Abstract
Zinc is concentrated in the hippocampus, particularly in the mossy fiber axons of the dentate gyrus, and has been hypothesized to be important in neurodegeneration and epilepsy. Previous studies have suggested that activity-dependent release of zinc from reorganized mossy fibers leads to collapse of granule-cell inhibition. Synaptically released zinc has been proposed to depress the function of the new “epileptic” GABAA receptors, which have subunits that are zinc-sensitive. Recent experiments by Molnar and Nadler have replicated the previous data, and further tested this hypothesis. Their work suggests that activated mossy fibers in hippocampal slices do not release adequate zinc to depress GABAA receptor function at nearby inhibitory synapses. These studies point to the complexity of this hypothesis, particularly in regard to zinc release in vitro versus in vivo and the diffusion of zinc in the extracellular space.
Introduction
The observation that zinc (Zn2+) is present at discrete sites in the nervous system, and that it may influence specific physiological mechanisms, has led to interesting speculations on its role in neural function and neurological disorders. Because zinc is highly concentrated in the hippocampus 1, particularly in the mossy fibers of the dentate gyrus, it has been proposed to play a role in hippocampal processing.
In vitro studies suggest that zinc modulates some of the receptors that participate in synaptic transmission 2. A series of reports have suggested that zinc depresses specific GABAA receptors in dentate granule cells from epileptic tissue, but not from normal tissue 3, 4, 5. This finding is of interest because it represents a potential mechanism of activity-dependent decrease in inhibition, which could contribute to the generation of seizures in the dentate gyrus.
Recently, Molnar and Nadler 6 replicated the zinc-induced modulation of GABAA-receptor function using exogenous zinc in solutions without polyvalent anions, and then further assessed the impact of activity-dependent zinc release on inhibition in the dentate gyrus. Their results suggest that strong electrical activation of mossy fibers in hippocampal slices bathed with normal medium does not induce a measurable decrease in GABAA-receptor-mediated inhibition. This article reviews the background on zinc and the hippocampus, and discusses the physiological components of the zinc-disinhibition hypothesis. It seems clear, however, that additional studies will be needed to evaluate whether the hypothesis remains tenable, and whether it represents an important mechanism of epileptogenesis.
The Distribution of Zinc in the Nervous System
Zinc is present in many areas of the nervous system, and in particular is concentrated in specific sites of the hippocampus 1. The use of the Timm stain, which serves as a marker of heavy metals including zinc, has facilitated analyses of the distribution of the zinc-rich axons in structures such as the hippocampus. The zinc in the mossy fibers of the dentate gyrus, which are the axons of the granule cells, has attracted the attention of many epileptologists. Several studies have suggested that zinc is released from the mossy fibers, particularly with intense electrical activity, as occurs during repeated seizures 7, 8, 9, 10, 11, 12. Thus, the question of how synaptically released zinc might alter neural function is an interesting issue, particularly because zinc has been hypothesized to be neurotoxic at high concentrations, and zinc-containing pathways undergo synaptic reorganization in temporal lobe epilepsy.
Neurodegeneration and Zinc
Release of zinc during intense synaptic excitation has been hypothesized to induce neurodegeneration 13, 14. Zinc is present with glutamate in synaptic vesicles, but it has not been found with GABA. Several lines of evidence suggest that co-release of zinc and glutamate during excessive activity of excitatory synapses leads to selective loss of neurons that have been shown to accumulate zinc. Although somewhat peripheral to the specific issue under consideration here, an important question is also whether zinc contributes to neuronal death after repeated seizures.
Modulation of Synaptic Receptors by Zinc
In vitro experiments, using either isolated neurons or brain-slice preparations, have shown that zinc can have a myriad of effects on both excitatory and inhibitory synapses and on several neuromodulatory systems. The effect of zinc on different types of glutamate and GABA receptors has attracted a great deal of attention in the last few years. Modulation of AMPA, NMDA, and GABAA receptors by zinc has been used to characterize their subunit composition 2. For example, zinc has been proposed to augment glutamate-receptor function via a reduction of desensitization of AMPA receptors 15. More relevant to the present issue, however, is the idea that the subunit composition of GABAA receptors determines the zinc sensitivity 2, 16.
The Hypothesis of Zinc-induced Collapse of Inhibition and Epilepsy
Zinc and GABA Receptors
Recent interest has been directed at the hypothesis that zinc, co-released with glutamate from mossy fiber terminals after synaptic reorganization, leads to a zinc-induced collapse of GABA-mediated inhibition of dentate granule cells during chronic epilepsy 3. This hypothesis provides a mechanism of activity-dependent disinhibition, which is a means by which seizure activity may be initiated or spread. If intense mossy fiber activation results in the release of large amounts of glutamate and zinc in a short period of time, then the zinc may diffuse from the mossy fiber synapse to block nearby GABAergic synapses (Fig. 1). This mechanism might be particularly effective at disinhibiting the dentate gyrus in chronically epileptic tissue, because zinc-containing mossy fibers of the dentate granule cells have been observed to sprout dense axon collaterals that return to the inner molecular layer of the dentate gyrus in both epileptic humans 17, 18, 19, 20 and animals 21, 22, 23, 24. If the sprouted mossy fibers synapse on the dendrites of either granule cells or interneurons, robust granule cell activation would cause the sprouted mossy fibers to release zinc that could block local GABA synapses that mediate feedforward inhibition, thus disinhibiting the dentate network.
FIGURE 1.
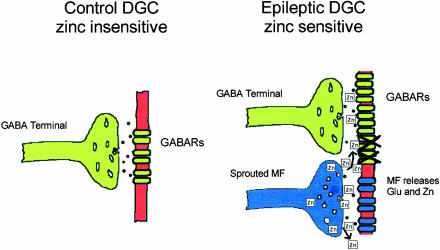
Hypothesis of zinc-induced collapse of inhibition after mossy fiber reorganization. In control dentate granule cells (DGCs), the GABA receptors (GABARs) are insensitive to zinc (left). In DGCs from tissue of animals or humans with temporal lobe epilepsy, the subsynaptic GABARs are hypothetically altered and inhibition is enhanced (right). One hypothetical mechanism for the altered inhibition is an epilepsy-associated change in the subunit composition of the GABARs, which results in new receptors that are sensitive to zinc. The reorganized mossy fiber (MF) terminals release zinc that can hypothetically block the new GABARs, particularly during intense activity (i.e., seizures). Modified with permission from Coulter 26.
Experiments by Buhl et al. 3 in hippocampal slices from kindled rats and by Gibbs et al. 4 in isolated dentate granule cells from pilocarpine-treated rats with spontaneous seizures have supported this hypothesis. These studies demonstrated that bath application of zinc depresses inhibitory postsynaptic currents (IPSCs) and responses to exogenous GABA, respectively. Further, the subunit composition of GABAA receptors on granule cells in the dentate gyrus was demonstrated to be primarily insensitive to zinc in normal tissue, but to be predominantly zinc-sensitive in epileptic tissue 4, 5, 25, 26. Thus, the zinc-mediated disinhibition of the dentate gyrus would be amplified in epileptic tissue both by the presence of a local source of zinc (the sprouted mossy fibers) and by the presence of zinc-sensitive GABAA receptors.
Zinc-Induced Collapse of Inhibition and Recurrent Excitation
Coulter et al. 25, 26 elaborated on the hypothesis of a zinc-induced collapse of inhibition by postulating that the activity-dependent reduction of inhibition might also increase the effectiveness of new recurrent excitatory circuits arising from mossy fiber sprouting 27. Several studies have provided converging evidence that depression of GABAA-receptor-mediated inhibition “unmasks” the recurrent excitatory circuits associated with mossy fiber sprouting 28, 29, 30, 31, 32. This concept derives from the observation that GABAA-receptor blockers reveal excitatory synaptic events and can induce epileptiform activity in preparations with mossy fiber sprouting, yet have comparatively little effect on normal tissue. This in turn is based on research on the local synaptic circuits of CA3 pyramidal cells from normal animals 33, 34, 35, 36, which suggest that GABA-mediated inhibition essentially shunts the effects of recurrent excitation. Elevation of extracellular potassium, which secondarily also depresses GABAA-mediated inhibition, is possibly even more effective at augmenting the effects of the new recurrent excitatory circuits 31, 32, 37, 38. Therefore, the hypothetical zinc-induced depression of inhibition would possibly augment the functional expression of these new excitatory circuits.
Recent Experiments Testing the Hypothesis
Molnar and Nadler 6 have tested the effect of zinc released from sprouted mossy fibers on granule-cell GABAA receptors in the pilocarpine model of temporal lobe epilepsy. In agreement with prior studies, exogenously applied zinc depressed GABAA-receptor-mediated currents in granule cells. The authors found that 200 μM zinc reduced the amplitude of muscimol-induced currents applied to the proximal portion of the granule cell dendrite, and also reduced the mean amplitude and frequency of miniature inhibitory postsynaptic currents (mIPSCs). Thus, the authors independently corroborated earlier findings concerning the effects of zinc on GABAA-receptor-mediated currents. However, this effect depended heavily on removal of polyvalent anions from the superfusion medium, and repetitive stimulation of the mossy fibers did not alter the currents evoked by photolysis of caged GABA on the proximal dendrites of the granule cells. In the Discussion, Molnar and Nadler 6 reported that they had replicated the results of Vogt et al. 39 in which electrical stimulation depressed the NMDA-receptor-mediated component of the mossy fiber synaptic response recorded in the CA3 area, which is considered to be a consequence of zinc blockade of the NMDA receptor. In a subsequent paper, Molnar and Nadler 40 found that calcium disodium EDTA, a high affinity membrane impermeant zinc chelator, significantly increased the size of the NMDA component of the recurrent mossy fiber EPSC. This treatment, however, did not significantly alter the non-NMDA receptor-mediated component of the recurrent mossy fiber EPSC, nor did it alter the NMDA-receptor-mediated component of the perforant path EPSC. Molnar and Nadler 6, 40 concluded that the inability of mossy fiber stimulation to diminish the response to photolysis of caged GABA did not result from a failure of the stimulation to release zinc. Their recent study 40 suggests that release of zinc from sprouted mossy fibers depresses the NMDA-mediated component of the recurrent excitation from mossy fibers. Thus, Molnar and Nadler 40 propose that zinc from sprouted mossy fibers may depress seizures in the dentate gyrus.
As outlined by Molnar and Nadler 6, the hypothesis that zinc release from mossy fiber terminals depresses GABAA receptors requires that zinc diffuse from the mossy fiber synapse at an adequate concentration to depress the activation of GABAA receptors at adjacent but different synapses. These authors emphasize that at least two mechanisms serve as impediments to the diffusion of zinc from the synaptic cleft of the mossy fibers to the GABAA receptors: 1 zinc transporters on the surface of the mossy fiber terminal, and 2 the presence of polyvalent anions in the medium that bind zinc. Thus, Molnar and Nadler 6 suggest that zinc is bound to molecules that essentially make it unavailable for regulating GABAA receptors distant from the synaptic site.
Molnar and Nadler 6 provide at least two limitations to their experiments. First, they did not activate the entire recurrent excitatory pathway from the mossy fibers back to the granule cells, as might be expected to occur during an electrographic seizure in an intact animal. However, electrical stimulation of the mossy fibers has been shown in several studies 29, 31, 41 to evoke excitatory synaptic postsynaptic potentials and currents in granule cells. The second possible limitation is that the laser-evoked photolysis of caged GABA would activate both synaptic and extrasynaptic GABAA receptors. Although this latter methodological problem is an issue, some of the previous studies that led to the hypothesis used bath application of GABA, which has the same limitation.
A final limitation relates to the amount of zinc that can be released in brain slice preparations. The preparation of brain slices causes loss of zinc, and low temperature depresses the synaptic release of the remainder of the zinc 42. Most of the experiments of Molnar and Nadler 6 were performed at room temperature, although some were conducted at 33°C. Molnar and Nadler 6 also attempted to replenish any zinc that may have been lost during slice preparation; nonetheless, it is possible that the amount of zinc released in their stimulation experiments was below what occurs in the intact brain.
Conclusions
The experiments of Molnar and Nadler 6 emphasize the potential complexity surrounding the hypothesis that zinc modulates GABAergic synapses under physiological or pathophysiological conditions. The critical question addressed in the work of Molnar and Nadler 6 is whether zinc is actually free in solution to alter the properties of the GABAA receptors that are present at different synapses. Several studies have suggested that the sprouted mossy fibers synapse on GABAergic interneurons 3, 29, 43, 44. Therefore, another important issue is whether zinc induces a collapse of inhibition onto inhibitory interneurons that may be zinc sensitive 45, which might counteract the proposed effect on granule cells. Although zinc is present in many neurons, the highest concentration is in the hippocampus, and particularly the mossy fibers. Thus, the present hypothesis is directed primarily at the dentate gyrus, which may not be uniquely critical for epileptogenesis and seizures. The hypothesis implies that release of zinc depends on powerful high-frequency activation of the mossy fibers, so if this mechanism is viable, it may be important at augmenting the seizure rather than actually generating it. The recent work of Molnar and Nadler 40, however, suggests that zinc depresses the NMDA-receptor component of recurrent excitation, and may thus reduce seizure activity in the dentate gyrus.
The hypothesis of zinc-induced collapse of inhibition in the dentate gyrus remains an interesting and potentially important issue for epilepsy researchers. Future work will be required to determine whether the hypothesis should be rejected on the basis of the results presented by Molnar and Nadler 6, or whether these recent data represent a “false negative” regarding an important mechanism for temporal lobe epilepsy.
Acknowledgments
Drs. D. Coulter, I. Mody, V. Nadler, K. Partin, K. Staley, and P. Williams kindly provided helpful comments on a previous draft.
References
- 1.Fredrickson CJ, Danscher G. Zinc containing neurons in hippocampus and related CNS structures. Prog. Brain Res 1990;83:71–74. [DOI] [PubMed] [Google Scholar]
- 2.Macdonald RL, Kapur J. Pharmacological properties of recombinant and dentate granule cell GABAA receptors. Adv. Neurol 1999;79:979–990. [PubMed] [Google Scholar]
- 3.Buhl EH, Otis TS, Mody I. Zinc-induced collapse of augmented inhibition by GABA in a temporal lobe epilepsy model. Science 1996;271:369–373. [DOI] [PubMed] [Google Scholar]
- 4.Gibbs JW, III, Shumate MD, Coulter DA. Differential epilepsy-associated alterations in postsynaptic GABAA receptor function in dentate granule and CA1 neurons. J Neurophysiol 1997;77:1924–1938. [DOI] [PubMed] [Google Scholar]
- 5.Shumate MD, Lin DD, Gibbs JW, III, Holloway KL, Coulter DA. GABAA receptor function in epileptic human dentate granule cells: comparison to epileptic and control rat. Epilepsy Res 1998;32:114–128. [DOI] [PubMed] [Google Scholar]
- 6.Molnar P, Nadler JV. Lack of effect of mossy fiber-released zinc on granule cell GABAA receptors in the pilocarpine model of epilepsy. J. Neurophysiol 2001;85:1932–1940. [DOI] [PubMed] [Google Scholar]
- 7.Aniksztein L, Charton G, Ben-Ari Y. Selective release of endogenous zinc from the hippocampal mossy fibers in situ. Brain Res 1987;404:58–64. [DOI] [PubMed] [Google Scholar]
- 8.Assaf SY, Chung S-H. Release of endogenous Zn2+ from brain tissue during activity. Nature 1984;308:734–735. [DOI] [PubMed] [Google Scholar]
- 9.Budde T, Minta A, White JA, Kay AR. Imaging free zinc in synaptic terminals in live hippocampal slices. Neuroscience 1997;79:347–358. [DOI] [PubMed] [Google Scholar]
- 10.Fredrickson CJ, Hernandez MD, Goik SA, Morton JD, McGinty JF. Loss of zinc staining from mossy fibers during kainic acid induced seizures a histofluorescence study. Brain Res 1988;446:383–386. [DOI] [PubMed] [Google Scholar]
- 11.Howell GA, Welch MG, Fredrickson CJ. Stimulation-induced uptake and release of zinc in hippocampal slices. Nature 1984;308:736–738. [DOI] [PubMed] [Google Scholar]
- 12.Takeda A, Hanajima T, Ijiro H, Ishige A, Iizuka S, Okada S, Oku N. Release of zinc from the brain of E1 (epilepsy) mice during seizure induction. Brain Res 1999;828:174–178. [DOI] [PubMed] [Google Scholar]
- 13.Choi DW, Koh JS. Zinc and brain injury. Ann Rev Neurosci 1998;21:347–375. [DOI] [PubMed] [Google Scholar]
- 14.Weiss JH, Sensi SL, Koh JY. Zn2+: a novel ionic mediator of neural injury in brain disease. Trends Pharmacol Sci 2000;21:395–401. [DOI] [PubMed] [Google Scholar]
- 15.Lin DD, Cohen AS, Coulter DA. Zinc-induced augmentation of excitatory synaptic currents and glutamate receptor responses in hippocampal CA3 neurons. J Neurophysiol 2001;85:1185–1196. [DOI] [PubMed] [Google Scholar]
- 16.Macdonald RL, Olsen RW. GABAA receptor channels. Ann Rev Neurosci 1994; 17:569–602. [DOI] [PubMed] [Google Scholar]
- 17.Babb TL, Kupfer WR, Pretorius JK, Crandall PH, Levesque MF. Synaptic reorganization by mossy fibers in human epileptic fascia dentate. Neuroscience 1991;42:351–363. [DOI] [PubMed] [Google Scholar]
- 18.de Lanerolle NC, Kim JH, Robbins RJ, Spencer DD. Hippocampal interneuron loss and plasticity in human temporal lobe epilepsy. Brain Res 1989;495:387–395. [DOI] [PubMed] [Google Scholar]
- 19.Houser CR, Miyashiro JE, Swartz BE, Walsh GO, Rich JR, Delgado-Escueta AV. Altered patterns of dynorphin immunoreactivity suggest mossy fiber reorganization in human hippocampal epilepsy. J. Neurosci 1990;10:267–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sutula T, Cascino G, Cavazos J, Parada I,. Ramirez L. Mossy fiber synaptic reorganization in the epileptic human temporal lobe. Ann Neurol 1989;26:321–330. [DOI] [PubMed] [Google Scholar]
- 21.Ben-Ari Y. Limbic seizures and brain damage produced by kainic acid: Mechanisms and relevance to human temporal lobe epilepsy. Neuroscience 1985;14:375–403. [DOI] [PubMed] [Google Scholar]
- 22.Cavazos JE, Das I, Sutula TP. Neuronal loss induced in limbic pathways by kindling: evidence for induction of hippocampal sclerosis by repeated brief seizures. J. Neurosci 1994;14:3106–3121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nadler JV. Kainic acid as a tool for the study of temporal lobe epilepsy. Life Sci 1981;29:2031–2042. [DOI] [PubMed] [Google Scholar]
- 24.Nadler JV, Perry BW, Cotman CW. Selective reinnervation of hippocampal area CA1 and the fascia dentata after destruction of CA3-CA4 afferents with kainic acid. Brain Res 1980;182:1–9. [DOI] [PubMed] [Google Scholar]
- 25.Coulter DA. Chronic epileptogenic cellular alterations in the limbic system after status epilepticus. Epilepsia 1999;40:S23–33. [DOI] [PubMed] [Google Scholar]
- 26.Coulter DA. Mossy fiber zinc and temporal lobe epilepsy: pathological association with altered “epileptic” gamma-aminobutyric acid A receptors in dentate granule cells. Epilepsia 2000;41:S96–99. [DOI] [PubMed] [Google Scholar]
- 27.Tauck DL, Nadler JV. Evidence of functional mossy fiber sprouting in hippocampal formation of kainic acid-treated rats. J Neurosci 1985;5:1016–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Buckmaster PS, Dudek FE. Network properties of the dentate gyrus in epileptic rats with hilar neuron loss and granule cell axon reorganization. J Neurophysiol 1997. a;77:2685–2696. [DOI] [PubMed] [Google Scholar]
- 29.Cronin J, Obenaus A, Houser CR, Dudek FE. Electrophysiology of dentate granule cells after kainate-induced synaptic reorganization of the mossy fibers. Brain Res 1992;573:305–310. [DOI] [PubMed] [Google Scholar]
- 30.Lynch M, Sutula T. Recurrent excitatory connectivity in the dentate gyrus of kindled and kainic acid-treated rats. J Neurophysiol 1996;83:693–704. [DOI] [PubMed] [Google Scholar]
- 31.Wuarin J-P, Dudek FE. Electrographic seizures and new recurrent excitatory circuits in the dentate gyrus of hippocampal slices from kainate-treated epileptic rats. J Neurosci 1996;16:4438–4448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wuarin J-P, Dudek FE. Excitatory synaptic input to granule cells increases with time after kainate treatment. J Neurophysiol 2001;85:1067–1077. [DOI] [PubMed] [Google Scholar]
- 33.Christian EP, Dudek FE. Characteristics of local excitatory circuits studied with glutamate microapplication in the CA3 area of rat hippocampal slices. J Neurophysiol 1988;59:90–109. [DOI] [PubMed] [Google Scholar]
- 34.Miles R, Wong RKS. Excitatory synaptic interactions between CA3 neurones in the guinea-pig hippocampus. J Physiol 1986;373:397–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miles R, Wong RKS. Inhibitory control of local excitatory circuits in the guinea-pig hippocampus. J Physiol 1987;388:611–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Traub RD, Wong RKS. Cellular mechanism of neuronal synchronization in epilepsy. Science 1982;216:745–747. [DOI] [PubMed] [Google Scholar]
- 37.Hardison JL, Okazaki MM, Nadler JV. Modest increase in extracellular potassium unmasks effect of recurrent mossy fiber growth. J Neurophysiol 2000;84:2380–2389. [DOI] [PubMed] [Google Scholar]
- 38.Patrylo PR, Dudek FE. Physiological unmasking of new glutamatergic pathways in the dentate gyrus of hippocampal slices from kainate-induced epileptic rats. J Neurophysiol 1998;79:418–429. [DOI] [PubMed] [Google Scholar]
- 39.Vogt K, Mellor J, Tong G, Nicoll R. The actions of synaptically released zinc at hippocampal mossy fiber synapses. Neuron 2000;26:187–196. [DOI] [PubMed] [Google Scholar]
- 40.Molnar P, Nadler JV. Synaptically-released zinc inhibits N-methyl-D-aspartate receptor activation at recurrent mossy fiber synapses. Brain Res 2001;910:205–207. [DOI] [PubMed] [Google Scholar]
- 41.Okazaki MM, Molnar P, Nadler JV. Recurrent mossy fiber pathway in rat dentate gyrus: synaptic currents evoked in presence and absence of seizure-induced growth. J Neurophysiol 1999;81:1645–1660. [DOI] [PubMed] [Google Scholar]
- 42.Suh SW, Danscher G, Jensen MS, Thompson R, Motamedi M, Frederickson CJ. Release of synaptic zinc is substantially depressed by conventional brain slice preparations. Brain Res 2000;879:7–12. [DOI] [PubMed] [Google Scholar]
- 43.Kotti T, Riekkinen PJ, Sr, Miettinen R. Characterization of target cells for aberrant mossy fiber collaterals in the dentate gyrus of epileptic rat. Exp Neurol 1997;146:323–330. [DOI] [PubMed] [Google Scholar]
- 44.Sloviter RS. Possible functional consequences of synaptic reorganization in the dentate gyrus of kainate-treated rats. Neurosci Lett 1992;137:91–96. [DOI] [PubMed] [Google Scholar]
- 45.Berger T, Schwarz C, Kraushaar U, Monyer H. Dentate gyrus basket cell GABAA receptors are blocked by Zn2+ via changes of their desensitization kinetics: an in situ patch-clamp and single-cell PCR study. J Neurosci 1998;18:2437–2448. [DOI] [PMC free article] [PubMed] [Google Scholar]


