Abstract
Abstract
Transcranial magnetic stimulation (TMS) has become a popular method to non-invasively stimulate the human brain. The opportunity to modify cortical excitability with repetitive stimulation (rTMS) has especially gained interest for its therapeutic potential. However, details of the cellular mechanisms of the effects of rTMS are scarce. Currently favoured are long-term changes in the efficiency of excitatory synaptic transmission, with low-frequency rTMS depressing it, but high-frequency rTMS augmenting. Only recently has modulation of cortical inhibition been considered as an alternative way to explain lasting changes in cortical excitability induced by rTMS. Adequate animal models help to highlight stimulation-induced changes in cellular processes which are not assessable in human rTMS studies. In this review article, we summarize findings obtained with our rat models which indicate that distinct inhibitory cell classes, like the fast-spiking cells characterized by parvalbumin expression, are most sensitive to certain stimulation protocols, e.g. intermittent theta burst stimulation. We discuss how our findings can support the recently suggested models of gating and homeostatic plasticity as possible mechanisms of rTMS-induced changes in cortical excitability.
Klaus Funke finished studying biology at the Ruhr-University in Bochum (RUB) with a diploma (1983) and doctoral thesis work (1988) on the central somatosensory system of pigeons. As a postdoc he joined the group of Ulf Eysel (Dept Neurophysiology, Medical School, RUB) studying the state-dependent modulation of retinogeniculate signal transmission (habilitation in physiology 1996). Since then he has built up a research team working on visual cortex plasticity and, currently, on the cellular mechanisms of transcranial magnetic stimulation. Alia Benali studied biology at the RUB. Her diploma (1996) and doctoral thesis (2001) were focused on somatosensory cortex plasticity in rats, while her postdoc studies (Ulf Eysel, Dept Neurophysiology) were devoted to lesion-induced visual cortex plasticity and the cellular effects of transcranial magnetic stimulation. In 2010 she joined the groups of Cornelius Schwarz (Werner Reichardt Centre for Integrative Neuroscience, University of Tübingen) and Jason Kerr (MPI for Biological Cybernetics, Tübingen), studying action perception and decision making in mice.
 |
Introduction
Besides other recent developments, repetitive transcranial magnetic stimulation (rTMS) has become a very popular non-invasive method of human brain stimulation, and an aid to inducing lasting changes in cortical excitability (Fitzgerald et al. 2006). The physiological mechanisms of this effect are largely unknown, but changes in synaptic transmission between neurons (synaptic plasticity) are discussed as the most likely process (Ahmed & Wierasko, 2003; Thickbroom, 2007; for a very detailed and critical review of the rTMS effects see Pell et al. 2010). Repeated low-frequency stimulation (∼1 Hz) of the cortical network and the continuous form of theta-burst stimulation (cTBS, Huang et al. 2005) may induce a suppression of excitatory synaptic transmission while high-frequency stimulation (regular 5–50 Hz, and the intermittent form of theta-burst stimulation, iTBS, Huang et al. 2005) may potentiate it (for review see Thickbroom, 2007; Ridding & Ziemann, 2010). However, as extensively discussed by Pell et al. (2010), changes in cortical excitability induced by rTMS differ much from the classical forms of long-term depression (LTD) and long-term potentiation (LTP) of synaptic transmission described by in vivo and in vitro studies of synaptic plasticity (for review see Malenka & Bear, 2004). This difference may primarily relate to the different conditions of stimulation: TMS activates a huge number of axons, presynaptic terminals and postsynaptic sites simultaneously (including antidromic activation of cell bodies), leading to a massive synaptic bombardment of excitatory and inhibitory cells. By contrast, pre- and postsynaptic activity is well controlled in in vitro experiments and limited to a small number of connections. The simultaneous potentiation or suppression of numerous inputs to a neuron increases the likelihood of inducing processes resembling synaptic scaling (Turrigiano, 2008) and homeostatic adjustments of further synaptic plasticity (Bienenstock et al. 1982; Ziemann & Siebner, 2008). Accordingly, rTMS-induced changes in cortical network activity can be expected to differ from classical induction of LTD or LTP.
Cortical inhibition
While excitatory neuronal transmission primarily transmits information from one place to another serving for further signal integration or the activation of effector organs, inhibitory activity appears to have numerous specific functions in modulating neuronal activity, like controlling the spatial and temporal structure of activity and its plasticity. This multiple function of inhibition is reflected by a great diversity of inhibitory interneurons with regard to both morphology and different firing behaviour and synaptic properties (Kawaguchi & Kondo, 2002; Freund, 2003; Markram et al. 2004). In principle, two major functional classes of interneurons can be discerned: (1) fast-spiking (FS) neurons of the large basket or chandelier type, which inhibit pyramidal cells perisomatically and thereby control the level and the temporal pattern of spiking activity (Freund, 2003), and (2) by contrast, diverse non-FS cells, which exhibit irregular, adaptive and low-threshold bursting activity, and modulate the dendritic integration of pyramidal cell inputs. The former express the calcium-binding protein (CaBP) parvalbumin (PV), while the latter primarily express the CaBP calbindin (CB) (Kawaguchi & Kondo, 2002; Blatow et al. 2003b; Markram et al. 2004). Some of these co-express the CaBP calretinin (CR), but the majority of CR-positive cells belong to a class of interneurons exerting inhibitory control of other inhibitory interneurons (Caputi et al. 2009).
Repetitive TMS, by using trains of short stimulus transients, may interfere with the natural firing patterns of distinct cell types and local circuits and – depending on stimulus frequency, train duration and duration of train intervals – might entrain activity in some neuronal classes and networks more than in others. For example, networks involving FS cells preferentially oscillate at theta and gamma frequency and may therefore be better entrained by theta-burst stimulation than by other, e.g. low-frequency, stimulation patterns. The contrary may apply to networks of interneurons preferring low-frequency firing. Moreover, rTMS-induced changes in synaptic strength may vary depending on type of synapse and post-synaptic target cell since excitatory synapses targeting distinct classes of inhibitory interneurons often show synaptic plasticity that differs from the classic hebbian rules (Kullmann & Lamsa, 2007; McBain & Kauer, 2009). Findings of human rTMS studies of cortical inhibition, testing short- and long-interval cortical inhibition (SICI, LICI) and the cortical silent period (CSP) in the primary motor cortex (for review see Fitzgerald et al. 2006; Pell et al. 2010), and paired-pulse afferent inhibition (PPAI) in the somatosensory cortex (Ragert et al. 2004, 2008), are quite inconsistent. Inhibitory synaptic interactions were mostly found to be reduced by conventional high-frequency rTMS (see Fitzgerald et al. 2006), but it increased following the facilitating action of intermittent theta-burst stimulation (iTBS) and decreased after the depressive protocol of continuous TBS (cTBS, Huang et al. 2005). This is at odds with the later finding of Stagg et al. (2009) of increased cortical levels of the inhibitory transmitter GABA following cTBS of human motor cortex. Studying rTMS effects in animal models will be one way to further highlight the cellular mechanisms related to stimulation-induced changes in cortical excitability.
Animal models of cellular cortical rTMS effects
A couple of animal experiments have been performed to study acute and chronic rTMS effects on memory performance (Wang et al. 2006; Li et al. 2007), hippocampal synaptic plasticity (Ahmed & Wierasko, 2003) and changes in neurotransmitter release (Yue et al. 2009), or with the intention to establish animal models suitable to study TMS evoked motor potentials (MEPs; Rotenberg et al. 2010) or to simulate distinct therapeutic interventions related to the modulation of cortical excitability (stroke: Gao et al. 2010; Parkinson's disease: Yang et al. 2010). Recently, Rotenberg's group were able to demonstrate that long-interval cortical inhibition (LICI) of MEPs can be evoked in rats to the same degree as in humans (Vahabzahdeh-Hagh et al. 2011), offering a way to study modulation of the motor cortex by TMS in a rat model. Our own rat studies – combining immunohistochemical, molecular, electrophysiological and behavioural studies – are primarily concerned with rTMS-induced changes of the physiological state of the neocortical network and particularly the role of inhibitory interneurons in this process.
The major advantage of animal TMS studies is the opportunity of applying invasive recording techniques and histological/molecular procedures to get closer to the cellular processes affected. In addition, these techniques can be directly combined with behavioural studies. On the other hand, the major disadvantage of animal studies of rTMS is the restricted spatial selectivity of stimulation as compared to stimulation of the human brain. Even the smallest coils capable of applying high-frequency rTMS protocols are too large to allow selective stimulation of one particular cortical area in the laboratory rat or in other small mammals. Demonstration of rTMS effects in such experiments is thus limited to those processes that may be unique to neocortical neuronal networks occurring in the same way even if several cortical areas will be stimulated simultaneously. Our recent experiments, partly described in the following, were intended to study the cellular effects of rTMS that relate to the highly synchronous activation of the cortical network. If distinct stimulation protocols drive some neocortical circuits or cell types more than others, leading to subsequent changes in neuronal activity and related protein expression, then these effects should be largely independent of the isolated or combined stimulation of different cortical areas according to the uniform canonical design and similar composition of cell populations in neocortical areas. To primarily stimulate cortical areas without stimulating also deep structures, we aimed to activate the long axons of the corpus callosum which could be achieved by a mediolateral orientation of the induced electric field and rather low stimulation strength (20–30% of maximum machine output). Some spatial specificity, however, can be achieved when combining the uniform stimulation of several cortical areas with additional procedures targeting only one specific cortical area as we did with a tactile learning task primarily involving the rat barrel cortex but no other sensory systems.
Effects of rTMS on inhibitory interneurons in the rat neocortex
Initially, we demonstrated that rTMS of the rat neocortex leads to specific changes in the expression of the immediate early gene products c-Fos and zif268 (Aydin-Abidin et al. 2008; Funke & Benali 2010) and that of the GABA-synthesizing enzymes GAD65 and GAD67 (Trippe et al. 2009; Funke & Benali 2010) resembling changes in the activity of primarily excitatory or inhibitory cells, respectively. While c-Fos expression was mainly increased by low-frequency stimulation (1 Hz), only the high-frequency protocols at 10 Hz, iTBS and cTBS significantly increased the expression of zif268 (Aydin-Abidin et al. 2008; Trippe et al. 2009), indicative of a kind of activation suitable to induce synaptic plasticity (Davis et al. 2003). Theta-burst stimulation (iTBS and cTBS) increased the GAD65 expression but reduced that of GAD67 (Trippe et al. 2009). The increase in GAD65 (and a concomitant increase in presynaptic GABA transporter GAT-1) appeared to be fast and rather transient, obviously signalling increased activation and short-term plasticity of GABAergic synapses as an acute consequence of stimulation. The decrease in GAD67 appeared to be slower and longer lasting (Trippe et al. 2009; Mix et al. 2010, and additional unpublished data of control experiments), most likely resembling a lowered electric and metabolic activity level of the GABAergic cells late (some hours) after stimulation (see Wei & Wu, 2008).
These findings demonstrate early and late effects of rTMS on cortical inhibitory neurons but do not allow distinguishing between classes of interneurons affected. Therefore, we next focused on the calcium-binding proteins PV, CB and CR, known to be expressed by different classes of inhibitory interneurons (Kawaguchi & Kondo, 2002; Markram et al. 2004). At least PV and CB have been shown to be expressed in an activity-dependent fashion (Bender et al. 2000; Patz et al. 2004; Tropea et al. 2006; Chaudhury et al. 2007; Nowicka et al. 2009). When comparing the effects of 1 Hz rTMS, iTBS and cTBS, a significant reduction in the number of PV-expressing cells was evident primarily following iTBS, while a reduction in CB expressing interneurons primarily occurred after 1 Hz stimulation and cTBS (Fig. 1, only iTBS and cTBS shown). CB expression recovered after about 1 day but PV expression remained lowered for at least a week. Control experiments revealed that cells were still vital even when completely losing PV expression (Benali et al. 2011). We propose that the reduced activity of inhibitory neurons, as reflected by the decrease in GAD67, PV and CB expression, is caused by depression of the excitatory inputs as a consequence of excessive synaptic drive during stimulation (see discussion below).
Figure 1. rTMS-induced changes in the cortical expression of PV and CB.
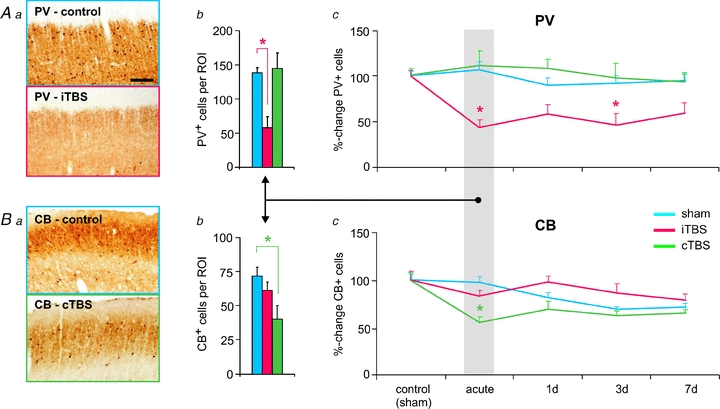
Theta-burst stimulation (TBS) of the rat brain affected the expression of the calcium-binding proteins parvalbumin (PV) and calbindin-D28k (CB). Intermittent TBS (iTBS, Huang et al. 2005) strongly reduced the number of cortical cells expressing PV both acutely (after about 2 h, Aa and b) and subchronically (1–7 days, Ac), while continuous TBS (cTBS, Huang et al. 2005) caused a transient (acute) reduction in the number of CB-expressing interneurons (Ba–c). Cortical slices in Aa and Ba were taken from rat frontal cortex. Scale bar corresponds to 100 μm. ROI: region of interest. *P < 0.05 compared to control (sham-treatment) condition (Tukey's post hoc test). Figures and diagrams partly taken and rearranged from Benali et al. (2011), with permission of the Society for Neuroscience.
The possible consequences of rTMS-induced reduction in PV and CB expression on cortical activity were studied by recording spontaneous multi-unit activity (MUA), EEG and sensory evoked responses (SEPs) in the anaesthetized rat prior to and after iTBS or cTBS. Following iTBS, but not cTBS, spontaneous MUA within layer 4 of rat somatosensory cortex and gamma power of the EEG recorded from frontal cortical regions increased, indicating enhanced ‘resting’ cortical excitability (Fig. 2). Regarding the class of inhibitory system affected, both effects can more likely be explained by a weakened perisomatic disinhibition mediated by PV-positive cells than by a reduced inhibition of dendritic excitation related to CB-positive interneurons. This conclusion is in accordance with the effects of iTBS and cTBS on the two calcium-binding proteins PV and CB, described above. Usually, enhanced gamma activity is associated with increased activity of PV-positive neurons (Cardin et al. 2009) and loss of these neurons is related to impaired human gamma activity as in schizophrenia (Lewis et al. 2005). However, the fact that reciprocal inhibitory (synaptic) and excitatory (gap-junctions) connectivity of these neurons controls the degree of synchronization (Gibson et al. 2005; Manseau et al. 2010) opens further options of plasticity that can lead to episodes of stronger gamma activity.
Figure 2. iTBS and cTBS induced changes in EEG gamma power and spontaneous multi-unit spiking activity (MUA).
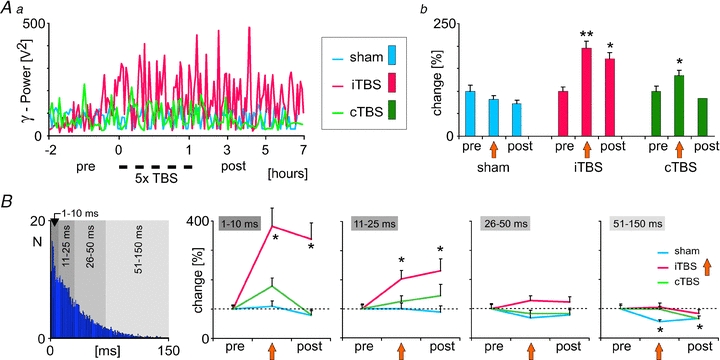
iTBS but not cTBS caused a lasting increase in the gamma power of the EEG recorded from frontal cortical areas in the anesthetized rat (Aa and b), which was accompanied by changes in spontaneous spiking activity recorded from layer IV of rat somatosensory cortex. B, increase in the rate of MUA following iTBS (not explicitly shown here, see Benali et al. 2011) was characterized by a stronger increase in short inter-spike intervals (1–10 and 11–25 ms), while longer intervals (>25 ms) were less changed. The left-most diagram shows a typical, Poisson-like, inter-spike interval distribution of cortical MUA. Results diagrams taken and rearranged from Benali et al. (2011), with permission of the Society for Neuroscience.
SEPs elicited by electric stimulation of the rat hindpaw were also differently affected by the two TBS protocols: the first response arising from resting cortical activity increased following both kinds of stimulation, similar to that described for human SEP recordings following iTBS (Katayama & Rothwell, 2007). However, the second of three responses evoked at intervals of 35 ms was differently affected (Fig. 3Ba and Ca). The second response is much smaller than the first response, likely to be due to the contribution of a volley of recurrent cortical inhibition induced by the first volley of afferent activity (Frasson et al. 2001; Ragert et al. 2008). Similarly, also a single TMS pulse applied to motor cortex evokes a series of inhibitory episodes, demonstrated as CSP, SICI and LICI (for review see Fitzgerald et al. 2006). Paired-pulse evoked cortical inhibition could further be demonstrated for rat motor cortex (Vahabzadeh-Hagh et al. 2011; see also Fig. 3D). This suppression, which we termed paired-pulse afferent inhibition (PPAI), was strengthened by iTBS but was less affected by cTBS, which increased first and second responses proportionally. On a first view, this finding is at odds with the disinhibition of resting cortical activity discussed above but can be explained by the action of the calcium-binding protein PV and its reduction found some time (1–2 h) after iTBS (Benali et al. 2011). GABAergic synaptic transmission shows paired-pulse suppression at short intervals (<100 ms) if PV is present in the presynaptic terminal but paired-pulse facilitation of transmitter release if this slow calcium buffer is experimentally reduced (Caillard et al. 2000; Collin et al. 2005; Lucas et al. 2010). As a consequence, GABA release is increased in PV-deficient neurons when active at high frequency, or when stimulated at short interval as we did for evoking the trains of SEPs. During low-frequency cortical resting activity, however, this mechanism is not active and depressed excitatory input to inhibitory interneurons results in increased cortical excitability (more details discussed below). A proportional increase of all SEPs within the train – as found following cTBS – is more likely to be related to a disinhibition of the dendritic sensory input (likely to be forward inhibition). Reduction in CB expression not only indicates reduced activity of dendrite-targeting interneurons but is also accompanied with a reduced paired-pulse facilitation of transmitter release (Rozov et al. 2001; Blatow et al. 2003a).
Figure 3. iTBS and cTBS induced changes in the amplitude of somatosensory evoked potentials (SEP).
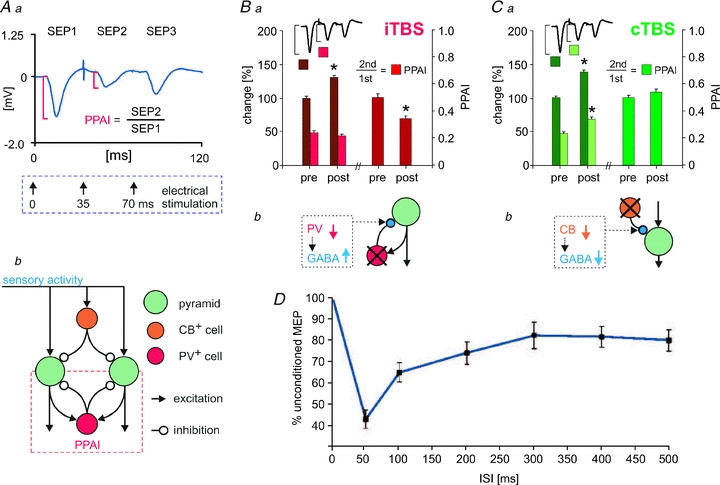
SEPs were elicited with a triple-pulse protocol of electrical stimulation from the toe of a rat hindpaw with inter-pulse intervals of 35 ms corresponding to about 30 Hz (Aa). Typically, the second response shows strong suppression, which is quantified by the ratio of the second to the first SEP amplitude, named paired-pulse afferent inhibition (PPAI, Aa). iTBS increased the first and further reduced the second response, leading to stronger PPAI (Ba), while cTBS increased all response components, leaving PPAI unchanged (Ca). For the relationship of these effects to distinct inhibitory cortical systems (Ab, Bb and Cb), see discussion of main text. For results of A–C refer to Benali et al. 2011. Figures Ba and Ca modified from Benali et al. (2011), with permission of the Society for Neuroscience. D, results of paired-pulse TMS of rat brain showing reduced MEP size around 50 ms intervals supposed to be related to strong intracortical inhibition (modified with permission of the American Physiological Society from Vahabzadeh-Hagh et al. 2011).
Functional consequences of rTMS-modulated cortical inhibition
Our next studies were intended to find a relationship between the rTMS-induced changes in cortical inhibition, as reflected by reduced PV and CB expression, and cortical processing. Therefore, we combined behavioural testing with subsequent analysis of protein expression (Mix et al. 2010). Rats had to learn an associative sensory discrimination task while repeatedly receiving iTBS, cTBS or sham-stimulation prior to each training trial (Fig. 4). This experimental design allowed the distinguishing of effects related to learning alone, to rTMS alone, and to the combination of both. rTMS almost equally affected all the dorsal cortical areas investigated (frontal, motor, somatosensory and visual cortex, see Aydin-Abidin et al. 2008; Trippe et al. 2009; Benali et al. 2011), while activity related to learning was restricted to one sensory system, the tactile barrel cortex system but involving also motor and frontal cortex. Within a special radial maze, rats had to find food rewards solely by using their whiskers, while other sensory cues (visual, olfactory) and also spatial orientating were prevented (for details see Mix et al. 2010). This way, the visual cortex could be used as an internal control region for rTMS effects and other global activities related to the behavioural task. Cortical protein expression in the experimental groups was normalized to control rats perfused directly after being anaesthetized. Furthermore, protein expression was analysed at two different time points during the training procedure, either directly after rTMS or after rTMS followed by a learning trial when rats just showed significant increase in learning performance (reaching the criterion of 75% correct trials, early group), or 24 h after 6 days of stabilized performance above threshold (late group). This was done to distinguish between acute and subchronic effects of rTMS and learning, respectively.
Figure 4. iTBS but not cTBS improved rat learning performance in relation to changes in cortical protein expression.
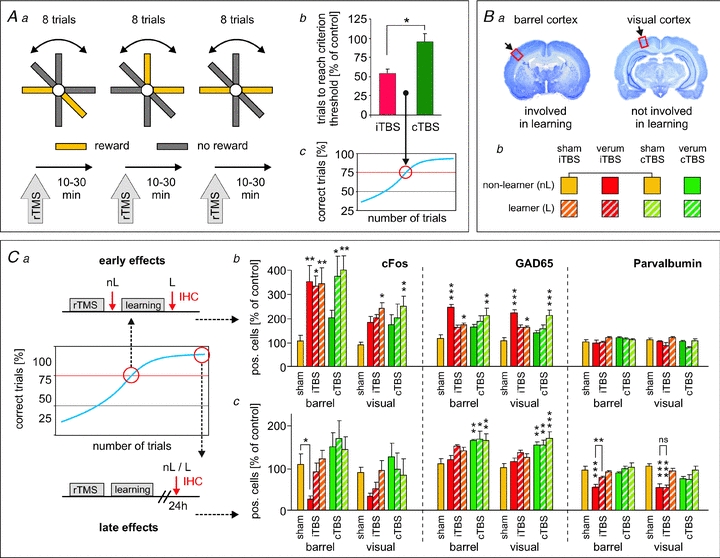
Rats were trained to distinguish rewarded from non-rewarded arms of a radial maze by tactile cues sensed via the whiskers of the rat (Aa). Other sensory cues, like visual, olfactory and spatial, were prevented (for details see Mix et al. 2010). Rats received iTBS, cTBS or sham-stimulation prior to each training block of 8 trials each. Intermittent TBS, but not cTBS, improved learning by reducing the number of trials needed by the rats to reach the threshold criterion of 75% correct choices (Ab and c). Subsequent to the learning experiments, rats were perfused either directly after the training when just reaching the threshold criterion (early effects, Ca) or 1 day after the last training block when reaching stable learning performance (late effects, Ca). Here, analysis of cortical protein expression is shown for the barrel cortex involved in learning task and for the visual cortex not involved (Ba). Eight groups of rats received different rTMS protocols and were either trained or not (learner, non-learner, Bb). Strong early (Cb) and late (Cc) changes in protein expression (c-Fos, GAD65, PV) were evident for iTBS treatment and learning but less for cTBS. For the relationship between changes in protein expression and learning performance, see discussion in the main text. Asterisks on top of bars indicate statistically significant differences from sham controls (yellow left-most bar) with *P < 0.05, **P < 0.01, ***P < 0.001 (ANOVA + Fisher's LSD). Results according to and figures taken and modified from Mix et al. (2010), with permission of Wiley-Blackwell.
Rats learned the task significantly faster when treated with iTBS as compared to sham-treated and cTBS-treated animals (Fig. 4Ab and c) while cTBS had no significant effect compared to sham treatment. Also analysis of protein expression revealed significant differences between iTBS and cTBS treated animals (Fig. 4Cb and c). Compared to animals receiving sham-rTMS and being transferred to a standard cage (yellow bars in Fig. 4Ca), cortical c-Fos and GAD65 expression generally increased directly after the learning trials (hatched bars) with particularly strong increase in c-Fos in the barrel cortex. The learning-related increase in c-Fos and GAD65 found to a lesser degree also in the visual cortex indicates that cortical activity may have been increased in more general terms by arousal effects and exploratory activity. Application of iTBS by itself (red bars) had about the same quantitative effect on c-Fos expression than learning without further increasing the number of c-Fos expressing cells during subsequent learning. A facilitating effect of iTBS on the learning activity cannot be simply derived from the c-Fos expression itself because c-Fos expression already induced by iTBS may not further increase with learning although synaptic plasticity may take place. As indicated by GAD65, inhibitory neurons were obviously driven even stronger by iTBS than by learning. The fact that the combination of iTBS and learning shows no higher GAD65 level than learning alone obviously relates to the very transient increase in GAD65 (Wei & Wu 2008), already declining during the training session. A clearly weaker effect on c-Fos and GAD65 expression was found with cTBS (green bars). These data indicate that iTBS more than cTBS is able to drive cortical excitatory (c-Fos, activation of cells) and inhibitory (GAD65) synaptic transmission quantitatively comparable to natural activity induced by the learning procedure although the pattern of activation may be different (see below). PV expression was not lowered with respect to sham-control levels when determined directly after iTBS although it has been found to be strongly lowered after 2 h and even later (see above) and may therefore be still lowered as a consequence of previous stimulations. Obviously, iTBS acutely first increases PV expression as a result of the strong activation of cortical cells during stimulation before it drops again some time later (see below). However, we never observed an increase in the number of PV-positive cells above control level, indicating that usually PV expression is high in all cells of this class.
Protein expression levels were completely different when measured in the late group after stabilized learning and 24 h after the last training trial (Fig. 4Cc): c-Fos and PV (and to a lesser degree CB and GAD67, not shown here but see Mix et al. 2010) were strongly reduced in all cortical areas in the rats receiving only iTBS (non-learner) indicating a hypoactive cortex. However, in those rats performing the learning task, c-Fos and PV expression were close to sham-control level in barrel, motor and frontal cortex but not in the visual cortex where both remained reduced. This means that learning-related activity has either largely prevented the iTBS effect or has induced a recovery process in the neurons involved in learning-related activity. By contrast, repeated cTBS had no late effect on c-Fos and PV expression but increased that of GAD65. Calretinin expression was affected neither by rTMS nor by learning, indicating that either the expression of this CaBP is not regulated by activity or this class of interneurons is less plastic.
In summary, these studies allow three major conclusions. (1) iTBS and cTBS (and also 1 Hz rTMS) differently modulate excitatory and inhibitory cortical activity early and late after stimulation. iTBS more strongly than cTBS acutely activates excitatory and inhibitory neurons and also leads to late, lasting changes in cortical inhibition. (2) Inhibitory interneurons belonging to different classes are not equally sensitive to the different rTMS protocols, with the PV-expressing FS-spiking interneurons reacting more strongly to repeated high-frequency stimulation than CB-positive interneurons, and with no obvious effect on CR-expressing neurons. (3) The varying expression of the calcium-binding proteins PV and CB indicates a possible contribution to short- and long-term plasticity of neuronal activity.
Model of improved learning performance with iTBS
As demonstrated above, iTBS had a strong and lasting suppressive effect on PV expression which was prevented by learning and led to a better learning performance. Therefore, it is obvious to conclude that changes in the activity of FS-spiking PV-expressing interneurons, which mainly control the strength and oscillatory pattern of pyramidal cell output activity (Blatow et al. 2003b; Cardin et al. 2009), contribute to the learning process. Moreover, iTBS increased cortical excitability and gamma power in the anesthetized rats. How could these effects of iTBS and learning-related activity interfere at the cellular level?Figure 5 demonstrates our working hypothesis, which is based on the aspects of gating and homeostatic plasticity already suggested by others with regard to the findings obtained from human rTMS studies (Thickbroom, 2007; Ziemann & Siebner, 2008; Siebner et al. 2009). Initially, iTBS elicits a high rate of activity within long-range projecting excitatory axons leading to strong drive of excitatory and inhibitory synapses and neurons, as reflected by the early increase in c-Fos and GAD65 expression (Fig. 5Aa). Very quickly this may lead to changes in synaptic transmission due to short-term and long-term synaptic plasticity. Despite a direct potentiation of excitatory synapses on excitatory neurons by iTBS (which we cannot demonstrate here), a depression of the glutamatergic synaptic inputs to FS interneurons is likely to occur with high-frequency stimulation (Reyes et al. 1998; Beierlein et al. 2003; Gonzalez-Burgos et al. 2005) as indicated by the strong reduction in PV expression (Fig. 5Ba). The details of this process are fully discussed in Benali et al. (2011) and relate to the non-classical features of plasticity demonstrated for excitatory synapses targeting inhibitory interneurons (reviewed in Kullmann & Lamsa, 2007) and the primarily depressing nature of inputs to FS neurons (Beierlein et al. 2003) compared to non-FS interneurons (Chen et al. 2009). Initially, this will weaken perisomatic/recurrent inhibition, and the resulting disinhibition of pyramidal cells could promote potentiation of those sensory pyramidal cell inputs (gating process) being activated during the training sessions (Fig. 5Ba and b, see further explanations in the legend). Subsequently, PV content rapidly decreases as a consequence of hypo-activity and leads to facilitation of GABA release (Fig. 5Ca), possibly as a compensatory mechanism to stabilize the balance of excitation and inhibition within the cortical network. Now, a process related to homeostatic metaplasticity may come into play additionally. While those FS neurons and their synapses not involved in the learning process seem to reside at this depressed state, learning-related sensory activity may re-potentiate the depressed synapses at the PV-type interneurons: induction of LTP may be alleviated at these synapses according to the homeostatic process described by Bienenstock, Cooper and Munro (1982; the BCM rule). The sensory active lines (Fig. 5Da) drive the excitatory synapses at PV cells which had been previously depressed by iTBS and – assuming that the threshold for inducing LTP had been lowered previously at these synapses according to the BMC-rule – now re-potentiate the depressed synapses with PV expression normalizing subsequently (Fig. 5Ca and Db). However, other synapses of this kind, being not involved in the learning-related activity, will stay at the depressed level and PV expression remains low. The process of learning-related re-potentiation may happen early after an iTBS-induced homeostatic shift in synaptic plasticity threshold, or much later during a training session following on the next training day. According to the long-lasting reduction in PV expression, a persistent depression of the inputs to PV cells can be expected as long as they are not reversed by appropriate activity (Fig. 5Ca).
Figure 5. Modell of iTBS-related improvement of learning performance according to changes in the activity of PV+ FS interneurons.
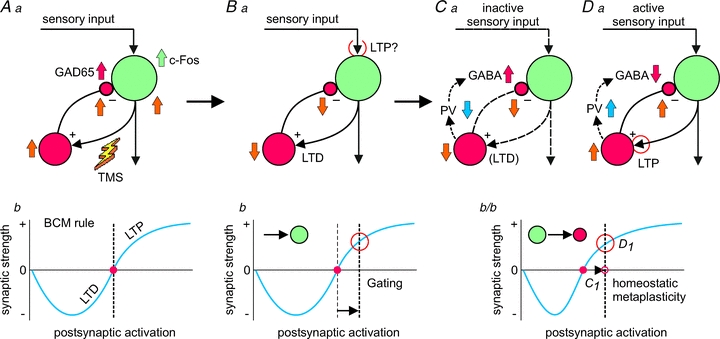
Aa, high-frequency activation of cortical axons by iTBS-rTMS will not only result in activation of excitatory neurons (pyramidal cells – green), but also of the excitatory synapses on inhibitory interneurons (here PV+ FS cells – red) and, secondary, their GABAergic synapses (yellow arrows indicate changes in electrical activity). Bb, as a consequence, these synapses are depressed (long-term depression, LTD) leading to hypoactivation of the interneurons and disinhibition of the pyramidal cell. As a gating process, this disinhibition may promote long-term potentiation (LTP) of active sensory inputs due to enhanced postsynaptic activity. Ab, the Bienenstock–Cooper–Munro model (BCM, Bienenstock et al. 1982) postulates that synaptic plasticity (LTD or LTP) is governed by a dynamic threshold (red dot) which adapts to the global mean rate of postsynaptic activation. In a balanced cell, this threshold is close to this activity level (vertical dotted line). Synaptic plasticity can be induced either if postsynaptic activity level at a particular synapse is significantly deviating from this threshold, as would be the case with gating by disinhibition (Bb), or if the plasticity threshold has been shifted by the history of postsynaptic activity (Cb). Postsynaptic hypoactivity induced via previous LTD at the excitatory synapses on PV+ cells would shift the plasticity threshold to a lower level of postsynaptic activity and would favour induction of LTP at sensory active synapses (Da and Cb/Db) while the inactive synapses remain depressed (Ca). For further explanations regarding the changes in PV expression and GABA release at either active or inactive circuits, see discussion in the main text.
This model cannot cover all pre- and postsynaptic mechanisms possibly contributing to the rTMS-induced changes in cortical activity and protein expression but focuses on those likely to be related to the profound decline in PV expression following iTBS. PV neurons may be more vulnerable to sustained high-frequency stimulation because of their abundant and strong excitatory inputs (Melchitzky & Lewis 2003) and their electric coupling via gap junctions (Gibson et al. 2005). Additionally, PV neurons have been shown to be involved in the generation of cortical gamma rhythms (Cardin et al. 2009) and the oscillatory coupling between hippocampus and prefrontal cortex (Hartwich et al. 2009). Changes in the spatiotemporal selectivity of these rhythms induced by sensory activity or rTMS may contribute to the improved learning performance. However, in our experimental condition, we can largely exclude considerable hippocampal contribution to the learning effect. First, the tactile learning paradigm largely prevented spatial and episodic cues to focus learning related activity to the barrel cortex system (see Mix et al. 2010). Secondly, rTMS was applied in a way that primarily evokes supragranular cortical activity via activation of callosal fibres (mediolateral orientation of induced electric field). The low stimulation intensity required to achieve that (∼23% of max. machine output with iTBS and cTBS) renders direct stimulation of the hippocampus less likely although possible for long axons oriented in the mediolateral direction (e.g. Schaffer collaterals). Also indirect activation of deep structures via subgranular cortical layers will be reduced, considering that no visible muscular activity had been induced at the limbs and the body and no significant change in immediate early gene expression occurred within the hippocampus (Aydin-Abidin et al. 2008). Another rat rTMS study by Li et al. (2007) focused on spatial memory performance: by using a circular coil centred above the brain and by applying stronger stimuli that induced muscle activity, more likely larger parts of the brain had been activated, including the hippocampus. Interestingly, this study demonstrated an impairment of short- and long-term memory retrieval when repeatedly applying inhibitory low-frequency rTMS (0.5 Hz) but no acute effects on acquisition and working memory which would be in accordance with plastic processes occurring late after rTMS. On the other hand, Wang et al. (2006) by applying facilitative 5 Hz rTMS to the auditory cortex of gerbils before and/or after a sound-cued foot-shock avoidance task found impaired short-term but not long-term memory.
Implication for human studies and therapeutic interventions
Modifying cortical inhibitory systems via artificial cortical stimulation would be of particular interest in the case of neurological and neuropsychiatric disorders related to disturbance of either the excitatory–inhibitory balance in general or the activity of a distinct inhibitory system in particular. To a different degree, the PV- and CB-expressing neurons, which were most affected by rTMS in rats, seem to play a role in some forms of schizophrenia (Lewis et al. 2005), in major depression (Croarkin et al. 2011), and in cortical dysplasia with associated disturbance of cortical activity (e.g. epilepsy, Zhou et al. 2009). It would be a great therapeutic advantage if one could specifically drive or inhibit distinct neuronal classes of interneurons. Our studies so far have primarily revealed a suppressive action of rTMS on the expression of PV and CB reflecting hypoactivity of the affected interneurons but also plasticity of the GABAergic synapse due to the reduced expression of the calcium-binding proteins PV and CB. It has to be tested further if modification of the stimulation protocols, like the number of pulses and train length in the case of cTBS and iTBS (300, 600, 1200; see Gentner et al. 2008 for effects on human cortical excitability), or the subsequent combination of protocols, like cTBS and iTBS, at distinct intervals may lead to opposite effects possibly expanding the therapeutic window (Gamboa et al. 2010).
Knowledge about the effects of rTMS is further beneficial if the cortical balance of excitation and inhibition is changed in a more general sense as opposed to the case in cortical regions affected by stroke (Bütefisch et al. 2008; Di Lazzaro et al. 2008) and when an excitatory drive is diminished as being the case in Morbus Parkinson. Weakening inhibitory systems either transiently or chronically could be beneficial in such cases. This, however, has to be done with particular care. Our results show that iTBS can induce a profound and long-lasting reduction in PV expression, which could itself cause dysfunction of the cortical network. We could, however, reverse or prevent the reduction in PV expression when combining rTMS with sensory training sessions and, surprisingly, this was associated with a better performance of the rats in the tactile discrimination task. Also recent human rTMS studies revealed beneficial effects of iTBS (Ragert et al. 2008) and high-frequency rTMS (Tegenthoff et al. 2005; Karim et al. 2006) on tactile discrimination performance when applied to the primary somatosensory cortex prior to training sessions. Otherwise, reports about improvement of cognitive and motor functions by TMS in healthy subjects are rare (Bütefisch et al. 2004; Luber et al. 2007; Galea et al. 2010; Yamanaka et al. 2010). TMS most likely disturbs memory consolidation, critically depending on the cortical area stimulated, the timing of stimulation, the current state of the cortex and the type of training procedure (for review see Censor & Cohen, 2011). Improvement of cortical function with rTMS seems to be more likely in disease states when cortical activity deviates from the physiological level (Pascual-Leone et al. 1994; Lefaucheur et al. 2004; Bütefisch et al. 2008; Di Lazzaro et al. 2008). Then, therapeutic rTMS interventions may be particularly effective and selective if appropriately combined with other appropriate therapies, like sensory, motor, or mental training, or pharmacological treatment.
Experimental
The experimental procedures involving animals were carried out according to the guidelines laid down by the animal welfare committee of the Ruhr-University of Bochum, and conformed to UK regulations, as described in Drummond (2009). In part, experiments were carried out under urethane anaesthesia (1.5 g kg−1, i.p.). Animals were killed by cardiovascular perfusion while in deep barbiturate anaesthesia (pentobarbital-sodium, 300 mg kg−1, i.p.).
Acknowledgments
The authors would like to thank Jennifer Endres, Dimitri Kullmann, Fahad Sultan and Ulf Ziemann for proof-reading and commenting on the manuscript. The authors are particularly grateful to all the co-workers contributing to the cited studies. This work was funded by grants (SFB 509, TP C12; FU 256/2-1, 3-1) of the Deutsche Forschungsgemeinschaft (DFG) to K. Funke and by the DFG graduate school ‘Development & Plasticity of the Central Nervous System’ (GRK 736).
Glossary
Abbreviations
- CaBP
calcium-binding protein
- CB
calbindin
- CR
calretinin
- CSP
cortical silent period
- c-Fos
cellular DNA-binding proteins encoded by the c-fos genes
- FS
fast-spiking cell
- GAD65/GAD67
65 kD and 67 kD isoforms of the glutamic acid decarboxylase
- GAT-1
GABA transporter 1
- LICI
long-interval cortical inhibition
- LTD
long-term depression
- LTP
long-term potentiation
- MEP
motor evoked potential
- MUA
multi-unit activity
- PPAI
paired-pulse afferent inhibition
- PV
parvalbumin
- SEP
somatosensory evoked potential
- SICI
short-interval cortical inhibition
- rTMS
repetitive transcranial magnetic stimulation
- TBS
theta-burst stimulation
- cTBS
continuous TBS
- iTBS
intermittent TBS
- zif268
zinc finger transcription factor
References
- Ahmed Z, Wieraszko A. Modulation of learning and hippocampal, neuronal plasticity by repetitive transcranial magnetic stimulation (rTMS) Bioelectromagn. 2003;27:288–294. doi: 10.1002/bem.20211. [DOI] [PubMed] [Google Scholar]
- Aydin-Abidin S, Trippe J, Funke K, Eysel UT, Benali A. High- and low-frequency repetitive transcranial magnetic stimulation differentially activates c-fos and zif268 protein expression in the rat brain. Exp Brain Res. 2008;188:249–261. doi: 10.1007/s00221-008-1356-2. [DOI] [PubMed] [Google Scholar]
- Beierlein M, Gibson JR, Connors BW. Distinct inhibitory networks in layer 4 of the neocortex. J Neurophysiol. 2003;90:2987–3000. doi: 10.1152/jn.00283.2003. [DOI] [PubMed] [Google Scholar]
- Benali A, Trippe J, Weiler E, Mix A, Petrasch-Parwez E, Girzalsky W, Eysel UT, Erdmann R, Funke K. Theta-burst transcranial magnetic stimulation alters cortical inhibition. J Neurosci. 2011;31:1193–1203. doi: 10.1523/JNEUROSCI.1379-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender R, Hoffmann MC, Frotscher M, Nitsch C. Species-specific expression of parvalbumin in the entorhinal cortex of the Mongolian gerbil: dependence on local activity but not extrinsic afferents. Neuroscience. 2000;99:423–431. doi: 10.1016/s0306-4522(00)00208-6. [DOI] [PubMed] [Google Scholar]
- Bienenstock EL, Cooper L, Munro P. Theory for the development of neuron selectivity: orientation specificity and binocular interaction in visual cortex. J Neurosci. 1982;2:32–48. doi: 10.1523/JNEUROSCI.02-01-00032.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatow M, Caputi A, Burnashev N, Monyer H, Rozov A. Ca2+ buffer saturation underlies paired pulse facilitation in calbindin-D28k-containing terminals. Neuron. 2003a;38:79–88. doi: 10.1016/s0896-6273(03)00196-x. [DOI] [PubMed] [Google Scholar]
- Blatow M, Rozov A, Katona I, Hormuzdi SG, Meyer AH, Whittington MA, Caputi A, Monyer H. A novel network of multipolar bursting interneurons generates theta frequency oscillations in neocortex. Neuron. 2003b;38:805–817. doi: 10.1016/s0896-6273(03)00300-3. [DOI] [PubMed] [Google Scholar]
- Bütefisch CM, Khurana V, Kopylev L, Cohen LG. Enhancing encoding of a motor memory in the primary motor cortex by cortical stimulation. J Neurophysiol. 2004;91:2110–2116. doi: 10.1152/jn.01038.2003. [DOI] [PubMed] [Google Scholar]
- Bütefisch CM, Wessling M, Netz J, Seitz RJ, Hömberg V. Relationship between interhemispheric inhibition and motor cortex excitability in subacute stroke patients. Neurorehabil Neural Repair. 2008;22:4–21. doi: 10.1177/1545968307301769. [DOI] [PubMed] [Google Scholar]
- Caillard O, Moreno H, Schwaller B, Llano I, Celio MR, Marty A. Role of the calcium-binding protein parvalbumin in short-term synaptic plasticity. Proc Natl Acad Sci U S A. 2000;97:13372–13377. doi: 10.1073/pnas.230362997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caputi A, Rozov A, Blatow M, Monyer H. Two calretinin-positive GABAergic cell types in layer 2/3 of the mouse neocortex provide different forms of inhibition. Cereb Cortex. 2009;19:1345–1359. doi: 10.1093/cercor/bhn175. [DOI] [PubMed] [Google Scholar]
- Cardin JA, Carlén M, Meletis K, Knoblich U, Zhang F, Deisseroth K, Tsai LH, Moore CI. Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature. 2009;459:663–668. doi: 10.1038/nature08002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Censor N, Cohen LG. Using repetitive transcranial magnetic stimulation to study the underlying neural mechanisms of human motor learning and memory. J Physiol. 2011;589:21–28. doi: 10.1113/jphysiol.2010.198077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhury S, Nag TC, Wadhwa S. Calbindin D-28K and parvalbumin expression in embryonic chick hippocampus is enhanced by prenatal auditory stimulation. Brain Res. 2007;1191:96–106. doi: 10.1016/j.brainres.2007.11.021. [DOI] [PubMed] [Google Scholar]
- Chen HX, Jiang M, Akakin D, Roper SN. Long-term potentiation of excitatory synapses on neocortical somatostatin-expressing interneurons. J Neurophysiol. 2009;102:3251–3259. doi: 10.1152/jn.00641.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collin T, Chat M, Lucas MG, Moreno H, Racay P, Schwaller B, Marty A, Llano I. Developmental changes in parvalbumin regulate presynaptic Ca2+ signaling. J Neurosci. 2005;25:96–107. doi: 10.1523/JNEUROSCI.3748-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croarkin PE, Levinson AJ, Daskalakis ZJ. Evidence for GABAergic inhibitory deficits in major depressive disorder. Neurosci Biobehav Rev. 2011;35:818–825. doi: 10.1016/j.neubiorev.2010.10.002. [DOI] [PubMed] [Google Scholar]
- Davis S, Bozon B, Laroche S. How necessary is the activation of the immediate early gene zif268 in synaptic plasticity and learning? Behav Brain Res. 2003;142:17–30. doi: 10.1016/s0166-4328(02)00421-7. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Pilato F, Dileone M, Profice P, Capone F, Ranieri F, Musumeci G, Cianfoni A, Pasqualetti P, Tonali PA. Modulating cortical excitability in acute stroke: a repetitive TMS study. Clin Neurophysiol. 2008;119:715–723. doi: 10.1016/j.clinph.2007.11.049. [DOI] [PubMed] [Google Scholar]
- Drummond GB. Reporting ethical matters in The Journal of Physiology: standards and advice. J Physiol. 2009;587:713–719. doi: 10.1113/jphysiol.2008.167387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frasson E, Priori A, Bertolasi L, Mauguière F, Fiaschi A, Tinazzi M. Somatosensory disinhibition in dystonia. Mov Disord. 2001;16:674–682. doi: 10.1002/mds.1142. [DOI] [PubMed] [Google Scholar]
- Fitzgerald PB, Fountain S, Daskalakis ZJ. A comprehensive review of the effects of rTMS on motor cortical excitability and inhibition. Clin Neurophysiol. 2006;117:2584–2596. doi: 10.1016/j.clinph.2006.06.712. [DOI] [PubMed] [Google Scholar]
- Freund TF. Interneuron diversity series: Rhythm and mood in perisomatic inhibition. Trends Neurosci. 2003;28:489–495. doi: 10.1016/S0166-2236(03)00227-3. [DOI] [PubMed] [Google Scholar]
- Funke K, Benali A. Cortical cellular actions of transcranial magnetic stimulation. Restor Neurol Neurosci. 2010;28:399–417. doi: 10.3233/RNN-2010-0566. [DOI] [PubMed] [Google Scholar]
- Galea JM, Albert NB, Ditye T, Miall RC. Disruption of the dorsolateral prefrontal cortex facilitates the consolidation of procedural skills. J Cogn Neurosci. 2010;22:1158–1164. doi: 10.1162/jocn.2009.21259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamboa OL, Antal A, Moliadze V, Paulus W. Simply longer is not better: reversal of theta burst after-effect with prolonged stimulation. Exp Brain Res. 2010;204:181–187. doi: 10.1007/s00221-010-2293-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao F, Wang S, Guo Y, Wang J, Lou M, Wu J, Ding M, Tian M, Zhang H. Protective effects of repetitive transcranial magnetic stimulation in a rat model of transient cerebral ischaemia: a microPET study. Eur J Nucl Med Mol Imaging. 2010;37:954–961. doi: 10.1007/s00259-009-1342-3. [DOI] [PubMed] [Google Scholar]
- Gentner R, Wankerl K, Reinsberger C, Zeller D, Classen J. Depression of human corticospinal excitability induced by magnetic theta-burst stimulation: evidence of rapid polarity-reversing metaplasticity. Cereb Cortex. 2008;18:2046–2053. doi: 10.1093/cercor/bhm239. [DOI] [PubMed] [Google Scholar]
- Gibson JR, Beierlein M, Connors BW. Functional properties of electrical synapses between inhibitory interneurons in neocortical layer 4. J Neurophysiol. 2005;93:467–480. doi: 10.1152/jn.00520.2004. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Burgos G, Kroener S, Seamans JK, Lewis DA, Barrionuevo G. Dopaminergic modulation of short-term synaptic plasticity in fast-spiking interneurons of primate dorsolateral prefrontal cortex. J Neurophysiol. 2005;94:4168–4177. doi: 10.1152/jn.00698.2005. [DOI] [PubMed] [Google Scholar]
- Hartwich K, Pollak T, Klausberger T. Distinct firing patterns of identified basket and dendrite-targeting interneurons in the prefrontal cortex during hippocampal theta and local spindle oscillations. J Neurosci. 2009;29:9563–9574. doi: 10.1523/JNEUROSCI.1397-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YZ, Edwards MJ, Rounis E, Bhatia KP, Rothwell JC. Theta burst stimulation of the human motor cortex. Neuron. 2005;45:201–206. doi: 10.1016/j.neuron.2004.12.033. [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y, Kondo S. Parvalbumin, somatostatin and cholecystokinin as chemical markers for specific GABAergic interneuron types in the rat frontal cortex. J Neurocytol. 2002;31:277–287. doi: 10.1023/a:1024126110356. [DOI] [PubMed] [Google Scholar]
- Kullmann DM, Lamsa KP. Long-term synaptic plasticity in hippocampal interneurons. Nat Rev Neurosci. 2007;8:687–699. doi: 10.1038/nrn2207. [DOI] [PubMed] [Google Scholar]
- Karim AA, Schuler A, Hegner YL, Friedel E, Godde B. Facilitating effect of 15-Hz repetitive transcranial magnetic stimulation on tactile perceptual learning. J Cogn Neurosci. 2006;18:1577–1585. doi: 10.1162/jocn.2006.18.9.1577. [DOI] [PubMed] [Google Scholar]
- Katayama T, Rothwell JC. Modulation of somatosensory evoked potentials using transcranial magnetic intermittent theta burst stimulation. Clin Neurophysiol. 2007;118:2506–2511. doi: 10.1016/j.clinph.2007.08.011. [DOI] [PubMed] [Google Scholar]
- Lefaucheur JP, Drouot X, Von Raison F, Ménard-Lefaucheur I, Cesaro P, Nguyen JP. Improvement of motor performance and modulation of cortical excitability by repetitive transcranial magnetic stimulation of the motor cortex in Parkinson's disease. Clin Neurophysiol. 2004;115:2530–2541. doi: 10.1016/j.clinph.2004.05.025. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory interneurons and schizophrenia. Trends Neurosci. 2005;6:312–324. doi: 10.1038/nrn1648. [DOI] [PubMed] [Google Scholar]
- Li W, Yang Y, Ye Q, Yang B, Wang Z. Effect of chronic and acute low-frequency transcanial magnetic stimulation on spatial memory in rats. Brain Res Bull. 2007;71:493–500. doi: 10.1016/j.brainresbull.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Luber B, Kinnunen LH, Rakitin BC, Ellsasser R, Stern Y, Lisanby SH. Facilitation of performance in a working memory task with rTMS stimulation of the precuneus: frequency- and time-dependent effects. Brain Res. 2007;1128:120–129. doi: 10.1016/j.brainres.2006.10.011. [DOI] [PubMed] [Google Scholar]
- Lucas EK, Markwardt SJ, Gupta S, Meador-Woodruff JH, Lin JD, Overstreet-Wadiche L, Cowell RM. Parvalbumin deficiency and GABAergic dysfunction in mice lacking PGC-1α. J Neurosci. 2010;30:7227–7235. doi: 10.1523/JNEUROSCI.0698-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malenka RC, Bear MF. LTP and LTD: an embarrassment of riches. Neuron. 2004;44:5–21. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Manseau F, Marinelli S, Méndez P, Schwaller B, Prince DA, Huguenard JR, Bacci A. Desynchronization of neocortical networks by asynchronous release of GABA at autaptic and synaptic contacts from fast-spiking interneurons. PLoS Biol. 2010;8:e1000492. doi: 10.1371/journal.pbio.1000492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markram H, Toledo-Rodriguez M, Wang Y, Gupta A, Silberberg G, Wu C. Interneurons of the neocortical inhibitory system. Nat Rev Neurosci. 2004;5:793–807. doi: 10.1038/nrn1519. [DOI] [PubMed] [Google Scholar]
- McBain CJ, Kauer JA. Presynaptic plasticity: targeted control of inhibitory networks. Curr Opin Neurobiol. 2009;19:254–262. doi: 10.1016/j.conb.2009.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melchitzky DS, Lewis DA. Pyramidal neuron local axon terminals in monkey prefrontal cortex: differential targeting of subclasses of GABA neurons. Cereb Cortex. 2003;13:452–460. doi: 10.1093/cercor/13.5.452. [DOI] [PubMed] [Google Scholar]
- Mix A, Benali A, Eysel UT, Funke K. Continuous and intermittent transcranial magnetic theta-burst stimulation differently modify tactile learning performance and cortical protein expression in the rat. Eur J Neurosci. 2010;32:1575–1586. doi: 10.1111/j.1460-9568.2010.07425.x. [DOI] [PubMed] [Google Scholar]
- Nowicka D, Soulsby S, Skangiel-Kramska J, Glazewski S. Parvalbumin-containing neurons, perineuronal nets and experience-dependent plasticity in murine barrel cortex. Eur J Neurosci. 2009;30:2053–2063. doi: 10.1111/j.1460-9568.2009.06996.x. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Valls-Solé J, Brasil-Neto JP, Cammarota A, Grafman J, Hallett M. Akinesia in Parkinson's disease. II. Effects of subthreshold repetitive transcranial motor cortex stimulation. Neurology. 1994;44:892–898. doi: 10.1212/wnl.44.5.892. [DOI] [PubMed] [Google Scholar]
- Patz S, Grabert J, Gorba T, Wirth MJ, Wahle P. Parvalbumin expression in visual cortical interneurons depends on neuronal activity and TrkB ligands during an early period of postnatal development. Cereb Cortex. 2004;14:342–351. doi: 10.1093/cercor/bhg132. [DOI] [PubMed] [Google Scholar]
- Pell GS, Roth Y, Zangen A. Modulation of cortical excitability induced by repetitive transcranial magnetic stimulation: Influence of timing and geometrical parameters and underlying mechanisms. Prog Neurobiol. 2010;93:59–98. doi: 10.1016/j.pneurobio.2010.10.003. [DOI] [PubMed] [Google Scholar]
- Ragert P, Becker M, Tegenthoff M, Pleger B, Dinse HR. Sustained increase of somatosensory cortex excitability by 5 Hz repetitive transcranial magnetic stimulation studied by paired median nerve stimulation in humans. Neurosci Lett. 2004;356:91–94. doi: 10.1016/j.neulet.2003.11.034. [DOI] [PubMed] [Google Scholar]
- Ragert P, Franzkowiak S, Schwenkreis P, Tegenthoff M, Dinse HR. Improvement of tactile perception and enhancement of cortical excitability through intermittent theta burst rTMS over human primary somatosensory cortex. Exp Brain Res. 2008;184:1–11. doi: 10.1007/s00221-007-1073-2. [DOI] [PubMed] [Google Scholar]
- Reyes A, Lujan R, Rozov A, Burnashev N, Somogyi P, Sakmann B. Target-cell-specific facilitation and depression in neocortical circuits. Nat Neurosci. 1998;1:279–285. doi: 10.1038/1092. [DOI] [PubMed] [Google Scholar]
- Ridding MC, Ziemann U. Determinants of the induction of cortical plasticity by non-invasive brain stimulation in healthy subjects. J Physiol. 2010;588:2291–2304. doi: 10.1113/jphysiol.2010.190314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotenberg A, Muller PA, Vahabzadeh-Hagh AM, Navarro X, López-Vales R, Pascual-Leone A, Jensen F. Lateralization of forelimb motor evoked potentials by transcranial magnetic stimulation in rats. Clin Neurophysiol. 2010;121:104–108. doi: 10.1016/j.clinph.2009.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozov A, Burnashev N, Sakmann B, Neher E. Transmitter release modulation by intracellular Ca2+ buffers in facilitating and depressing nerve terminals of pyramidal cells in layer 2/3 of the rat neocortex indicates a target cell-specific difference in presynaptic calcium dynamics. J Physiol. 2001;531:807–826. doi: 10.1111/j.1469-7793.2001.0807h.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebner HR, Hartwigsen G, Kassuba T, Rothwell JC. How does transcranial magnetic stimulation modify neuronal activity in the brain? Implications for studies of cognition. Cortex. 2009;45:1035–1042. doi: 10.1016/j.cortex.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stagg CJ, Wylezinska M, Matthews PM, Johansen-Berg H, Jezzard P, Rothwell JC, Bestmann S. Neurochemical effects of theta burst stimulation as assessed by magnetic resonance spectroscopy. J Neurophysiol. 2009;101:2872–2877. doi: 10.1152/jn.91060.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegenthoff M, Ragert P, Pleger B, Schwenkreis P, Förster AF, Nicolas V, Dinse HR. Improvement of tactile discrimination performance and enlargement of cortical somatosensory maps after 5 Hz rTMS. PLoS Biol. 2005;3:e362. doi: 10.1371/journal.pbio.0030362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thickbroom GW. Transcranial magnetic stimulation and synaptic plasticity: experimental framework and human models. Exp Brain Res. 2007;180:583–593. doi: 10.1007/s00221-007-0991-3. [DOI] [PubMed] [Google Scholar]
- Trippe J, Mix A, Aydin-Abidin S, Funke K, Benali A. Theta burst and conventional low frequency rTMS differentially affect GABAergic neurotransmission in the rat cortex. Exp Brain Res. 2009;199:411–421. doi: 10.1007/s00221-009-1961-8. [DOI] [PubMed] [Google Scholar]
- Tropea D, Kreiman G, Lyckman A, Mukherjee S, Yu H, Horng S, Sur M. Gene expression changes and molecular pathways mediating activity-dependent plasticity in visual cortex. Nat Neurosci. 2006;9:660–668. doi: 10.1038/nn1689. [DOI] [PubMed] [Google Scholar]
- Turrigiano GG. The self-tuning neuron: synaptic scaling of excitatory synapses. Cell. 2008;135:422–435. doi: 10.1016/j.cell.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vahabzadeh-Hagh AM, Muller PA, Pascual-Leone A, Jensen FE, Rotenberg A. Measures of cortical inhibition by paired-pulse transcranial magnetic stimulation in anesthetized rats. J Neurophysiol. 2011;105:615–624. doi: 10.1152/jn.00660.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Wang X, Wetzel W, Scheich H. Rapid-rate transcranial magnetic stimulation of animal auditory cortex impairs short-term but not long-term memory formation. Eur J Neurosci. 2006;23:2176–2184. doi: 10.1111/j.1460-9568.2006.04745.x. [DOI] [PubMed] [Google Scholar]
- Wei J, Wu JY. Post-translational regulation of L-glutamic acid decarboxylase in the brain. Neurochem Res. 2008;33:1459–1465. doi: 10.1007/s11064-008-9600-5. [DOI] [PubMed] [Google Scholar]
- Yamanaka K, Yamagata B, Tomioka H, Kawasaki S, Mimura M. Transcranial magnetic stimulation of the parietal cortex facilitates spatial working memory: near-infrared spectroscopy study. Cereb Cortex. 2010;20:1037–1045. doi: 10.1093/cercor/bhp163. [DOI] [PubMed] [Google Scholar]
- Yang X, Song L, Liu Z. The effect of repetitive transcranial magnetic stimulation on a model rat of Parkinson's disease. Neuroreport. 2010;21:268–272. doi: 10.1097/WNR.0b013e328335b411. [DOI] [PubMed] [Google Scholar]
- Yue L, Xiao-Lin H, Tao S. The effects of chronic repetitive transcranial magnetic stimulation on glutamate and gamma-aminobutyric acid in rat brain. Brain Res. 2009;1260:94–99. doi: 10.1016/j.brainres.2009.01.009. [DOI] [PubMed] [Google Scholar]
- Zhou FW, Chen HX, Roper SN. Balance of inhibitory and excitatory synaptic activity is altered in fast-spiking interneurons in experimental cortical dysplasia. J Neurophysiol. 2009;102:2514–2525. doi: 10.1152/jn.00557.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemann U, Siebner HR. Modifying motor learning through gating and homeostatic metaplasticity. Brain Stimul. 2008;1:60–66. doi: 10.1016/j.brs.2007.08.003. [DOI] [PubMed] [Google Scholar]


