Abstract
Background
Some reports have shown that co-inheritance of α-thalassemia and sickle cell disease improves hematological parameters and results in a relatively mild clinical picture for patients; however, the exact molecular basis and clinical significance of the interaction between α-thalassemia and sickle cell disease in India has not yet been described. There is little agreement on the clinical effects of α-thalassemia on the phenotype of sickle cell disease.
Methods
Complete blood count and red cell indices were measured by an automated cell analyzer. Quantitative assessment of hemoglobin variants HbF, HbA, HbA2, and HbS was performed by high performance liquid chromatography (HPLC). DNA extraction was performed using the phenol-chloroform method, and molecular study for common α-deletions was done by gap-PCR.
Results
Out of 60 sickle cell anemia patients, the α-thalassemia genotype was found in 18 patients. Three patients had the triplicated α-genotype (Anti α-3.7 kb), and the remaining patients did not have α-deletions. This study indicates that patients with co-existing α-thalassemia and sickle cell disease had a mild phenotype, significantly improved hematological parameters, and fewer blood transfusions than the patients with sickle cell anemia without co-existing α-deletions.
Conclusion
Co-existence of α-thalassemia and sickle cell anemia has significant effects on the phenotype of Indian sickle cell patients.
Keywords: α-thalasemia, Sickle cell anemia, Hemoglobinopathies
INTRODUCTION
The interaction of α-thalassemia with sickle cell anemia (hemoglobin SS disease, HbSS) is known to influence hematological indices, reduce hemolytic rate and intravascular sickling, and increase deformation of the red blood cells. Decreased synthesis of the hemoglobin variant HbS is due to decreased availability of α chains to combine with βs chains. α-thalassemia is a heterogeneous group of disorders that are characterized by impaired synthesis of α-globin chains because of deletions involving one or both of the α-globin genes. It is predicted that the lower concentration of HbS associated with α-thalassemias would mitigate the clinical severity of sickle cell disease.
α-thalassemia is the most common single gene disorder in the world. DNA analysis carried out in certain regions of India has shown that α3.7 and α4.2 are the most common single gene deletions [1]. Some larger deletions like 20.5 kb, SA, and SEA have been reported in the Indian population. Eight α-chain deletions causing α-thalassemia (α3.7, α4.2, SEA, THAI, SA, 20.5 kb, MED, and FI) have been described. Hemoglobin Koya Dora is found at high frequency (10%) in certain communities of Andhra Pradesh [2]. The α0 alleles are less common in India compared to neighboring Southeast Asian countries like China and Taiwan. α-thalassemia is highly prevalent in the tribal populations with a frequency of 1-40% in Andhra Pradesh and Gujarat. The genotype of α-thalassemia that is prevalent in India is α+, which is clinically silent both in the heterozygous and homozygous states. Hence, it is free from morbidity [3].
A clinically important feature of α-thalassemia is its interaction with sickle cell disease. It has been shown that co-inheritance of α-thalassemia and sickle cell disease improves the hematological parameters of heterozygous β-thalassemia and results in a relatively mild clinical picture of homozygous β-thalassemia [4, 5]. The influence of α-thalassemia on the features of sickle cell disease has not been well characterized. The exact molecular basis of the interaction of α-thalassemia with sickle cell disease in India has not yet been described. The two most common forms of α-thalassemia are 3.7 and 4.2 kb deletions; these forms are geographically widespread. α-thalassemia is found in a high proportion of a tribal population in south and east India, and a study indicated an estimated gene frequency of 0.32 among patients with sickle cell disease [6, 7]. A few studies state that sickle cell patients with α-thalassemia have higher total hemoglobin levels and changes that are compatible with slower hemolysis than patients with sickle cell anemia alone [8-10]. However the clinical significance of the interaction of α-thalassemia with sickle cell disease remains controversial. Thus, the objective of our study was to investigate the effect of α-thalassemia genotypes on clinical and hematological parameters in patients with sickle cell anemia.
MATERIALS AND METHODS
Subjects in this study included patients with sickle cell anemia who were recruited from the Hematology Outpatient Department at the All India Institute of Medical Sciences in New Delhi, India. This study was approved by the institutional ethical committee. About 5 mL of venous blood was drawn after obtaining signed consent from the patient. DNA was extracted by the phenol-chloroform method. Complete blood count and red cell indices were measured by an automated cell analyzer (SYSMEX K-4500, Kobe, Japan). A cation exchange high performance liquid chromatography (HPLC-Bio-Rad-Variant™, Bio Rad, Hercules, CA, USA) was used for the quantitative assessment of hemoglobin variants HbA, HbF, HbA2, and HbS and the diagnosis of sickle cell anemia in patients. Identification of α-deletions and triplication was performed by gap-PCR. α-3.7 and α-4.2 kb deletions and triplication ααα (anti 3.7 kb) were identified using the methods described by Baysal and Huisman [11] and Smetanina and Huisman [12]. Identification of South African deletions was performed according to Shaji et al. [1], while Southeast Asian deletion was identified according to Chang et al. [13]. Mean values, standard deviations, and frequency distributions were used to evaluate the hematological and clinical data. Student's t-test was used to compare the means of groups using GraphPad software (version 3.06). P<0.05 was considered statistically significant.
RESULTS
Blood samples were collected and characterized from a total of 60 patients with sickle cell anemia. After identification of α mutations, subjects were categorized in 3 groups according to the α-thalassemia genotype. Eighteen patients, including 10 males and 8 females with a mean age of 10.4±6.7 years, had α-deletions. Three patients, including 2 males and 1 female with a mean age of 18±6.1 years, had anti α-3.7 kb (α-triplication). The remaining 39 patients did not have an α-deletion, had a mean age of 11.41±8.1 years, and included 24 males and 16 females.
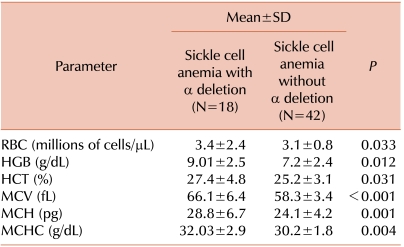
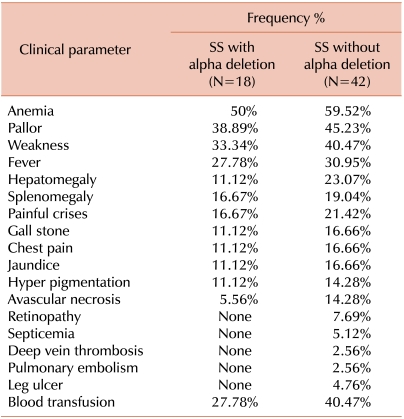
Patients with α-deletions had higher hemoglobin, mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH) levels, and mean corpuscular hemoglobin concentration (MCHC) than patients without α-deletions. Mean differences of hematological variables among patients with α-deletions and patients without α-deletions were statistically significant. Details are provided in Table 1. Patients without α-deletions had greater severity and higher frequency of clinical manifestations. Comparative clinical frequency details are given in Table 2.
Table 1.
Comparative hematological parameters with and without the co-existence of α-thalassemia genotype in sickle cell anemia patients.
Table 2.
Comparative clinical parameter with and without alpha thalassemia genotype in sickle cell anemia patients.
Screening of single (α3.7 and α4.2 kb) deletions, double (--SA and --SEA) deletions, and α-triplication (anti 3.7 kb) was performed by gap-PCR in all subjects. The most frequent mutation in patients with sickle cell anemia was α-3.7 heterozygous (50%), followed by α-3.7 homozygous (38.9%), and α-4.2 heterozygous (11.1%), while α-triplication was found in 3 (5%) patients. None of the patients had double (--SA and --SEA) deletions.
DISCUSSION
Most cases of α-thalassemia result from deletions that originate from unequal crossover events in the α-globin gene cluster (α-3.7 and α-4.2) and large deletions probably resulting from illegitimate recombination, while non-deletional forms are geographically more localized and less common [14, 15]. Higgs et al. [8] reported that homozygotes had significantly lower chronic leg ulceration and acute chest syndrome and a significantly greater persistence of splenomegaly among Jamaican patients. Because of this finding, one might have expected that the hematologic effect of α-thalassemia in Indians would be also reflected by a greater prevalence of splenomegaly in the α-thalassemia group. However, in Indians, splenomegaly was common and occurred at a later age than in the Jamaican patients [6]; this was probably related to the raised HbF levels in Indian patients, and an additional effect of α-thalassemia on the rate of splenomegaly was not observed. This might suggest that the effect of α-thalassemia is not large enough to be noticeable in a population with another strong genetic ameliorating factor like raised HbF levels [16].
Our cases of sickle cell anemia present only single α-chain deletions (α-3.7 and α-4.2 kb) and α-triplication. The α-4.2 deletion was present only in the heterozygous state. Overall, 30% of patients with sickle cell anemia had an α-thalassemia genotype. Patients with sickle cell anemia and homozygous α-deletion showed significant differences in hematological as well as clinical presentation. There is considerable evidence that patients with sickle cell and α-thalassemia have increased prevalence of splenomegaly with episodes of splenic sequestration, increased retinopathy, increased avascular necrosis, fewer leg ulcers, and fewer symptomatic cerebro-vascular accidents [8, 17-26]. The effect of α-thalassemia on the incidence of acute chest syndrome and acute painful episodes is controversial. Some investigators reported reduced incidence of acute chest syndrome [8, 24], but other studies refuted this finding [17, 27]. The effect of α-thalassemia on the frequency of acute painful episodes is even more controversial. One group of investigators reported a decreased incidence of acute painful episodes in patients with sickle cell and α-thalassemia [28], Another group reported higher incidence of acute painful episodes [24, 29], and a third group found no difference in the frequency of acute painful episodes in the presence or absence of α-thalassemia [30]. We report a higher frequency of splenomegaly (19.0%), acute chest pain (16.7%), and painful crisis (21.4%) in patients with sickle cell anemia without co-existent α-deletions than patients with sickle cell anemia and α-thalassemia. This discrepancy among reports may be due to several factors. One possibility is that subjects with α-thalassemia have higher hematocrit (HCT) readings and hence have increased blood viscosity that predisposes patients to vaso-occlusion and painful episodes. Along the same lines, increased blood viscosity predisposes patients to a higher incidence of another painful condition, avascular necrosis, which has been well documented [19, 20].
The cases of sickle cell homozygous patients with a co-existing α-deletion but without a characterized α-thalassemia genotype showed great variability in clinical and hematological parameters. Our sickle cell anemia patients without an α-thalassemia genotype had lower mean hemoglobin, MCV, MCH, HCT, and MCHC, and these parameters were improved in patients with α-deletions. Red cell indices were statistically significant in an entire group of patients. Clinical symptoms occurred at higher frequency and were more severe in patients without α-deletions compared to patients who had α-deletions. Due to the low number of α-triplicated patients, we did not evaluate these patients separately, and instead included them in the without α-deletion group of patients. We observed that sickle cell anemia patients with α-deletions presented milder symptoms, improved hematological parameters, and less dependency on blood transfusion than sickle cell anemia patients without α-deletions. This finding suggested that the presence of α-thalassemia genotypes influenced clinical manifestations of Indian sickle cell anemia patients.
ACKNOWLEDGEMENTS
Sincere thanks to technical staff of Department of Hematology, All India Institute of Medical Sciences for expert assistance.
Footnotes
This study was supported by the Indian Council of Medical Research (ICMR) and Department of Hematology, All India Institute of Medical Sciences, New Delhi, India.
References
- 1.Shaji RV, Eunice SE, Baidya S, Srivastava A, Chandy M. Determination of the breakpoint and molecular diagnosis of a common alpha-thalassaemia-1 deletion in the Indian population. Br J Haematol. 2003;123:942–947. doi: 10.1046/j.1365-141.2003.04704.x. [DOI] [PubMed] [Google Scholar]
- 2.Fodde R, Losekoot M, van den Broek MH, et al. Prevalence and molecular heterogeneity of alfa+ thalassemia in two tribal populations from Andhra Pradesh, India. Hum Genet. 1988;80:157–160. doi: 10.1007/BF00702860. [DOI] [PubMed] [Google Scholar]
- 3.Mukherjee MB, Lu CY, Ducrocq R, et al. Effect of alpha-thalassemia on sickle-cell anemia linked to the Arab-Indian haplotype in India. Am J Hematol. 1997;55:104–109. doi: 10.1002/(sici)1096-8652(199706)55:2<104::aid-ajh9>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 4.Kanavakis E, Wainscoat JS, Wood WG, et al. The interaction of alpha thalassaemia with heterozygous beta thalassaemia. Br J Haematol. 1982;52:465–473. doi: 10.1111/j.1365-2141.1982.tb03916.x. [DOI] [PubMed] [Google Scholar]
- 5.Wainscoat JS, Kanavakis E, Wood WG, et al. Thalassaemia intermedia in Cyprus: the interaction of alpha and beta thalassaemia. Br J Haematol. 1983;53:411–416. doi: 10.1111/j.1365-2141.1983.tb02041.x. [DOI] [PubMed] [Google Scholar]
- 6.Kar BC, Satapathy RK, Kulozik AE, et al. Sickle cell disease in Orissa State, India. Lancet. 1986;2:1198–1201. doi: 10.1016/s0140-6736(86)92205-1. [DOI] [PubMed] [Google Scholar]
- 7.Brittenham G, Lozoff B, Harris JW, Kan YW, Dozy AM, Nayudu NV. Alpha globin gene number: population and restriction endonuclease studies. Blood. 1980;55:706–708. [PubMed] [Google Scholar]
- 8.Higgs DR, Aldridge BE, Lamb J, et al. The interaction of alpha-thalassemia and homozygous sickle-cell disease. N Engl J Med. 1982;306:1441–1446. doi: 10.1056/NEJM198206173062402. [DOI] [PubMed] [Google Scholar]
- 9.Embury SH, Dozy AM, Miller J, et al. Concurrent sickle-cell anemia and alpha-thalassemia: effect on severity of anemia. N Engl J Med. 1982;306:270–274. doi: 10.1056/NEJM198202043060504. [DOI] [PubMed] [Google Scholar]
- 10.de Ceulaer K, Higgs DR, Weatherall DJ, Hayes RJ, Serjeant BE, Serjeant GR. Alpha-Thalassemia reduces the hemolytic rate in homozygous sickle-cell disease. N Engl J Med. 1983;309:189–190. doi: 10.1056/NEJM198307213090320. [DOI] [PubMed] [Google Scholar]
- 11.Baysal E, Huisman TH. Detection of common deletional alpha-thalassemia-2 determinants by PCR. Am J Hematol. 1994;46:208–213. doi: 10.1002/ajh.2830460309. [DOI] [PubMed] [Google Scholar]
- 12.Smetanina NS, Huisman TH. Detection of alpha-thalassemia-2 (-3.7 kb) and its corresponding triplication ααα (anti-3.7 kb) by PCR: an improved technical change. Am J Hematol. 1996;53:202–203. doi: 10.1002/(SICI)1096-8652(199611)53:3<202::AID-AJH11>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 13.Chang JG, Lee LS, Lin CP, Chen PH, Chen CP. Rapid diagnosis of alpha-thalassemia-1 of southeast Asia type and hydrops fetalis by polymerase chain reaction. Blood. 1991;78:853–854. [PubMed] [Google Scholar]
- 14.Higgs DR, Weathenall DJ. Alpha thalassemia. In: Piomelli S, Yachnin S, editors. Current topics in hematology. New York, NY: Wiley-Liss; 1983. p. 37. [PubMed] [Google Scholar]
- 15.Nicholls RD, Fischel-Ghodsian N, Higgs DR. Recombination at the human alpha-globin gene cluster: sequence features and topological constraints. Cell. 1987;49:369–378. doi: 10.1016/0092-8674(87)90289-3. [DOI] [PubMed] [Google Scholar]
- 16.Kulozik AE, Kar BC, Satapathy RK, Serjeant BE, Serjeant GR, Weatherall DJ. Fetal hemoglobin levels and beta (s) globin haplotypes in an Indian populations with sickle cell disease. Blood. 1987;69:1742–1746. [PubMed] [Google Scholar]
- 17.Steinberg MH, Rosenstock W, Coleman MB, et al. Effects of thalassemia and microcytosis on the hematologic and vasoocclusive severity of sickle cell anemia. Blood. 1984;63:1353–1360. [PubMed] [Google Scholar]
- 18.Koshy M, Entsuah R, Koranda A, et al. Leg ulcers in patients with sickle cell disease. Blood. 1989;74:1403–1408. [PubMed] [Google Scholar]
- 19.Ballas SK, Talacki CA, Rao VM, Steiner RM. The prevalence of avascular necrosis in sickle cell anemia: correlation with alpha-thalassemia. Hemoglobin. 1989;13:649–655. doi: 10.3109/03630268908998842. [DOI] [PubMed] [Google Scholar]
- 20.Milner PF, Kraus AP, Sebes JI, et al. Sickle cell disease as a cause of osteonecrosis of the femoral head. N Engl J Med. 1991;325:1476–1481. doi: 10.1056/NEJM199111213252104. [DOI] [PubMed] [Google Scholar]
- 21.Platt OS, Thorington BD, Brambilla DJ, et al. Pain in sickle cell disease. Rates and risk factors. N Engl J Med. 1991;325:11–16. doi: 10.1056/NEJM199107043250103. [DOI] [PubMed] [Google Scholar]
- 22.Hawker H, Neilson H, Hayes RJ, Serjeant GR. Haematological factors associated with avascular necrosis of the femoral head in homozygous sickle cell disease. Br J Haematol. 1982;50:29–34. doi: 10.1111/j.1365-2141.1982.tb01887.x. [DOI] [PubMed] [Google Scholar]
- 23.Hayes RJ, Condon PI, Serjeant GR. Haematological factors associated with proliferative retinopathy in homozygous sickle cell disease. Br J Ophthalmol. 1981;65:29–35. doi: 10.1136/bjo.65.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gill FM, Sleeper LA, Weiner SJ, et al. Clinical events in the first decade in a cohort of infants with sickle cell disease. Cooperative Study of Sickle Cell Disease. Blood. 1995;86:776–783. [PubMed] [Google Scholar]
- 25.Adams RJ, Kutlar A, McKie V, et al. Alpha thalassemia and stroke risk in sickle cell anemia. Am J Hematol. 1994;45:279–282. doi: 10.1002/ajh.2830450402. [DOI] [PubMed] [Google Scholar]
- 26.Miller ST, Rieder RF, Rao SP, Brown AK. Cerebrovascular accidents in children with sickle-cell disease and alpha-thalassemia. J Pediatr. 1988;113:847–849. doi: 10.1016/s0022-3476(88)80013-1. [DOI] [PubMed] [Google Scholar]
- 27.Castro O, Brambilla DJ, Thorington B, et al. The acute chest syndrome in sickle cell disease: incidence and risk factors. The Cooperative Study of Sickle Cell Disease. Blood. 1994;84:643–649. [PubMed] [Google Scholar]
- 28.Mukherjee MB, Colah RB, Ghosh K, Mohanty D, Krishnamoorthy R. Milder clinical course of sickle cell disease in patients with alpha thalassemia in the Indian subcontinent. Blood. 1997;89:732. [PubMed] [Google Scholar]
- 29.Billett HH, Nagel RL, Fabry ME. Paradoxical increase of painful crises in sickle cell patients with alpha-thalassemia. Blood. 1995;86:4382. [PubMed] [Google Scholar]
- 30.Ballas SK, Larner J, Smith ED, Surrey S, Schwartz E, Rappaport EF. Rheologic predictors of the severity of the painful sickle cell crisis. Blood. 1988;72:1216–1223. [PubMed] [Google Scholar]