Abstract
Chronic GVHD was recognized as a complication of allogeneic hematopoietic cell transplantation more than 30 years ago, but progress has been slowed by the limited insight into the pathogenesis of the disease and the mechanisms that lead to development of immunological tolerance. Only 6 randomized phase III treatment studies have been reported. Results of retrospective studies and prospective phase II clinical trials suggested overall benefit from treatment with mycophenolate mofetil or thalidomide, but these results were not substantiated by phase III studies of initial systemic treatment for chronic GVHD. A comprehensive review of published reports showed numerous deficiencies in studies of secondary treatment for chronic GVHD. Fewer than 10% of reports documented an effort to minimize patient selection bias, used a consistent treatment regimen, or tested a formal statistical hypothesis that was based on a contemporaneous or historical benchmark. In order to enable valid comparison of the results from different studies, eligibility criteria, definitions of individual organ and overall response, and time of assessment should be standardized. Improved treatments are more likely to emerge if reviewers and journal editors hold authors to higher standards in evaluating manuscripts for publication.
Keywords: Chronic graft-versus-host disease, Treatment, Phase II clinical trials, Review
INTRODUCTION - THE PAST
Allogeneic hematopoietic cell transplantation (HCT) is frequently complicated by acute and chronic graft-versus-host disease (GVHD) [1, 2]. Although considerable progress has been made in the development of methods to prevent or treat acute GVHD, similar progress in chronic GVHD has languished by comparison after the clinical and pathologic features of this syndrome were first described in 1980 [3]. Interest in this debilitating complication of HCT was rejuvenated when recommendations of the National Institutes of Health Consensus Conference on Criteria for Clinical Trials in Chronic Graft-versus-host disease were published in 2005-2006 [4-9].
The Consensus Conference recognized two major categories of GVHD, each with 2 subcategories [4]. Acute GVHD with onset before day 100 was defined as "classic GVHD." A separate category was recognized for persistent, recurrent or late-onset acute GVHD beyond day 100 after HCT. Chronic GVHD was separated from acute GVHD not by time from HCT but by the presence of diagnostic criteria or by distinctive findings supported by biopsy or other procedures. Classic chronic GVHD was defined by unambiguous chronic GVHD manifestations in the absence of abnormalities such as cutaneous erythema, liver function abnormalities, or gastrointestinal manifestations typical of acute GVHD. "Overlap syndrome" is a subcategory of chronic GVHD characterized by chronic GVHD in the presence of one or more manifestations typical of acute GVHD.
Chronic GVHD is a pleomorphic syndrome with "autoimmune" features that sometimes resemble clinical findings in scleroderma and Sjögren syndrome. The onset usually occurs between 3 and 15 months after HCT [10-13]. Risk factors associated with an increased risk of chronic GVHD include the use of a mobilized blood cell graft or an HLA-mismatched or unrelated donor, older patient age and a history of acute GVHD [12]. The risk of chronic GVHD can be decreased by exhaustive depletion of T cells from the graft or by treatment of the recipient with rabbit antibodies specific for human T cells as part of the conditioning regimen before HCT [12, 14, 15]. Without these measures, approximately 30% to 50% of HCT recipients develop chronic GVHD [2, 16].
Chronic GVHD can affect multiple organs and sites, including the skin and subcutaneous connective tissues, lacrimal and salivary glands, oral mucosa, lungs, esophagus, joints, gastrointestinal tract and liver. The disease is characterized by immune dysfunction with an increased risk of infections and a 30% to 50% risk of mortality during the first 5 years after diagnosis [10, 11]. Chronic GVHD has been associated with a reduced risk of recurrent malignancy after HCT [17-22], but despite this benefit, survival is not improved [22]. A prognostic scoring system has recently been proposed based on factors present at the time of chronic GVHD diagnosis [13].
PRINCIPLES OF TREATMENT - THE PRESENT
To date, only 6 randomized phase III studies have ever been reported for initial treatment of chronic GVHD [23-28]. The study by Koc et al. [26] was the only one that indicated benefit. Results of this study suggested that treatment with cyclosporine reduced the amount of glucocorticoid treatment needed to control the disease, as indicated by a decreased frequency of avascular necrosis. The generally recommended approach for treatment of chronic GVHD involves continued administration of the calcineurin inhibitor used for GVHD prophylaxis together with prednisone initially at 1 mg/kg/day [2, 29, 30]. Strategies for tapering the dose of prednisone vary considerably, but as a general principle, efforts should be made to use the minimum dose that is sufficient to control GVHD manifestations. At our center, the dose of prednisone is tapered to an alternate-day schedule of administration after initial clinical improvement, which generally occurs within 6 weeks after starting treatment. The dose of prednisone is then tapered to 0.5 mg/kg every other day and generally continued until reversible manifestations of the disease resolve. The dose of prednisone is then gradually tapered with careful monitoring for recurrent manifestations of chronic GVHD. Doses of the calcineurin inhibitor are gradually decreased after treatment with prednisone has been withdrawn.
The median duration of treatment for chronic GVHD is approximately 2 years in patients who had HCT with marrow cells and approximately 3.5 years in those who had HCT with growth factor-mobilized blood cells [31]. The current therapeutic approach functions primarily to prevent immune-mediated damage, while awaiting the development of tolerance. Evidence to suggest that current treatments accelerate the development of immunological tolerance is lacking. The mechanisms that facilitate development of tolerance have not been defined.
Administration of medications to prevent infection with Pneumocystis jirovecii and encapsulated bacteria is necessary during treatment for chronic GVHD [32]. Some patients may need topical or systemic treatment to prevent mucocutaneous candida infection. Patients at risk of Varicella zoster activation should be given antiviral prophylaxis, and CMV monitoring and preemptive treatment is necessary in patients at risk of CMV infection [33]. Activation of CMV during the first 3 months after HCT suggests an increased risk of subsequent reactivation in patients with chronic GVHD. Systemic immunosuppressive treatment should be administered at the lowest effective dose in order to minimize the risk of infections and other complications. Many steroid-related complications can be avoided or at least minimized by an alternate-day schedule of administration [34], and topical treatment can be used to minimize the need for systemic treatment [35]. Bone mineral density should be monitored yearly, and losses should be minimized through weight bearing exercise, administration of calcium and vitamin D supplements and hormone replacement.
Indications for secondary treatment include worsening manifestations in a previously affected organ, development of manifestations in a previously unaffected organ, absence of improvement after 1 month of treatment, or inability to decrease the dose of prednisone below 1.0 mg/kg/day within 2 months [30, 36]. Numerous clinical trials have been carried out to evaluate approaches for secondary treatment of chronic GVHD. To date, no consensus has been reached regarding the optimal choice of agents for secondary treatment, and clinical management is generally approached through empirical trial and error [36]. Treatment choices are based on physician experience, ease of use, need for monitoring, risk of toxicity and potential exacerbation of pre-existing co-morbidity.
MYCOPHENOLATE MOFETIL: A CASE STUDY ILLUSTRATING CURRENT PROBLEMS
Progress in the clinical management of chronic GVHD has been slowed by limited insight into the pathogenesis of the disease and the mechanisms that lead to development of immunological tolerance. In the absence of pathophysiologic understanding, physicians must rely on personal or published empirical experience in making decisions regarding treatment. In principle, results of treatment in patients with "steroid-refractory" or "steroid-resistant" chronic GVHD could be used to identify promising agents for initial treatment. Effective agents would be expected to decrease reliance on glucocorticoids and could conceivably decrease the duration of time needed for resolution of the disease.
A variety of retrospective and phase II studies suggested that MMF could be used successfully for secondary treatment of chronic GVHD. In results of a survey published in 2002, nearly 80% of clinicians reported that they had used mycophenolate mofetil (MMF) with great success or at least some success for treatment of chronic GVHD [37]. In another survey proposing a hypothetical scenario describing a case of high-risk chronic GVHD, 54% of the respondents indicated that they would add MMF for initial treatment of chronic GVHD [38]. These results supported a formal test of the hypothesis that the addition of MMF to standard initial treatment could improve outcomes for patients with chronic GVHD.
We therefore conducted a prospective, double-blind, randomized phase III clinical trial to test this hypothesis [27]. The primary endpoint was resolution of reversible manifestations of chronic GVHD within 2 years after enrollment, before death or the onset of recurrent malignancy and without the need for secondary systemic treatment. It was expected that the use of MMF would shorten the time to response, decrease systemic steroid exposure, and decrease the risk of transplant-related mortality without increasing the risk of recurrent malignancy, thereby potentially improving overall survival. Results of the trial, however, did not show any benefits of treatment with MMF. Potential reasons for the negative results were thoroughly explored. The absence of success in this randomized trial could not be attributed to an imbalance of risk factors between the arms, sub-optimal dosing of MMF or non-adherence with administration of the study drug. Hence, this clinical trial definitively demonstrated that addition of MMF to standard initial treatment did not improve outcomes for patients with chronic GVHD.
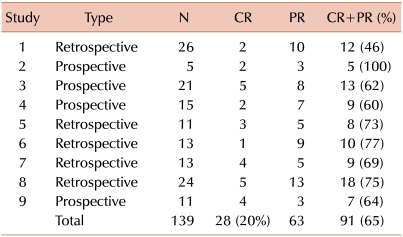
These unexpected results conflicted with previously prevailing clinical impressions and motivated a careful review of prior reports evaluating the use of MMF for treatment of chronic GVHD. Overall results of 9 such studies suggested that secondary treatment with MMF produced a 20% complete response rate and a 65% complete or partial response rate (Table 1) [39-47]. One of these studies also evaluated results in 10 patients who received MMF as part of the initial treatment regimen for chronic GVHD [45]. Seven of the 10 patients had a complete response, and 2 had a partial response, yielding an overall 90% rate of complete or partial response. In addition, a further study from our center had shown that the proportion of patients who discontinued systemic immunosuppressive treatment after resolution of reversible abnormalities increased progressively from 9% to 17% and 26% at 1, 2 and 3 years, respectively, after starting treatment with MMF [48].
Table 1.
Response rates in prior studies of mycophenolate mofetil.
Similar discrepancies have been observed in studies to evaluate the efficacy of thalidomide for treatment of chronic GVHD. Results of 6 retrospective studies and prospective phase II clinical trials suggested favorable outcomes with the use of thalidomide for secondary treatment of chronic GVHD [49-54]. The two randomized prospective studies testing the use of thalidomide for primary treatment of chronic GVHD, however, showed no benefit [24, 25].
Results of the randomized trials defied expectations coming from at least 16 studies evaluating the use of MMF or thalidomide for treatment of chronic GVHD. At least 2 explanations could be proposed to explain this discrepancy. 1) Results of secondary treatment might not predict efficacy as an added agent for primary treatment, perhaps because most patients do not need additional agents in order to gain maximal benefit from initial treatment. Experience at our center, however, has indicated that systemic treatment is changed in approximately 60% of patients during the first 3 years because of inadequate response to primary therapy for chronic GVHD [55]. 2) Alternatively, previous studies might have had unrecognized limitations leading to overstated expectations.
PROPOSED QUALITY INDICATORS FOR EVALUATING REPORTS
Previous publications have identified quality indicators for evaluation of phase III clinical trials [56, 57], but to our knowledge, similar quality criteria have not been previously proposed for phase II trials and retrospective studies. Therefore, before embarking on a detailed review of the 10 previous studies evaluating the use of MMF, we developed a list of 10 quality indicators that could be used to characterize an ideal prospective phase II clinical trial or retrospective study of treatment for GVHD. The proposed quality indicators are summarized below.
1. Adequately defined eligibility criteria
Inclusion and exclusion criteria should specify affected sites, severity of manifestations, and prior treatment used to define the cohort. Exclusion criteria should indicate whether factors such as the presence of infection, inability to tolerate the study treatment, presence of persistent malignancy or low performance score were used to define the cohort. Studies intended to evaluate treatment of "steroid-refractory" GVHD should indicate the glucocorticoid dose and duration of treatment used to define the cohort. Eligibility criteria are typically more precisely defined for prospective studies than for retrospective studies. Data from retrospective studies describing all patients who received the study treatment of interest are difficult to interpret unless additional selection criteria are applied to improve homogeneity within the study cohort.
2. Documented minimization of bias in the selection of patients
Readers should be given enough information to determine whether the characteristics of the patients included in a study are representative of the more general population of patients with chronic GVHD. Risk factors that could affect outcome should be delineated. Ideally, either an historical or contemporaneous cohort should be identified for comparison, and any differences in the prevalence of risk factors between the study cohort and the comparison cohort should be noted. The use of randomization to define cohorts helps to ensure the absence of bias, but this procedure does not ensure that the study cohort is representative of the more general population of patients with the indication of interest. Enrollment of all consecutive patients who meet eligibility criteria can ensure that the cohort is representative of the more general population, but this approach would raise concerns about the adequacy of informed consent. Thus, comparisons of demographics and risk factors between patients who participated and those who did not are crucial.
3. Consistent treatment regimen
The study treatment of interest should be administered in a consistent manner in dose, schedule and duration of administration. Differences in dose, schedule or duration of administration can be addressed by stratified analysis of each specific subgroup. As much as possible, concomitant treatment with immunosuppressive agents other than glucocorticoids should also be administered in a consistent manner in order to facilitate the interpretation of results. Such consistency greatly improves the ability to interpret results and to confirm the results in subsequent studies. Concomitant treatment can be standardized more easily in studies of initial therapy for standard or high-risk disease and for secondary therapy than in studies of subsequent therapies. For third-line or subsequent therapy, such consistency is feasible only if prior treatment with agents other than glucocorticoids is discontinued.
4. Objective criteria for organ response
Categorical criteria should be defined for complete response, partial response, no change, and worsening for each organ or site affected by GVHD, even if organ response criteria have not been validated, since conclusions of the study are based on response rates. Definitions require formal assessment at baseline and at the comparison time point. In many studies, partial response was defined as "at least 50% improvement" in disease manifestations. This terse, and likely oversimplified, definition meets the formal criterion of objectivity but suggests that the response assessment actually reflects a general overall impression, as opposed to a detailed comparison of changes in chronic GVHD manifestations in each organ between baseline and the assessment time.
5. Unambiguous criteria for overall response
The definition of overall response is distinct from the criteria for organ response. Overall responses are often defined according to the overall pattern of organ responses. At a minimum, overall partial response indicates improvement in at least one organ. The category assigned for patients with improvement in one organ but deterioration in another organ should be clearly stated.
6. Specified time for assessment of response
To facilitate comparisons between studies, at least one specified time point should be used for assessment of response, and the data for this assessment should be shown. Additional information can be shown as a time to event analysis. The number of patients who died or had recurrent malignancy before the assessment time point should be specified, and results should clearly indicate whether these patients were excluded from consideration in the assessment of response or whether they were included as non-responders. Tabulation of results according to "best response" or "last value carried forward" is not appropriate, since these categories do not reflect clinical benefit at a specific time point.
7. Concomitant treatment taken into account
New systemic treatment for GVHD added after enrollment but before the assessment time point because of inadequately controlled disease manifestations should be categorized as non-response. Even in studies that use "best response" as the endpoint, the text should state whether response was evaluated before any new systemic treatment was added. Changes in glucocorticoid dose should be described, but a temporary small increase in glucocorticoid dose during a taper should not be categorized as non-response, because temporary flares of GVHD activity cannot be avoided when conscientious efforts are made to determine the minimum glucocorticoid dose needed to control GVHD.
8. Well-established control benchmark
A specific historical or concurrent control benchmark should be used to establish a null hypothesis for the primary endpoint. Response criteria for the benchmark and study cohorts should be identical or closely similar.
9. Statistical hypothesis and power estimate
The methods should provide values for the null and alternative hypotheses and for the one-sided or two-sided type 1 error, together with estimates of statistical power and the necessary sample size. Although these considerations might be difficult to apply in retrospective studies, they should always be applied in prospective studies.
10. Survival
The results should show survival of the cohort from the onset of study treatment. Kaplan-Meier curves should show tic marks depicting end of follow-up, especially if the minimum follow-up time for surviving patients is less than 6 months. Alternatively, results can be shown in tables indicating time to death or last follow-up for each patient. When response definitions differ, survival data provide the only gauge that can be used as a simple and universally applicable method for comparisons with other studies.
EVALUATION OF PRIOR REPORTS FOR STUDIES OF MMF
Two individuals (YI and PM) independently reviewed the 10 prior reports of studies testing the use of MMF for secondary treatment of chronic GVHD [39-48]. Reports were evaluated according to whether each quality criterion was met or not, based on careful reading of the text. Differences in scores were reconciled by joint review to arrive at a consensus. Since the purpose of publication is to persuade others, application of the criteria was very strict, and no credit was given if the text did not address the criterion or if the text was not clear. Therefore, in many cases, deficiencies in the report might not have been representative of a study as it was actually conducted.
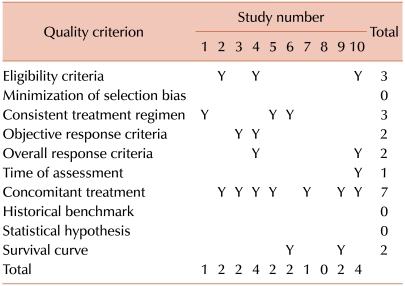
Results for the 10 studies of MMF are summarized in Table 2. Scores at the bottom of the table represent the total number of criteria met by each report. One report failed to meet any of the 10 criteria. Two reports met 4 criteria, and none had higher scores. The mean score for the 10 reported studies was 2.0. Scores at the right margin of the table represent the number of reports that met each criterion. None of the reports attempted to demonstrate that bias had been minimized in the selection of patients, used an historical or contemporaneous benchmark or tested a statistical hypothesis. Only one report had a specified time of assessment, and only two had objective response criteria and well-defined overall response criteria. Three reports employed a consistent treatment regimen, while 7 accounted for possible effects of concomitant treatment.
Table 2.
Quality of prior reports of studies testing mycophenolate mofetil.
COMPREHENSIVE REVIEW OF PRIOR REPORTS
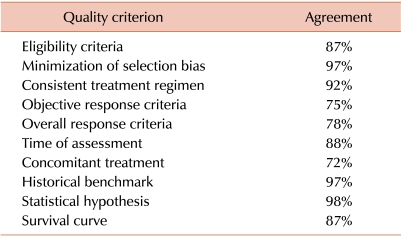
Results of the review of reports for studies testing MMF prompted a more comprehensive review of studies testing systemic agents for secondary treatment of chronic GVHD published between 1990 and 2011. We searched the Medline (PubMed) database using a broad search strategy to identify studies evaluating secondary treatment of chronic GVHD. The search was conducted using the terms "Chronic graft versus host disease" and "Treatment" excluding "Review." Relevant references in the publications identified were also reviewed. Both retrospective and prospective studies were included, but studies with cohorts containing fewer than 10 patients (N=26), phase III studies and case reports were excluded. A total of 60 studies were selected for review [39-54, 58-101]. Initial agreement between the two reviewers was high, ranging between 72% and 98% (Table 3).
Table 3.
Initial agreement between evaluators.a)
a)Each of the 60 selected reports was independently evaluated by 2 reviewers. Results in the table indicate the percent agreement between the 2 reviewers for each quality criterion.
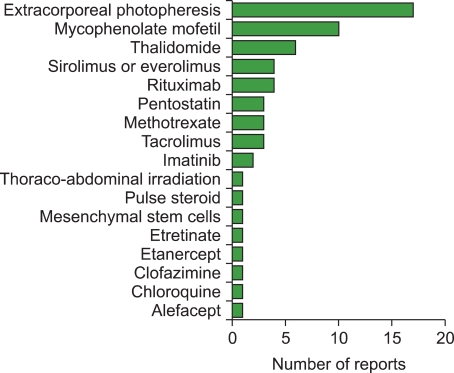
Across the 60 studies, 17 different agents were evaluated (Fig. 1). Extracorporeal photopheresis was the most frequently studied agent (N=17) followed by mycophenolate mofetil (N=10), thalidomide (N=6), sirolimus or everolimus (N=4) and rituximab (N=4). The distribution of scores representing the total number of criteria met by each report ranged from a low of 0 (N=6) to 8 (N=1) [61] (Fig. 2). The mean score for all 60 reports was 2.5. The mean score for prospective studies (N=31) was 3.1, compared to 1.8 for retrospective studies (N=29). The mean score for multicenter studies (N=7) was 3.6, compared to 2.3 for single-center studies (N=53).
Fig. 1.
Treatments evaluated in prior reports. Treatments are listed in order of frequency among the 60 reports included in the literature review.
Fig. 2.
Distribution of scores representing the total number of criteria met by each report for the 60 studies included in the literature review.
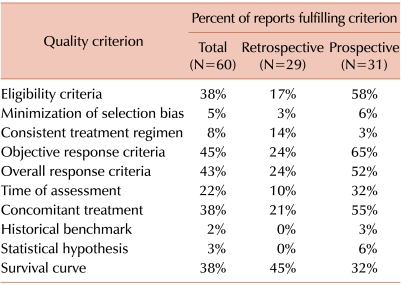
Approximately 35% to 45% of all reports provided adequate information regarding eligibility criteria, organ response criteria, overall response criteria, concomitant treatment and overall survival (Table 4). Only 22% of the reports had a specified time for assessment of response, and less than 10% of the reports documented an absence of bias in the selection of patients, used a consistent treatment regimen, or tested a formal statistical hypothesis on the basis of a benchmark from a contemporaneous or historical cohort. The percentage of reports fulfilling quality indicators was generally higher for prospective studies than for retrospective studies (Table 4).
Table 4.
Quality of prior reports.a)
a)Data in the table indicate the percentage of reports in each category that were judged to meet each of the indicated quality criteria.
SHORTCOMINGS IN THE CURRENT STATE OF AFFAIRS
Despite their many shortcomings, all 10 reports evaluating MMF offered favorable overall assessments, 8 in the abstract, and 2 in the discussion. All 10 reports called for additional studies, 3 in the abstract, and 7 in the discussion. The contrast with results of the prospective phase III trial testing MMF for initial treatment of chronic GVHD raises a general concern that other previously tested agents also do not provide as much benefit as suggested in the reports. The approach used in most reports relies on the assumption that any improvement after new treatment must have resulted from the new treatment, but most of the studies did not attempt to assess the durability of response. Taken as a whole, the collection of reports does not facilitate comparisons of efficacy from one agent to the next, and readers are left to conclude that everything works, more or less.
Investigators prefer new treatment to be effective, and under the "publish or perish" pressures of academic life, authors may lose objectivity and attempt to portray results as positively as possible. None of the 60 reviewed results indicated negative overall results, strongly suggesting a powerful bias by authors and journals to publish only the results of "positive" studies. Conclusions from retrospective studies and phase II clinical trials should be stated more cautiously. For example, we suggest that an appropriate conclusion from the studies of MMF would be the following: "Our results demonstrate the feasibility of using MMF to treat chronic GVHD. The true merits of using MMF for this indication can be evaluated only in a prospective controlled trial". Small retrospective studies have very limited value for assessing results of a new treatment, and the distinction between retrospective studies and prospective studies is important. Nonetheless, many prospective phase II studies still fall far short of the ideal.
Progress would be enhanced if studies could be conducted in a way that allows results to be compared from one study to the next in a more informative way. Aggregation of results for secondary therapy with those for third, fourth and subsequent lines of treatment makes such comparisons impossible, due to large variation in prior treatment and concomitant therapy. Comparisons are also impeded by an inability to estimate the baseline prognosis of patients enrolled in any given study as compared to those enrolled in other studies.
The current state of affairs has many harmful effects. Most reviews that summarize previous literature regarding treatment of chronic GVHD focus on overall complete and partial responses, leading readers to uncritical acceptance of conclusions that agents are effective, when in fact, they are not. Agents that are accepted as effective could actually cause unrecognized harm, as suggested by results of the phase III MMF study [27]. Clinicians who believe that they already know what is best have little incentive to participate in clinical trials. As a consequence, progress has stalled, and no one is able to identify new treatments that are truly effective. Progress would be enhanced if investigators could identify truly promising results from phase II trials and move forward more quickly to testing in definitive phase III trials.
THE NEED FOR STANDARDIZATION - A PROPOSAL FOR THE FUTURE
Progress would be greatly enhanced by standardization in 4 areas: eligibility criteria, organ response criteria, overall response criteria, and time of assessment. Eligibility criteria should focus on true secondary systemic therapy, rather than allowing enrollment at any point beyond primary therapy. This strategy will provide a more homogeneous population and more interpretable results than one that allows enrollment at any point in the development of the disease. Criteria defining "steroid-resistant" and "steroid-refractory" chronic GVHD should be standardized. Lack of adequate improvement after at least one month of treatment with prednisone at 1 mg/kg per day represents one possible definition, although some chronic GVHD manifestations would not be expected to improve within a month after starting treatment.
Standardized response criteria have been proposed but have not been used because of their complexity and lack of validation [7]. Death, recurrent malignancy or a further change in systemic treatment other than the dose of prednisone before the assessment point should not qualify as a response. Measures of response will have to be simplified and validated in order to gain wider acceptance. For example, the NIH scales for mild (score 1), moderate (score 2) and severe (score 3) chronic GVHD could be used to standardize organ response criteria. The same scale could be used to standardize overall response criteria, although the appropriate classification for cases with improvement in one organ with progression in another would still pose difficulty. Alternatively, changes measured according to the NIH scale for global severity of chronic GVHD could be used to standardize overall response, since several studies have shown that the NIH global severity at initial diagnosis correlates with survival [102, 103], although changes in global severity have not yet been correlated with survival. Further evidence from retrospective or prospective studies will be needed to reach consensus on standards for assessment of treatment response in patients with chronic GVHD.
Many studies have used short-term response to assess new therapies for chronic GVHD, but at least one prior study showed that response at 3 or 6 months does not predict resolution of the disease [104]. On the other hand, results of this study showed that several definitions of response could be used together with the additional criterion of a prednisone dose ≤0.25 mg/kg/day to predict the risk of subsequent failure, defined as death, onset of bronchiolitis obliterans, or introduction of a new systemic treatment because of new or progressive manifestations of chronic GVHD. Hence, patients with response by the proposed composite definitions had lower risks of subsequent failure, as compared to those who did not have responses by the same definitions.
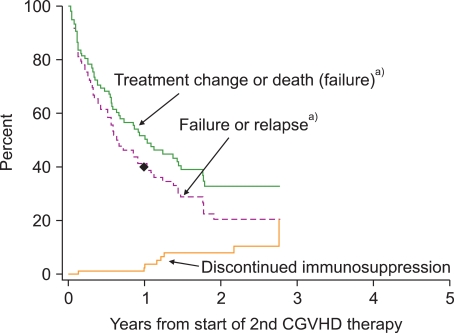
Progress has been hampered by the absence of any established benchmark of success that could be used as a comparison point for studies of new treatment. We have previously reviewed outcomes after secondary systemic treatment for chronic GVHD at our center [29]. Approximately 50% of patients died or had a qualitative change in systemic therapy during the first year, and an additional 10% had recurrent malignancy (Fig. 3). The proportion of patients who were alive without a subsequent change in systemic therapy and without recurrent malignancy was approximately 60% at 6 months and 40% at 1 year. The proportion of patients with complete or partial response at 1 year was not determined, but it cannot exceed 40%. These results might not be representative of current outcomes, since historical criteria were used to define chronic GVHD.
Fig. 3.
Historical outcomes after secondary systemic therapy for chronic GVHD. The upper solid curve shows time to treatment failure defined as a qualitative change in systemic therapy or death during secondary therapy. The dashed curve shows time to treatment failure or recurrent malignancy during secondary therapy. The lower solid curve shows the cumulative incidence of discontinued systemic treatment after resolution of chronic GVHD. The dot on the dashed line indicates that approximately 40% of patients were alive at 1 year after the onset of secondary treatment without a qualitative change in systemic therapy and without recurrent malignancy. Chronic GVHD was defined according to historical criteria and might not reflect results to be expected for patients with chronic GVHD defined according to NIH criteria. The figure is adapted from reference [29]. a)During secondary therapy.
HIGHER STANDARDS FOR PUBLICATION - A PROPOSAL FOR THE FUTURE
Editors of the Journal of Clinical Oncology have recognized the importance of improving the conduct and reporting of phase II trials testing treatments for cancer [105]. As a guideline for authors, the editors have summarized criteria for the types of phase II studies that would be most appropriate for consideration by the journal. The editors also expressed the hope that their views might assist other journals that may be struggling to prioritize the most important types of phase II trials for publication.
The editorial position at the Journal of Clinical Oncology is that phase II studies will be considered only if they include 1) a clear definition of the primary end point, 2) a hypothesized value of the primary end point that justified the planned sample size, and 3) a discussion of possible weaknesses, such as any comparison to historical controls [106]. Only one of the 31 prospective studies of secondary treatment for chronic GVHD had a formal estimation of sample size with a well-justified historical benchmark. The Journal of Clinical Oncology also requires on-line publication of a redacted version of the study protocol, thereby enabling reviewers and readers to recognize any differences between the reported results and the study as originally planned. Improved treatment for chronic GVHD is more likely to emerge if reviewers and journal editors hold authors to higher standards in evaluating manuscripts for publication.
CONCLUSION - THE FUTURE
Standardization of methods for clinical trials would enable comparison of results in different trials and thereby accelerate progress in evaluation of new treatments for chronic GVHD. An urgent priority is the development of a benchmark of success based on results of unbiased retrospective reviews or prospective studies that include all patients who received secondary systemic therapy for chronic GVHD. Robust phase II studies could then be carried out to evaluate whether new therapies offer any genuine improvement compared to the benchmark. For example, a clinical trial could be designed to test whether a new treatment improves outcomes compared to the 40% historical response rate at 1 year, as described above. If the true response rate with the new treatment were 60%, enrollment of 42 patients would offer 80% statistical power with a 0.05 one-side type I error, and successful outcomes in at least 22 patients would encourage further studies. Alternatively, randomized trials with a "pick-the-winner" design could be used to identify approaches that truly warrant further evaluation.
Promising candidate treatments identified in robust phase II studies could be taken forward in phase III studies of secondary treatment, and successful results in such a study would establish a new benchmark for future phase II studies of secondary therapy. Promising candidate treatments could also be tested in phase II studies of primary treatment. Most importantly, successful results in phase III studies of either secondary or primary treatment would improve patient outcomes and establish new standards of care.
Footnotes
This study was supported by research grant CA98906 from the National Institutes of Health, Department of Health and Human Services. Y.I. is a recipient of the JSPS Postdoctoral Fellowships for Research Abroad.
References
- 1.Cutler C, Antin JH. Manifestations and treatment of acute graft-versus-host disease. In: Appelbaum FR, Forman SJ, Negrin RS, Blume KG, editors. Thomas' hematopoietic cell transplantation. 4th ed. Oxford, UK: Wiley-Blackwell; 2009. pp. 1287–1303. [Google Scholar]
- 2.Pavletic SZ, Vogelsang GB. Chronic graft-versus-host disease: clinical manifestations and therapy. In: Appelbaum FR, Forman SJ, Negrin RS, Blume KG, editors. Thomas' hematopoietic cell transplantation. 4th ed. Oxford, UK: Wiley-Blackwell; 2009. pp. 1304–1324. [Google Scholar]
- 3.Shulman HM, Sullivan KM, Weiden PL, et al. Chronic graftversus-host syndrome in man. A long-term clinicopathologic study of 20 Seattle patients. Am J Med. 1980;69:204–217. doi: 10.1016/0002-9343(80)90380-0. [DOI] [PubMed] [Google Scholar]
- 4.Filipovich AH, Weisdorf D, Pavletic S, et al. National Institutes of Health Consensus Development Project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and Staging Working Group Report. Biol Blood Marrow Transplant. 2005;11:945–956. doi: 10.1016/j.bbmt.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 5.Shulman HM, Kleiner D, Lee SJ, et al. Histopathologic diagnosis of chronic graft-versus-host disease: National Institutes of Health Consensus Development Project on criteria for clinical trials in chronic graft-versus-host disease: II. Pathology Working Group Report. Biol Blood Marrow Transplant. 2006;12:31–47. doi: 10.1016/j.bbmt.2005.10.023. [DOI] [PubMed] [Google Scholar]
- 6.Schultz KR, Miklos DB, Fowler D, et al. Toward biomarkers for chronic graft-versus-host disease: National Institutes of Health Consensus Development Project on criteria for clinical trials in chronic graft-versus-host disease: III. Biomarker Working Group Report. Biol Blood Marrow Transplant. 2006;12:126–137. doi: 10.1016/j.bbmt.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 7.Pavletic SZ, Martin P, Lee SJ, et al. Measuring therapeutic response in chronic graft-versus-host disease: National Institutes of Health Consensus Development Project on criteria for clinical trials in chronic graft-versus-host disease: IV. Response Criteria Working Group Report. Biol Blood Marrow Transplant. 2006;12:252–266. doi: 10.1016/j.bbmt.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 8.Couriel D, Carpenter PA, Cutler C, et al. Ancillary therapy and supportive care of chronic graft-versus-host disease: National Institutes of Health Consensus Development Project on criteria for clinical trials in chronic graft-versus-host disease: V. Ancillary Therapy and Supportive Care Working Group Report. Biol Blood Marrow Transplant. 2006;12:375–396. doi: 10.1016/j.bbmt.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 9.Martin PJ, Weisdorf D, Przepiorka D, et al. National Institutes of Health Consensus Development Project on criteria for clinical trials in chronic graft-versus-host disease: VI. Design of Clinical Trials Working Group Report. Biol Blood Marrow Transplant. 2006;12:491–505. doi: 10.1016/j.bbmt.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 10.Stewart BL, Storer B, Storek J, et al. Duration of immunosuppressive treatment for chronic graft-versus-host disease. Blood. 2004;104:3501–3506. doi: 10.1182/blood-2004-01-0200. [DOI] [PubMed] [Google Scholar]
- 11.Vigorito AC, Campregher PV, Storer BE, et al. Evaluation of NIH consensus criteria for classification of late acute and chronic GVHD. Blood. 2009;114:702–708. doi: 10.1182/blood-2009-03-208983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Flowers ME, Inamoto Y, Carpenter PA, et al. Comparative analysis of risk factors for acute graft-versus-host disease and for chronic graft-versus-host disease according to National Institutes of Health consensus criteria. Blood. 2011;117:3214–3219. doi: 10.1182/blood-2010-08-302109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arora M, Klein JP, Weisdorf DJ, et al. Chronic GVHD risk score: a Center for International Blood and Marrow Transplant Research Analysis. Blood. 2011;117:6714–6720. doi: 10.1182/blood-2010-12-323824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Finke J, Bethge WA, Schmoor C, et al. Standard graft-versus-host disease prophylaxis with or without anti-T-cell globulin in haematopoietic cell transplantation from matched unrelated donors: a randomised, open-label, multicentre phase 3 trial. Lancet Oncol. 2009;10:855–864. doi: 10.1016/S1470-2045(09)70225-6. [DOI] [PubMed] [Google Scholar]
- 15.Mohty M, Labopin M, Balère ML, et al. Antithymocyte globulins and chronic graft-vs-host disease after myeloablative allogeneic stem cell transplantation from HLA-matched unrelated donors: a report from the Sociéte Française de Greffe de Moelle et de Thérapie Cellulaire. Leukemia. 2010;24:1867–1874. doi: 10.1038/leu.2010.200. [DOI] [PubMed] [Google Scholar]
- 16.Lee SJ, Vogelsang G, Flowers ME. Chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2003;9:215–233. doi: 10.1053/bbmt.2003.50026. [DOI] [PubMed] [Google Scholar]
- 17.Weiden PL, Sullivan KM, Flournoy N, Storb R, Thomas ED. Antileukemic effect of chronic graft-versus-host disease: contribution to improved survival after allogeneic marrow transplantation. N Engl J Med. 1981;304:1529–1533. doi: 10.1056/NEJM198106183042507. [DOI] [PubMed] [Google Scholar]
- 18.Sullivan KM, Weiden PL, Storb R, et al. Influence of acute and chronic graft-versus-host disease on relapse and survival after bone marrow transplantation from HLA-identical siblings as treatment of acute and chronic leukemia. Blood. 1989;73:1720–1728. [PubMed] [Google Scholar]
- 19.Horowitz MM, Gale RP, Sondel PM, et al. Graft-versus-leukemia reactions after bone marrow transplantation. Blood. 1990;75:555–562. [PubMed] [Google Scholar]
- 20.Baron F, Maris MB, Sandmaier BM, et al. Graft-versus-tumor effects after allogeneic hematopoietic cell transplantation with nonmyeloablative conditioning. J Clin Oncol. 2005;23:1993–2003. doi: 10.1200/JCO.2005.08.136. [DOI] [PubMed] [Google Scholar]
- 21.Thepot S, Zhou J, Perrot A, et al. The graft-versus-leukemia effect is mainly restricted to NIH-defined chronic graft-versus-host disease after reduced intensity conditioning before allogeneic stem cell transplantation. Leukemia. 2010;24:1852–1858. doi: 10.1038/leu.2010.187. [DOI] [PubMed] [Google Scholar]
- 22.Inamoto Y, Flowers ME, Lee SJ, et al. Influence of immunosuppressive treatment on risk of recurrent malignancy after allogeneic hematopoietic cell transplantation. Blood. 2011;118:456–463. doi: 10.1182/blood-2011-01-330217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sullivan KM, Witherspoon RP, Storb R, et al. Prednisone and azathioprine compared with prednisone and placebo for treatment of chronic graft-v-host disease: prognostic influence of prolonged thrombocytopenia after allogeneic marrow transplantation. Blood. 1988;72:546–554. [PubMed] [Google Scholar]
- 24.Koc S, Leisenring W, Flowers ME, et al. Thalidomide for treatment of patients with chronic graft-versus-host disease. Blood. 2000;96:3995–3996. [PubMed] [Google Scholar]
- 25.Arora M, Wagner JE, Davies SM, et al. Randomized clinical trial of thalidomide, cyclosporine, and prednisone versus cyclosporine and prednisone as initial therapy for chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2001;7:265–273. doi: 10.1053/bbmt.2001.v7.pm11400948. [DOI] [PubMed] [Google Scholar]
- 26.Koc S, Leisenring W, Flowers ME, et al. Therapy for chronic graft-versus-host disease: a randomized trial comparing cyclosporine plus prednisone versus prednisone alone. Blood. 2002;100:48–51. doi: 10.1182/blood.v100.1.48. [DOI] [PubMed] [Google Scholar]
- 27.Martin PJ, Storer BE, Rowley SD, et al. Evaluation of mycophenolate mofetil for initial treatment of chronic graftversus-host disease. Blood. 2009;113:5074–5082. doi: 10.1182/blood-2009-02-202937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gilman AL, Schultz KR, Goldman FD, et al. Randomized trial of hydroxychloroquine for newly diagnosed chronic graftversus-host disease in children: a children's oncology group study. Biol Blood Marrow Transplant. 2011 doi: 10.1016/j.bbmt.2011.05.016. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martin PJ, Carpenter PA, Sanders JE, Flowers ME. Diagnosis and clinical management of chronic graft-versus-host disease. Int J Hematol. 2004;79:221–228. doi: 10.1532/ijh97.03176. [DOI] [PubMed] [Google Scholar]
- 30.Martin P, Gilman AL. Front line treatment of chronic GVHD. In: Vogelsang GB, Pavletic SZ, editors. Chronic graft versus host disease: interdisciplinary management. New York, NY: Cambridge University Press; 2009. pp. 124–133. [Google Scholar]
- 31.Flowers ME, Parker PM, Johnston LJ, et al. Comparison of chronic graft-versus-host disease after transplantation of peripheral blood stem cells versus bone marrow in allogeneic recipients: long-term follow-up of a randomized trial. Blood. 2002;100:415–419. doi: 10.1182/blood-2002-01-0011. [DOI] [PubMed] [Google Scholar]
- 32.Tomblyn M, Chiller T, Einsele H, et al. Guidelines for preventing infectious complications among hematopoietic cell transplantation recipients: a global perspective. Biol Blood Marrow Transplant. 2009;15:1143–1238. doi: 10.1016/j.bbmt.2009.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boeckh M, Leisenring W, Riddell SR, et al. Late cytomegalovirus disease and mortality in recipients of allogeneic hematopoietic stem cell transplants: importance of viral load and T-cell immunity. Blood. 2003;101:407–414. doi: 10.1182/blood-2002-03-0993. [DOI] [PubMed] [Google Scholar]
- 34.Harter JG, Reddy WJ, Thorn GW. Studies on an intermittent corticosteroid dosage regimen. N Engl J Med. 1963;269:591–596. doi: 10.1056/NEJM196309192691201. [DOI] [PubMed] [Google Scholar]
- 35.Wolff D, Gerbitz A, Ayuk F, et al. Consensus conference on clinical practice in chronic graft-versus-host disease (GVHD): first-line and topical treatment of chronic GVHD. Biol Blood Marrow Transplant. 2010;16:1611–1628. doi: 10.1016/j.bbmt.2010.06.015. [DOI] [PubMed] [Google Scholar]
- 36.Wolff D, Schleuning M, von Harsdorf S, et al. Consensus conference on clinical practice in chronic GVHD: second-line treatment of chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2011;17:1–17. doi: 10.1016/j.bbmt.2010.05.011. [DOI] [PubMed] [Google Scholar]
- 37.Lee SJ, Vogelsang G, Gilman A, et al. A survey of diagnosis, management, and grading of chronic GVHD. Biol Blood Marrow Transplant. 2002;8:32–39. doi: 10.1053/bbmt.2002.v8.pm11846354. [DOI] [PubMed] [Google Scholar]
- 38.ASBMT eNEWS Survey. Arlington Heights, IL: American Society for Blood and Marrow Transplantation; 2008. [Accessed June 15, 2011]. at http://www.news-source.org/ASBMT/Archive/2008/asbmtonline04-01-08.htm. [Google Scholar]
- 39.Mookerjee B, Altomonte V, Vogelsang G. Salvage therapy for refractory chronic graft-versus-host disease with mycophenolate mofetil and tacrolimus. Bone Marrow Transplant. 1999;24:517–520. doi: 10.1038/sj.bmt.1701936. [DOI] [PubMed] [Google Scholar]
- 40.Busca A, Saroglia EM, Lanino E, et al. Mycophenolate mofetil (MMF) as therapy for refractory chronic GVHD (cGVHD) in children receiving bone marrow transplantation. Bone Marrow Transplant. 2000;25:1067–1071. doi: 10.1038/sj.bmt.1702410. [DOI] [PubMed] [Google Scholar]
- 41.Baudard M, Vincent A, Moreau P, Kergueris MF, Harousseau JL, Milpied N. Mycophenolate mofetil for the treatment of acute and chronic GVHD is effective and well tolerated but induces a high risk of infectious complications: a series of 21 BM or PBSC transplant patients. Bone Marrow Transplant. 2002;30:287–295. doi: 10.1038/sj.bmt.1703633. [DOI] [PubMed] [Google Scholar]
- 42.Busca A, Locatelli F, Marmont F, Audisio E, Falda M. Response to mycophenolate mofetil therapy in refractory chronic graft-versus-host disease. Haematologica. 2003;88:837–839. [PubMed] [Google Scholar]
- 43.Kim JG, Sohn SK, Kim DH, et al. Different efficacy of mycophenolate mofetil as salvage treatment for acute and chronic GVHD after allogeneic stem cell transplant. Eur J Haematol. 2004;73:56–61. doi: 10.1111/j.1600-0609.2004.00247.x. [DOI] [PubMed] [Google Scholar]
- 44.Krejci M, Doubek M, Buchler T, Brychtova Y, Vorlicek J, Mayer J. Mycophenolate mofetil for the treatment of acute and chronic steroid-refractory graft-versus-host disease. Ann Hematol. 2005;84:681–685. doi: 10.1007/s00277-005-1070-0. [DOI] [PubMed] [Google Scholar]
- 45.Lopez F, Parker P, Nademanee A, et al. Efficacy of mycophenolate mofetil in the treatment of chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2005;11:307–313. doi: 10.1016/j.bbmt.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 46.Takami A, Mochizuki K, Okumura H, et al. Mycophenolate mofetil is effective and well tolerated in the treatment of refractory acute and chronic graft-versus-host disease. Int J Hematol. 2006;83:80–85. doi: 10.1532/IJH97.05111. [DOI] [PubMed] [Google Scholar]
- 47.Onishi C, Ohashi K, Sawada T, et al. A high risk of life-threatening infectious complications in mycophenolate mofetil treatment for acute or chronic graft-versus-host disease. Int J Hematol. 2010;91:464–470. doi: 10.1007/s12185-010-0516-x. [DOI] [PubMed] [Google Scholar]
- 48.Furlong T, Martin P, Flowers ME, et al. Therapy with mycophenolate mofetil for refractory acute and chronic GVHD. Bone Marrow Transplant. 2009;44:739–748. doi: 10.1038/bmt.2009.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vogelsang GB, Farmer ER, Hess AD, et al. Thalidomide for the treatment of chronic graft-versus-host disease. N Engl J Med. 1992;326:1055–1058. doi: 10.1056/NEJM199204163261604. [DOI] [PubMed] [Google Scholar]
- 50.Parker PM, Chao N, Nademanee A, et al. Thalidomide as salvage therapy for chronic graft-versus-host disease. Blood. 1995;86:3604–3609. [PubMed] [Google Scholar]
- 51.Rovelli A, Arrigo C, Nesi F, et al. The role of thalidomide in the treatment of refractory chronic graft-versus-host disease following bone marrow transplantation in children. Bone Marrow Transplant. 1998;21:577–581. doi: 10.1038/sj.bmt.1701138. [DOI] [PubMed] [Google Scholar]
- 52.Browne PV, Weisdorf DJ, DeFor T, et al. Response to thalidomide therapy in refractory chronic graft-versus-host disease. Bone Marrow Transplant. 2000;26:865–869. doi: 10.1038/sj.bmt.1702626. [DOI] [PubMed] [Google Scholar]
- 53.van de Poel MH, Pasman PC, Schouten HC. The use of thalidomide in chronic refractory graft versus host disease. Neth J Med. 2001;59:45–49. doi: 10.1016/s0300-2977(01)00133-4. [DOI] [PubMed] [Google Scholar]
- 54.Kulkarni S, Powles R, Sirohi B, et al. Thalidomide after allogeneic haematopoietic stem cell transplantation: activity in chronic but not in acute graft-versus-host disease. Bone Marrow Transplant. 2003;32:165–170. doi: 10.1038/sj.bmt.1704033. [DOI] [PubMed] [Google Scholar]
- 55.Flowers ME, Storer B, Carpenter P, et al. Treatment change as a predictor of outcome among patients with classic chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2008;14:1380–1384. doi: 10.1016/j.bbmt.2008.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Atkins D, Best D, Briss PA, et al. Grading quality of evidence and strength of recommendations. BMJ. 2004;328:1490. doi: 10.1136/bmj.328.7454.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Neunert C, Lim W, Crowther M, Cohen A, Solberg L, Jr, Crowther MA. The American Society of Hematology 2011 evidence-based practice guideline for immune thrombocytopenia. Blood. 2011;117:4190–4207. doi: 10.1182/blood-2010-08-302984. [DOI] [PubMed] [Google Scholar]
- 58.Cutler C, Miklos D, Kim HT, et al. Rituximab for steroidrefractory chronic graft-versus-host disease. Blood. 2006;108:756–762. doi: 10.1182/blood-2006-01-0233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zaja F, Bacigalupo A, Patriarca F, et al. Treatment of refractory chronic GVHD with rituximab. Bone Marrow Transplant. 2007;40:273–277. doi: 10.1038/sj.bmt.1705725. [DOI] [PubMed] [Google Scholar]
- 60.von Bonin M, Oelschlägel U, Radke J, et al. Treatment of chronic steroid-refractory graft-versus-host disease with low-dose rituximab. Transplantation. 2008;86:875–879. doi: 10.1097/TP.0b013e318183f662. [DOI] [PubMed] [Google Scholar]
- 61.Kim SJ, Lee JW, Jung CW, et al. Weekly rituximab followed by monthly rituximab treatment for steroid-refractory chronic graft-versus-host disease: results from a prospective, multicenter, phase II study. Haematologica. 2010;95:1935–1942. doi: 10.3324/haematol.2010.026104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Magro L, Mohty M, Catteau B, et al. Imatinib mesylate as salvage therapy for refractory sclerotic chronic graft-versus-host disease. Blood. 2009;114:719–722. doi: 10.1182/blood-2009-02-204750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Olivieri A, Locatelli F, Zecca M, et al. Imatinib for refractory chronic graft-versus-host disease with fibrotic features. Blood. 2009;114:709–718. doi: 10.1182/blood-2009-02-204156. [DOI] [PubMed] [Google Scholar]
- 64.Jacobsohn DA, Chen AR, Zahurak M, et al. Phase II study of pentostatin in patients with corticosteroid-refractory chronic graft-versus-host disease. J Clin Oncol. 2007;25:4255–4261. doi: 10.1200/JCO.2007.10.8456. [DOI] [PubMed] [Google Scholar]
- 65.Jacobsohn DA, Gilman AL, Rademaker A, et al. Evaluation of pentostatin in corticosteroid-refractory chronic graft-versus-host disease in children: a Pediatric Blood and Marrow Transplant Consortium Study. Blood. 2009;114:4354–4360. doi: 10.1182/blood-2009-05-224840. [DOI] [PubMed] [Google Scholar]
- 66.Pidala J, Kim J, Roman-Diaz J, et al. Pentostatin as rescue therapy for glucocorticoid-refractory acute and chronic graft-versus-host disease. Ann Transplant. 2010;15:21–29. [PubMed] [Google Scholar]
- 67.Weng JY, Du X, Geng SX, et al. Mesenchymal stem cell as salvage treatment for refractory chronic GVHD. Bone Marrow Transplant. 2010;45:1732–1740. doi: 10.1038/bmt.2010.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Greinix HT, Volc-Platzer B, Rabitsch W, et al. Successful use of extracorporeal photochemotherapy in the treatment of severe acute and chronic graft-versus-host disease. Blood. 1998;92:3098–3104. [PubMed] [Google Scholar]
- 69.Smith EP, Sniecinski I, Dagis AC, et al. Extracorporeal photochemotherapy for treatment of drug-resistant graft-vs.-host disease. Biol Blood Marrow Transplant. 1998;4:27–37. doi: 10.1016/s1083-8791(98)90007-6. [DOI] [PubMed] [Google Scholar]
- 70.Child FJ, Ratnavel R, Watkins P, et al. Extracorporeal photopheresis (ECP) in the treatment of chronic graft-versus-host disease (GVHD) Bone Marrow Transplant. 1999;23:881–887. doi: 10.1038/sj.bmt.1701733. [DOI] [PubMed] [Google Scholar]
- 71.Alcindor T, Gorgun G, Miller KB, et al. Immunomodulatory effects of extracorporeal photochemotherapy in patients with extensive chronic graft-versus-host disease. Blood. 2001;98:1622–1625. doi: 10.1182/blood.v98.5.1622. [DOI] [PubMed] [Google Scholar]
- 72.Salvaneschi L, Perotti C, Zecca M, et al. Extracorporeal photochemotherapy for treatment of acute and chronic GVHD in childhood. Transfusion. 2001;41:1299–1305. doi: 10.1046/j.1537-2995.2001.41101299.x. [DOI] [PubMed] [Google Scholar]
- 73.Apisarnthanarax N, Donato M, Körbling M, et al. Extracorporeal photopheresis therapy in the management of steroid-refractory or steroid-dependent cutaneous chronic graft-versus-host disease after allogeneic stem cell transplantation: feasibility and results. Bone Marrow Transplant. 2003;31:459–465. doi: 10.1038/sj.bmt.1703871. [DOI] [PubMed] [Google Scholar]
- 74.Messina C, Locatelli F, Lanino E, et al. Extracorporeal photochemotherapy for paediatric patients with graft-versus-host disease after haematopoietic stem cell transplantation. Br J Haematol. 2003;122:118–127. doi: 10.1046/j.1365-2141.2003.04401.x. [DOI] [PubMed] [Google Scholar]
- 75.Seaton ED, Szydlo RM, Kanfer E, Apperley JF, Russell-Jones R. Influence of extracorporeal photopheresis on clinical and laboratory parameters in chronic graft-versus-host disease and analysis of predictors of response. Blood. 2003;102:1217–1223. doi: 10.1182/blood-2002-11-3351. [DOI] [PubMed] [Google Scholar]
- 76.Foss FM, DiVenuti GM, Chin K, et al. Prospective study of extracorporeal photopheresis in steroid-refractory or steroid-resistant extensive chronic graft-versus-host disease: analysis of response and survival incorporating prognostic factors. Bone Marrow Transplant. 2005;35:1187–1193. doi: 10.1038/sj.bmt.1704984. [DOI] [PubMed] [Google Scholar]
- 77.Rubegni P, Cuccia A, Sbano P, et al. Role of extracorporeal photochemotherapy in patients with refractory chronic graft-versus-host disease. Br J Haematol. 2005;130:271–275. doi: 10.1111/j.1365-2141.2005.05586.x. [DOI] [PubMed] [Google Scholar]
- 78.Bisaccia E, Palangio M, Gonzalez J, et al. Treatment of extensive chronic graft-versus-host disease with extracorporeal photochemotherapy. J Clin Apher. 2006;21:181–187. doi: 10.1002/jca.20084. [DOI] [PubMed] [Google Scholar]
- 79.Couriel DR, Hosing C, Saliba R, et al. Extracorporeal photochemotherapy for the treatment of steroid-resistant chronic GVHD. Blood. 2006;107:3074–3080. doi: 10.1182/blood-2005-09-3907. [DOI] [PubMed] [Google Scholar]
- 80.Berger M, Pessolano R, Albiani R, et al. Extracorporeal photopheresis for steroid resistant graft versus host disease in pediatric patients: a pilot single institution report. J Pediatr Hematol Oncol. 2007;29:678–687. doi: 10.1097/MPH.0b013e31814d66f5. [DOI] [PubMed] [Google Scholar]
- 81.Perseghin P, Galimberti S, Balduzzi A, et al. Extracorporeal photochemotherapy for the treatment of chronic graft-versus-host disease: trend for a possible cell dose-related effect? Ther Apher Dial. 2007;11:85–93. doi: 10.1111/j.1744-9987.2007.00421.x. [DOI] [PubMed] [Google Scholar]
- 82.Flowers ME, Apperley JF, van Besien K, et al. A multicenter prospective phase 2 randomized study of extracorporeal photopheresis for treatment of chronic graft-versus-host disease. Blood. 2008;112:2667–2674. doi: 10.1182/blood-2008-03-141481. [DOI] [PubMed] [Google Scholar]
- 83.Jagasia MH, Savani BN, Stricklin G, et al. Classic and overlap chronic graft-versus-host disease (cGVHD) is associated with superior outcome after extracorporeal photopheresis (ECP) Biol Blood Marrow Transplant. 2009;15:1288–1295. doi: 10.1016/j.bbmt.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Perotti C, Del Fante C, Tinelli C, et al. Extracorporeal photochemotherapy in graft-versus-host disease: a longitudinal study on factors influencing the response and survival in pediatric patients. Transfusion. 2010;50:1359–1369. doi: 10.1111/j.1537-2995.2009.02577.x. [DOI] [PubMed] [Google Scholar]
- 85.Tzakis AG, Abu-Elmagd K, Fung JJ, et al. FK 506 rescue in chronic graft-versus-host-disease after bone marrow transplantation. Transplant Proc. 1991;23:3225–3227. [PMC free article] [PubMed] [Google Scholar]
- 86.Kanamaru A, Takemoto Y, Kakishita E, et al. FK506 treatment of graft-versus-host disease developing or exacerbating during prophylaxis and therapy with cyclosporin and/or other immunosuppressants. Japanese FK506 BMT Study Group. Bone Marrow Transplant. 1995;15:885–889. [PubMed] [Google Scholar]
- 87.Carnevale-Schianca F, Martin P, Sullivan K, et al. Changing from cyclosporine to tacrolimus as salvage therapy for chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2000;6:613–620. doi: 10.1016/s1083-8791(00)70026-7. [DOI] [PubMed] [Google Scholar]
- 88.Lee SJ, Wegner SA, McGarigle CJ, Bierer BE, Antin JH. Treatment of chronic graft-versus-host disease with clofazimine. Blood. 1997;89:2298–2302. [PubMed] [Google Scholar]
- 89.Couriel DR, Saliba R, Escalón MP, et al. Sirolimus in combination with tacrolimus and corticosteroids for the treatment of resistant chronic graft-versus-host disease. Br J Haematol. 2005;130:409–417. doi: 10.1111/j.1365-2141.2005.05616.x. [DOI] [PubMed] [Google Scholar]
- 90.Johnston LJ, Brown J, Shizuru JA, et al. Rapamycin (sirolimus) for treatment of chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2005;11:47–55. doi: 10.1016/j.bbmt.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 91.Jurado M, Vallejo C, Pérez-Simón JA, et al. Sirolimus as part of immunosuppressive therapy for refractory chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2007;13:701–706. doi: 10.1016/j.bbmt.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 92.Jedlickova Z, Burlakova I, Bug G, Baurmann H, Schwerdtfeger R, Schleuning M. Therapy of sclerodermatous chronic graft-versus-host disease with mammalian target of rapamycin inhibitors. Biol Blood Marrow Transplant. 2011;17:657–663. doi: 10.1016/j.bbmt.2010.07.025. [DOI] [PubMed] [Google Scholar]
- 93.Chiang KY, Abhyankar S, Bridges K, Godder K, Henslee-Downey JP. Recombinant human tumor necrosis factor receptor fusion protein as complementary treatment for chronic graft-versus-host disease. Transplantation. 2002;73:665–667. doi: 10.1097/00007890-200202270-00035. [DOI] [PubMed] [Google Scholar]
- 94.Giaccone L, Martin P, Carpenter P, et al. Safety and potential efficacy of low-dose methotrexate for treatment of chronic graft-versus-host disease. Bone Marrow Transplant. 2005;36:337–341. doi: 10.1038/sj.bmt.1705022. [DOI] [PubMed] [Google Scholar]
- 95.Huang XJ, Jiang Q, Chen H, et al. Low-dose methotrexate for the treatment of graft-versus-host disease after allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 2005;36:343–348. doi: 10.1038/sj.bmt.1705034. [DOI] [PubMed] [Google Scholar]
- 96.Inagaki J, Nagatoshi Y, Hatano M, Isomura N, Sakiyama M, Okamura J. Low-dose MTX for the treatment of acute and chronic graft-versus-host disease in children. Bone Marrow Transplant. 2008;41:571–577. doi: 10.1038/sj.bmt.1705922. [DOI] [PubMed] [Google Scholar]
- 97.Robin M, Guardiola P, Girinsky T, et al. Low-dose thoracoabdominal irradiation for the treatment of refractory chronic graft-versus-host disease. Transplantation. 2005;80:634–642. doi: 10.1097/01.tp.0000173436.99153.78. [DOI] [PubMed] [Google Scholar]
- 98.Akpek G, Lee SM, Anders V, Vogelsang GB. A high-dose pulse steroid regimen for controlling active chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2001;7:495–502. doi: 10.1053/bbmt.2001.v7.pm11669216. [DOI] [PubMed] [Google Scholar]
- 99.Gilman AL, Chan KW, Mogul A, et al. Hydroxychloroquine for the treatment of chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2000;6:327–334. doi: 10.1016/s1083-8791(00)70058-9. [DOI] [PubMed] [Google Scholar]
- 100.Shapira MY, Abdul-Hai A, Resnick IB, et al. Alefacept treatment for refractory chronic extensive GVHD. Bone Marrow Transplant. 2009;43:339–343. doi: 10.1038/bmt.2008.324. [DOI] [PubMed] [Google Scholar]
- 101.Marcellus DC, Altomonte VL, Farmer ER, et al. Etretinate therapy for refractory sclerodermatous chronic graft-versus-host disease. Blood. 1999;93:66–70. [PubMed] [Google Scholar]
- 102.Cho BS, Min CK, Eom KS, et al. Feasibility of NIH consensus criteria for chronic graft-versus-host disease. Leukemia. 2009;23:78–84. doi: 10.1038/leu.2008.276. [DOI] [PubMed] [Google Scholar]
- 103.Arai S, Jagasia M, Storer B, et al. Global and organ-specific chronic graft-versus-host disease severity according to the 2005 NIH Consensus Criteria. Blood. 2011 doi: 10.1182/blood-2011-03-344390. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Martin PJ, Storer BE, Carpenter PA, et al. Comparison of short-term response and long-term outcomes after initial systemic treatment of chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2011;17:124–132. doi: 10.1016/j.bbmt.2010.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cannistra SA. Phase II trials in journal of clinical oncology. J Clin Oncol. 2009;27:3073–3076. doi: 10.1200/JCO.2009.23.1811. [DOI] [PubMed] [Google Scholar]
- 106.Journal of Clinical Oncology Information for Contributors. Alexandria, VA: The American Society of Clinical Oncology; [Accessed July 20, 2011]. at http://jco.ascopubs.org/site/ifc/categories.xhtml. [Google Scholar]