Non-technical summary
An organizing principle of the visual system is the segregation of ON and OFF responses into parallel streams to signal light increment and decrement. This segregation begins in the retina where the output ganglion cells can be divided into ON and OFF subtypes based on their responses to light. Here we show that blockade of GABAergic inhibition reveals opposite polarity responses in ganglion cells whereby OFF cells display ON responses and ON cells display OFF responses. This crossover excitation was found in both the rabbit and mouse, indicating that it is a common synaptic mechanism in the mammalian retina. Overall, these results challenge the idea that light increment and decrement is signalled by different visual pathways. Moreover, our findings suggest that release of inhibition under certain light conditions can enable single ganglion cells to carry both ON and OFF signals, thereby allowing additional information to be propagated across the limited bandwidth of the optic nerve.
Abstract
Abstract
A fundamental organizing feature of the visual system is the segregation of ON and OFF responses into parallel streams to signal light increment and decrement. However, we found that blockade of GABAergic inhibition unmasks robust ON responses in OFF α-ganglion cells (α-GCs). These ON responses had the same centre-mediated structure as the classic OFF responses of OFF α-GCs, but were abolished following disruption of the ON pathway with l-AP4. Experiments showed that both GABAA and GABAC receptors are involved in the masking inhibition of this ON response, located at presynaptic inhibitory synapses on bipolar cell axon terminals and possibly amacrine cell dendrites. Since the dendrites of OFF α-GCs are not positioned to receive excitatory inputs from ON bipolar cell axon terminals in sublamina-b of the inner plexiform layer (IPL), we investigated the possibility that gap junction-mediated electrical synapses made with neighbouring amacrine cells form the avenue for reception of ON signals. We found that the application of gap junction blockers eliminated the unmasked ON responses in OFF α-GCs, while the classic OFF responses remained. Furthermore, we found that amacrine cells coupled to OFF α-GCs display processes in both sublaminae of the IPL, thus forming a plausible substrate for the reception and delivery of ON signals to OFF α-GCs. Finally, using a multielectrode array, we found that masked ON and OFF signals are displayed by over one-third of ganglion cells in the rabbit and mouse retinas, suggesting that masked crossover excitation is a widespread phenomenon in the inner mammalian retina.
Introduction
A key organizing feature of the vertebrate retina is the segregation of responses signalling light increment and decrement into parallel ON and OFF pathways (reviewed by Wässle, 2004). Hartline (1938) first classified retinal ganglion cells as ON, OFF, or ON–OFF, based on their excitatory responses to the onset and/or offset of light stimuli. It is now clear that the ON and OFF pathways are generated at the very first synapse in the retina, resulting from the differential expression of ionotropic and metabotropic glutamate receptors on the second-order bipolar cells (Nomura et al. 1994; Masu et al. 1995; Vardi & Morigiwa, 1997; DeVries, 2000). In the inner retina, ON and OFF bipolar cell axons terminate in different sublaminae of the inner plexiform layer (IPL) where they selectively innervate ON and OFF ganglion cells (Famiglietti & Kolb, 1976; Nelson et al. 1978; Peichl & Wässle, 1981; Bloomfield & Miller, 1986). The ON and OFF signals generated in the retina thereby apparently remain separate as they propagate to the lateral geniculate nucleus and finally converge at the level of the visual cortex (Schiller, 1982; Knapp & Mistler, 1983; Horton & Sherk, 1984; Thurlow et al. 1993).
While the segregation of ON and OFF signals is supported by both morphological and physiological data, there is emerging evidence for significant inhibitory interactions between the two pathways. This ‘crossover’ inhibition between the ON and OFF streams has been found at the level of bipolar cells, amacrine cells and ganglion cells (Zaghloul et al. 2003; Roska et al. 2006; Molnar & Werblin, 2007; Hsueh et al. 2008; Manookin et al. 2008; Murphy & Rieke, 2008). These inhibitory interactions are thought to be mediated mainly by glycinergic, multistratified amacrine cells whose dendrites receive excitatory bipolar cell synaptic input in one sublamina of the IPL, but provide inhibition to ganglion cells stratifying in the other sublamina.
In addition, examples of excitatory interactions between the ON and OFF pathways in the mammalian retina have been reported sporadically over the years. However, these interactions, which have been reported to date only in ON ganglion cells, were often revealed after blockade of GABAergic inhibition (Ariel & Daw, 1982; Nirenberg & Meister, 1997; Roska & Werblin, 2001; Rentería et al. 2006; Ackert et al. 2009), thereby suggesting that the mixing of excitatory ON and OFF signals in retinal ganglion cells may be masked under common experimental conditions. Consequently, such excitatory interactions were often reported anecdotally as simple observations without follow-up analysis or even attributed to technical artifact.
Therefore, in the present study, we have investigated excitatory crosstalk between the ON and OFF pathways in the rabbit and mouse retinas, including the circuitry responsible for its generation and masking. We initially focused on OFF α-ganglion cells (α-GCs), finding that blockade of GABAergic inhibition reveals a robust centre-mediated ON response sensitive to l-2-amino-4-phosphonobutyric acid (l-AP4). The ON response was abolished by gap junction blockers, suggesting that the electrical synapses between α-GCs and neighbouring multistratified amacrine cells form the pathway for excitatory crosstalk between the ON and OFF pathways. We also found that the inhibition responsible for masking of ON responses in OFF α-GCs is subserved by both GABAA and GABAC receptors on presynaptic bipolar and possibly amacrine cells, suggesting that the masked excitatory crosstalk may occur in many ganglion cell subtypes. Indeed, recordings made with a multielectrode array showed that blockade of GABAergic inhibition unmasks opposite polarity responses in over one-third of ON and OFF ganglion cells examined in both rabbit and mouse retinas. Our results therefore challenge the view that excitatory ON and OFF signals are transmitted centrally via strictly segregated parallel streams subserved by different subtypes of ganglion cells.
Methods
Flattened retina–sclera preparation
The rabbit and mouse retina–sclera preparations used in this study have been previously described (Hu et al. 2000; Hu & Bloomfield, 2003; Völgyi et al. 2004). Adult New Zealand White rabbits were anaesthetized with an intraperitoneal injection of 40% ethyl carbamate (2.0 g (kg body weight)−1). For mouse experiments, wild-type adult C57BL/6 mice were anaesthetized with an intraperitoneal injection of sodium pentobarbital (0.08 g (g body weight)−1). Following a local injection of 2% lidocaine hydrochloride to the eyelids and surrounding tissue, the eyes were removed under dim red illumination and hemisected anterior to the ora serrata. The vitreous humour was removed with an ophthalmic sponge (Bausch & Lomb, Rochester, NY, USA), and the resultant retina-eyecup was flattened by making radial cuts at the periphery in a Maltese-cross configuration. For all recordings from the rabbit, retina-eyecups or isolated retinas were placed in a superfusion chamber, which was mounted on the stage of an upright light microscope (Olympus BX51-WI; Olympus, Centre Valley, PA, USA) within a light-tight Faraday cage. The tissue was superfused at a rate of 30 ml min−1 with a mammalian Ringer solution (in mm): 120.0 NaCl, 5.0 KCl, 25.0 NaHCO3, 0.8 Na2HPO4, 0.1 NaH2PO4, 10.0 glucose, 0.01 ascorbate, 1.0 MgSO4 and 2.0 CaCl2. All chemicals were obtained from Fisher Scientific (Waltham, MA, USA) or Sigma-Aldrich (St Louis, MO, USA). The superfusate was kept at a constant temperature of 34°C and a pH of 7.4 was maintained by bubbling with a gaseous mixture of 95% O2–5% CO2. After enucleations, animals were killed with either an intracardial injection of ethyl carbamate (rabbits) or cervical dislocation (mice). All surgical procedures were approved by the Institutional Animal Care and Use Committee at NYU School of Medicine. These procedures comply with The Journal of Physiology policy and UK regulations on animal experimentation. Animals were maintained in 12/12 h day–night cycle and all experiments were performed during daylight hours.
Visualization of cells
To visualize ganglion cells in the rabbit retina, the superfusion was temporarily halted and 3–5 drops of 0.1% Azure B (Sigma), dissolved in Ringer solution, were placed on the retinal surface. After 60–90 s the superfusion was resumed and the Azure B suctioned off the retina and discarded. Optimal staining density occurred within 10–15 min and cells remained visible for the entire duration of the experiment (up to 10 h). A 780 nm cut-off filter allowed transmission of infrared (IR) light from below the stage and then up through a condenser and the glass coverslip mounted in the superfusion chamber. An IR sensitive CCD camera (IR-1000; Dage-MTI, Michigan City, IN, USA) captured the retinal image that was displayed on a video monitor outside the Faraday cage. This protocol allowed retinas to remain in the dark-adapted state during targeting and recording of cells.
Extracellular and intracellular recordings
Extracellular recordings from rabbit retina neurons were made using carbon fibre microelectrodes (World Precision Instruments, Sarasota, FL, USA) attached to an isolated AC differential amplifier (ISO-80; World Precision Instruments). Intracellular recordings were obtained from neurons using sharp microelectrodes fashioned from borosilicate glass tubing with filament. Electrodes were filled with 4%N-(2-amino-ethyl)-biotinamide hydrochloride (Neurobiotin; Vector Laboratories, Burlingame, CA, USA) or 10 mm Po-Pro-1 iodide (Invitrogen, Carlsbad, CA, USA) in 0.1 m Tris buffer, pH 7.6, and then backfilled with a small amount of 3 m potassium chloride to produce a reversible junction with the Ag–AgCl connector. Final DC resistances of these electrodes ranged from 200 to 450 MΩ. After physiological characterization of a cell, Neurobiotin was injected into the cell with a combination of sinusoidal (3 Hz; 0.8 nA; peak-to-peak) and direct current (0.4 nA) applied simultaneously; this method allowed for passage of tracer through the microelectrode without polarization. For some experiments, we added the chloride channel blocker 4,4′-dinitro-stilbene-2,2′-disulphonic acid (DNDS; Pfaltz and Bauer Inc., Waterbury, CT, USA) to the microelectrode (500 μm in 0.1 m Tris). All recordings were digitized online with an analog-to-digital board (Digidata 1200; Molecular Devices, Sunnyvale, CA, USA) and stored for offline analysis. All spike records were sorted and time-stamped offline using commercially available software (Offline Sorter; Plexon, Dallas, TX, USA). Peristimulus time histograms were generated using the NeuroExplorer software (Nex Technologies, Littleton, MA, USA).
Whole cell recordings
Whole-cell patch-clamp recordings were made from ganglion cells in an isolated rabbit retina. A piece of retina was transferred to the recording chamber and placed flat, ganglion cell layer up, over a cellulose acetate/nitrate membrane filter (Millipore, Billerica, MA, USA) that was mounted in the superfusion chamber. Responses to full-field light stimulation were recorded with low-resistance electrodes (5–6 MΩ), filled with a pipette solution consisting of (in mm): 100 potassium gluconate, 10 NaCl, 0.5 CaCl2, 2 MgCl2, 0.5 EGTA, 2 ATP, 0.1 GTP and 10 Hepes, buffered to pH 7.4. The retina was superfused at a rate of 4–5 ml min−1 with a Ringer solution maintained at 34°C and equilibrated with 95% O2–5% CO2 to pH 7.4. Voltage clamp data were acquired at a sampling rate of 2 kHz using pCLAMP 9.2 (Molecular Devices) software, low-pass filtered at 500 Hz and digitized. Series resistance was compensated 50–80% with the series resistance compensation circuitry of the patch-clamp amplifier (Axopatch 200B; Molecular Devices).
Multielectrode array recordings
For multielectrode array experiments on rabbit and mouse, retinas were isolated, mounted on filter paper (8 μm pore size; Millipore), and placed ganglion side down on the grid of a 60-channel electrode array (Multi Channel Systems, Reutlingen, Germany). The isolated retinas were superfused with a Ringer solution at a rate of 5–7 ml min−1. Spike data were sorted and time-stamped using commercially available software as described above.
Pharmacology
For pharmacological experiments, drugs were applied to the retina by switching from the control Ringer solution to one containing the drug. We utilized the following drugs in our experiments: picrotoxin (PTX; Tocris Bioscience, Ellisville, MO, USA), l-2-amino-4-phosphonobutyric acid (l-AP4; Tocris Bioscience), SR-95531 (Tocris Bioscience), 1,2,5,6-tetrahydropyridin-4-yl-methylphosphinic acid (TPMPA; Sigma), baclofen (Sigma), CGP-55845 (Tocris Bioscience), strychnine (Sigma), 18β-glycyrrhetinic acid (18β-GA; Sigma), and meclofenamic acid (MFA; Sigma).
Light stimulation
Light stimulus intensities were kept within the scotopic range and retinas were maintained in a dark-adapted state. For most experiments, a green light emitting diode (λmax = 525 nm) focused onto the retinal surface provided full-field illumination. In some experiments, white light provided by a tungsten-halogen lamp was used to stimulate the retina. Light intensities used in different experiments are provided in the figure legends.
For area summation experiments, we utilized concentric green spots of varying sizes, but constant intensity, centred on the soma of the targeted cell. The stimuli were generated on a DLP projector (Samsung, Ridgefield Park, NJ, USA) using Matlab (The MathWorks, Natick, MA, USA) and Psychtoolbox-3 extensions (Brainard, 1997; Pelli, 1997). The stimuli were delivered to the camera port of the microscope by means of a precision, coherent fibre optic and projected onto the retina through the objective.
To measure the receptive field size of cellular responses, a 50 μm wide/1.0 mm long rectangular slit of light was moved along its minor axis (parallel to the visual streak) in discrete steps in both directions from the central position. The position of the slit at which it evoked the largest response was considered to be centred over the cell. Peak spike frequency responses were plotted against stimulus position and the extent of a neuron's centre-receptive field was taken as the diameter of the Gaussian function fitted to the data using Origin software (OriginLab Corp., Northampton, MA, USA). The Gaussian diameter was defined as 0.849 times the width (w) of the Gaussian at half height (w≈ 2σ).
Histology and immunocytochemistry
After electrophysiological experiments, retinas were fixed at room temperature in a solution of 4% paraformaldehyde in 0.9% phosphate buffered saline (PBS), pH 7.4, for 12–15 min. The retina was then detached, trimmed, and washed overnight in PBS at 4°C. Neurobiotin injections in retinas were visualized using a Cy3-conjugated streptavidin reagent (Sigma). After labelling with streptavidin-Cy3, retinas were washed in PBS for 1 h followed by incubation in a primary antibody solution of goat anti-choline acetyltransferase (ChAT) antibody (Chemicon, Temecula, CA, USA) at a concentration of 1:100 for 72 h at 4°C. Retinas were then washed in PBS, before incubation in a secondary antibody solution of donkey anti-goat Cy2 (Jackson ImmunoResearch Laboratories, West Grove, PA, USA) at a concentration of 1:200 overnight at 4°C. Retinas were subsequently washed in PBS and mounted in Vectashield mounting medium (Vector Laboratories). Retinas were imaged using a Zeiss 510 Meta confocal microscope (Zeiss, Thornwood, NY, USA).
Results
To visually target α-GCs for electrophysiological recordings it was necessary to unequivocally identify them in the superfused rabbit retina-eyecup. As reported previously, Azure B was found to stain a subset of the somata within the ganglion cell layer (GCL) of the living rabbit retina (Hu et al. 2000; Hu & Bloomfield, 2003). These included mosaics of cells easily identified by their regular spacing and particularly large somata when viewed under IR illumination. In this study, we targeted the largest somata in the GCL that, when stained with Neurobiotin, displayed the morphological features described previously for α-GCs in a number of mammalian species, including the rabbit (Boycott & Wässle, 1974; Wässle et al. 1975, 1981; Peichl et al. 1987; Peichl, 1991; Hu & Bloomfield, 2003) (Fig. 1A). These features included: (1) relatively large somata (diameter range of 19–28 μm) and dendritic fields (diameter range of 585–1010 μm); (2) 4–6 stout primary dendrites; (3) dendrites with up to sixth-order radiate branching at acute angles; (4) rare overlap of dendrites; (5) relatively long terminal dendrites; (6) a narrowly stratified arbour in either sublamina a or b of the inner plexiform layer (IPL), which corresponded to the cells’ OFF or ON receptive fields, respectively (Famiglietti et al. 1977; Nelson et al. 1978; Bloomfield & Miller, 1986) (Fig. 1B); and (7) tracer coupling to neighbouring α-GCs, as well as an array of different amacrine cell subtypes (Xin & Bloomfield, 1997; Hu & Bloomfield, 2003; Mills et al. 2007). We recorded from a total of 124 OFF α-GCs in this study.
Figure 1. Coupling pattern and dendritic stratification of OFF α-GCs in the rabbit retina.
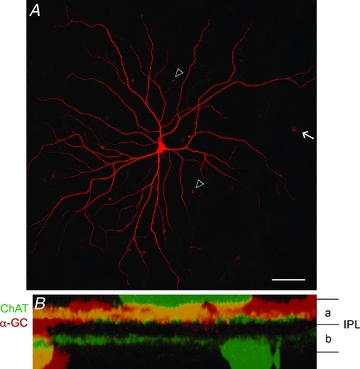
A, photomicrograph showing the flatmount image of an OFF α-GC injected with Neurobiotin. The somata of tracer-coupled amacrine cells (arrowheads) and α-GCs (arrow) can be visualized. Scale bar: 100 μm. B, a z-stack (vertical) confocal image of the dendrites of a Neurobiotin-injected OFF α-GC (red). The dendrites stratify within sublamina-a of the IPL. The two ChAT bands (green) were labelled using an antibody to provide landmarks for the boundaries of sublamina-a and -b.
GABA blockade unmasks an ON response in OFF α-GCs
Under control, dark-adapted conditions, recordings from OFF α-GCs (n = 92) showed a brisk and transient response to the offset of light stimuli (19 ± 11 spikes) (Fig. 2A and E) as previously described in a number of mammalian species (Peichl & Wässle, 1981; Saito, 1983; Fukuda et al. 1984; Stanford & Sherman, 1984; Hu & Bloomfield, 2003). The OFF α-GCs showed little to no spontaneous activity in dark-adapted retinas and therefore the light-evoked responses were unambiguous. Application of the non-specific GABA blocker picrotoxin (PTX; 100 μm) had two prominent and reversible effects on the responses of OFF α-GCs (Fig. 2B and F). First, it produced an increase in the response to light offset, indicating a release from tonic inhibition. Second, PTX revealed a robust response at light onset that had brisk and transient properties similar to those of the OFF response (14 ± 9 spikes). We found that PTX unmasked an ON response in every OFF α-GC examined in this study (n = 41). The unmasked ON response showed a latency of 180 ± 28 ms.
Figure 2. Blockade of GABAergic inhibition unmasks a robust ON response in OFF α-GCs in the rabbit retina.
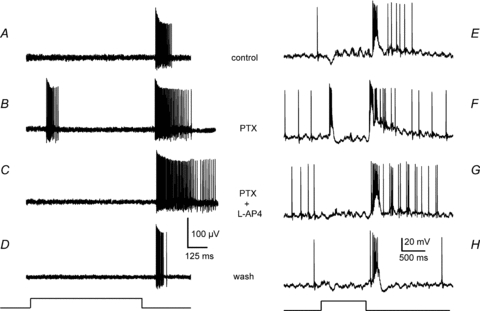
A, extracellularly recorded responses of an OFF α-GC consists of a brisk and transient burst of spike activity at stimulus offset. Presentation of the light stimulus is indicated by the stimulus trace at the bottom of the figure. The increase in spike activity at light offset is likely to reflect the removal of a tonic inhibition at light offset. B, blockade of GABAergic inhibition with PTX (100 μm) unmasks a robust brisk and transient ON response at stimulus onset. The increase in spike activity at light offset likely reflects the removal of a tonic inhibition. C, blockade of the ON pathway using the mGluR6 receptor agonist l-AP4 (100 μm) abolishes the unmasked ON response, indicating that the ON component is produced by the ON pathway. The increase in spike activity at stimulus offset is likely to reflect the removal of a crossover inhibition by ON amacrine cells; see text for details. D, after wash, the OFF α-GC once again shows the classic response at stimulus offset. Light intensity of green stimulus for extracellular recordings was 0.21 Rh* rod−1 s−1. E–H, current clamp recordings from an OFF α-GC using the same pharmacological protocol as in A–D. Light intensity for white light stimulus = log –5.5. Maximum irradiance (log 0.0) = 2.37 mW cm−2.
To determine whether the unmasked ON response is in fact generated by the ON pathway we applied the mGluR6 agonist l-AP4 (100 μm), which effectively abolishes the responses of ON bipolar cells (Slaughter & Miller, 1981). Whereas the application of l-AP4 reversibly blocked the ON response of OFF α-GCs (n = 13), the OFF response remained (Fig. 2C). These results indicate that the unmasked ON response is dependent on a functionality of the ON pathway. Interestingly, we found that l-AP4 often enhanced the OFF response of OFF α-GCs, a finding consistent with crossover inhibitory interactions between the ON and OFF pathways (Zaghloul et al. 2003; Rentería et al. 2006; Roska et al. 2006; Molnar & Werblin, 2007; Hsueh et al. 2008; Manookin et al. 2008; Molnar et al. 2009).
To determine the receptive field structure of the ON and OFF responses of OFF α-GCs, we computed the area summations by evoking them with concentric spots of light of changing diameter, but of constant dim intensity (n = 5) (Fig. 3A). Both ON and OFF responses were saturated by spots of light between about 500 and 750 μm, whereas larger spots produced a slight attenuation of activity, presumably due to activation of a reduced antagonistic surround under our dark-adapted conditions (Jensen, 1991; Muller & Dacheux, 1997). The similar profiles of the ON and OFF responses suggest that the former is not a surround response, but rather that both signals are mediated by centre receptive field mechanisms.
Figure 3. The classic OFF and unmasked ON responses of OFF α-GCs show similar area summation and receptive field profiles.
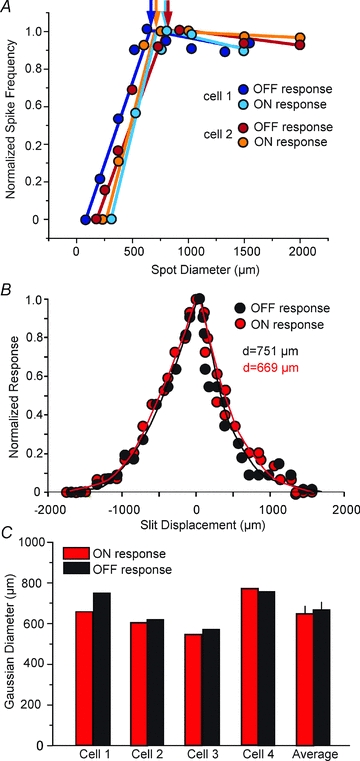
A, area summations were computed from extracellular spike recordings evoked by concentric spots of light of varying diameter, but of constant intensity. The curves show the area summation profiles of the ON (unmasked by PTX) and OFF responses of two OFF α-GCs in the rabbit retina. The area summation of all the responses fell within a common range suggesting that both ON and OFF signals were centre mediated. Reductions in the response profiles to larger spots of light are likely due to surround inhibitory mechanisms. Light intensities for green stimulus = 0.21 Rh* rod−1 s−1. B, receptive field profiles of the OFF and unmasked ON response following application of PTX to an OFF α-GC. The computed Gaussian diameters were very similar for the two responses. C, the receptive field Gaussian diameters computed for the OFF and unmasked ON responses for 4 OFF α-GCs and the composition averages. Like for the area summation measures, the receptive fields of the ON and OFF responses were very similar. Light intensity for white light stimulus = log –5.0. Maximum irradiance (log 0.0) = 2.37 mW cm−2
To further evaluate the receptive field sizes of the ON and OFF responses, we computed Gaussian widths using a 50 μm wide/1 mm long slit of light displaced in discrete lengths along its minor axis. Consistent with the area summation measures, we found that the ON and OFF receptive fields of individual OFF α-GCs were comparable in size (Fig. 3B and C). These data indicate that the ON and OFF receptive fields were co-extensive and occupied similar space, consistent with the idea that they are both centre-mediated.
Pharmacology of the unmasked ON response
Since PTX blocks both GABAA and GABAC receptors, we used antagonists specific for the receptors to determine the role of each in the inhibition masking ON responses. Application of the GABAA receptor blocker SR-95531 in doses of 5–20 μm (n = 7) did not unmask any ON spikes in OFF α-GCs (Fig. 4B). Similarly, blockade of GABAC receptors with TPMPA in doses of 50–100 μm (n = 5) also failed to unmask the ON response (Fig. 4C). In current clamp experiments, we did find that application of SR-95531 (20 μm) and TPMPA (100 μm) produced a small depolarization at light onset, 4.2 ± 1.3 mV and 5.6 ± 1.8 mV, respectively. Thus, while the selective GABA blockers could unmask a subthreshold, excitatory synaptic response at light onset, neither alone could unmask the robust spike response revealed by PTX. However, when SR-95531 and TPMPA were applied together at the lowest doses (5 μm and 50 μm, respectively), the robust ON spike response was reversibly unmasked in OFF α-GCs (n = 7) (Fig. 4D). These results indicate that whereas both GABAA and GABAC receptors subserve the inhibition masking the ON response in OFF α-GCs, activation of each receptor alone was sufficient to mask it. We also examined whether metabotropic GABAB receptors are involved in the masking of the ON response. However, neither the application of the GABAB receptor agonist baclofen (100 μm; n = 8) nor the GABAB receptor antagonist CGP-55845 (50 μm; n = 6) was able to unmask an ON response in OFF α-GCs (Fig. 4G and H).
Figure 4. Both GABAA and GABAC receptors are involved in inhibitory circuits responsible for masking of the ON responses of OFF α-GCs.
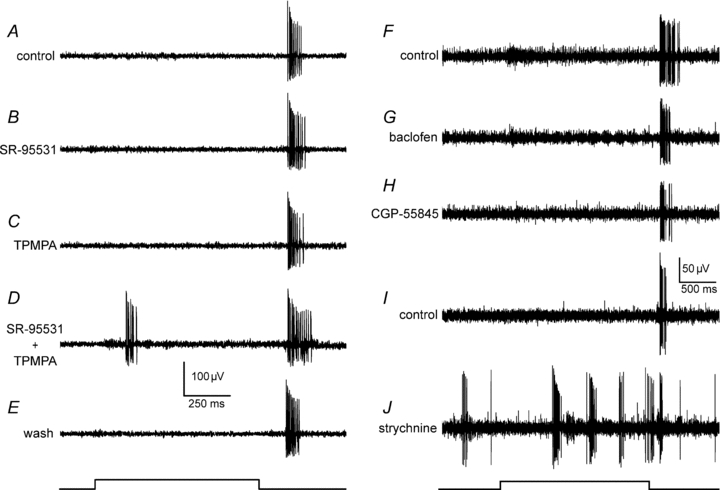
A, response of an OFF α-GC in the rabbit retina under control conditions. Trace at bottom indicates onset and offset of the light stimulus. Neither the blockade of GABAA receptors using SR-95531 (B, 5 μm), nor the blockade of GABAC receptors using TPMPA (C, 50 μm) alone unmasks an ON response in the OFF α-GC. D, however, the simultaneous blockade of both receptors unmasks the ON response. E, the effects of the selective GABA receptor blockers were reversible after wash. F, response of another OFF α-GC under control conditions. Neither the GABAB receptor agonist baclofen (G, 100 μm), nor the GABAB receptor antagonist CGP-55845 (H, 50 μm) unmasks an ON response and neither has a significant effect on the OFF light response of the OFF α-GC. I, response of another OFF α-GC under control conditions. J, blockade of glycine receptors using strychnine (1 μm) increased spontaneous burst activity of the OFF α-GC, but failed to unmask an ON response at stimulus onset. Light intensity of green stimulus = 0.21 Rh* rod−1 s−1.
Picrotoxin has been shown to block glycine receptors in retinal neurons (Wang & Slaughter, 2005; Li & Slaughter, 2007), suggesting that at least part of the effects of PTX could have reflected blockade of glycinergic inhibition. However, while application of strychnine (1 μm; n = 9) increased the spontaneous activity of OFF α-GCs and evoked bursts of spikes, it never unmasked an ON response (Fig. 4J).
Localization of inhibitory circuits masking the ON response
There are two major types of inhibition found in the inner retina: feedforward inhibition in which amacrine cells synapse directly onto ganglion cells and feedback inhibition where amacrine cells synapse onto the axon terminals of bipolar cells or dendrites of other amacrine cells (Dowling & Boycott, 1966). The finding that GABAC receptors play a role in the masking of ON responses in OFF α-GCs implicates feedback inhibition to bipolar cells, whereas GABAA receptors can subserve either feedback or feedforward inhibition (Enz et al. 1996; Wässle et al. 1998; Rotolo & Dacheux, 2003; Zhou & Dacheux, 2005; Eggers et al. 2007; Eggers & Lukasiewicz, 2010). To differentiate between these two possibilities we examined whether the chloride channel blocker DNDS could unmask the ON response in OFF α-GCs. Delivered to OFF α-GCs via an intracellular microelectrode, DNDS eliminates the effects of feedforward GABAA receptor activation by blocking the chloride channel complex (Dudek & Friedlander, 1996; Shao & Burkhalter, 1996; Völgyi et al. 2002). Therefore, if GABAA-mediated feedforward inhibition to OFF α-GCs was responsible for masking the ON response, then intracellular application of DNDS, together with bath-applied TPMPA, to block GABAC-mediated inhibition, should abolish the GABAergic inhibition and reveal the response. Conversely, if feedback inhibition was responsible for masking the ON response, then application of DNDS should have no effect.
The intracellularly recorded responses of OFF α-GCs consisted of a hyperpolarization at light onset, likely to be due to glycinergic crossover inhibition from the ON pathway (Manookin et al. 2008; Murphy & Rieke, 2008) and a depolarizing response at light offset (Fig. 5A). Application of DNDS (500 μm; n = 5) had two major effects including an increase in spontaneous spike activity, presumably due to release from a tonic inhibition, and a reduction of the inhibitory ON hyperpolarization (Fig. 5B). However, DNDS did not unmask an ON response in OFF α-GCs (n = 5) indicating that blockade of feedforward inhibition via GABAA receptors was insufficient to produce unmasking. To determine whether a combination of GABAC-mediated feedback inhibition and GABAA-mediated feedforward inhibition masked the ON response, we applied TPMPA (50 μm) together with DNDS (500 μm) (n = 3). This combination also did not unmask an ON response in OFF α-GCs (Fig. 5C). In contrast, application of DNDS together with PTX (n = 2), which blocks both the GABAA and GABAC receptors presynaptic to the OFF α-GCs, successfully unmasked the ON response (Fig. 5D). Taken together, these results suggest that feedback inhibition, mediated by both GABAA and GABAC receptors, is responsible for masking ON responses in OFF α-GCs and that feedforward inhibition plays little or no role.
Figure 5. The inhibition responsible for masking the ON response acts presynaptic to OFF α-GCs.
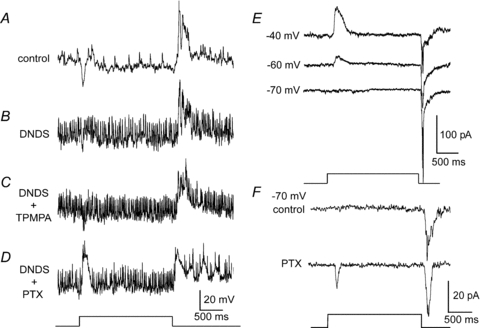
A, intracellular recording of an OFF α-GC shows a hyperpolarizing component at light onset and a depolarization at light offset. Light trace indicating stimulus onset and offset is below panel D. B, intracellular blockade of chloride channels using a DNDS-filled (500 μm) microelectrode increases spontaneous spiking and reduces the hyperpolarization at stimulus onset indicating a blockade of tonic and light-evoked inhibition of the OFF α-GC. However, DNDS did not unmask an excitatory ON response. C, simultaneous application of intracellular DNDS and bath-applied TPMPA (50 μm), which blockaded GABAA-mediated feedforward inhibition and GABAC-mediated feedback inhibition, did not unmask an ON response in the OFF α-GC. D, however, the simultaneous application of intracellular DNDS and bath-applied PTX (100 μm), which blocked both GABAA- and GABAC-mediated feedback inhibition, unmasks a robust excitatory ON depolarization at light onset. Resting membrane potentials for recordings in panels A–D were 50–55 mV. E, whole cell recording from an OFF α-GC voltage clamped to –40, –60, and –70 mV. At a membrane potential of –40 mV, the cell shows an outward inhibitory current at light onset and an inward excitatory current at light offset. At the approximate reversal potential for chloride of –70 mV, the outward current at light onset is eliminated, but no excitatory inward current is unmasked. Light-evoked excitatory postsynaptic currents recorded from an OFF α-GC indicate the presence of excitatory inputs at light offset, but not light onset, under control conditions. F, whole cell recording of an OFF α-GC voltage clamped at –70 mV before and after bath application of PTX (100 μm). PTX unmasked an excitatory inward current in response to light onset. Light intensity of green stimulus = 0.21 Rh* rod−1 s−1.
To further verify the presynaptic origin of the inhibition masking the ON responses, we performed whole-cell voltage clamp recordings from OFF α-GCs (n = 4). At –60 mV and more positive holding potentials, the light evoked response included an inhibitory outward current at light onset as previously reported (Manookin et al. 2008; Murphy & Rieke, 2008) and an excitatory inward current at offset (Fig. 5E). Under voltage clamp conditions, with the membrane potential held at –70 mV, near the reversal potential for chloride, the outward current at light onset was eliminated, but this did not reveal an excitatory inward current (Fig. 5E). Our results are consistent with those of Molnar et al. (2009) who reported that OFF α-GCs in the rabbit do not receive excitation at light onset. In contrast, bath application of PTX (100 μm) or a combination, but not separate, application of SR-95531 (20 μm) and TPMPA (100 μm) (data not shown) unmasked an excitatory current in response to light onset in these cells (Fig. 5F). These findings indicate that OFF α-GCs only receive an excitatory ON input after the blockade of GABAergic inhibition with PTX, further supporting the conclusion that the excitatory ON signals are masked by inhibition positioned presynaptically.
Coupling via gap junctions is required for expression of the ON response
Since the dendrites of OFF α-GCs are restricted to sublamina-a of the IPL, they are positioned to receive excitatory inputs only from OFF bipolar cells and not from ON bipolar cells whose axons stratify in sublamina-b (Nelson et al. 1978). This organization raises the important question: how do OFF α-GCs receive excitatory signals from the ON pathway? Although it would appear that OFF α-GCs cannot receive excitatory ON inputs via conventional chemical circuitry, an alternative pathway is the gap junctions formed with a number of amacrine cell subtypes (Xin & Bloomfield, 1997; Hu & Bloomfield, 2003; Mills et al. 2007).
To test this hypothesis we used 18β-glycyrrhetinic acid (18β-GA), which has been shown to effectively block gap junctional transmission in the nervous system (Davidson & Baumgarten, 1988; Ackert et al. 2009). We first unmasked the ON response in OFF α-GCs with PTX (100 μm) and then applied 18β-GA (25 μm) to block gap junctions (Fig. 6A–E). We found that 18β-GA reversibly abolished the unmasked ON response, but had no significant effect on the OFF response (n = 7). We have recently shown that it takes approximately 20 min for 18β-GA to block gap junctions in the retina (Ackert et al. 2009). Consistent with the timing of the uncoupling action of 18β-GA, we found that it took about 20 min of exposure of the drug to completely block the ON response in OFF α-GCs. To verify the results with 18β-GA, we utilized a second gap junction blocker, meclofenamic acid (MFA; Pan et al. 2007). Identical to the effects of 18β-GA, administration of MFA (100 μm) completely abolished the ON response unmasked with PTX, yet had no effect on the OFF response (n = 3) (Fig. 6F–H).
Figure 6. Gap junctions are required for the presence of excitatory ON responses in OFF α-GCs.
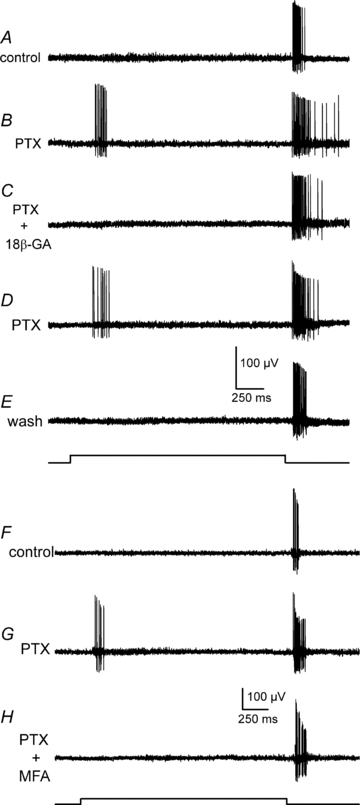
A, the typical extracellularly recorded response of an OFF α-GC in the rabbit retina under control conditions. B, application of PTX (100 μm) unmasks a robust ON response in the OFF α-GC. C, application of the gap junction blocker 18β-GA (25 μm) blocks the unmasked ON response, but has no significant effect on the OFF response. D, the ON response returns after washout of 18β-GA. E, the typical response of the OFF α-GC is restored when all drugs are washed out. F, the typical extracellularly recorded response of another OFF α-GC in the rabbit retina under control conditions. G, application of PTX (100 μm) unmasked an ON response at stimulus onset. H, application of the gap junction blocker MFA (100 μm) blocks the unmasked ON response, but has no significant effect on the OFF response. Light intensity of green stimulus = 0.21 Rh* rod−1 s−1.
These results suggest that electrical synapses form the pathway for crossover excitation of OFF α-GCs. To further test this idea, we investigated crossover excitation in ON α-GCs, which, unlike OFF α-GCs, are not electrically coupled (Hu & Bloomfield, 2003). If gap junctions are required for reception of excitatory crossover inputs, we would expect that ON α-GCs would not display an OFF response under GABAergic blockade. Indeed, we found that blockade of GABAergic inhibition with PTX (100 μm) did not unmask an excitatory OFF response in ON α-GCs (n = 4) (Fig. 7).
Figure 7. PTX does not unmask crossover excitation in ON α-GCs.
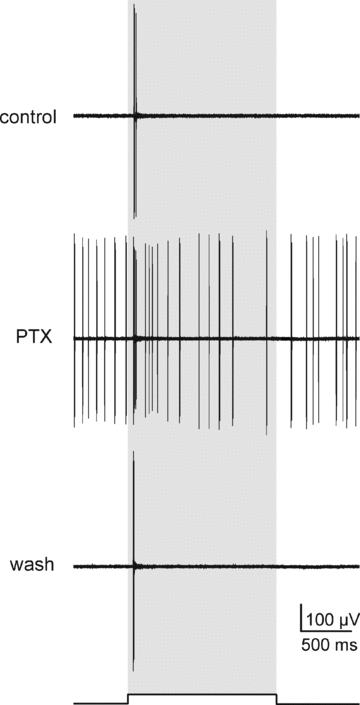
Extracellular recordings from an ON α-GC before, during and after application of PTX (100 μm). PTX increased the spontaneous and light-evoked spike activity of the cell, but did not unmask an OFF response at stimulus offset. Shaded bar indicates duration of the light stimulus. Light intensity of green stimulus = 0.21 Rh* rod−1 s−1.
Our results indicate that functional gap junctions, presumably those between OFF α-GCs and amacrine cells, are required for the transmission of excitatory ON signals to OFF α-GCs. In this scheme, amacrine cells receive excitatory inputs from ON bipolar cell terminals in sublamina-b of the IPL and subsequently relay them to OFF α-GCs via gap junctions that lie in sublamina-a. To test this idea, we examined the stratification of amacrine cells coupled to OFF α-GCs. We first injected OFF α-GCs with the gap junction-permeant fluorescent dye Po-Pro-1 (Hoshi et al. 2006) to visualize the somata of coupled amacrine cells in the living rabbit retina (Fig. 8A). We have shown previously that OFF α-GCs in the rabbit are coupled to a number of different amacrine cell subtypes (Hu et al. 2010). Here, we targeted the Po-Pro-1-labelled amacrine cell somata of intermediate size, and injected them with Neurobiotin to determine their dendritic/axonal morphology (n = 12). Figure 8B shows an example of a Neurobiotin-injected intermediate amacrine cell coupled to an OFF α-GC. The amacrine cell displays radiate dendritic branching that narrows to form thin axon-like structures running for nearly 2 mm. A vertical rotation of the confocal Z-stack image of the amacrine cell shows that the cell maintains processes that stratify in sublamina-a (Fig. 8C) and sublamina-b (Fig. 8D) of the IPL. We examined the dendritic morphology of 12 intermediate-sized amacrine cells coupled to OFF α-GCs and found that each maintained processes in both sublaminae of the IPL. These results indicate that the amacrine cells coupled to OFF α-GCs can serve as the necessary anatomical substrate for receiving excitatory inputs from ON bipolar cell terminals in sublamina-b of the IPL and the subsequent transfer of these ON signals to OFF α-GCs via gap junctions made in sublamina-a.
Figure 8. OFF α-GCs are coupled to amacrine cells that stratify within both sublamina-a and sublamina-b of the IPL.
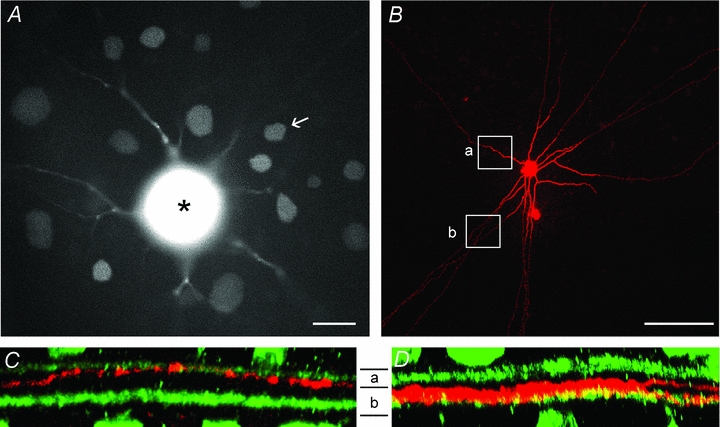
A, photomicrograph of the living rabbit retina showing an OFF α-GC injected with the gap junction-permeant fluorescent tracer Po-Pro-1. The soma of the OFF α-GC with bloomed fluorescence is indicated by the asterisk. The somata of coupled amacrine cell and ganglion cell neighbours can also be clearly visualized. One amacrine cell (arrow) was labelled with Neurobiotin. Scale bar = 25 μm. B, confocal micrograph of the amacrine cell in panel A labelled with Neurobiotin after post hoc histological processing. This subtype of amacrine cell typically showed long radiate branches that became narrow and axon-like, running for up to 2 mm. The rectangles show the portion of the processes in the confocal images in the subsequent panels. Scale bar = 100 μm. C, Z-stack vertical confocal images of the amacrine cell process (red) in rectangle ‘a’ in panel B showing that it stratifies in sublamina-a of the IPL. ChAT bands are shown in green. D, Z-stack vertical confocal image of the amacrine cell process (red) in rectangle ‘b’ in panel B showing that it stratifies within sublamina-b of the IPL.
Excitatory crosstalk between the ON and OFF pathways is widespread in mammalian ganglion cells
Considering the fact that many ganglion cell subtypes are coupled to neighbouring amacrine cells (Vaney, 1991; Xin & Bloomfield, 1997; Völgyi et al. 2009), our results for OFF α-GCs indicating that these gap junctions may form the conduit for the mixing of excitatory ON and OFF signals suggest that excitatory crosstalk between the ON and OFF pathways may be widespread amongst mammalian ganglion cells. To test this idea we used a 60-channel multielectrode array to determine the effects of PTX on a large number of ganglion cells in both the rabbit and mouse retinas. In the rabbit, we found that the blockade of GABA receptors with PTX (100 μm) unmasked robust ON responses in 44% of OFF ganglion cells we recorded (54 ± 13 spikes; n = 34) and unmasked an OFF response in 31% of ON ganglion cells (49 ± 9 spikes; n = 35) (Fig. 9A). Latencies of the unmasked ON and OFF responses were 174 ± 24 ms and 125 ± 23 ms to stimulus onset and offset, respectively. In the mouse retina, PTX (100 μm) revealed an ON response in 19% of OFF (26 ± 15 spikes; n = 21) ganglion cells, whereas PTX unmasked OFF responses in 44% of ON ganglion cells (17 ± 13 spikes; n = 25) examined (Fig. 9B). Latencies of the unmasked ON and OFF responses were 187 ± 34 ms and 155 ± 32 ms to stimulus onset and offset, respectively. Overall, these results indicate that a significant number of mammalian ganglion cells receive masked excitatory crossover inputs.
Figure 9. Crossover excitation is displayed by many ganglion cells in the rabbit and mouse retinas.
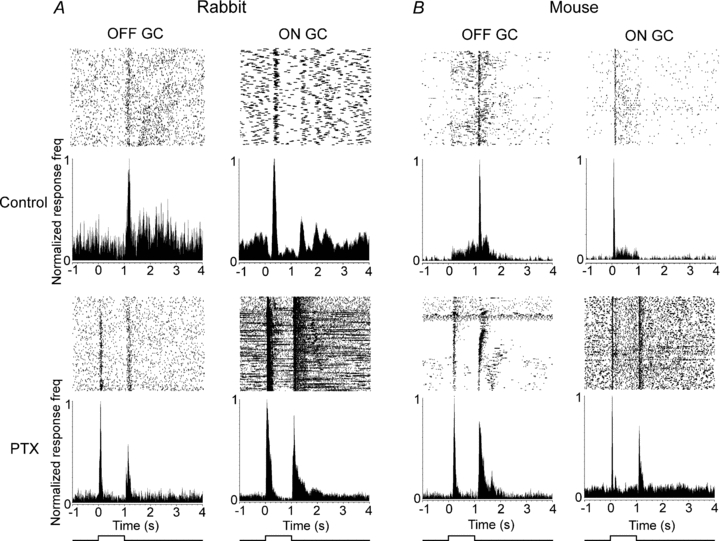
A, raster plots and peristimulus time histograms (PSTHs) of an OFF and an ON ganglion cell in the rabbit retina recorded with a multielectrode array. Blockade of GABAergic inhibition with PTX (100 μm) unmasks robust responses of opposite polarity. B, raster plots and PSTHs of an OFF and an ON ganglion cell in the wild-type mouse retina recorded with a multielectrode array. Similar to the rabbit, blockade of GABAergic inhibition with PTX unmasks robust responses with polarity opposite to that of the cells’ classic centre-mediated responses. Light intensity of green stimulus = 0.18 Rh* rod−1 s−1.
Discussion
Work over the past 70 years has shown the segregation of visual information into parallel ON and OFF pathways to be a principal organizing feature of the visual system (Hartline, 1938; Werblin & Dowling, 1969; Famiglietti & Kolb, 1976). Here, we found that over one-third of the ganglion cells in the rabbit and mouse retinas with classic ON and OFF responses also display robust opposite polarity responses following blockade of GABAergic inhibition. Interestingly, reports of masked opposite polarity responses in mammalian ganglion cells have surfaced intermittently over the years (Ariel & Daw, 1982; Nirenberg & Meister, 1997; Roska & Werblin, 2001; Rentería et al. 2006; Ackert et al. 2009). However, these results were often cited anecdotally, even attributed to technical artifact, and so the extent of crossover excitation in the retina has remained unclear. Further, previous investigations had all focused on the masked OFF inputs of ON ganglion cells and it therefore remained an open question as to whether OFF ganglion cells could receive excitatory crossover ON inputs. The present report is thus the first to examine crossover excitation in both ON and OFF ganglion cells in the mammalian retina and to examine the synaptic circuitry underlying its generation and masking. Overall, our results indicate a significant interaction between excitatory ON and OFF signals in the inner retina and thus call into question the traditional view that these signals are largely segregated within parallel retinal circuits innervating different ganglion cell subtypes.
Our spatial measures showed that the area summation and receptive fields of the ON responses in OFF α-GCs were co-extensive with those of the OFF responses, suggesting that the unmasked ON response was centre-mediated. This assertion is supported by our finding that the ON responses were blocked by application of l-AP4, indicating that they were generated by the ON pathway. Consistent with our findings, Roska & Werblin (2001) found that, following PTX application, OFF responses could be evoked in certain ON ganglion cells in rabbit with a light stimulus restricted to the centre receptive field.
Recently, Rentería et al. (2006) reported a late ON response in certain OFF ganglion cells in the mouse retina. However, those responses were not blocked by application of l-AP4, indicating that they were not generated by the ON pathway, and showed latencies >1000 ms from stimulus onset. The unmasked ON responses reported here were sensitive to l-AP4 and showed latencies <200 ms and thus were different from the late ON responses reported previously.
Feedback inhibition masks crossover excitation
Our results show that a GABAergic inhibition, subserved by both GABAA and GABAC receptors, masks the ON signals in OFF α-GCs under our experimental conditions. Two lines of evidence indicate that the site(s) of the masking inhibition is likely to be presynaptic to ganglion cells. First, we found that application of the intracellular chloride channel blocker DNDS to OFF α-GCs, either alone or together with TPMPA, did not unmask an ON response. Second, no excitatory ON signals were recorded in voltage-clamped OFF α-GCs even when held at the reversal potential for the inhibitory response evoked at stimulus onset. Consistent with our finding, Molnar et al. (2009) also reported that OFF α-GCs in the rabbit do not receive excitation at light onset. Taken together, these data suggest feedback and not feedforward inhibition in the masking of crossover excitation.
While there is both anatomical (Enz et al. 1996; Wässle et al. 1998), and physiological (Rotolo & Dacheux, 2003; Zhou & Dacheux, 2005; Eggers et al. 2007; Eggers & Lukasiewicz, 2010) evidence showing that GABAC receptors are expressed exclusively on bipolar cell terminals, GABAA receptors are found on both bipolar cell terminals and amacrine cell processes (Greferath et al. 1994). This indicates that the masking inhibition can be limited to ON bipolar cells that provide excitatory drive to the amacrine cells that are coupled to OFF α-GCs and, in addition, could involve direct inhibition of the coupled amacrine cells. Irrespective of which of these scenarios is correct, it is important to note that the blockade of both GABAA and GABAC receptors is necessary for unmasking the ON response in OFF α-GCs. This suggests a certain level of redundancy in the masking inhibitory circuit in that activation of either receptor alone is sufficient to mask the crossover excitation.
Finally, the fact that the masking inhibition appears not to be directly on α-GCs, but is acting presynaptically on bipolar cell terminals and possibly amacrine cells suggests that crossover excitation is a phenomenon expressed by numerous ganglion cell subtypes due to the divergent circuitry subserved by these presynaptic cells in the IPL. This idea is clearly supported by our finding that many of the ganglion cells recorded in the rabbit and mouse retinas showed crossover excitation when GABAergic inhibition was blocked.
Gap junctional coupling with amacrine cells mediates crossover excitation in ganglion cells
Although our results indicate a convergence of ON and OFF signals within individual ganglion cells, there is no question, regarding the anatomical and physiological evidence, of the segregation of ON and OFF signals in presynaptic circuits within the retina. This is most clearly exemplified by the parallel ON and OFF bipolar cells, whose terminals end within different sublaminae of the IPL.
The fact that OFF α-GCs’ dendrites adhere to this sublamination scheme, stratifying exclusively in sublamina-a to receive excitatory drive from OFF bipolar cells (Fig. 1A), raises the issue as to how these cells receive crossover ON excitation. Although it is plausible that OFF α-GCs dendrites receive direct synaptic drive from ON bipolar cells as they ascend through sublamina-b, such inputs have not been observed in electron microscopic studies (Kolb & Nelson, 1993; Owczarzak & Pourcho, 1999). In addition, it has recently been shown that certain ON bipolar cells can make ectopic synapses in the OFF sublamina-a of the IPL (Dumitrescu et al. 2009; Hoshi et al. 2009), providing a second plausible route by which the coupled amacrine cells in this study could receive ON inputs. Although this possibility cannot be completely discounted, our present results provide strong evidence that the ON excitation is derived from electrical synapses, likely those formed between OFF α-GCs and wide-field amacrine cells. First, we found that application of gap junction blockers abolished the unmasked ON response in OFF α-GCs, but had no significant effect on the classic OFF response. Second, we found that it took approximately 20 min for gap junction blockers to abolish the ON response, which is the same time necessary to show uncoupling between ganglion cells and amacrine cells (Ackert et al. 2009). Third, we found that ON α-GCs, which are not electrically coupled, do not receive excitatory crossover inputs. Finally, coupled amacrine cell dendrites were found to be bistratified within both sublamina-a and -b, and thus can provide the morphological substrate for the crosstalk between ON and OFF channels. We therefore posit that the gap junctions between ganglion cells and amacrine cells form the conduit for ON signal transmission to OFF α-GCs. In this scheme, multistratified amacrine cells receive ON signals via chemical synapses with ON bipolar cell axon terminals in sublamina-b of the IPL and then deliver these signals to OFF α-GC dendrites in sublamina-a.
A number of functional roles have been attributed to retinal gap junctions (Bloomfield & Völgyi, 2009), and our present results suggest a novel one: forming conduits for excitatory interaction between ON and OFF channels in the inner retina. Recently, we found a similar function for gap junctions as mediators of excitatory OFF inputs to ON direction selective GCs (Ackert et al. 2009). Taken together, these results suggest that gap junctions between amacrine cells and ganglion cells form symmetrical circuits for the crossover excitation of both ON and OFF ganglion cells. Many ganglion cell subtypes in mammalian retinas have been shown to be coupled to neighbouring amacrine cells (Vaney, 1991; Xin & Bloomfield, 1997; Völgyi et al. 2009), including 40% of ganglion cells in the mouse retina. An extensive morphological substrate therefore exists for the interaction between ON and OFF channels in the inner retina, clearly consistent with our finding that many ganglion cells in rabbit and mouse retinas show crossover excitation.
In addition to the crossover excitation described here, amacrine cells also play an important role in crossover inhibition between the ON and OFF pathways (Molnar & Werblin, 2007; Manookin et al. 2008; Murphy & Rieke, 2008; Liang & Freed, 2010; Werblin, 2010). This inhibition is mediated by amacrine cells that carry signals across the ON and OFF sublaminar boundary of the IPL. It has been suggested that crossover inhibition can compensate for non-linear rectification at chemical synapses, can suppress anomalous ON signals in OFF ganglion cells, and can extend the light responses of OFF ganglion cells via disinhibition (Rentería et al, 2006; Manookin et al. 2008; Liang & Freed, 2010; Werblin, 2010). It is at present uncertain whether the inhibition responsible for masking the crossover excitation is also a form of crossover inhibition. However, crossover inhibition appears to be mediated by glycinergic amacrine cells (Werblin, 2010) and so it is likely the GABAergic masking inhibition is mediated by amacrine cells with the same ON or OFF physiology as their target neurons. In any event, it appears that amacrine cells play extensive roles in communication between the ON and OFF pathways, with small-field glycinergic cells affording crossover inhibition via conventional chemical synapses and wide-field, presumably GABAergic, cells subserving crossover excitation via gap junction-mediated electrical synapses.
Masking of excitatory inputs is widespread in the CNS
Overall, our results show that ganglion cells with classic ON and OFF responses can express excitatory opposite polarity signals when GABAergic inhibition is blocked. This raises the obvious question concerning the wiring strategy of the retina: why is the excitatory crosstalk between the ON and OFF pathways masked by inhibition under control experimental conditions? To address this question, it is important to note that the masking of synaptic inputs is a phenomenon found throughout the CNS and appears to be highly plastic. For example, manipulation of sensory afferents has been found to produce rapid and dramatic changes in the receptive field structure of neurons in somatosensory cortex (Metzler & Marks, 1979; Merzenich et al. 1983; Calford & Tweedale, 1988; Turnbull & Rasmusson, 1990; Calford, 2002; Foeller & Feldman, 2004), visual cortex (Gilbert & Wiesel, 1992), inferior colliculus (Snyder et al. 2000), motor cortex (Sanes et al. 1988), thalamus (Nicolelis et al. 1993; Faggin et al. 1997) and brainstem (Dostrovsky et al. 1976; Faggin et al. 1997). The rapid timeframe, within minutes, in which the receptive field changes occur virtually eliminates axonal sprouting and creation of new synapses as potential underlying mechanisms. Instead, it is believed that the receptive field changes reflect a release from tonic inhibition that unmasks previously latent synaptic inputs (Batuev et al. 1982; Garraghty et al. 1991; Jacobs & Donoghue, 1991; Wellman et al. 2002; Foeller & Feldman, 2004). Masking can therefore be considered a component of the normal dynamics of the plastic brain in which neuronal responses are modified under changing stimulus conditions.
In the retina, Geffen et al. (2007) observed that many salamander OFF ganglion cells can reversibly switch their response polarity to ON following a peripheral image shift. Importantly, their study implicated inhibitory synaptic circuitry in the mechanism underlying the change in response polarity. In addition, Sagdullaev & McCall (2005) reported that certain mouse OFF ganglion cells could be changed to ON–OFF or ON with changes in the size or intensity of the light stimulus, possibly due to alterations in inhibition. We therefore posit that GABAergic inhibition is relieved under certain stimulus conditions allowing crosstalk between the ON and OFF channels in the inner retina. What would be the consequence of this crosstalk? One idea is that the unmasking of crossover excitation in the retina would result in ON and OFF signals being carried by a larger number of output ganglion cells. Since the optic nerve forms a bottleneck in the visual system, the multiplexing of signals across a larger contingent of ganglion cell subtypes can increase valuable bandwidth. Crossover excitation can thereby enhance the efficiency and capacity of visual information flow to the brain.
Acknowledgments
This work was supported by NIH Grants EY007360 and EY017832.
Glossary
Abbreviations
- l-AP4
l-2-amino-4-phosphonobutyric acid
- α-GC
α-ganglion cell
- CNS
central nervous system
- DNDS
4,4′-dinitro-stilbene-2,2′-disulphonic acid
- 18β-GA
18β-glycyrrhetinic acid
- INL
inner nuclear layer
- IPL
inner plexiform layer
- MFA
meclofenamic acid
- PTX
picrotoxin
- TPMPA
1,2,5,6-tetrahydropyridin-4-yl-methylphosphinic acid
Author contributions
Conception and design of experiments: R.F., S.B. Collection, analysis and interpretation of data: R.F., F.P., A.A., B.V., S.B. Drafting the article or revising it critically for important intellectual content: R.F., F.P., A.A., B.V., S.B. All authors approved the final version of the manuscript.
References
- Ackert JM, Farajian R, Völgyi B, Bloomfield SA. GABA blockade unmasks an OFF response in ON direction selective ganglion cells in the mammalian retina. J Physiol. 2009;587:4481–4495. doi: 10.1113/jphysiol.2009.173344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariel M, Daw NW. Pharmacological analysis of directionally sensitive rabbit retinal ganglion cells. J Physiol. 1982;324:161–185. doi: 10.1113/jphysiol.1982.sp014105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batuev AS, Alexandrov AA, Scheynikov NA. Picrotoxin action on the receptive fields of the cat sensorimotor cortex neurons. J Neurosci Res. 1982;7:49–55. doi: 10.1002/jnr.490070106. [DOI] [PubMed] [Google Scholar]
- Bloomfield SA, Miller RF. A functional organization of ON and OFF pathways in the rabbit retina. J Neurosci. 1986;6:1–13. doi: 10.1523/JNEUROSCI.06-01-00001.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloomfield SA, Völgyi B. The diverse functional roles and regulation of neuronal gap junctions in the retina. Nat Rev Neurosci. 2009;10:495–506. doi: 10.1038/nrn2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boycott BB, Wässle H. The morphological types of ganglion cells of the domestic cat's retina. J Physiol. 1974;240:397–419. doi: 10.1113/jphysiol.1974.sp010616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brainard DH. The Psychophysics Toolbox. Spat Vis. 1997;10:433–436. [PubMed] [Google Scholar]
- Calford MB. Dynamic representational plasticity in sensory cortex. Neuroscience. 2002;111:709–738. doi: 10.1016/s0306-4522(02)00022-2. [DOI] [PubMed] [Google Scholar]
- Calford MB, Tweedale R. Immediate and chronic changes in responses of somatosensory cortex in adult flying-fox after digit amputation. Nature. 1988;332:446–448. doi: 10.1038/332446a0. [DOI] [PubMed] [Google Scholar]
- Davidson JS, Baumgarten IM. Glycyrrhetinic acid derivatives: a novel class of inhibitors of gap-junctional intercellular communication. Structure-activity relationships. J Pharmacol Exp Ther. 1988;246:1104–1107. [PubMed] [Google Scholar]
- DeVries SH. Bipolar cells use kainate and AMPA receptors to filter visual information into separate channels. Neuron. 2000;28:847–856. doi: 10.1016/s0896-6273(00)00158-6. [DOI] [PubMed] [Google Scholar]
- Dostrovsky JO, Millar J, Wall PD. The immediate shift of afferent drive to dorsal column nucleus cells following deafferentation: a comparison of acute and chronic deafferentation in gracile nucleus and spinal cord. Exp Neurol. 1976;52:480–495. doi: 10.1016/0014-4886(76)90219-3. [DOI] [PubMed] [Google Scholar]
- Dowling JE, Boycott BB. Organization of the primate retina: electron microscopy. Proc R Soc Lond B Biol Sci. 1966;166:80–111. doi: 10.1098/rspb.1966.0086. [DOI] [PubMed] [Google Scholar]
- Dudek SM, Friedlander MJ. Intracellular blockade of inhibitory synaptic responses in visual cortical layer IV neurons. J Neurophysiol. 1996;75:2167–2173. doi: 10.1152/jn.1996.75.5.2167. [DOI] [PubMed] [Google Scholar]
- Dumitrescu ON, Pucci FG, Wong KY, Berson DM. Ectopic retinal ON bipolar cell synapses in the OFF inner plexiform layer: contacts with dopaminergic amacrine cells and melanopsin ganglion cells. J Comp Neurol. 2009;517:226–244. doi: 10.1002/cne.22158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggers ED, Lukasiewicz PD. Interneuron circuits tune inhibition in retinal bipolar cells. J Neurophysiol. 2010;103:25–37. doi: 10.1152/jn.00458.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggers ED, McCall MA, Lukasiewicz PD. Presynaptic inhibition differentially shapes transmission in distinct circuits in the mouse retina. J Physiol. 2007;582:569–582. doi: 10.1113/jphysiol.2007.131763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enz R, Brandstatter JH, Wässle H, Bormann J. Immunocytochemical localization of the GABAc receptor rho subunits in the mammalian retina. J Neurosci. 1996;16:4479–4490. doi: 10.1523/JNEUROSCI.16-14-04479.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faggin BM, Nguyen KT, Nicolelis MA. Immediate and simultaneous sensory reorganization at cortical and subcortical levels of the somatosensory system. Proc Natl Acad Sci U S A. 1997;94:9428–9433. doi: 10.1073/pnas.94.17.9428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Famiglietti EV, Jr, Kaneko A, Tachibana M. Neuronal architecture of on and off pathways to ganglion cells in carp retina. Science. 1977;198:1267–1269. doi: 10.1126/science.73223. [DOI] [PubMed] [Google Scholar]
- Famiglietti EV, Jr, Kolb H. Structural basis for ON-and OFF-center responses in retinal ganglion cells. Science. 1976;194:193–195. doi: 10.1126/science.959847. [DOI] [PubMed] [Google Scholar]
- Foeller E, Feldman DE. Synaptic basis for developmental plasticity in somatosensory cortex. Curr Opin Neurobiol. 2004;14:89–95. doi: 10.1016/j.conb.2004.01.011. [DOI] [PubMed] [Google Scholar]
- Fukuda Y, Hsiao CF, Watanabe M, Ito H. Morphological correlates of physiologically identified Y-, X-, and W-cells in cat retina. J Neurophysiol. 1984;52:999–1013. doi: 10.1152/jn.1984.52.6.999. [DOI] [PubMed] [Google Scholar]
- Garraghty PE, LaChica EA, Kaas JH. Injury-induced reorganization of somatosensory cortex is accompanied by reductions in GABA staining. Somatosens Mot Res. 1991;8:347–354. doi: 10.3109/08990229109144757. [DOI] [PubMed] [Google Scholar]
- Geffen MN, de Vries SE, Meister M. Retinal ganglion cells can rapidly change polarity from Off to On. PLoS Biol. 2007;5:e65. doi: 10.1371/journal.pbio.0050065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert CD, Wiesel TN. Receptive field dynamics in adult primary visual cortex. Nature. 1992;356:150–152. doi: 10.1038/356150a0. [DOI] [PubMed] [Google Scholar]
- Greferath U, Grünert U, Müller F, Wässle H. Localization of GABAA receptors in the rabbit retina. Cell Tissue Res. 1994;276:295–307. doi: 10.1007/BF00306115. [DOI] [PubMed] [Google Scholar]
- Hartline HK. The response of single optic nerve fibers of the vertebrate eye to illumination of the retina. Am J Physiol. 1938;121:400–415. [Google Scholar]
- Horton JC, Sherk H. Receptive field properties in the cat's lateral geniculate nucleus in the absence of on-center retinal input. J Neurosci. 1984;4:374–380. doi: 10.1523/JNEUROSCI.04-02-00374.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshi H, Massey SC, Mills SL. ON inputs to the OFF layer: bipolar cells that break the stratification rules of the retina. J Neurosci. 2009;29:8875–8883. doi: 10.1523/JNEUROSCI.0912-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshi H, O'Brien J, Mills SL. A novel fluorescent tracer for visualizing coupled cells in neural circuits of living tissue. J Histochem Cytochem. 2006;54:1169–1176. doi: 10.1369/jhc.6A6935.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsueh HA, Molnar A, Werblin FS. Amacrine-to-amacrine cell inhibition in the rabbit retina. J Neurophysiol. 2008;100:2077–2088. doi: 10.1152/jn.90417.2008. [DOI] [PubMed] [Google Scholar]
- Hu EH, Bloomfield SA. Gap junctional coupling underlies the short-latency spike synchrony of retinal α ganglion cells. J Neurosci. 2003;23:6768–6777. doi: 10.1523/JNEUROSCI.23-17-06768.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu EH, Dacheux RF, Bloomfield SA. A flattened retina-eyecup preparation suitable for electrophysiological studies of neurons visualized with trans-scleral infrared illumination. J Neurosci Methods. 2000;103:209–216. doi: 10.1016/s0165-0270(00)00319-8. [DOI] [PubMed] [Google Scholar]
- Hu EH, Pan F, Völgyi B, Bloomfield SA. Light increases the gap junctional coupling of retinal ganglion cells. J Physiol. 2010;588:4145–4163. doi: 10.1113/jphysiol.2010.193268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs KM, Donoghue JP. Reshaping the cortical motor map by unmasking latent intracortical connections. Science. 1991;251:944–947. doi: 10.1126/science.2000496. [DOI] [PubMed] [Google Scholar]
- Jensen RJ. Involvement of glycinergic neurons in the diminished surround activity of ganglion cells in the dark-adapted rabbit retina. Vis Neurosci. 1991;6:43–53. doi: 10.1017/s0952523800000894. [DOI] [PubMed] [Google Scholar]
- Knapp AG, Mistler LA. Response properties of cells in rabbit's lateral geniculate nucleus during reversible blockade of retinal on-center channel. J Neurophysiol. 1983;50:1236–1245. doi: 10.1152/jn.1983.50.5.1236. [DOI] [PubMed] [Google Scholar]
- Kolb H, Nelson R. OFF-alpha and OFF-beta ganglion cells in cat retina: II. Neural circuitry as revealed by electron microscopy of HRP stains. J Comp Neurol. 1993;329:85–110. doi: 10.1002/cne.903290107. [DOI] [PubMed] [Google Scholar]
- Li P, Slaughter M. Glycine receptor subunit composition alters the action of GABA antagonists. Vis Neurosci. 2007;24:513–521. doi: 10.1017/S0952523807070368. [DOI] [PubMed] [Google Scholar]
- Liang Z, Freed MA. The ON pathway rectifies the OFF pathway of the mammalian retina. J Neurosci. 2010;30:5533–5543. doi: 10.1523/JNEUROSCI.4733-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manookin MB, Beaudoin DL, Ernst ZR, Flagel LJ, Demb JB. Disinhibition combines with excitation to extend the operating range of the OFF visual pathway in daylight. J Neurosci. 2008;28:4136–4150. doi: 10.1523/JNEUROSCI.4274-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masu M, Iwakabe H, Tagawa Y, Miyoshi T, Yamashita M, Fukuda Y, Sasaki H, Hiroi K, Nakamura Y, Shigemoto R, et al. Specific deficit of the ON response in visual transmission by targeted disruption of the mGluR6 gene. Cell. 1995;80:757–765. doi: 10.1016/0092-8674(95)90354-2. [DOI] [PubMed] [Google Scholar]
- Merzenich MM, Kaas JH, Wall JT, Sur M, Nelson RJ, Felleman DJ. Progression of change following median nerve section in the cortical representation of the hand in areas 3b and 1 in adult owl and squirrel monkeys. Neuroscience. 1983;10:639–665. doi: 10.1016/0306-4522(83)90208-7. [DOI] [PubMed] [Google Scholar]
- Metzler J, Marks PS. Functional changes in cat somatic sensory-motor cortex during short-term reversible epidural blocks. Brain Res. 1979;177:379–383. doi: 10.1016/0006-8993(79)90790-x. [DOI] [PubMed] [Google Scholar]
- Mills SL, Xia XB, Hoshi H, Firth SI, Rice ME, Frishman LJ, Marshak DW. Dopaminergic modulation of tracer coupling in a ganglion-amacrine cell network. Vis Neurosci. 2007;24:593–608. doi: 10.1017/S0952523807070575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molnar A, Hsueh HA, Roska B, Werblin FS. Crossover inhibition in the retina: circuitry that compensates for nonlinear rectifying synaptic transmission. J Comput Neurosci. 2009;27:569–590. doi: 10.1007/s10827-009-0170-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molnar A, Werblin F. Inhibitory feedback shapes bipolar cell responses in the rabbit retina. J Neurophysiol. 2007;98:3423–3435. doi: 10.1152/jn.00838.2007. [DOI] [PubMed] [Google Scholar]
- Muller JF, Dacheux RF. Alpha ganglion cells of the rabbit retina lose antagonistic surround responses under dark adaptation. Vis Neurosci. 1997;14:395–401. doi: 10.1017/s0952523800011512. [DOI] [PubMed] [Google Scholar]
- Murphy GJ, Rieke F. Signals and noise in an inhibitory interneuron diverge to control activity in nearby retinal ganglion cells. Nat Neurosci. 2008;11:318–326. doi: 10.1038/nn2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson R, Famiglietti EV, Jr, Kolb H. Intracellular staining reveals different levels of stratification for on- and off-center ganglion cells in cat retina. J Neurophysiol. 1978;41:472–483. doi: 10.1152/jn.1978.41.2.472. [DOI] [PubMed] [Google Scholar]
- Nicolelis MA, Lin RC, Woodward DJ, Chapin JK. Induction of immediate spatiotemporal changes in thalamic networks by peripheral block of ascending cutaneous information. Nature. 1993;361:533–536. doi: 10.1038/361533a0. [DOI] [PubMed] [Google Scholar]
- Nirenberg S, Meister M. The light response of retinal ganglion cells is truncated by a displaced amacrine circuit. Neuron. 1997;18:637–650. doi: 10.1016/s0896-6273(00)80304-9. [DOI] [PubMed] [Google Scholar]
- Nomura A, Shigemoto R, Nakamura Y, Okamoto N, Mizuno N, Nakanishi S. Developmentally regulated postsynaptic localization of a metabotropic glutamate receptor in rat rod bipolar cells. Cell. 1994;77:361–369. doi: 10.1016/0092-8674(94)90151-1. [DOI] [PubMed] [Google Scholar]
- Owczarzak MT, Pourcho RG. Transmitter-specific input to OFF-alpha ganglion cells in the cat retina. Anat Rec. 1999;255:363–373. doi: 10.1002/(SICI)1097-0185(19990801)255:4<363::AID-AR1>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Pan F, Mills SL, Massey SC. Screening of gap junction antagonists on dye coupling in the rabbit retina. Vis Neurosci. 2007;24:609–618. doi: 10.1017/S0952523807070472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peichl L. Alpha ganglion cells in mammalian retinae: common properties, species differences, and some comments on other ganglion cells. Vis Neurosci. 1991;7:155–169. doi: 10.1017/s0952523800011020. [DOI] [PubMed] [Google Scholar]
- Peichl L, Ott H, Boycott BB. Alpha ganglion cells in mammalian retinae. Proc R Soc Lond B Biol Sci. 1987;231:169–197. doi: 10.1098/rspb.1987.0040. [DOI] [PubMed] [Google Scholar]
- Peichl L, Wässle H. Morphological identification of on- and off-centre brisk transient (Y) cells in the cat retina. Proc R Soc Lond B Biol Sci. 1981;212:139–153. doi: 10.1098/rspb.1981.0030. [DOI] [PubMed] [Google Scholar]
- Pelli DG. The VideoToolbox software for visual psychophysics: transforming numbers into movies. Spat Vis. 1997;10:437–442. [PubMed] [Google Scholar]
- Rentería RC, Tian N, Cang J, Nakanishi S, Stryker MP, Copenhagen DR. Intrinsic ON responses of the retinal OFF pathway are suppressed by the ON pathway. J Neurosci. 2006;26:11857–11869. doi: 10.1523/JNEUROSCI.1718-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roska B, Molnar A, Werblin FS. Parallel processing in retinal ganglion cells: how integration of space-time patterns of excitation and inhibition form the spiking output. J Neurophysiol. 2006;95:3810–3822. doi: 10.1152/jn.00113.2006. [DOI] [PubMed] [Google Scholar]
- Roska B, Werblin F. Vertical interactions across ten parallel, stacked representations in the mammalian retina. Nature. 2001;410:583–587. doi: 10.1038/35069068. [DOI] [PubMed] [Google Scholar]
- Rotolo TC, Dacheux RF. Evidence for glycine, GABAA, and GABAB receptors on rabbit OFF-alpha ganglion cells. Vis Neurosci. 2003;20:285–296. doi: 10.1017/s0952523803203072. [DOI] [PubMed] [Google Scholar]
- Sagdullaev BT, McCall MA. Stimulus size and intensity alter fundamental receptive-field properties of mouse retinal ganglion cells in vivo. Vis Neurosci. 2005;22:649–659. doi: 10.1017/S0952523805225142. [DOI] [PubMed] [Google Scholar]
- Saito HA. Morphology of physiologically identified X-, Y-, and W-type retinal ganglion cells of the cat. J Comp Neurol. 1983;221:279–288. doi: 10.1002/cne.902210304. [DOI] [PubMed] [Google Scholar]
- Sanes JN, Suner S, Lando JF, Donoghue JP. Rapid reorganization of adult rat motor cortex somatic representation patterns after motor nerve injury. Proc Natl Acad Sci U S A. 1988;85:2003–2007. doi: 10.1073/pnas.85.6.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller PH. Central connections of the retinal ON and OFF pathways. Nature. 1982;297:580–583. doi: 10.1038/297580a0. [DOI] [PubMed] [Google Scholar]
- Shao Z, Burkhalter A. Different balance of excitation and inhibition in forward and feedback circuits of rat visual cortex. J Neurosci. 1996;16:7353–7365. doi: 10.1523/JNEUROSCI.16-22-07353.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slaughter MM, Miller RF. 2-Amino-4-phosphonobutyric acid: a new pharmacological tool for retina research. Science. 1981;211:182–185. doi: 10.1126/science.6255566. [DOI] [PubMed] [Google Scholar]
- Snyder RL, Sinex DG, McGee JD, Walsh EW. Acute spiral ganglion lesions change the tuning and tonotopic organization of cat inferior colliculus neurons. Hear Res. 2000;147:200–220. doi: 10.1016/s0378-5955(00)00132-5. [DOI] [PubMed] [Google Scholar]
- Stanford LR, Sherman SM. Structure/function relationships of retinal ganglion cells in the cat. Brain Res. 1984;297:381–386. doi: 10.1016/0006-8993(84)90580-8. [DOI] [PubMed] [Google Scholar]
- Thurlow GA, Bowling DB, Cooper RM. ON and OFF activity gradients in the lateral geniculate nucleus of the cat: a combined 14C 2-deoxyglucose and D,L-2-amino-4-phosphonobutyric acid study. Vis Neurosci. 1993;10:1027–1033. doi: 10.1017/s0952523800010130. [DOI] [PubMed] [Google Scholar]
- Turnbull BG, Rasmusson DD. Acute effects of total or partial digit denervation on raccoon somatosensory cortex. Somatosens Mot Res. 1990;7:365–389. doi: 10.3109/08990229009144714. [DOI] [PubMed] [Google Scholar]
- Vaney DI. Many diverse types of retinal neurons show tracer coupling when injected with biocytin or Neurobiotin. Neurosci Lett. 1991;125:187–190. doi: 10.1016/0304-3940(91)90024-n. [DOI] [PubMed] [Google Scholar]
- Vardi N, Morigiwa K. ON cone bipolar cells in rat express the metabotropic receptor mGluR6. Vis Neurosci. 1997;14:789–794. doi: 10.1017/s0952523800012736. [DOI] [PubMed] [Google Scholar]
- Völgyi B, Chheda S, Bloomfield SA. Tracer coupling patterns of the ganglion cell subtypes in the mouse retina. J Comp Neurol. 2009;512:664–687. doi: 10.1002/cne.21912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Völgyi B, Deans MR, Paul DL, Bloomfield SA. Convergence and segregation of the multiple rod pathways in mammalian retina. J Neurosci. 2004;24:11182–11192. doi: 10.1523/JNEUROSCI.3096-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Völgyi B, Xin D, Bloomfield SA. Feedback inhibition in the inner plexiform layer underlies the surround-mediated responses of AII amacrine cells in the mammalian retina. J Physiol. 2002;539:603–614. doi: 10.1113/jphysiol.2001.013133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P, Slaughter MM. Effects of GABA receptor antagonists on retinal glycine receptors and on homomeric glycine receptor α subunits. J Neurophysiol. 2005;93:3120–3126. doi: 10.1152/jn.01228.2004. [DOI] [PubMed] [Google Scholar]
- Wässle H. Parallel processing in the mammalian retina. Nat Rev Neurosci. 2004;5:747–757. doi: 10.1038/nrn1497. [DOI] [PubMed] [Google Scholar]
- Wässle H, Koulen P, Brandstatter JH, Fletcher EL, Becker CM. Glycine and GABA receptors in the mammalian retina. Vision Res. 1998;38:1411–1430. doi: 10.1016/s0042-6989(97)00300-3. [DOI] [PubMed] [Google Scholar]
- Wässle H, Levick WR, Cleland BG. The distribution of the alpha type of ganglion cells in the cat's retina. J Comp Neurol. 1975;159:419–438. doi: 10.1002/cne.901590308. [DOI] [PubMed] [Google Scholar]
- Wässle H, Peichl L, Boycott BB. Morphology and topography of on- and off-alpha cells in the cat retina. Proc R Soc Lond B Biol Sci. 1981;212:157–175. doi: 10.1098/rspb.1981.0032. [DOI] [PubMed] [Google Scholar]
- Wellman CL, Arnold LL, Garman EE, Garraghty PE. Acute reductions in GABAA receptor binding in layer IV of adult primate somatosensory cortex after peripheral nerve injury. Brain Res. 2002;954:68–72. doi: 10.1016/s0006-8993(02)03343-7. [DOI] [PubMed] [Google Scholar]
- Werblin FS. Six different roles for crossover inhibition in the retina: correcting the nonlinearities of synaptic transmission. Vis Neurosci. 2010;27:1–8. doi: 10.1017/S0952523810000076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werblin FS, Dowling JE. Organization of the retina of the mudpuppy, Necturus maculosus. II. Intracellular recording. J Neurophysiol. 1969;32:339–355. doi: 10.1152/jn.1969.32.3.339. [DOI] [PubMed] [Google Scholar]
- Xin D, Bloomfield SA. Tracer coupling pattern of amacrine and ganglion cells in the rabbit retina. J Comp Neurol. 1997;383:512–528. doi: 10.1002/(sici)1096-9861(19970714)383:4<512::aid-cne8>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Zaghloul KA, Boahen K, Demb JB. Different circuits for ON and OFF retinal ganglion cells cause different contrast sensitivities. J Neurosci. 2003;23:2645–2654. doi: 10.1523/JNEUROSCI.23-07-02645.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou C, Dacheux RF. Glycine- and GABA-activated inhibitory currents on axon terminals of rabbit cone bipolar cells. Vis Neurosci. 2005;22:759–767. doi: 10.1017/S095252380522607X. [DOI] [PubMed] [Google Scholar]


