Abstract
Objective: In order to overcome the defects of chemical hydrolysis approach to prepare glucosamine, an enzymatic hydrolysis method was developed. Methods: Glucosamine was prepared by hydrolyzing chitosan, employing α-amylase initially, and subsequently, glucoamylase. Results: The optimal hydrolyzing conditions were as follows: reaction time, 4 h; pH, 5.0; temperature, 50 °C; and, α-amylase, 80 U/g for the initial reaction. Subsequently, glucoamylase was added in the presence of α-amylase. The optimal reaction conditions were found to be: reaction time, 8 h; pH, 4.5; temperature, 55 °C; and, glucoamylase, 4 000 U/g. The hydrolysates were subject to filtrating, concentrating to about 20% (w/w), precipitating with five volumes of ethanol, and drying at 60 °C for 2 h. The content and the yield of glucosamine in the dried precipitate were 91.3% (w/w) and 86.2% (w/w), respectively. Conclusions: The method developed in this study is a promising option in the preparation of glucosamine.
Keywords: Glucosamine, α-Amylase, Glucoamylase, Hydrolysis
1. Introduction
Glucosamine, an amino monosaccharide, has been used for the treatment of arthritis (Reginster et al., 2001; Nakamura, 2011), inflammation (Nagaoka et al., 2011), cancer (Oh et al., 2007), and orthopedic diseases (Minami et al., 2011). Glucosamine can usually be obtained by chemical hydrolysis of chitosan (Zhang et al., 2007; Xing et al., 2009). The chemical hydrolysis often involves acid and has some defects: harsh hydrolysis conditions, chemical modifications of glucose ring, and others (Wang et al., 2008). Enzymatic methods have advantages over chemical approach, such as mild reaction conditions and high specificity, as well as high yield, in the products of glucosamine, without modifications of glucose ring.
Many enzymes with different specificities have been reported for their abilities to hydrolyze chitosan at a comparable level, such as cellulase, pectinase, pepsin, papain, neutral protease, lipase, and α-amylase (Xia et al., 2008; Wu, 2011). Based on our previous studies, glucoamylase, which belongs to exo-amylase, also has the ability to hydrolyze chitosan (Pan and Wu, 2011).
In this paper, we report the preparation of high glucosamine-containing product by hydrolyzing chitosan with α-amylase and glucoamylase. The optimal reaction conditions were investigated, and the composition of the product produced by hydrolysis was studied.
2. Materials and methods
2.1. Materials
Chitosan was obtained from Nantong Biochemical Co. (Jiangsu, China). Its degree of de-acetylation and molecular weight (M w) were 91.7% and 4.1×105, respectively. α-Amylase, with activity at 4 000 U/mg, and glucoamylase, with activity at 50 000 U/mg were obtained from Fuchen Chemical reagents Co. (Tianjin, China).
2.2. Hydrolysis of chitosan using α-amylase followed by glucoamylase
Chitosan was dissolved in acetic acid (HAc) solution (1%, v/v) to make a solution of a concentration of 1% (w/w). The pH was adjusted to 5.0 using 1 mol/L NaOH. α-Amylase (80 U/g) was added into a reactor containing 500 ml of chitosan solution and the reactor was kept in a thermostatic water bath at 50 °C for up to 6 h. To the above hydrolysates, 4 000 U/g glucoamylase was added and the pH was adjusted to 4.5 using 1 mol/L HAc. The hydrolysis was continued at 55 °C for additional 12 h. Aliquots were periodically taken from the reaction mixture, filtrated, and heated to 95 °C for 15 min to terminate the reaction.
2.3. Precipitating with ethanol
The reaction mixture was filtrated through Whatman GF/A filter paper, concentrated to about 20% (w/w) in a vacuum rotary evaporator (Jiangyin Dry Equipment Manufacture Co., Ltd., Jiangyin, Jiangsu, China) at 60 °C, and then precipitated with five volumes of ethanol. The precipitates were dried at 60 °C for 2 h (Wu et al., 2009).
2.4. Analytical methods
The pH of the solution was recorded using a digital pH meter (Model PHS-3C; CD Instruments, China). The reducing sugars were estimated by the method of Somogyi and expressed by dextrose equivalent (DE) value (Nelson, 1944). DE value was used as an index of enzyme activity in the reaction mixture. The composition of the sugar in the resulting product was analyzed employing Waters 600 high-performance liquid chromatography (HPLC) equipped with a double column system. The first column (Sugarpark 1, 6.5 mm i.d.×300 mm) used pure water as mobile phase at a flow rate of 0.5 ml/min and the column temperature was maintained at 85 °C. The second column (SpherisorbNH2, 4.6 mm i.d.×250 mm) used acetonitrile/water (70/30, v/v) as mobile phase at a flow rate of 1.0 ml/min and the column temperature was 30 °C. The detector used was a high sensitive refractive index detector (Model ERC-7515 A, ERC Inc., Japan). The detector sensitivity was 4 μRIU and the inject volume was 10 μl (Wu et al., 2009).
3. Results and discussion
3.1. Hydrolysis of chitosan by α-amylase and glucoamylase
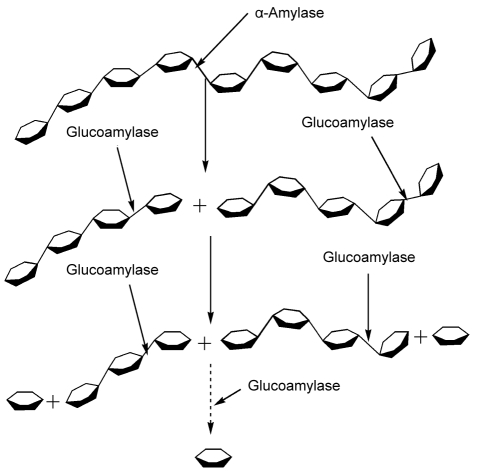
α-Amylase, an endo-acting hydrolase, was expected to hydrolyze β-1,4 linkages in chitosan in a random fashion and to yield water-soluble chitooligosaccharides and glucosamine. Subsequently added enzyme, glucoamylase, an exo-acting hydrolase, was expected to attack the substrates from the non-reducing end, producing glucosamine. Mechanism of hydrolysis of chitosan by α-amylase and glucoamylase to produce glucosamine can be illustrated as shown in Fig. 1. First, chitosan is hydrolyzed by α-amylase to produce chitooligosaccharides. Second, chitooligosaccharides are hydrolyzed by glucoamylase to produce the final glucosamine.
Fig. 1.
Schematic diagram of hydrolysis of chitosan by α-amylase and glucoamylase
3.2. Effect of time on hydrolyzing chitosan
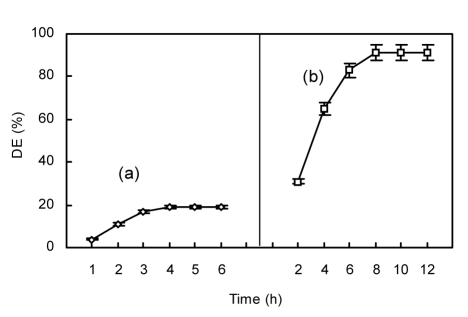
As shown in Fig. 2a, DE values increased sharply within 3 h, slightly from 3 h to 4 h, and did not increase further after 4 h. Therefore, the optimal reaction time with α-amylase was judged to be 4 h. When glucoamylase was added to this reaction mixture, the maximum DE value was achieved at 8 h reaction time, and there was no subsequent increase in DE value between 8 and 12 h. Therefore, the optimal reaction time with glucoamylase was considered to be 8 h (Fig. 2b).
Fig. 2.
Effect of reaction time on hydrolysis of chitosan employing α-amylase initially (a) and then glucoamylase (b)
(a) Initial reaction conditions with α-amylase: pH 5.0, 50 °C, 80 U/g of α-amylase; (b) Subsequent reaction conditions with glucoamylase: pH 4.5, 55 °C, 4 000 U/g of glucoamylase. Data are shown as mean±SD (n=9)
3.3. Effects of pH, temperature, and enzyme amount on hydrolyzing chitosan
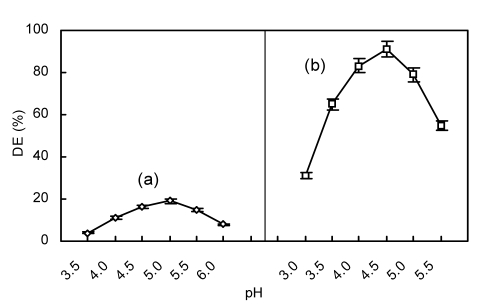
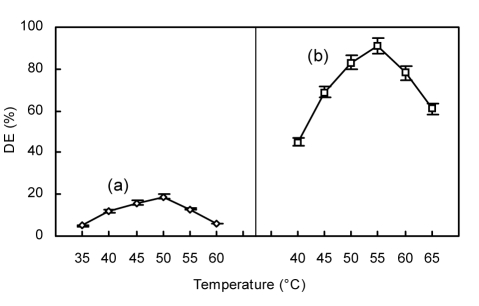
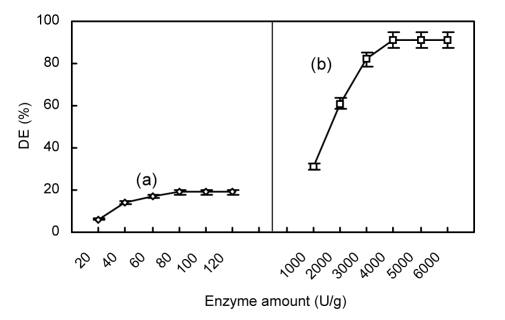
The pH and temperature of a reaction mixture can influence α-amylase and glucoamylase activities, and subsequently chitosan hydrolysis. Enzyme concentration may also be important for efficient hydrolysis. In the initial hydrolysis by α-amylase, the reaction was found to be optimal at pH 5.0 (Fig. 3a), 50°C (Fig. 4a), and 80 U/g α-amylase of reaction mixture (Fig. 5a). In contrast, other reports have described optimal conditions for chitosan hydrolysis using α-amylase at pH 5.4 (Zhou et al., 2003) and 7.0 (Yu and Wang, 2008), and temperatures of 45 °C (Yu and Wang, 2008) and 50 °C (Zhou et al., 2003). These varying reported optimal pH and temperatures may be due to differences in substrates, α-amylase sources, and reaction time. In the second stage of hydrolysis by the addition of glucoamylase, the reaction was found to be optimal at pH 4.5 (Fig. 3b), 55 °C (Fig. 4b), and 4 000 U/g glucoamylase of reaction mixture (Fig. 5b).
Fig. 3.
Effect of pH on hydrolysis of chitosan employing α-amylase initially (a) and then glucoamylase (b)
(a) Initial reaction conditions with α-amylase: 4 h, 50 °C, 80 U/g of α-amylase; (b) Subsequent reaction conditions with glucoamylase: 8 h, 55 °C, 4 000 U/g of glucoamylase. Data are shown as mean±SD (n=9)
Fig. 4.
Effect of temperature on hydrolysis of chitosan employing α-amylase initially (a) and then glucoamylase (b)
(a) Initial reaction conditions with α-amylase: 4 h, pH 5.0, 80 U/g of α-amylase; (b) Subsequent reaction conditions with glucoamylase: 8 h, pH 4.5, 4000 U/g of glucoamylase. Data are shown as mean±SD (n=9)
Fig. 5.
Effect of enzyme amount on hydrolysis of chitosan employing α-amylase initially (a) and then glucoamylase (b)
(a) Initial reaction conditions with α-amylase: 4 h, pH 5.0, 50 °C; (b) Subsequent reaction conditions with glucoamylase: 8 h, pH 4.5, 55 °C. Data are shown as mean±SD (n=9)
3.4. Characterization of the product in the resulting hydrolysate
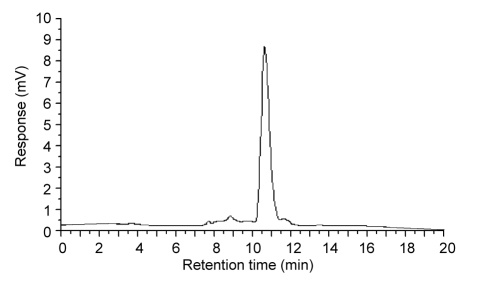
The sugars in the resulting hydrolysate were glucosamine (91.3%, w/w), N-acetyl-glucosamine (7.2%, w/w), and chitooligomers (1.5%, w/w) (Fig. 6), indicating that the amount of glucosamine in the product was very high. The yield of glucosamine was 86.2% (w/w). Although the glucosamine content was comparable to that obtained by Zhang et al. (2007) (89.14%–97.78%, w/w), the yield achieved in this study was higher than that achieved by those researchers (64.86%, w/w).
Fig. 6.
HPLC spectrum of the resulting glucosamine sample
4. Conclusions
Glucosamine can be prepared by hydrolyzing chitosan, using α-amylase followed by glucoamylase. The optimal conditions with α-amylase were as follows: reaction time, 4 h; pH, 5.0; temperature, 50 °C; and α-amylase, 80 U/g. In the subsequent hydrolysis by glucoamylase in the presence of α-amylase, the optimum conditions were found to be: reaction time, 8 h; pH, 4.5; temperature, 55 °C; and glucoamylase, 4 000 U/g. The hydrolysates were subject to filtrating, concentrating to 20% (w/w), and precipitating with five volumes of ethanol. The precipitates obtained were dried at 60 °C for 2 h. The content of glucosamine in the product and the yield of glucosamine were 91.3% (w/w) and 86.2% (w/w), respectively.
Footnotes
Project (No. 2009HS07) supported by the Open Fund of Jiangsu Key Laboratory of Marine Biotechnology, China
References
- 1.Minami C, Hata M, Tamai Y, Hashida M, Takayama T, Yamamoto S, Okada M, Funatsu T, Tsuka T, Imagawa T, et al. Clinical application of D-glucosamine and scale collagen peptide on canine and feline orthopedic diseases and spondylitis deformans. Carbohydr Polym. 2011;84(2):831–834. doi: 10.1016/j.carbpol.2010.06.021. [DOI] [Google Scholar]
- 2.Nagaoka I, Igarashi M, Hua J, Ju Y, Yomogida S, Sakamoto K. Recent aspects of the anti-inflammatory actions of glucosamine. Carbohydr Polym. 2011;84(2):825–830. doi: 10.1016/j.carbpol.2010.04.007. [DOI] [Google Scholar]
- 3.Nakamura H. Application of glucosamine on human disease—osteoarthritis. Carbohydr Polym. 2011;84(2):835–839. doi: 10.1016/j.carbpol.2010.08.078. [DOI] [Google Scholar]
- 4.Nelson N. A photometric adaptation of the Somogyi method for the determination of glucose. J Biol Chem. 1944;153(3):375–380. [Google Scholar]
- 5.Oh HJ, Lee JS, Song DK, Shin DH, Jang BC, Suh SI, Park JW, Suh MH, Baek WK. D-Glucosamine inhibits proliferation of human cancer cells through inhibition of p70S6K. Biochem Biophys Res Commun. 2007;360(4):840–845. doi: 10.1016/j.bbrc.2007.06.137. [DOI] [PubMed] [Google Scholar]
- 6.Pan SK, Wu SJ. Preparation of water soluble chitosan by hydrolysis with commercial glucoamylase containing chitosanase activity. Eur Food Res Technol. 2011;233(2):325–329. doi: 10.1007/s00217-011-1524-7. [DOI] [Google Scholar]
- 7.Reginster JY, Deroisy R, Rovati LC, Lee RL, Lejeane E, Bruyere O, Giacovelli G, Henrotin Y, Daore JE, Gossett C. Long-term effects of glucosamine sulphate on osteoarthritis progression: a randomized, placebo-controlled clinical trial. Lancet. 2001;357(9252):251–256. doi: 10.1016/S0140-6736(00)03610-2. [DOI] [PubMed] [Google Scholar]
- 8.Wang WP, Dua YM, Qiu YL, Wang XY, Hu YJ, Yang JH, Cai J, Kennedy JF. A new green technology for direct production of low molecular weight chitosan. Carbohydr Polym. 2008;74(1):127–132. doi: 10.1016/j.carbpol.2008.01.025. [DOI] [Google Scholar]
- 9.Wu SJ. Preparation of water soluble chitosan by hydrolysis with commercial α-amylase containing chitosanase activity. Food Chem. 2011;128(3):769–772. doi: 10.1016/j.foodchem.2011.03.111. [DOI] [Google Scholar]
- 10.Wu SJ, Chen HQ, Tong QY, Xu XM, Jin ZY. Preparation of maltotriose by hydrolyzing of pullulan with pullulanase. Eur Food Res Technol. 2009;229(5):821–824. doi: 10.1007/s00217-009-1118-9. [DOI] [Google Scholar]
- 11.Xia WS, Liu P, Liu J. Advance in chitosan hydrolysis by non-specific cellulases. Bioresour Technol. 2008;99(15):6751–6762. doi: 10.1016/j.biortech.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 12.Xing RE, Liu S, Wang L, Cai SB, Yu HH, Feng JH, Li PC. The preparation and antioxidant activity of glucosamine sulfate. Chin J Oceanol Limnol. 2009;27(2):283–287. doi: 10.1007/s00343-009-9135-x. [DOI] [Google Scholar]
- 13.Yu HY, Wang S. Study on enzymatic production of low-molecular weight chitosan. Food Sci. 2008;29:464–466. (in Chinese) [Google Scholar]
- 14.Zhang LJ, Hou DY, Xin G, Gu H, Zhao SY. Study on preparation and antioxidation effects of D-glucosamine hydrochloride. Food Sci. 2007;28:103–105. (in Chinese) [Google Scholar]
- 15.Zhou G, He ZP, Dun GH, Huang ZY, Tan XC. Enzyme kinetics of amylase and cellulose on hydrolyzing chitosan. Mar Sci. 2003;27(11):59–62. (in Chinese) [Google Scholar]