Non-technical summary
Advancing age is a major risk factor for the development of cardiovascular disease. A key characteristic of older arteries that may lead to cardiovascular disease is reduced endothelial function, characterized by blunted endothelium-dependent dilatation. Sirtuins, specifically sirtuin-1, are proteins linked to increases in lifespan and lower incidence of age-related diseases. We hypothesized that diminished sirtuin-1 with advancing age may alter regulation of a key endothelium dilatory enzyme, nitric oxide synthase. Our findings provide novel translational evidence that sirtuin-1 expression and activity contribute to arterial endothelial dysfunction with ageing and that this may be due to altered nitric oxide synthase activation. Importantly, our results provide further compelling support for sirtuin-1 as a potential therapeutic target for lifestyle and pharmacological interventions aimed at the prevention and treatment of arterial ageing and age-associated cardiovascular diseases.
Abstract
Abstract
We tested the hypothesis that reductions in the cellular deacetylase, sirtuin-1 (SIRT-1), contribute to vascular endothelial dysfunction with ageing via modulation of endothelial nitric oxide synthase (eNOS) acetylation/activation-associated nitric oxide (NO) production. In older (30 months, n = 14) vs. young (5–7 months, n = 16) B6D2F1 mice, aortic protein expression of SIRT-1 and eNOS phosphorylated at serine 1177 were lower (both P < 0.05), and acetylated eNOS was 6-fold higher (P < 0.05), whereas total eNOS did not differ (P = 0.65). Acetylcholine (ACh)-induced peak endothelium-dependent dilatation (EDD) was lower in isolated femoral arteries with ageing (P < 0.001). Incubation with sirtinol, a SIRT-1 inhibitor, reduced EDD in both young and older mice, abolishing age-related differences, whereas co-administration with l-NAME, an eNOS inhibitor, further reduced EDD similarly in both groups. Endothelium-independent dilatation to sodium nitroprusside (EID), was not altered by age or sirtinol treatment. In older (64 ± 1 years, n = 22) vs. young (25 ± 1 years, n = 16) healthy humans, ACh-induced forearm EDD was impaired (P = 0.01) and SIRT-1 protein expression was 37% lower in endothelial cells obtained from the brachial artery (P < 0.05), whereas EID did not differ. In the overall group, EDD was positively related to endothelial cell SIRT-1 protein expression (r = 0.44, P < 0.01). Reductions in SIRT-1 may play an important role in vascular endothelial dysfunction with ageing. SIRT-1 may be a key therapeutic target to treat arterial ageing.
Introduction
Arterial endothelial function declines with advancing age, as indicated by a reduction in endothelium-dependent dilatation (EDD) with ageing in humans and animals (Celermajer et al. 1994; Taddei et al. 1996; Spier et al. 2004; Lesniewski et al. 2009; Seals et al. 2011). The age-related decline in EDD is predictive of future cardiovascular events in older adults without cardiovascular disease (Yeboah et al. 2007; Lind et al. 2011). The decrease in EDD with ageing is mediated by reduced bioavailability of nitric oxide (NO) (van der Loo et al. 2000; Taddei et al. 2001), a dilating molecule produced by the enzyme endothelial NO synthase (eNOS) (Fleming & Busse, 1999). As such, understanding the mechanisms regulating eNOS and NO-mediated EDD with ageing has important implications for age-associated cardiovascular disease.
The sirtuin family of NAD+-dependent protein deacetylases and ADP-ribosyltransferases are involved in the physiological responses to altered energy metabolism and stress (Cohen et al. 2004; Guarente, 2006; Dali-Youcef et al. 2007). In mammals, sirtuins (SIRT) 1–4 are observed in multiple tissues. SIRT-1 is expressed in the nucleus and cytoplasm, whereas SIRT-2, 3 and 4 are predominantly expressed in mitochondria (Guarente, 2006; Whittle et al. 2007; Rodgers et al. 2008). Arterial SIRT-1 expression is reduced in older rodents (Ungvari et al. 2008; Rippe et al. 2010), but no clear mechanistic link with endothelial dysfunction has been established. Recent evidence suggests that reductions in SIRT-1 deacetylation of eNOS can impair NO-dependent EDD (Mattagajasingh et al. 2007).
We tested the hypothesis that reductions in SIRT-1 contribute to impaired NO-mediated EDD with ageing. To do so, we used a translational approach to elucidate the role of SIRT-1 in age-related endothelial dysfunction. We first sought to establish that inhibiting SIRT-1 activity in excised murine arteries would eliminate age-related reductions in NO-dependent EDD. We then determined if impaired EDD with ageing is associated with reduced SIRT-1 expression in endothelial cells obtained from peripheral arteries of humans.
Methods
Experiments in young and older B6D2F1 mice
Animals
Sixteen young (5–7 months) and 14 older (30 months) male B6D2F1 mice were obtained from the National Institute on Aging rodent colony. All mice were housed in an animal care facility at the University of Colorado at Boulder on a 12:12 light–dark cycle and fed standard rodent chow ad libitum. All animal procedures conformed to the Guide to the Care and Use of Laboratory Animals (NIH publication no. 85–23, revised 1996), conform to the principles of UK regulations as described by Drummond (2009) and were approved by the University of Colorado at Boulder and University of Utah Animal Care and Use Committees.
EDD and endothelium-independent dilatation (EID)
Measures of EDD and EID were made in isolated vessels studied ex vivo as described previously (Donato et al. 2005, 2007b, 2009; Lesniewski et al. 2009, 2011). Mice were killed by exsanguinations via cardiac puncture while under inhaled isoflurane anesthesia (1–5%). Femoral arteries were excised and placed in myograph chambers (DMT Inc., Aarhus, Denmark) containing EDTA-buffered physiological saline solution (PSS), cannulated onto glass micropipettes and secured with nylon (11–0) suture. Once cannulated, the femoral arteries were warmed to 37°C and pressurized to 50 mmHg intraluminal pressure and allowed to equilibrate for 1 h. All arteries then were submaximally preconstricted with phenylephrine (2 μm). Increases in luminal diameter in response to increasing concentrations of the endothelium-dependent dilator acetylcholine (Ach; 1 × 10−9 to 1 × 10−4m) and endothelium-independent dilator sodium nitroprusside (SNP; 1 × 10−10 to 1 × 10−4m) were determined. To assess the influence of SIRT-1, dose responses to ACh were repeated in the presence of the sirtinol, a SIRT-1 antagonist (10−4m, 60 min incubation), (Ota et al. 2008, 2010; Zhang et al. 2008). These concentrations have been established to increase acetylated eNOS in vitro (Ota et al. 2010). To determine the contribution of NO to dilatation, responses to ACh and sirtinol were repeated in the presence of NG-nitro-l-arginine methyl ester (l-NAME) (10−4m, 30 min incubation) (Durrant et al. 2009; Lesniewski et al. 2009).
Arterial protein expression
The thoracic aorta was excised, cleared of surrounding tissues while maintained in 4°C PSS, and frozen in liquid nitrogen. Whole artery lysates were prepared as previously described (Durrant et al. 2009; Lesniewski et al. 2009; Lesniewski et al. 2011). For measures of protein expression in aortic lysates, 15 μg of protein with 2 mol l−1 dithiothreitol were loaded into polyacrylamide gels, separated by electrophoresis and transferred onto a nitrocellulose membrane. The membrane was blocked in 5% non-fat dry milk in Tris-buffered saline with 0.05% Tween (TBS-T) overnight at 4°C. After blocking, the membrane was washed with TBS-T and incubated overnight at 4°C in primary antibody. eNOS (1:1000; 140 kDa; BD Biosciences, Franklin Lakes, NJ, USA), Ser1177-phosphorylated eNOS (peNOS, 1:1000; 140 kDa; Cell Signaling Technology, Inc., Danvers, MA, USA) and SIRT-1 (1:1000; 110 kDa; Abcam Inc., Cambridge, MA, USA) abundance were measured by standard Western blotting techniques using an HRP-conjugated secondary antibody (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA, USA) and Supersignal ECL (Pierce, Rockford, IL, USA). Bands were visualized using a digital acquisition system (ChemiDoc-It, UVP, Upland, CA, USA) and quantified using ImageJ software (NIH). Expression is presented normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH 1:1000; 37 kDa; Cell Signaling), to account for differences in protein loading. The quantifications of total protein were normalized to GAPDH of the same band after stripping and re-probing, and are expressed relative to the mean of the young control bands on a given blot. The ratio of phoshorylated to total protein was calculated from the bands for a given sample lysate run on the two gels/membranes, with each normalized to its own GAPDH. These data also are expressed as a ratio of the mean of the young control group within a given set of lysates.
eNOS acetylation expression
To determine acetylation state of eNOS for which there is no commercially available acetylated specific antibody, acetyl-lysine (1:1000 crosslinked to DYNAL (Invitrogen) magnetic beads for extraction) was immunoprecipitated from 20 μg of aortic protein lysate and the association of the acetyl-lysine with eNOS was determined by immunoblotting (as described above) with the primary antibody for eNOS (eNOS, 1:1000; 140 kDa; BD Biosciences).
Experiments in human subjects
Subjects
Data were obtained on 38 healthy men and women: 16 young (aged 18–30 years) and 22 older (59–76 years). All subjects had resting blood pressure less than 140/90 mmHg, body mass index less than 30 kg m−2, and were free of cardiovascular diseases (CVD), diabetes, and other clinical disorders as assessed by medical history, physical examination, and blood chemistries. Subjects greater than 40 years of age were further screened for CVD using electrocardiogram and blood pressure responses to incremental treadmill exercise performed to volitional exhaustion. Subjects were non-smokers, not taking dietary supplements, and not regularly exercising. All procedures were approved by the Human Research Committee of the University of Colorado at Boulder. The nature, benefits, and risks of the study were explained to the volunteers and their written informed consent was obtained prior to participation.
Study procedures
All measurements were performed at the University of Colorado at Boulder Clinical and Translational Research Center after an overnight fast and a 24 h abstention from alcohol and physical activity.
Subject characteristics
Body mass, body mass index, resting arterial blood pressure, fasting blood chemistries, habitual physical activity, oxidized low-density lipoprotein (LDL), total antioxidant status and C reactive protein were measured as previously described (Moreau et al. 2005; Donato et al. 2007a).
EDD and endothelium-independent dilatation
EDD and endothelium-independent dilatation were determined as the peak forearm blood flow (measured by venous occlusion plethysmography) responses to an incremental intra-brachial artery infusion of ACh (1.0, 2.0, 4.0 and 8.0 μg (dl forearm tissue)−1 min−1) and SNP (0.5, 1 and 2.0 μg (dl forearm tissue)−1 min−1), respectively, as described previously (Donato et al. 2008b, 2009; Pierce et al. 2008).
Endothelial cell protein expression
The procedures used for collection of endothelial cells and measurement of protein expression were originally described by Feng et al. (1999) and Colombo et al. (2002), and more recently by our laboratory (Eskurza et al. 2006; Gates et al. 2007; Silver et al. 2007; Donato et al. 2007a, 2008a, 2009). Briefly, J-wires were advanced into a brachial artery ∼4 cm beyond the tip of the catheter and withdrawn, and cells were recovered by washing and centrifugation. Collected cells were fixed with 3.7% formaldehyde, plated on slides and stored at –80°C.
For immunofluorescence staining, two control cultured human umbilical vein endothelial cell (HUVEC: passage 6–9 processed identically to the human ECs) slides and eight subject slides were selected (balanced for age) for each staining batch. After blocking non-specific binding sites with 5% donkey serum (Jackson Immunoresearch, West Grove, PA, USA), cells were incubated with monoclonal antibody against SIRT-1 (1:150; Abcam, Cambridge, MA, USA) and a specific AlexaFlour555-conjugated secondary antibody (Research Diagnostics, Acton, MA, USA). Next, cells were incubated with vWF (von Willebrand factor; 1:1000; Dako, Carpinteria, CA, USA) and a specific AlexaFlour488-conjugated secondary antibody (Research Diagnostics). Slides were then cover slipped with a Vectashield DAPI (4′,6′-diamidino-2-phenylindole hydrochloride) fluorescent mounting medium (Vector Laboratories, Inc., Burlingame, CA, USA) and stored at 4°C overnight.
During analysis, slides were viewed using a fluorescence microscope (Eclipse 600, Nikon) and 30 individual endothelial cell images were digitally captured by a Photometrics CoolSNAPfx digital camera (Roper Scientific, Tuscon, AZ, USA). These endothelial cells were documented by cell staining of vWF and nuclear integrity was confirmed using DAPI staining. Once endothelial cells with intact nuclei were identified, they were analyzed using Metamorph Software (Universal Imageing, Downingtown, PA, USA) to quantify the intensity of SIRT-1 dependent AlexaFlour555 staining (i.e., average pixel intensity). The software program allows for systematic quantification of staining intensity and eliminates the potential for subjective error during analysis.
Values are reported as ratios of subject's SIRT-1 EC protein expression to HUVEC control. Reporting ratios to a ‘standard’ (cultured HUVEC) control minimizes the possible confounding effects of differences in intensity of staining among different staining sessions. A single technician who was blinded to the subject's identity analysed each batch of slides.
Statistical analysis
Experiments in mice
For animal and vessel characteristics (maximum dilatation and EC50), group differences were determined by one-way analysis of variance (ANOVA) or t test where appropriate. For all dose responses, group differences were determined by repeated measures ANOVA. Data are presented as means ± SEM. Significance was set at P < 0.05. Data analyses were performed with SPSS (version 16.0).
Experiments in human subjects
Group differences were determined by t tests for independent sample comparisons and forearm blood flow responses and ex vivo vascular responses to incremental doses of ACh and SNP were analyzed by repeated measures ANOVA. Pearson correlation analysis was used to determine relations of interest. Statistical significance for all analyses was set at P < 0.05. Data analyses were performed with SPSS (version 16.0).
Results
Studies in young and older B6D2F1 mice
Animal and artery characteristics
Ageing did not alter mouse body (older: 36 ± 1 vs. young: 34 ± 1 g, P > 0.05), soleus (older: 0.012 ± 0.003 vs. young: 0.014 ± 0.001 g, P > 0.05) or gastrocnemius (older: 0.188 ± 0.010 vs. young: 0.205 ± 0.009 g, P > 0.05) muscle masses. Femoral artery maximal diameter was similar in the two groups (older: 404 ± 10 vs. young: 409 ± 9 μm, P > 0.05), as was the vascular tone after preconstriction (older: 33 ± 3 vs. young: 33 ± 2% tone from maximum diameter, P > 0.05).
Arterial protein expression
Total eNOS did not differ with age (older: 0.81 ± 0.18 vs. young: 1.00 ± 0.09 eNOS arbitrary units (AU), P > 0.05; Fig. 1A), but phosphorylation of eNOS at serine 1177 (peNOS) was lower (older: 0.59 ± 0.09 vs. young: 1.00 ± 0.08 peNOS AU, P < 0.05; Fig. 1B) and the ratio of peNOS to eNOS tended to be lower (older: 0.81 ± 0.10 vs. young: 1.00 ± 0.10 peNOS/eNOS ratio, P = 0.06; Fig. 1C) in aorta from the older mice. SIRT-1 protein expression was markedly lower in aorta from the older mice (older: 0.31 ± 0.09 vs. young: 1.00 ± 0.13 SIRT-1 AU, P < 0.05; Fig. 1D) and this was associated with a 6-fold greater eNOS acetylation (older: 6.01 ± 1.95 vs. young: 1.00 ± 0.30 acetyl-eNOS AU, P < 0.05; Fig. 1E).
Figure 1. Protein expression in aortas from young and older mice for eNOS, eNOS phosphorylated at serine 1177, ratio of peNOS to eNOS, SIRT-1 and acetylated eNOS.
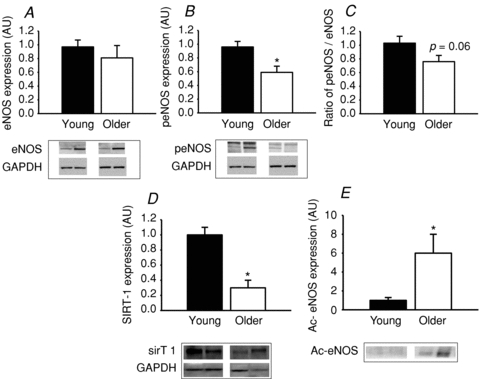
Protein expression is shown in aortas from young and older mice (n = 7–9 per group) for eNOS (A), eNOS phosphorylated at serine 1177 (peNOS) (B), ratio of peNOS to eNOS (C), SIRT-1 (D) and acetylated (Ac)-eNOS (E). Protein expression of eNOS, peNOS and SIRT-1 is expressed relative to GAPDH and immunopreciptated (IP) Ac-eNOS is expressed relative to no antibody IP control eNOS expression. Representative images are shown below the summary data. GAPDH images are from the same membrane after stripping and re-probing. Data are shown normalized to the young control mean values. Values are means ± SEM. *P < 0.05 vs. young.
Femoral artery EDD and endothelium-independent dilatation
Peak ACh-mediated dilatation (EDD) was lower in femoral arteries of the older mice (maximum dilatation to ACh, older: 85 ± 1%, vs. young: 95 ± 1%, P < 0.001; Fig. 2A). Sensitivity to ACh (EC50) was not different between young and older arteries (Table 1, P = 0.12). Inhibition of the SIRT-1 with sirtinol reduced EDD in both age groups (P < 0.01) and eliminated the age-related differences (maximum dilatation, older: 33 ± 7%vs. young: 33 ± 5%, P > 0.05; Fig. 2A). EC50 was significantly reduced with sirtinol treatment in young (P < 0.05), but not older arteries (P > 0.05) (Table 1). Addition of the eNOS inhibitor l-NAME combined with sirtinol pretreatment further reduced EDD (P < 0.01) similarly in the two age groups (maximum dilatation, older: 5 ± 6%vs. young: 10 ± 3%, P > 0.05; Fig. 2A), but did not alter EC50 (P > 0.05). There were no differences in femoral artery dilatation or EC50 to SNP in the young and older mice with or without sirtinol treatment (all P > 0.05; Fig. 2B).
Figure 2. Endothelium-dependent and -independent dilatation shown in untreated, sirtinol treated and sirtinol and l-NAME treated femoral arteries from young and older mice.
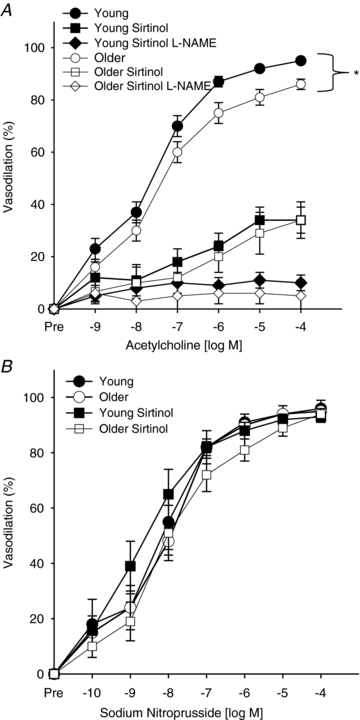
Endothelium-dependent dilatation (acetylcholine, 1 × 10−9 to 1 × 10−4m; A) and endothelium-independent dilatation (sodium nitroprusside, 1 × 10−10 to 1 × 10−4m; B) are shown in untreated (n = 16, young, filled circles; n = 14, older, open circles), sirtinol treated (n = 14, young, filled squares; n = 13, older, open squares) and sirtinol and l-NAME (n = 15, young, filled diamonds; n = 13, older, open diamonds) treated femoral arteries from young and older mice. Values are means ± SEM. *P < 0.05 vs. young.
Table 1.
Sensitivity (EC50) to ACh and SNP
| EC50 | Young | Older |
|---|---|---|
| Acetylcholine | (6.6 ± 2.9) × 10−8 | (41.3 ± 34.9) × 10−8 |
| Acetylcholine + sirtinol | (1.8 ± 1.2) × 10−6* | (12.0 ± 7.7) × 10−6 |
| Acetylcholine + sirtinol +l-NAME | (1.2 ± 0.5) × 10−6 | (1.6 ± 0.8) × 10−6 |
| Sodium nitroprusside | (2.5 ± 1.7) × 10−8 | (1.2 ± 0.2) × 10−8 |
| Sodium nitroprusside + sirtinol | (8.7 ± 5.1) × 10−9 | (22.0 ± 7.2) × 10−9 |
Values are means ± SEM; EC50, acetylcholine dose at which yields 50% vasodilation; l-NAME, NG-monomethyl-l-arginine
P < 0.05 vs. Young acetylcholine.
Studies in young and older human subjects
Subject characteristics
Characteristics of the young and older subjects are shown in Table 2. Body mass index, resting blood pressure, fasting plasma glucose and total and LDL cholesterol concentrations were higher in the older adults (all P < 0.05), but all values were within clinically normal ranges. Body mass, plasma HDL cholesterol and habitual physical activity were not significantly different between the groups. Plasma oxidized LDL was greater (P < 0.01), total antioxidant status was lower (P < 0.01) and C-reactive protein was not different in the older vs. young subjects.
Table 2.
Subject charateristics
| Young (n = 16) | Older (n = 22) | P | |
|---|---|---|---|
| Male/female | 12/4 | 15/7 | |
| Age (years) | 25 ± 1 | 64 ± 1 | <0.01 |
| Body mass (kg) | 75 ± 3 | 82 ± 3 | 0.06 |
| BMI (kg m−2) | 23.8 ± 0.7 | 27.6 ± 1.0 | <0.01 |
| Blood Pressure (mmHg) | |||
| Systolic | 111 ± 3 | 126 ± 3 | <0.01 |
| Diastolic | 71 ± 2 | 78 ± 2 | <0.01 |
| Cholesterol (mg dl−1) | |||
| Total | 162 ± 6 | 202 ± 5 | <0.01 |
| LDL | 92 ± 5 | 124 ± 4 | <0.01 |
| HDL | 51 ± 3 | 57 ± 3 | 0.08 |
| Fasting glucose (mg dl−1) | 87 ± 2 | 95 ± 2 | <0.01 |
| Leisure physical activity (MET h wk−1) | 24 ± 7 | 31 ± 6 | 0.23 |
| Oxidized LDL (U l−1) | 49.8 ± 4.3 | 64.7 ± 2.3 | <0.01 |
| Total antioxidant status (mmol l−1) | 1.46 ± 0.03 | 1.32 ± 0.04 | <0.01 |
| C-reactive protein (mg l−1) | 0.92 ± 0.30 | 1.19 ± 0.25 | 0.25 |
Values are means ± SEM; n, no. of subjects; BMI, body mass index; MET, metabolic equivalent; HDL, high-density lipoprotein; LDL, low-density lipoprotein.
EDD and endothelium-independent dilatation
The forearm blood flow response to ACh was lower (P < 0.05) in the older subjects (area under the curve (AUC), older: 67 ± 9 vs. young: 99 ± 12 AU, P < 0.01; Fig. 3A), whereas the response to SNP was similar in the groups (Fig. 3B) (AUC, older: 27 ± 2 vs. young: 31 ± 4 AU, P > 0.05).
Figure 3. Endothelium-dependent and -independent dilatation shown in young and older healthy humans.
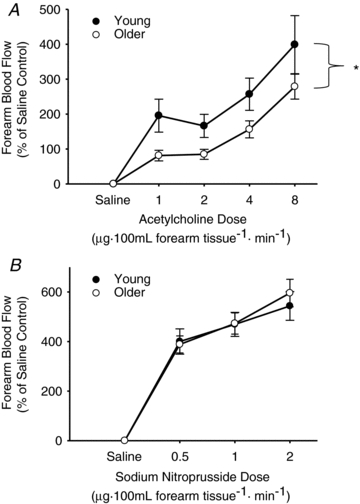
Endothelium-dependent dilatation (forearm blood flow to intra-brachial artery infusion of acetylcholine; 1.0, 2.0, 4.0, and 8.0 μg 100−1 ml forearm tissue; A) and endothelium-independent dilatation (forearm blood flow to intra-brachial artery infusion of sodium nitroprusside; 0.5, 1.0, 2.0 μg 100 ml−1 forearm tissue; B) are shown in young (n = 16, filled circles) and older (n = 22, open circles) healthy humans. Values are means ± SEM. *P = 0.01 vs. young.
SIRT-1 protein expression
Vascular endothelial cell expression of SIRT-1 was lower in the older subjects (older: 0.37 ± 0.03 vs. young: 0.51 ± 0.05, SIRT-1/HUVEC, P < 0.05; Fig. 4A). In the overall sample, forearm blood flow AUC to ACh was positively related to SIRT-1 expression in endothelial cells (r = 0.44, P < 0.01; Fig. 4B). There was no relation between forearm blood flow AUC to SNP and SIRT-1 (r = 0.17, P > 0.05). The age group differences in SIRT-1 expression were not related to age-associated differences in any other factor (all P > 0.20).
Figure 4. Arterial endothelial cell SIRT-1 protein expression and relation to endothelial dependent dilation.
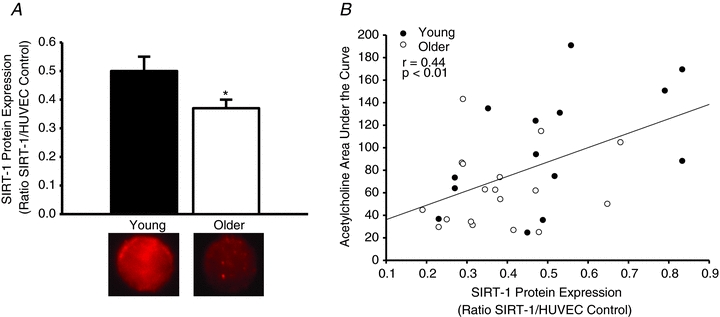
SIRT-1 protein expression is shown in endothelial cells obtained from brachial arteries of young (n = 14) and older (n = 18) healthy humans (A). Representative images of the immunofluorescence images of SIRT-1 from individual young and older subjects are shown below the group mean bars. The relation between acetylcholine-induced endothelium-dependent dilatation (area under the curve) and SIRT-1 endothelial cell protein expression is shown for all subjects (B: young, filled circles; older, open circles). Values are means ± SEM. *P < 0.05 vs. young.
Discussion
The novel findings of the present studies are as follows. First, the reduced arterial expression of SIRT-1, a cellular deacetylase, with ageing is associated with increased acetylated eNOS. Second, a reduction in SIRT-1 activity in arteries is an important mechanism mediating the age-associated impairment in NO-dependent EDD, without affecting endothelium-independent dilatation. Third, expression of SIRT-1 is lower in endothelial cells from older compared with young healthy adults, extending previous observations in arteries of rodents to humans. Lastly, among healthy adults of increasing age, EDD is positively related to SIRT-1 protein expression in vascular endothelial cells. Collectively, these results provide experimental evidence that decreases in sirtuins, specifically SIRT-1 expression and activity, play a key role in vascular endothelial dysfunction with ageing and that this is associated with increases in eNOS acetylation.
The present results confirm previous observations of our laboratory (Rippe et al. 2010) and others (Ungvari et al. 2008) that arterial SIRT-1 expression is reduced with age in rodents, and that this occurs in the absence of changes in total eNOS (Woodman et al. 2002; Spier et al. 2004; Durrant et al. 2009; Lesniewski et al. 2009). The present findings extend insight on this issue by showing that the reduction in arterial SIRT-1 with ageing is associated with an increase in acetylated eNOS. This is consistent with the fact that both pharmacological (sirtinol) and viral (siRNA) inhibition of SIRT-1 expression and activity directly acetylate eNOS, which inhibits eNOS activity and the production of NO in cultured endothelial cells (Mattagajasingh et al. 2007; Ota et al. 2010). Likewise, endothelial cells in culture that are exposed to shear stress, a potent physiological stimulus for NO production, exhibit enhanced colocalization of SIRT-1 and eNOS, resulting in deacetylation of eNOS and an associated increase in NO production (Chen et al. 2010). These results support the role of SIRT-1 expression and activity in regulation of eNOS (via eNOS acetylation) and NO bioavailability. In the present study, we found that reductions in SIRT-1 and increased acetylation of eNOS were associated with a reduction in peNOS and the peNOS:eNOS ratio, indicators of eNOS activation state, as we have reported previously in this model (Durrant et al. 2009; Rippe et al. 2010). Taken together, these results indicate that in mice, reductions in arterial SIRT-1 expression and activity are associated with acetylation and deactivation of eNOS, and impaired NO-mediated vascular endothelial dysfunction.
To establish more direct evidence linking reductions in SIRT-1 to endothelial dysfunction with ageing, we utilized an in vitro artery model to inhibit SIRT-1 activity using sirtinol. Treatment with sirtinol eliminated the age-related difference in NO-dependent EDD, without affecting vascular smooth muscle sensitivity to NO (endothelium-independent dilatation). These results are the first to demonstrate that diminished SIRT-1 is involved in vascular endothelial dysfunction with ageing. Our data are consistent with other observations in models of SIRT-1 inhibition in which adenoviral transfection of the endothelium with a dominant negative SIRT-1 mutant in aortic rings was found to impair NO-mediated EDD (Mattagajasingh et al. 2007), and transgenic mice with SIRT-1 overexpression demonstrated preserved EDD compared with wild-type mice in response to a high fat diet (Zhang et al. 2008).
In the present study, the fact that SIRT-1 inhibition reduced EDD in both age groups of mice (with a greater reduction in young arteries) and that the addition of l-NAME further inhibited EDD, indicates that inhibition of SIRT-1 decreases eNOS activity, but does not completely eliminate it. This is in contrast to the results of a previous study that showed complete inhibition of eNOS-mediated EDD with the andenoviral knock down of SIRT-1 in rat aorta (Mattagajasingh et al. 2007). These differences could be due to the method of SIRT-1 inhibition used or differences in the species studied, but in both cases support a strong role of SIRT-1 in regulating NO-dependent endothelial function.
To provide translational insight into the potential role of SIRT-1 in age-associated vascular endothelial dysfunction, we determined the forearm blood flow responses to ACh, a measure of NO-mediated EDD with ageing (Taddei et al. 2001; Seals et al. 2011), and the expression of SIRT-1 in endothelial cells acquired from arteries of young and older healthy adults. Our results are the first to demonstrate in humans that SIRT-1 is lower in vascular endothelial cells of older adults and that this is positively related to in vivo differences in EDD. These results extend our in vitro observations in mice to suggest a possible role for reduced SIRT-1 in mediating vascular endothelial dysfunction with ageing in humans. These findings also provide evidence that SIRT-1 activation may have therapeutic potential for the treatment of age-associated vascular dysfunction in humans.
We recognize several limitations of our study. First, because of the limited number of cells available from our sampling procedure, we were not able to immuno-precipitate and measure acetylated eNOS in our human endothelial cell samples. Second, although we focused on the role of SIRT-1 in acutely modulating endothelial function and the putative role of acetylated eNOS, altering SIRT-1 function may have influenced the acetylation status of other proteins affecting NO bioavailability. Finally, we used different arteries in our mouse (femoral, thoracic aorta) and human (brachial) studies. It is possible that the modulatory influence of SIRT-1 differs in the vascular endothelium of these arteries, although we found reduced expression of SIRT-1 in arterial tissue from both mice and humans in the present study. Nevertheless, eNOS expression and activation with ageing can be specific to the model used and species studied (Muller-Delp, 2006; Donato et al. 2009), so we cannot rule out the possibility of such specificity for SIRT-1.
In conclusion, the results of the present study provide new insight into the molecular events that contribute to vascular endothelial dysfunction with ageing. Specifically, here we provide direct evidence for a role for reduced SIRT-1 expression and activity in age-associated endothelial dysfunction, and suggest that increased acetylation of eNOS may be among the key mechanisms involved. We also show for the first time that SIRT-1 expression is reduced in endothelial cells obtained from arteries of older compared with young adult humans and is related to differences in vascular endothelial function. Together, these findings provide novel translational evidence that SIRT-1 expression and activity contribute to vascular endothelial dysfunction with ageing and that this may be due to altered eNOS acetylation. Importantly, our results provide further compelling support for SIRT-1 as a potential therapeutic target for lifestyle, nutraceutical and pharmacological interventions aimed at the prevention and treatment of arterial ageing and age-associated cardiovascular diseases.
Acknowledgments
This work was supported by the National Institutes of Health: AG029337, AG013038, AG000279, AG040297, AG033196, AG033755, HL007851, RR025780 and RR00051. We would like to thank Rhea Chiang, Cassandra Roeca and Melanie Connell for their technical assistance. We have no conflicts of interest or disclosures.
Glossary
Abbreviations
- ACh
acetylcholine
- EDD
endothelium-dependent dilatation
- EID
endothelium-independent dilatation
- eNOS
endothelial nitric oxide synthase
- HDL
high-density lipoprotein
- LDL
low-density lipoprotein
- l-NAME
NG-monomethyl-l-arginine
- NO
nitric oxide
- peNOS
phosphorylated endothelial nitric oxide synthase
- SIRT
sirtuin
- SNP
sodium nitroprusside
- vWF
von Willebrand factor
Author contributions
A.J.D., K.A.M., B.R.L., J.R.D., L.A.L., and D.R.S. contributed to the conception and design, analysis and interpretation of data. A.J.D., L.A.L., and D.R.S. contributed to the drafting and revision of the article. All authors provided final approval of the version to be published. All experiments were carried out at the University of Colorado at Boulder and the University of Utah.
References
- Celermajer D, Sorensen K, Bull C, Robinson J, Deanfield J. Endothelium-dependent dilation in the systemic arteries of asymptomatic subjects relates to coronary risk factors and their interaction. J Am Coll Cardiol. 1994;24:1468–1474. doi: 10.1016/0735-1097(94)90141-4. [DOI] [PubMed] [Google Scholar]
- Chen Z, Peng IC, Cui X, Li YS, Chien S, Shyy JY. Shear stress, SIRT1, and vascular homeostasis. Proc Natl Acad Sci U S A. 2010;107:10268–10273. doi: 10.1073/pnas.1003833107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen HY, Miller C, Bitterman KJ, Wall NR, Hekking B, Kessler B, Howitz KT, Gorospe M, de Cabo R, Sinclair DA. Calorie restriction promotes mammalian cell survival by inducing the SIRT1 deacetylase. Science. 2004;305:390–392. doi: 10.1126/science.1099196. [DOI] [PubMed] [Google Scholar]
- Colombo PC, Ashton AW, Celaj S, Talreja A, Banchs JE, Dubois NB, Marinaccio M, Malla S, Lachmann J, Ware JA, Le Jemtel TH. Biopsy coupled to quantitative immunofluorescence: a new method to study the human vascular endothelium. J Appl Physiol. 2002;92:1331–1338. doi: 10.1152/japplphysiol.00680.2001. [DOI] [PubMed] [Google Scholar]
- Dali-Youcef N, Lagouge M, Froelich S, Koehl C, Schoonjans K, Auwerx J. Sirtuins: the ‘magnificent seven’, function, metabolism and longevity. Ann Med. 2007;39:335–345. doi: 10.1080/07853890701408194. [DOI] [PubMed] [Google Scholar]
- Donato AJ, Black AD, Jablonski KL, Gano LB, Seals DR. Aging is associated with greater nuclear NFκB, reduced IκBα, and increased expression of proinflammatory cytokines in vascular endothelial cells of healthy humans. Aging Cell. 2008a;7:805–812. doi: 10.1111/j.1474-9726.2008.00438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donato AJ, Eskurza I, Jablonski KL, Gano LB, Pierce GL, Seals DR. Cytochrome P-450 2C9 signaling does not contribute to age-associated vascular endothelial dysfunction in humans. J Appl Physiol. 2008b;105:1359–1363. doi: 10.1152/japplphysiol.90629.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donato AJ, Eskurza I, Silver AE, Levy AS, Pierce GL, Gates PE, Seals DR. Direct evidence of endothelial oxidative stress with aging in humans: relation to impaired endothelium-dependent dilation and upregulation of nuclear factor-κB. Circ Res. 2007a;100:1659–1666. doi: 10.1161/01.RES.0000269183.13937.e8. [DOI] [PubMed] [Google Scholar]
- Donato AJ, Gano LB, Eskurza I, Silver AE, Gates PE, Jablonski K, Seals DR. Vascular endothelial dysfunction with aging: endothelin-1 and endothelial nitric oxide synthase. Am J Physiol Heart Circ Physiol. 2009;297:H425–432. doi: 10.1152/ajpheart.00689.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donato AJ, Lesniewski LA, Delp MD. The effects of aging and exercise training on endothelin-1 vasoconstrictor responses in rat skeletal muscle arterioles. Cardiovasc Res. 2005;66:393–401. doi: 10.1016/j.cardiores.2004.10.023. [DOI] [PubMed] [Google Scholar]
- Donato AJ, Lesniewski LA, Delp MD. Ageing and exercise training alter adrenergic vasomotor responses of rat skeletal muscle arterioles. J Physiol. 2007b;579:115–125. doi: 10.1113/jphysiol.2006.120055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond GB. Reporting ethical matters in The Journal of Physiology: standards and advice. J Physiol. 2009;587:713–719. doi: 10.1113/jphysiol.2008.167387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durrant JR, Seals DR, Connell ML, Russell MJ, Lawson BR, Folian BJ, Donato AJ, Lesniewski LA. Voluntary wheel running restores endothelial function in conduit arteries of old mice: direct evidence for reduced oxidative stress, increased superoxide dismutase activity and down-regulation of NADPH oxidase. J Physiol. 2009;587:3271–3285. doi: 10.1113/jphysiol.2009.169771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskurza I, Kahn Z, Seals D. Xanthine oxidase does not contribute to impaired peripheral artery conduit artery endothelium-dependent dilatation with ageing. J Physiol. 2006;571:661–668. doi: 10.1113/jphysiol.2005.102566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng L, Stern DM, Pile-Spellman J. Human endothelium: endovascular biopsy and molecular analysis. Radiology. 1999;212:655–664. doi: 10.1148/radiology.212.3.r99au28655. [DOI] [PubMed] [Google Scholar]
- Fleming I, Busse R. NO: the primary EDRF. J Mol Cell Cardiol. 1999;31:5–14. doi: 10.1006/jmcc.1998.0839. [DOI] [PubMed] [Google Scholar]
- Gates PE, Boucher ML, Silver AE, Monahan KD, Seals DR. Impaired flow-mediated dilation with age is not explained by l-arginine bioavailability or endothelial asymmetric dimethylarginine protein expression. J Appl Physiol. 2007;102:63–71. doi: 10.1152/japplphysiol.00660.2006. [DOI] [PubMed] [Google Scholar]
- Guarente L. Sirtuins as potential targets for metabolic syndrome. Nature. 2006;444:868–874. doi: 10.1038/nature05486. [DOI] [PubMed] [Google Scholar]
- Lesniewski LA, Connell ML, Durrant JR, Folian BJ, Anderson MC, Donato AJ, Seals DR. B6D2F1 Mice are a suitable model of oxidative stress-mediated impaired endothelium-dependent dilation with aging. J Gerontol A Biol Sci Med Sci. 2009;64:9–20. doi: 10.1093/gerona/gln049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesniewski LA, Durrant JR, Connell ML, Folian BJ, Donato AJ, Seals DR. Salicylate treatment improves age-associated vascular endothelial dysfunction: potential role of nuclear factor κB and forkhead box O phosphorylation. J Gerontol A Biol Sci Med Sci. 2011;66:409–418. doi: 10.1093/gerona/glq233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lind L, Berglund L, Larsson A, Sundstrom J. Endothelial function in resistance and conduit arteries and 5-year risk of cardiovascular disease. Circulation. 2011;123:1545–1551. doi: 10.1161/CIRCULATIONAHA.110.984047. [DOI] [PubMed] [Google Scholar]
- Mattagajasingh I, Kim CS, Naqvi A, Yamamori T, Hoffman TA, Jung SB, DeRicco J, Kasuno K, Irani K. SIRT1 promotes endothelium-dependent vascular relaxation by activating endothelial nitric oxide synthase. Proc Natl Acad Sci U S A. 2007;104:14855–14860. doi: 10.1073/pnas.0704329104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau KL, Gavin KM, Plum AE, Seals DR. Ascorbic acid selectively improves large elastic artery compliance in postmenopausal women. Hypertension. 2005;45:1107–1112. doi: 10.1161/01.HYP.0000165678.63373.8c. [DOI] [PubMed] [Google Scholar]
- Muller-Delp JM. Aging-induced adaptations of microvascular reactivity. Microcirculation. 2006;13:301–314. doi: 10.1080/10739680600619023. [DOI] [PubMed] [Google Scholar]
- Ota H, Eto M, Kano MR, Kahyo T, Setou M, Ogawa S, Iijima K, Akishita M, Ouchi Y. Induction of endothelial nitric oxide synthase, SIRT1, and catalase by statins inhibits endothelial senescence through the Akt pathway. Arterioscler Thromb Vasc Biol. 2010;30:2205–2211. doi: 10.1161/ATVBAHA.110.210500. [DOI] [PubMed] [Google Scholar]
- Ota H, Eto M, Kano MR, Ogawa S, Iijima K, Akishita M, Ouchi Y. Cilostazol inhibits oxidative stress-induced premature senescence via upregulation of Sirt1 in human endothelial cells. Arterioscler Thromb Vasc Biol. 2008;28:1634–1639. doi: 10.1161/ATVBAHA.108.164368. [DOI] [PubMed] [Google Scholar]
- Pierce GL, Beske SD, Lawson BR, Southall KL, Benay FJ, Donato AJ, Seals DR. Weight loss alone improves conduit and resistance artery endothelial function in young and older overweight/obese adults. Hypertension. 2008;52:72–79. doi: 10.1161/HYPERTENSIONAHA.108.111427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rippe C, Lesniewski L, Connell M, Larocca T, Donato A, Seals D. Short-term calorie restriction reverses vascular endothelial dysfunction in old mice by increasing nitric oxide and reducing oxidative stress. Aging Cell. 2010;9:304–312. doi: 10.1111/j.1474-9726.2010.00557.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers JT, Lerin C, Gerhart-Hines Z, Puigserver P. Metabolic adaptations through the PGC-1α and SIRT1 pathways. FEBS Lett. 2008;582:46–53. doi: 10.1016/j.febslet.2007.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seals DR, Jablonski KL, Donato AJ. Aging and vascular endothelial function in humans. Clin Sci (Lond) 2011;120:357–375. doi: 10.1042/CS20100476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver AE, Beske SD, Christou DD, Donato AJ, Moreau KL, Eskurza I, Gates PE, Seals DR. Overweight and obese humans demonstrate increased vascular endothelial NAD(P)H oxidase-p47(phox) expression and evidence of endothelial oxidative stress. Circulation. 2007;115:627–637. doi: 10.1161/CIRCULATIONAHA.106.657486. [DOI] [PubMed] [Google Scholar]
- Spier SA, Delp MD, Meininger CJ, Donato AJ, Ramsey MW, Muller-Delp JM. Effects of ageing and exercise training on endothelium-dependent vasodilatation and structure of rat skeletal muscle arterioles. J Physiol. 2004;556:947–958. doi: 10.1113/jphysiol.2003.060301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taddei S, Virdis A, Ghiadoni L, Mattei P, Sudano I, Bernini G, Pinto S, Salvetti A. Menopause is associated with endothelial dysfunction in women. Hypertension. 1996;28:576–582. doi: 10.1161/01.hyp.28.4.576. [DOI] [PubMed] [Google Scholar]
- Taddei S, Virdis A, Ghiadoni L, Salvetti G, Bernini G, Magagna A, Salvetti A. Age-related reduction of NO availability and oxidative stress in humans. Hypertension. 2001;38:274–279. doi: 10.1161/01.hyp.38.2.274. [DOI] [PubMed] [Google Scholar]
- Ungvari Z, Parrado-Fernandez C, Csiszar A, de Cabo R. Mechanisms underlying caloric restriction and lifespan regulation: implications for vascular aging. Circ Res. 2008;102:519–528. doi: 10.1161/CIRCRESAHA.107.168369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Loo B, Labugger R, Skepper JN, Bachschmid M, Kilo J, Powell JM, Palacios-Callender M, Erusalimsky JD, Quaschning T, Malinski T, Gygi D, Ullrich V, Luscher TF. Enhanced peroxynitrite formation is associated with vascular aging. J Exp Med. 2000;192:1731–1744. doi: 10.1084/jem.192.12.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittle JR, Powell MJ, Popov VM, Shirley LA, Wang C, Pestell RG. Sirtuins, nuclear hormone receptor acetylation and transcriptional regulation. Trends Endocrinol Metab. 2007;18:356–364. doi: 10.1016/j.tem.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Woodman CR, Price EM, Laughlin MH. Aging induces muscle-specific impairment of endothelium-dependent dilation in skeletal muscle feed arteries. J Appl Physiol. 2002;93:1685–1690. doi: 10.1152/japplphysiol.00461.2002. [DOI] [PubMed] [Google Scholar]
- Yeboah J, Crouse JR, Hsu FC, Burke GL, Herrington DM. Brachial flow-mediated dilation predicts incident cardiovascular events in older adults: the Cardiovascular Health Study. Circulation. 2007;115:2390–2397. doi: 10.1161/CIRCULATIONAHA.106.678276. [DOI] [PubMed] [Google Scholar]
- Zhang QJ, Wang Z, Chen HZ, Zhou S, Zheng W, Liu G, Wei YS, Cai H, Liu DP, Liang CC. Endothelium-specific overexpression of class III deacetylase SIRT1 decreases atherosclerosis in apolipoprotein E-deficient mice. Cardiovasc Res. 2008;80:191–199. doi: 10.1093/cvr/cvn224. [DOI] [PMC free article] [PubMed] [Google Scholar]


