p53 in confluent human malignant melanoma cells after ionizing radiation. FASEB J. 2000;14:379–390. doi: 10.1096/fasebj.14.2.379. [DOI] [PubMed] [Google Scholar]


