Non-technical summary
The visual system requires the generation of a variety of eye movements ranging from extremely rapid saccades to slow pursuit movements which all necessitate great precision and fatigue resistance. The extraocular muscles, which are the final generators of eye movements, must be adapted to fulfill these expectations. Therefore, it is not surprising that these muscles contain a diversity of myosin heavy chains, which specify the contraction speed of a muscle. Here, we show the transcription factor Pitx2 is critical in the establishment of the mature myosin heavy chain composition of extraocular muscle. We also demonstrate the absence of Pitx2 after birth leads to the loss of multi-innervation, a unique feature of the adult extraocular muscle phenotype. Knowledge of how expression of contractile proteins is regulated and their influence on eye movements may lead to potential therapeutics for strabismus and other disorders of ocular motility.
Abstract
Abstract
Extraocular muscle is fundamentally distinct from other skeletal muscle and demonstrates specific anatomical divisions, unique innervation, diverse myosin isoform expression patterns, a distinct genomic profile and differential involvement in neuromuscular disorders. The paired-like homeodomain transcription factor 2 (Pitx2) is known to regulate the formation of extraocular muscle development and in this report we show that its expression in adulthood also defines certain extraocular muscle traits. We found that expression of slow-MyHC and slow-tonic MyHC, along with contractile regulatory proteins troponin I and troponin T, is reduced during the first 3 weeks after birth in mice with conditional knockout of Pitx2, designated Pitx2Δflox/Δflox. En grappe endplates, which are normally only found on slow-MyHC expressing fibres, were not identified in the Pitx2Δflox/Δflox extraocular muscle, suggesting that altered innervation was responsible for the loss in slow-MyHC expression. Extraocular muscle (EOM)-specific MyHC expressing fibres were dramatically reduced at P14 and rarely detected at 3 months in the Pitx2Δflox/Δflox mice. 2A-MyHC fibres, which are excluded from mid-belly region in wild-type mice, dominated the orbital layer with no apparent longitudinal variation in the Pitx2Δflox/Δflox mice. Pure 2X-MyHC fibres, only present at distal ends in the wild-type mice, populated the outer global layer in the mid-belly region of the Pitx2Δflox/Δflox mice. Pitx2 influences slow-MyHC, slow-tonic MyHC and EOM-MyHC expression in extraocular muscle and its absence leads to increased expression of 2X-MyHC and 2A-MyHC. Precise definition of the regulation of MyHC isoforms in extraocular muscle may allow their rational manipulation, in order to alter muscle contractility for therapeutic purposes.
Introduction
The paired-like homeodomain transcription factor 2 (Pitx2) is a critical developmental regulator for a number of tissues, including heart, brain and eye (Piedra et al. 1998; Ryan et al. 1998; Gage et al. 1999; Lin et al. 1999; Martin et al. 2002; Martin et al. 2004; Diehl et al. 2006). Pitx2's critical role for extraocular muscle is confirmed by the muscle's absence if Pitx2 expression is eliminated during mouse ontogeny (Gage et al. 1999; Lin et al. 1999) and postnatal knockout of Pitx2 modifies gene expression, muscle fibre histology, and contractile characteristics of mature extraocular muscle (Zhou et al. 2009). Axenfeld–Rieger syndrome, which is caused by haploinsufficiency of PITX2 in humans, leads to abnormal development of the anterior segment and patients being at high risk for glaucoma (Hjalt & Semina, 2005). Extraocular muscle and ocular motility defects in this syndrome have not been well-characterized, and their exact relationship to the Pitx2 mutation is uncertain, since abnormalities of vision could alter extraocular muscle development. Beyond the observations of Pitx2's influence on the mature extraocular phenotype, understanding of genetic and environmental factors that modulate the extraocular muscle properties is limited. Dissection of the regulatory programmes that define the mature extraocular muscle contractile properties and their influence on eye movements could one day lead to novel therapeutics for strabismus and other disorders of ocular motility.
Extraocular muscle contracts rapidly with low levels of force generation. The conditional knockout of Pitx2 at the time of birth leads to extraocular muscle that contract with greater force and speed (Zhou et al. 2009). Contractile characteristics of a skeletal muscle are largely determined by the composition of myosin heavy chain (MyHC) isoforms (Bottinelli et al. 1991; Bottinelli et al. 1994). An unusual feature of extraocular muscle is the complexity of its MyHC isoform expression (Spencer & Porter, 2006). In addition to the Myh1 (2X), Myh2 (2A), Myh4 (2B) and Myh7 (slow) that are commonly found in most skeletal muscles, mature extraocular muscle retains two developmental isoforms, Myh3 (embryonic) and Myh8 (neonatal), as well as the cardiac isoform Myh6 and Myh13 (EOM-MyHC) (Wieczorek et al. 1985; Sartore et al. 1987; Jung et al. 1998; Lim et al. 2006). Recently, another two MyHC isoforms, Myh14 and Myh15, were found to be expressed by extraocular muscle (Rossi et al. 2010).
In addition to expression of virtually all known MyHC isoforms, each isoform has a unique longitudinal and cross-sectional expression pattern and more than one isoform may be expressed in a single fibre (Rubinstein & Hoh, 2000; Briggs & Schachat, 2002; Zhou et al. 2010). In mice, expression of emb-MyHC, neo-MyHC, and 2A-MyHC is restricted to the orbital layer, that of 2B-MyHC to the global layer. Furthermore, although slow-MyHC and 2B-MyHC do not exhibit obvious longitudinal variations, emb-MyHC, neo-MyHC and 2A-MyHC are more abundant at both tips of the muscle and excluded from the innervation zone, while EOM-MyHC is most abundant in the mid-belly region of the orbital and global layers. In a typical skeletal muscle, fibres express only one MyHC isoform except during development, ageing, or injury (Pette & Staron, 1997). Expression of two or more MyHC isoforms in single fibres is common in extraocular muscle (Wieczorek et al. 1985; Jacoby et al. 1990; Kranjc et al. 2000; Rubinstein & Hoh, 2000; Briggs & Schachat, 2002; Rubinstein et al. 2004; Bicer & Reiser, 2009; Zhou et al. 2010), particularly in the orbital layer, where at least five MyHC isoforms are found to have overlapping expression with at least one other isoform.
In addition to the complex MyHC expression pattern, extraocular muscle demonstrates structural diversity of neuromuscular junctions. In addition to twitch muscle fibres with a single en plaque point of innervation, multi-innervated fibres exist with several en grappe endplates on individual muscle fibres. The multi-innervated fibres possess unique contractile characteristics in that they contract in a tonic fashion to repetitive stimulation rather than a twitch as the singly innervated fibres do (Ruff et al. 1989). In rodents and humans, antibodies directed towards the slow MyHC isoform have been used to identify these slow tonic fibres (Bormioli et al. 1980; Rubinstein & Hoh, 2000; Khanna et al. 2003; Kjellgren et al. 2003; Fraterman et al. 2006). Further expanding the complexity of the MyHC expression, Rossi et al. (2010) found that the slow-tonic MyHC, the gene product of MYH14 is expressed in the slow tonic fibres of rat and human extraocular muscle.
After our preliminary identification of altered MyHC expression with Pitx2 knockout, we performed a detailed analysis of the temporal and anatomical expression pattern of the MyHC isoforms in the extraocular muscle of Pitx2Δflox/Δflox mice. Because of the identification of a loss of slow-MyHC expression, which is influenced by innervation and electrical stimulation (DiMario & Stockdale, 1997; Crew et al. 2010), we evaluated whether an alteration in innervation may have occurred in the Pitx2Δflox/Δflox mice.
Methods
Animal husbandry
Two mouse strains were crossed to generate the Pitx2 conditional knockout mice: muscle creatine kinase (MCK)-Cre mouse strain and the Pitx2flox/flox mouse strain, as previously described (Zhou et al. 2009). Genotyping was performed by PCR using genomic DNA isolated from tail tips. Mice that were both Pitx2flox/flox and Cre positive were referred to as the conditional knockout Pitx2Δflox/Δflox mice; their littermates, Pitx2flox/flox mice, were used as controls. Animals were maintained in accordance with National Institutes of Health (NIH) guidelines for animal care. All procedures involving mice were approved by the Institutional Animal Use and Care Committee at Saint Louis University. All experiments were conducted in accordance with the principles and procedures established by the NIH and the Association for Assessment and Accreditation of Laboratory Animal Care and in the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
Antibodies
We used a panel of isoform specific antibodies to examine the developmental transition as well as the longitudinal and cross-sectional expression patterns of MyHC isoforms in the Pitx2Δflox/Δflox and Pitx2flox/flox extraocular muscle. The sources and dilutions for antibodies against MyHC isoforms were as follows: mouse anti-embryonic MyHC (emb-MyHC, IgG, F1.652, 1:20 dilution), mouse anti-neonatal MyHC (neo-MyHC, IgM, N1.551;1:5 dilution), mouse anti-EOM specific MyHC (EOM-MyHC, 4A6, IgM; 1:20 dilution), mouse anti-slow-tonic MyHC (S46, IgG; 1:20), mouse anti-all MyHC except 2X (BF-35, IgG, 1:10 dilution), and mouse anti-cardiac troponin T (CT3, IgG, 1:30 dilution) were obtained from Developmental Studies Hybridoma Bank (DSHB) developed under the auspices of the National Institute of Child Health and Human Development and maintained by the University of Iowa, Department of Biology, Iowa City, IA, USA. Antibody 7A10 (against dystrophin protein, 1:30 dilution) was obtained from DSHB and used to identify myofibre boundaries. Mouse anti-skeletal MyHC (pan fast) (fast-MyHC, IgG, 1:500 dilution, My32) were purchased from Sigma (St Louis, MO, USA); mouse anti-slow muscle MyHC (slow-MyHC, IgG, 1:1000 dilution) were from Chemicon (Temecula, CA, USA); mouse anti-MyHC 2A (2A-MyHC, IgG, SC-71) and mouse anti-MyHC 2B (2B-MyHC, IgM, BF-F3) were obtained as a hybridoma from ATCC (Manassas, VA, USA) and supernatants from cell cultures were used. We also used mouse anti-cardiac troponin T (CT3, IgG, 1:30 dilution) from DSHB and mouse anti- skeletal muscle slow troponin I (ab8293, IgG, 1:500) purchased from Abcam Inc. (Cambridge, MA, USA) to evaluated troponins usually expressed with slow-MyHC. All secondary antibodies including Alexa Fluor 350, 488 and 594 goat anti-mouse IgG or IgM are from Invitrogen (Carlsbad, CA, USA) and used at 1:500 dilution.
Tissue preparation and immunohistochemistry
Eyes with all four rectus muscles attached were dissected from the Pitx2Δflox/Δflox and their control littermate Pitxflox/flox mice after killing by CO2 asphyxiation at P0, P7, P14, P21 and 3 months of age. After dissection, extraocular muscle were mounted on cork with 8% tragacanth (Sigma) and immediately frozen in liquid N2-cooled 2-methylbutane and stored at −80°C until use. Ten-micrometre serial sections of the extraocular muscle were collected and designated as follows: proximal sections (in the posterior aspect of the orbit at the annulus of Zinn), mid-belly sections (innervation zone) and distal sections (near the myotendinous junction at the attachment to the globe). The sections were air-dried for 30 min, rinsed with phosphate buffered saline (PBS), pH 7.4 before block with 3% normal goat serum for at least 1 h. Sections were incubated in primary antibody for 1 h at room temperature, washed with PBS before application of secondary antibody. For double immunostaining of mouse antibodies on mouse extraocular muscle, slides were incubated for 1 h at room temperature with Vector M.O.M. Blocking Reagent (Vector Laboratories, Burlingame, CA, USA) according to the manufacturer's instruction, before the next primary antibody was applied. This M.O.M reagent serves to block endogenous as well as previously applied exogenous mouse IgG antibodies. After staining, sections were examined with a fluorescence microscope (Olympus America Inc., Centre Valley, PA, USA) and images of superior rectus were captured with a digital camera (Spot; Diagnostic Instruments, Sterling Heights, MI, USA, USA) and software (Spot Advanced; Diagnostic Instruments) before processing with Adobe Photoshop.
Neuromuscular junction identification
The distribution of neuromuscular junctions was assessed by double immunostaining of 10 μm longitudinal sections with antibodies to slow-MyHC and dystrophin (with alexa 488 goat anti-mouse IgG as secondary antibody) and counterstained with Alexa 594 α-bungarotoxin (α-Bgt). Slow-MyHC immunostaining was performed to evaluate the hypothesis that an alteration in innervation was occurring in the multi-innervated fibres. To count the number and measure the size of α-Bgt clusters in the mid-belly and ends of the muscles, 10 μm serial cross-sections were immunostained with α-Bgt. Twelve-bit white and black images were captured. The area of each α-Bgt cluster from every 10th section was determined by outlining the area of α-Bgt fluorescence using ImagePro software (Media Cybernetics, Inc., Bethesda, MD, USA) and categorized as either greater or less than 100 (arbitrary units). This cutoff was chosen because it appeared to distinguish en grappe and en plaque endplates. The distribution was graphed based on anatomical region. The experiment was performed in triplicate on independent samples.
Muscle fibre number
Since there was a decrease in slow-MyHC fibres, we wondered whether there was disappearance of fibres or a transformation of slow-MyHC-expressing fibres to a different MyHC isoform. Serial cross sectioning of superior rectus was performed and every 10th section taken for double immunostaining with mouse anti-2A-MyHC antibody (SC-71 for 2A-MyHC myofibre counting) or mouse anti-all MyHC except 2X (BF-35 for pure 2X-MyHC myofibre counting) and mouse anti-dystrophin antibody to mark the fibre boundary. Twelve-bit white and black images were captured for both channels with fluorescence microscopy and Spot camera using ImagePro software. The images from the two channels were merged and the number of 2A-MyHC positive myofibres or negatively immunostained pure 2X-MyHC myofibres and total number of myofibres in each section were manually tagged and counted and distribution plots produced. The percentage of myofibres from each cross-section was determined and compared between the Pitx2flox/flox and Pitx2Δflox/Δflox mice.
Results
Loss of slow-MyHC expressing myofibres with Pitx2 elimination
Slow-MyHC expressing fibres are the primary fibres from which the secondary fibres are derived (Brueckner et al. 1996). These fibres were found through the longitudinal extent of the extraocular muscle without variation and were scattered with no obvious orbital and global layer distinction in wild-type extraocular muscle (Zhou et al. 2010). At P0 and P7 there was no difference in the number and distribution of slow-MyHC myofibres in Pitx2Δflox/Δflox compared to Pitx2flox/flox extraocular muscle (Fig. 1A and B). At P14 and after, the number of slow-MyHC myofibres decreased and these fibres appeared smaller in diameter at P14 and P21 (Fig. 1D). However, this observation was not quantitatively assessed. By 3 months of age, only rare slow-MyHC expressing fibres were observed in the Pitx2Δflox/Δflox extraocular muscle (Fig. 1F compared to Fig. 1E).
Figure 1. Loss of slow-MyHC expressing myofibres in Pitx2Δflox/Δflox mice.

Mid-belly cross-sections of P7 (A and B), P21 (C and D) and 3 months (E and F) extraocular muscle of Pitx2Δflox/Δflox mice (B, D and F) and Pitx2flox/flox control littermates (A, C and E) were immunolabelled with antibody to slow-MyHC. The number of slow-MyHC positive myofibres was comparable at P7 between wild-type Pitx2flox/flox (A) and mutant Pitx2Δflox/Δflox (B) mice but was reduced by 3 weeks of age (compare D for the Pitx2Δflox/Δflox mice to C for the Pitx2flox/flox mice). At 3 months, only occasional slow-MyHC positive myofibres were detected in the Pitx2Δflox/Δflox mice (F) while the wild-type Pitx2flox/flox extraocular muscle maintains a normal number of positive fibres. Orientation: orbital layer to the top left and global layer to the bottom right. Scale bars = 100 μm.
Loss of slow-tonic MyHC expressing myofibres in the Pitx2Δflox/Δflox extraocular muscle
The loss of slow MyHC fibres in Pitx2Δflox/Δflox extraocular muscle prompted us to examine the expression of the slow-tonic MyHC isoform, which is reported to be expressed in multiply-innervated fibres (Bormioli et al. 1979, 1980). The slow-tonic MyHC isoform has been identified to be the product of Myh14 gene (Rossi et al. 2010). The slow-tonic MyHC immunoreactivity was identified equally at P0 in Pitx2Δflox/Δflox and Pitx2flox/flox extraocular muscle (Fig. 2A and B). However, the intensity of slow-tonic MyHC immunostaining was already much weaker at P7 in Pitx2Δflox/Δflox extraocular muscle (compare Fig. 2D to C). At P21 (data not shown) and in the adult, very few slow-tonic MyHC expressing fibres were detected in the midbelly of Pitx2Δflox/Δflox extraocular muscle (Fig. 2F), while the proximal and distal region contain rare fibres which were weakly positive for the slow-tonic MyHC (Supplemental Material, Fig. S1).
Figure 2. Loss of slow-tonic MyHC expressing myofibres in Pitx2Δflox/Δflox mice.

Mid-belly cross-sections of P0 (A and B), P7 (C and D) and 3 months (E and F) extraocular muscle of Pitx2Δflox/Δflox mice (B, D and F) and Pitx2flox/flox control litter mates (A, C and E) were immunolabelled with antibody to slow-tonic MyHC. The expression of slow-tonic MyHC isoform was comparable at P0 between wild-type Pitx2flox/flox (A) and mutant Pitx2Δflox/Δflox (B) mice but was reduced at P7 (compare D to C). At 3 months, only occasional slow-tonic MyHC positive myofibres were detected in the Pitx2Δflox/Δflox mice (F) while the wild-type Pitx2flox/flox extraocular muscle maintains a normal number of positive fibres. Orientation: orbital layer to the top left and global layer to the bottom right. Scale bars = 100 μm.
Loss of troponin I and T associated with slow-MyHC expressing myofibres in the Pitx2Δflox/Δflox extraocular muscle
Troponins are proteins that associate with actin filaments and modulate muscle contraction. Troponin I, T or C each has fast, slow, or cardiac isoforms, expressed differentially among muscle fibres (Gomes et al. 2002). Since slow-MyHC is no longer expressed during maturation of the Pitx2Δflox/Δflox extraocular muscle, we evaluated the expression of slow troponin I. In the Pitx2flox/flox extraocular muscle, all slow-MyHC myofibres expressed the slow skeletal muscle troponin I (Fig. 3A–C). At 3 months the Pitx2Δflox/Δflox extraocular muscle had a marked reduction of slow troponin I expressing fibres when most of slow-MyHC fibres were absent (Fig. 3D–F). A few troponin I positive fibres (arrows in Fig. 3D and F) were found which did not express slow-MyHC, and some of the slow-MyHC positive fibres did not express troponin I (dashed arrow in Fig. 3E). Cardiac troponin T, like slow skeletal muscle troponin I, was exclusively expressed in slow-MyHC myofibres in the Pitx2flox/flox extraocular muscle but was totally absent from the Pitx2Δflox/Δflox extraocular muscle except in very few orbital fibres (Supplemental Fig. S2). However, we cannot be completely certain that cardiac troponin T was expressed in wild-type extraocular muscle as this antibody CT3 from DSHB also stained slow-MyHC myofibres in diaphragm (data not shown).
Figure 3. Loss of muscle regulatory protein troponin I in Pitx2Δflox/Δflox extraocular muscle.

Mid-belly cross-sections of 3-month-old Pitx2Δflox/Δflox extraocular muscle (D, E and F) and Pitx2flox/flox control littermates (A, B and C) were doubly immunolabelled with antibody to slow-MyHC (A and D) and skeletal muscle troponin I (B and E). In the wild-type control Pitx2flox/flox mice, skeletal muscle TnnI is colocalized mainly in slow-MyHC positive myofibres (merged image C). A dramatic decrease in TnnI expression is observed in the Pitx2Δflox/Δflox mice (E). Arrows point to the slow-MyHC positive only myofibres while dashed arrow points to the troponin I expressing only myofibres in the Pitx2Δflox/Δflox mice. Orientation: orbital layer to the top left and global layer to the bottom right. Scale bars = 100 μm.
Pitx2 ablation leads to loss of en grappe endplates and a reduction of fibre number
We evaluated extraocular muscle for the presence of en grappe endplates, which are only present on slow-MyHC expressing fibres of normal extraocular muscle (Rubinstein & Hoh, 2000; Khanna et al. 2003; Fraterman et al. 2006). At 3 months, the en grappe endplates were present on the slow-MyHC expressing fibres in the proximal and distal regions of the Pitx2flox/flox extraocular muscle (Fig. 4A and B), but not detected in the Pitx2Δflox/Δflox extraocular muscle (Fig. 4C and D). The en plaque endplates were present in the mid-belly region of both Pitx2flox/flox (Fig. 4A and B) and Pitx2Δflox/Δflox extraocular muscle (Fig. 4C and D), although the endplate zone in the Pitx2Δflox/Δflox mice appeared broader.
Figure 4. Loss of multi-innervation in Pitx2Δflox/Δflox extraocular muscle.
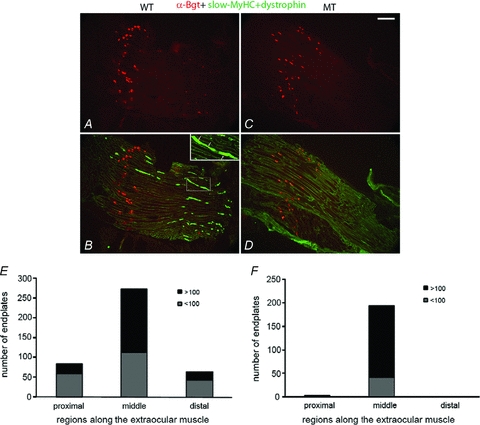
Longitudinal sections of extraocular muscle from Pitx2flox/flox mice (A and B) or Pitx2Δflox/Δflox (C and D) were immunostained with antibodies to slow-MyHC and dystrophin to outline all myofibre boundaries (both are in green) and counterstained with alexa-594 labelled α-Bgt (red). The large, en plaque endplates are present in the midbelly regions of both Pitx2flox/flox (A and B) and Pitx2Δflox/Δflox (C and D) mice. However, the small, en grappe endplates that were located in the distal region and associated with slow-MyHC myofibres in Pitx2flox/flox extraocular muscle (B, also see inset in B) were not detected in the Pitx2Δflox/Δflox mice (D). Analysis for size and location of endplates are consistent with the result that the small, en grappe endplates from the two distal regions were lost in Pitx2Δflox/Δflox mice (F) but not in Pitx2flox/flox mice (E). Three groups of independent mice were analysed with similar results. Scale bars = 100 μm.
To further confirm the loss of en grappe endplates, we counted the number and measured the sizes of α-Bgt clusters in cross-sections through the length of the superior rectus muscle. Many small endplates, typical in morphology of en grappe endings, were present in the proximal, mid-belly, and distal regions in the Pitx2flox/flox extraocular muscle (Fig. 4E). Only rare α-Bgt-labelled clusters were found in the proximal and distal regions of the Pitx2Δflox/Δflox extraocular muscle (Fig. 4F).
In contrast to our original report (Zhou et al. 2009), we identified a 12% decrease of total fibre numbers in 3-month-old Pitx2Δflox/Δflox (Pitx2flox/flox 859 ± 3 VS Pitx2Δflox/Δflox 753 ± 25, n = 3, P = 0.002). The method in the first report used an automated computer program to identify and count fibres; in contrast in this study we used manual tagging to identify fibres, which we now think is a more reliable method.
Loss of Pitx2 leads to loss of EOM-MyHC expression
EOM-MyHC is not expressed until P5 in wild-type extraocular muscle (Zhou et al. 2010), but during maturation the number of EOM-MyHC expressing fibres increases along with the intensity of immunoreactivity (Fig. 5A, C and E). By P21, EOM-MyHC had an expression pattern similar to 3 months (compare Fig. 5C to E). In Pitx2Δflox/Δflox extraocular muscle, the number of EOM-MyHC expressing myofibres was less than that in the Pitx2flox/flox mice as early as P7 (Fig. 5B) and continued to decrease over time (Fig. 5B, D and F). By 3 months, only a few positive EOM-MyHC reactive fibres were detected in the Pitx2Δflox/Δflox mice (Fig. 5F).
Figure 5. Loss of EOM-MyHC expressing myofibres in Pitx2Δflox/Δflox mice.

Mid-belly cross-sections of extraocular muscle from Pitx2Δflox/Δflox mice (B, D and F) and Pitx2flox/flox control littermates (A, C and E) at P14 (A and B), P21 (C and D) and 3 months (E and F) were immunolabelled with antibody to EOM-MyHC. The number of EOM-MyHC positive myofibres was already reduced in Pitx2Δflox/Δflox mice at P7 (B compared to A for the wild-type Pitx2flox/flox extraocular muscle) and continued to decrease with time that at 3 months of age, only a few positive fibres were observed in the Pitx2Δflox/Δflox mice (F compared to E for wild-type Pitx2flox/flox extraocular muscle). Orientation: orbital layer to the top left and global layer to the bottom right. Scale bars = 100 μm.
2A-MyHC myofibres increase in the Pitx2Δflox/Δflox extraocular muscle
In the Pitx2flox/flox extraocular muscle, the expression of 2A-MyHC exhibited longitudinal variation with primary expression in the orbital layer at both the distal and proximal ends (Fig. 6A and E) and only a few fibres in the mid-belly zone (Fig. 6C). In contrast, the Pitx2Δflox/Δflox extraocular muscle had a greatly increased number of 2A-MyHC myofibres in the mid-belly region (Fig. 6D) and 2A-MyHC expression was similar to that in Pitx2flox/flox extraocular muscle at the muscle tips (Fig. 6B and F).
Figure 6. Increase of 2A-MyHC positive fibres in mid-belly region of Pitx2Δflox/Δflox extraocular muscle.

Cross-sections of extraocular muscle from proximal (A and B), mid-belly (C and D) and distal region (E and F) of Pitx2Δflox/Δflox mice (B, D and F) and control littermates Pitx2flox/flox mice (A, C and E) were immunolabelled with antibody to 2A-MyHC. 2A-MyHC positive myofibres are nearly absent in the mid-belly region in the control littermate Pitx2flox/flox mice (C) but are retained in the Pitx2Δflox/Δflox mice. 2A-MyHC positive fibres are predominantly present in orbital layers at proximal and distal regions in both Pitx2Δflox/Δflox mice and Pitx2flox/flox mice. Counting of 2A-MyHC positive and negative myofibres in Pitx2flox/flox (G) and Pitx2Δflox/Δflox (H) mice and their corresponding percentages (I) showed similar results. Shown is a representative example of myofibre counting with data repeated 3 times with similar results. Orientation in A–H: orbital layer to the top left and global layer to the bottom right. Scale bar: 100 μm
We counted 2A-MyHC positive and negative myofibres through the length of the extraocular muscle. In Pitx2flox/flox extraocular muscle (Fig. 6G), there was a gradual decrease in the number of 2A-MyHC positive myofibres from proximal toward mid-belly region and then a gradual increase in the number of 2A-MyHC positive fibres away from the mid-belly region toward the distal end except for the very tips of the muscle, where the total number of myofibres was less than in the middle region. The same pattern existed for the percentage of 2A-MyHC expressing myofibres (Fig. 6I). In contrast, in Pitx2Δflox/Δflox extraocular muscle the number and percentage of 2A-MyHC positive myofibres remained relatively constant in the middle sections (Fig. 6H and I).
2A-MyHC myofibres replace some of the slow-MyHC myofibres in the Pitx2Δflox/Δflox mice
Because of the reduction in the number of slow-MyHC fibres and EOM-MyHC fibres, the increase in the number of 2A-MyHC myofibres, and the absence of degenerating myofibres in the Pitx2Δflox/Δflox extraocular muscle (Zhou et al. 2009), we speculated whether slow-MyHC and EOM-MyHC myofibres switched expression to other MyHC isoforms. We took advantage of our previous observation that 2A-MyHC and slow-MyHC are never coexpressed in single myofibres in the normal mouse extraocular muscle (Zhou et al. 2010). Double immunostaining was performed on cross-sections at P18, at which time the slow-MyHC myofibres were still relatively abundant in the Pitx2Δflox/Δflox extraocular muscle. 2A-MHC and slow-MHC were not found in the same fibres of the P18 Pitxflox/flox extraocular muscle (Fig. 7A–C; blue arrows point to the slow-MyHC expressing only fibres). In contrast, 2A-MyHC and slow-MyHC were coexpressed in some fibres of the Pitx2Δflox/Δflox extraocular muscle (white arrows in Fig. 7D–F), suggesting that certain myofibres were in the process of transition from slow-MyHC to 2A-MyHC expression.
Figure 7. Coexpression of slow-MyHC and 2A-MyHC in single myofibres in Pitx2Δflox/Δflox mice.

Cross-sections of Pitx2Δflox/Δflox (D, E and F) and Pitx2flox/flox extraocular muscles (A, B and C) from P18 mice were doubly immunolabelled with antibodies to slow-MyHC (green) and 2A-MyHC (red). 2A-MyHC and slow-MyHC are normally expressed in separate myofibres in wild-type Pitx2Δflox/Δflox extraocular muscle (A, B and C, blue arrows point to a few slow-MyHC positive but 2A-MyHC negative fibres). However, many doubly immunolabelled myofibres (white arrows in D, E and F) were detected in the Pitx2Δflox/Δflox mice extraocular muscle at P18, suggesting that some of slow-MyHC myofibres are replaced by 2A-MyHC fibres. There were some slow-MyHC fibres that were not colocalized with 2A-MyHC expression in the Pitx2Δflox/Δflox extraocular muscle (blue arrows in D, E and F). Orientation: orbital layer to the top left and global layer to the bottom right. * denotes the position of retractor muscle. Scale bar: 100 μm.
Increased number of pure 2X-MHC myofibres with Pitx2 absence
Although some of the slow-MyHC myofibres in the Pitx2Δflox/Δflox extraocular muscle were replaced by 2A-MyHC myofibres, the conversion to 2A-MyHC myofibres could not account for the loss of all slow-MyHC expression. Also, we had no explanation for the reduction of EOM-MyHC expressing fibres. Previously, we found that only four MyHC isoforms (slow-MyHC, EOM-MyHC, 2B- and 2X-MyHC) were present in the global layer (Zhou et al. 2010). While 2B-MyHC did not display an obvious alteration in expression in Pitx2Δflox/Δflox extraocular muscle (see below), we evaluated whether the number of 2X-MyHC expressing myofibres was altered. Pure 2X-MyHC expression exhibited longitudinal variations along the extraocular muscle and was excluded from the mid-belly region of the Pitxflox/flox extraocular muscle (Fig. 8A, C and E). However, in the Pitx2Δflox/Δflox mice, pure 2X-MyHC myofibres were found in the mid-belly region (Fig. 8D) in addition to the 2X-MyHC fibres at the two ends. Quantitative analysis of 2X-MyHC expressing fibres (Fig. 8G–I) was consistent with the hypothesis that 2X-MyHC expression may substitute for slow-MHC and EOM-MyHC expression in some myofibres.
Figure 8. Pure 2X-MyHC myofibres are present in mid-belly region in Pitx2Δflox/Δflox extraocular muscle.

Cross-sections of extraocular muscle from proximal (A and B), mid-belly (C and D) and distal region (E and F) of Pitx2Δflox/Δflox mice (B, D and F) and control littermates Pitx2flox/flox mice (A, C and E) were doubly immunolabelled with an antibody against all fibres except 2X-MyHC (BF-35) and antibody to dystrophin to outline myofibre boundary. Pure 2X-MyHC fibres are represented by the negatively immunolabelled fibres (arrows point to a few pure 2X-MyHC fibres). Pure 2X-MyHC fibres are present in the global layer in the proximal (A and B) and distal (E and F) regions in both Pitx2Δflox/Δflox (B and F) and Pitx2flox/flox (A and E) mice. Pure 2X-MyHC myofibres are not detected in the mid-belly region in Pitx2flox/flox mice (C) but are present in the same region in the Pitx2Δflox/Δflox mice (D) and populate predominantly the outer global layer, suggesting that some of the slow-MyHC and EOM-MyHC myofibres are replaced by pure 2X-MyHC fibres. Counting of pure 2X-MyHC and non-pure 2X-MyHC fibres in Pitx2flox/flox (G) and Pitx2Δflox/Δflox (H) mice and their corresponding percentages (I) showed similar results. Shown is a representative example of myofibre counting with data repeated 3 times with similar results.Orientation in A–H: orbital layer to the top left and global layer to the bottom right. * denotes the position of retractor muscle. Scale bar: 100 μm.
Ptix2 loss does not alter expression of emb-MyHC and 2B-MyHC
We did not detect a difference in the expression of emb-MyHC and 2B-MyHC between Pitx2flox/flox and Pitx2Δflox/Δflox extraocular muscle. Emb-MyHC positive myofibres were observed in the orbital layer at the distal and proximal ends in both Pitx2flox/flox and Pitx2Δflox/Δflox extraocular muscle. 2B-MyHC isoforms was predominantly expressed in the global layer with no longitudinal variations (data not shown), the same pattern as in the Pitx2flox/flox extraocular muscle.
Discussion
We found that MyHC isoform expression in postnatal extraocular muscle is under the partial control of Pitx2 transcriptional factor based on the fundamental alterations of expression of slow-MyHC, EOM-MyHC, 2A-MyHC and 2X-MyHC isoforms in Pitx2Δflox/Δflox extraocular muscle, in which Pitx2 gene expression is knocked out at P0 and Pitx2 protein significantly reduced by P21 (Zhou et al. 2009). First, slow-MyHC myofibres that were present in early postnatal extraocular muscle were nearly gone along with the associated regulatory molecules slow troponin I and troponin T by 3 months of age in the Pitx2Δflox/Δflox extraocular muscle. Second, EOM-MyHC expression began to decrease in the Pitx2Δflox/Δflox extraocular muscle by P7 and continued to decrease to 3 months when very few myofibres were immunoreactive for EOM-MyHC. Third, the longitudinal variation of 2A-MyHC and pure 2X-MyHC expressions was lost in Pitx2Δflox/Δflox extraocular muscle. Instead, both the number of 2A-MyHC and of pure 2X-MyHC positive fibres increased, most prominently in the mid-belly region, an area in which the two MyHC isoforms are excluded in wild-type extraocular muscle. Since there are no degenerating fibres detected in the Pitx2Δflox/Δflox extraocular muscle (Zhou et al. 2009), the slow- and EOM-MyHC expression appears to be down-regulated, these fibers may switch expression to 2A-MyHC and 2X-MyHC. Fourth, Pitx2 does not influence emb-MyHC and 2B-MyHC expression. These data indicate that Pitx2 plays a fundamental regulatory role in the expression of MyHC isoforms in the adult extraocular muscle of the mouse.
Slow-MyHC myofibres were present in Pitx2Δflox/Δflox extraocular muscle at a level equivalent to that in Pitx2flox/flox extraocular muscle during early postnatal stages at the time Pitx2 expression is beginning to be inactivated (Zhou et al. 2009). However, slow-MyHC expression began to decrease after P7, during which time Pitx2 immunoreactivity begins to decrease in the Pitx2Δflox/Δflox extraocular muscle. Our results indicate that Pitx2 is required to maintain slow-MyHC expression in extraocular muscle throughout life. This is consistent with the fact that there are four bicoid binding sites (5′-TAATCC-3′) 3000 bp upstream to the Myh7 mRNA start site (results from analysis of Myh7 promotor region), suggesting that Pitx2 regulates Myh7 expression by binding directly to the promoter region of Myh7 gene. In mammalian and avian muscle, electrical stimulation and innervation has been shown to influence slow-MyHC expression (DiMario & Stockdale, 1997; Pette, 2002; Crew et al. 2010) and therefore we evaluated in the Pitx2Δflox/Δflox extraocular muscle for acetylcholine receptor clusters. We were surprised to observe the loss of en grappe endplates in the Pitx2Δflox/Δflox extraocular muscle because there was no prior expectation for Pitx2 to play a role in muscle innervation. It appears that Pitx2 expression within the muscle is a critical determinant of the unique multi-innervational pattern of extraocular muscle. We are actively evaluating the mechanisms underlying this observation. From our present analysis, we cannot absolutely determine what is happening to the slow-MyHc fibres with certainty. Because of the reduction in the number of slow-MyHC fibres and EOM-MyHC fibres, the increase in the number of 2A-MyHC myofibres, and the absence of degenerating myofibres in the Pitx2Δflox/Δflox extraocular muscle (Zhou et al. 2009), we speculated that slow-MyHC and EOM-MyHC myofibres were switching expression to other MyHC isoforms. We did observe a lower number of fibres in the Pitx2Δflox/Δflox extraocular muscle suggesting a true loss of fibres; however, the extraocular muscle fibres are not continuous (McLoon et al. 1999) and therefore, we cannot be absolutely certain that the fibre number is reduced.
Slow-MyHC expression has been used as a marker for multi-innervated fibres (Bormioli et al. 1980; Rubinstein & Hoh, 2000; Khanna et al. 2003; Kjellgren et al. 2003; Fraterman et al. 2006). Previous studies have indicated that the muscle fibres expressing the slow-tonic MyHC isoform in rat and human extraocular muscle are the fibres that co-express slow-MyHC, and so are likely to be multi-innervated (Kjellgren et al. 2003; Rossi et al. 2010). However, we found that some slow-tonic MyHC immunoreactive myofibres from the orbital layer do not co-express slow-MyHC isoform in the mouse extraocular muscle (Fig. S1). In the Pitx2Δflox/Δflox extraocular muscle, the number of slow-tonic MyHC positive fibres is greatly reduced in the mid-belly region but there are a few weakly positive fibres in the orbital layer from the proximal and distal end, which corresponds to the location of fibres that express slow-tonic MyHC but not slow-MyHC (Fig. S1). This suggests that slow-tonic MyHC expression is independently regulated by Pitx2 from slow-MyHC expression. To further support this notion, we found that the earliest time that a reduction of slow-tonic MyHC expression was detected in the Pitx2Δflox/Δflox was P7, a time when slow-MyHC is still abundantly expressed with no differences compared to the Pitx2flox/flox extraocular muscle (Fig. 1). Since the multi-innervation, en grappe endplates are lost from the proximal and distal ends of the Pitx2Δflox/Δflox muscle while some slow-tonic MyHC expressing fibres are still present, we suggest that expression of slow-tonic MyHC in these remaining myofibres is not subject to innervational regulation.
Unlike slow-MyHC isoform, EOM-MyHC is not normally expressed until around P5 in wild-type extraocular muscle (Zhou et al. 2010). At P7, the number of EOM-MyHC myofibres is already reduced in Pitx2Δflox/Δflox extraocular muscle compared with Pitx2flox/flox extraocular muscle. This observation suggests that the initiation of EOM-MyHC expression is dependent on Pitx2 expression. Consistent with this observation is that the Myh13 gene, which encodes the EOM-MyHC isoform, contains a potential Pitx2 binding domain in its cis-regulatory region (Schachat & Briggs, 2002). The presence of some EOM-MyHC positive fibres between P7 and P21 in Pitx2Δflox/Δflox extraocular muscle may be due to the presence of residual Pitx2 protein during this time period despite the Pitx2 gene having been recombined and inactivated by Cre-recombinase by P0 (Zhou et al. 2009). At 3 months, only a few EOM-MyHC positive myofibres are detected in Pitx2Δflox/Δflox extraocular muscle. The maintenance of both slow-MyHC and EOM-MyHC expression appears to require the expression of Pitx2.
The dramatic loss of slow-MyHC and EOM-MyHC fibres is not accompanied by degenerating myofibres (Zhou et al. 2009), suggesting that these fibres have undergone an alteration of MyHC isoform expression. To support this conclusion, we found that in the Pitx2Δflox/Δflox extraocular muscle at P18 some slow-MyHC fibres coexpress 2A-MyHC, which was never observed in Pitx2flox/flox or pure wild-type mice (Zhou et al. 2010). This indicates that slow-MyHC fibres may move to expression of the 2A-MyHC isoform. However, the pure conversion to 2A-MyHC expression appears not to completely explain the loss of slow-MyHC expressing fibres. First, the number of 2A-MyHC fibres is increased most obviously in the mid-belly region, while loss of slow-MyHC expressing fibres occurs throughout the length of the muscle. Second, even though we observed some of the global slow-MyHC myofibres coexpressing 2A-MyHC at P18 (Fig. 7), 2A-MyHC fibres are predominant in the orbital layer and very few 2A-MyHC myofibres are observed in the global layer at 3 months (see Fig. 6). These observations suggest that the slow-MyHC expressing fibres switch expression not only to 2A-MyHC but also other isoforms, and some of the slow-MyHC to 2A-MyHC fibres further switch to other isoforms.
The EOM-MyHC fibres are essentially lost with the conditional knockout of Pitx2, but we do not have a full explanation for what is happening with these previously expressing EOM-MyHC fibres. Again, there is no evidence of fibre degeneration indicating that conversion to a different MyHC isoform must be occurring. To support this idea, we found that pure 2X-MyHC fibres, which were detected only at the proximal and distal ends in the wild-type (Zhou et al. 2010) and Pitx2flox/flox extraocular muscle, were present in the mid-belly region in Pitx2Δflox/Δflox extraocular muscle. However, since EOM-MyHC is expressed in fibres of both the orbital and global layers in Pitx2flox/flox extraocular muscle and that pure 2X-MyHC myofibres are found only in the orbital and global boundary areas in the Pitx2Δflox/Δflox extraocular muscle, conversion to 2X-MyHC expression cannot account for all the ‘lost’ EOM-MyHC expressing fibres. Although Pitx2 down-regulation is likely to account for disappearance of EOM-MyHC, there must be other regulatory programmes accounting for conversion of expression to 2X-MyHC in some fibres and an undetermined isoform(s) in other fibres. These programmes become apparent with the disappearance of the dominant Pitx2 pathway.
In contrast to the altered expression of slow-MyHC, EOM-specific, 2X- and 2A-MyHC isoforms, we found that the expression of emb-MyHC and 2B-MyHC isoforms, was not altered with Pitx2 ablation. The persistent presence of developmental isoforms in adult extraocular muscle indicates that non-Pitx2 related mechanisms regulate expression of this MyHC isoform.
As isoforms are functionally different (Allen et al. 2000), the fibre type switch of the Pitx2Δflox/Δflox extraocular muscle is expected to result in altered physiological properties (Zhou et al. 2009). In vitro functional studies have demonstrated that the Pitx2Δflox/Δflox extraocular muscle had higher velocity of shortening and greater peak tetanic force but fatigued more rapidly (Zhou et al. 2009). The increased shortening velocity is consistent with the observed decrease in the slow MHC isoform, and increase in the expression of MyHC-2A and MyHC-2X. (Resnicow et al. 2010)
Conclusions
The findings identify that in vivo Pitx2 serves to modulate expression of certain MyHC isoforms. The conditional knockout of Pitx2 at the time of birth leads to suppression of EOM-MyHC and slow-MyHC likely through its direct binding to these genes' promoter region. In addition, loss of slow-MyHC expression is also influenced through the absence of multi-innervation consistent with other studies that demonstrate slow-MyHC expression is regulated by innervation and electrical activity. The results of our present and previous work (Zhou et al. 2009) suggest that educated manipulation of MyHC expression could one day be exploited to modify extraocular muscle contractile characteristics and a therapeutic modality.
Acknowledgments
This work was supported by NIH grant R01 EY-015306.
Glossary
Abbreviations
- α-Bgt
α-bungarotoxin
- emb
embryonic
- EOM
extraocular muscle
- neo
neonatal
- MyHC
myosin heavy chain
- Pitx2
paired-like homeodomain transcription factor 2
- Pitx2Δflox/Δflox
Pitx2 conditional knockout mice
Author contributions
Y.Z. and H.J.K designed research; Y.Z. and D.L. performed experiments; Y.Z. and H.J.K. reviewed all results and wrote the manuscript together. All authors read and approved the manuscript for publication.
Supplementary material
Figure S1
Figure S2
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer-reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors
References
- Allen DL, Harrison BC, Leinwand LA. Inactivation of myosin heavy chain genes in the mouse: diverse and unexpected phenotypes. Microsc Res Tech. 2000;50:492–499. doi: 10.1002/1097-0029(20000915)50:6<492::AID-JEMT6>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Bicer S, Reiser PJ. Myosin isoform expression in dog rectus muscles: patterns in global and orbital layers and among single fibers. Invest Ophthalmol Vis Sci. 2009;50:157–167. doi: 10.1167/iovs.08-2416. [DOI] [PubMed] [Google Scholar]
- Bormioli SP, Sartore S, Vitadello M, Schiaffino S. “Slow” myosins in vertebrae skeletal muscle. An immunofluorescence study. J Cell Biol. 1980;85:672–681. doi: 10.1083/jcb.85.3.672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bormioli SP, Torresan P, Sartore S, Moschini GB, Schiaffino S. Immunohistochemical identification of slow-tonic fibers in human extrinsic eye muscles. Invest Ophthalmol Vis Sci. 1979;18:303–306. [PubMed] [Google Scholar]
- Bottinelli R, Betto R, Schiaffino S, Reggiani C. Unloaded shortening velocity and myosin heavy chain and alkali light chain isoform composition in rat skeletal muscle fibres. J Physiol. 1994;478:341–349. doi: 10.1113/jphysiol.1994.sp020254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottinelli R, Schiaffino S, Reggiani C. Force–velocity relations and myosin heavy chain isoform compositions of skinned fibres from rat skeletal muscle. J Physiol. 1991;437:655–672. doi: 10.1113/jphysiol.1991.sp018617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs MM, Schachat F. The superfast extraocular myosin (MYH13) is localized to the innervation zone in both the global and orbital layers of rabbit extraocular muscle. J Exp Biol. 2002;205:3133–3142. doi: 10.1242/jeb.205.20.3133. [DOI] [PubMed] [Google Scholar]
- Brueckner JK, Itkis O, Porter JD. Spatial and temporal patterns of myosin heavy chain expression in developing rat extraocular muscle. J Muscle Res Cell Motil. 1996;17:297–312. doi: 10.1007/BF00240928. [DOI] [PubMed] [Google Scholar]
- Crew JR, Falzari K, DiMario JX. Muscle fiber type specific induction of slow myosin heavy chain 2 gene expression by electrical stimulation. Exp Cell Res. 2010;316:1039–1049. doi: 10.1016/j.yexcr.2010.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diehl AG, Zareparsi S, Qian M, Khanna R, Angeles R, Gage PJ. Extraocular muscle morphogenesis and gene expression are regulated by Pitx2 gene dose. Invest Ophthalmol Vis Sci. 2006;47:1785–1793. doi: 10.1167/iovs.05-1424. [DOI] [PubMed] [Google Scholar]
- DiMario JX, Stockdale FE. Both myoblast lineage and innervation determine fiber type and are required for expression of the slow myosin heavy chain 2 gene. Dev Biol. 1997;188:167–180. doi: 10.1006/dbio.1997.8619. [DOI] [PubMed] [Google Scholar]
- Fraterman S, Khurana TS, Rubinstein NA. Identification of acetylcholine receptor subunits differentially expressed in singly and multiply innervated fibers of extraocular muscles. Invest Ophthalmol Vis Sci. 2006;47:3828–3834. doi: 10.1167/iovs.06-0073. [DOI] [PubMed] [Google Scholar]
- Gage PJ, Suh H, Camper SA. Dosage requirement of Pitx2 for development of multiple organs. Development. 1999;126:4643–4651. doi: 10.1242/dev.126.20.4643. [DOI] [PubMed] [Google Scholar]
- Gomes AV, Potter JD, Szczesna-Cordary D. The role of troponins in muscle contraction. IUBMB Life. 2002;54:323–333. doi: 10.1080/15216540216037. [DOI] [PubMed] [Google Scholar]
- Hjalt TA, Semina EV. Current molecular understanding of Axenfeld-Rieger syndrome. Expert Rev Mol Med. 2005;7:1–17. doi: 10.1017/S1462399405010082. [DOI] [PubMed] [Google Scholar]
- Jacoby J, Ko K, Weiss C, Rushbrook JI. Systematic variation in myosin expression along extraocular muscle fibres of the adult rat. J Muscle Res Cell Motil. 1990;11:25–40. doi: 10.1007/BF01833323. [DOI] [PubMed] [Google Scholar]
- Jung HH, Lieber RL, Ryan AF. Quantification of myosin heavy chain mRNA in somatic and branchial arch muscles using competitive PCR. Am J Physiol Cell Physiol. 1998;275:C68–74. doi: 10.1152/ajpcell.1998.275.1.C68. [DOI] [PubMed] [Google Scholar]
- Khanna S, Richmonds CR, Kaminski HJ, Porter JD. Molecular organization of the extraocular muscle neuromuscular junction: partial conservation of and divergence from the skeletal muscle prototype. Invest Ophthalmol Vis Sci. 2003;44:1918–1926. doi: 10.1167/iovs.02-0890. [DOI] [PubMed] [Google Scholar]
- Kjellgren D, Thornell LE, Andersen J, Pedrosa-Domellof F. Myosin heavy chain isoforms in human extraocular muscles. Invest Ophthalmol Vis Sci. 2003;44:1419–1425. doi: 10.1167/iovs.02-0638. [DOI] [PubMed] [Google Scholar]
- Kranjc BS, Sketelj J, Albis AD, Ambroz M, Erzen I. Fibre types and myosin heavy chain expression in the ocular medial rectus muscle of the adult rat. J Muscle Res Cell Motil. 2000;21:753–761. doi: 10.1023/a:1010362926221. [DOI] [PubMed] [Google Scholar]
- Lim SJ, Jung HH, Cho YA. Postnatal development of myosin heavy chain isoforms in rat extraocular muscles. Mol Vis. 2006;12:243–250. [PubMed] [Google Scholar]
- Lin CR, Kioussi C, O'Connell S, Briata P, Szeto D, Liu F, Izpisua-Belmonte JC, Rosenfeld MG. Pitx2 regulates lung asymmetry, cardiac positioning and pituitary and tooth morphogenesis. Nature. 1999;401:279–282. doi: 10.1038/45803. [DOI] [PubMed] [Google Scholar]
- Martin DM, Skidmore JM, Fox SE, Gage PJ, Camper SA. Pitx2 distinguishes subtypes of terminally differentiated neurons in the developing mouse neuroepithelium. Dev Biol. 2002;252:84–99. doi: 10.1006/dbio.2002.0835. [DOI] [PubMed] [Google Scholar]
- Martin DM, Skidmore JM, Philips ST, Vieira C, Gage PJ, Condie BG, Raphael Y, Martinez S, Camper SA. PITX2 is required for normal development of neurons in the mouse subthalamic nucleus and midbrain. Dev Biol. 2004;267:93–108. doi: 10.1016/j.ydbio.2003.10.035. [DOI] [PubMed] [Google Scholar]
- McLoon LK, Rios L, Wirtschafter JD. Complex three-dimensional patterns of myosin isoform expression: differences between and within specific extraocular muscles. J Muscle Res Cell Motil. 1999;20:771–783. doi: 10.1023/a:1005656312518. [DOI] [PubMed] [Google Scholar]
- Pette D. The adaptive potential of skeletal muscle fibers. Can J Appl Physiol. 2002;27:423–448. doi: 10.1139/h02-023. [DOI] [PubMed] [Google Scholar]
- Pette D, Staron RS. Mammalian skeletal muscle fiber type transitions. Int Rev Cytol. 1997;170:143–223. doi: 10.1016/s0074-7696(08)61622-8. [DOI] [PubMed] [Google Scholar]
- Piedra ME, Icardo JM, Albajar M, Rodriguez-Rey JC, Ros MA. Pitx2 participates in the late phase of the pathway controlling left-right asymmetry. Cell. 1998;94:319–324. doi: 10.1016/s0092-8674(00)81475-0. [DOI] [PubMed] [Google Scholar]
- Resnicow DI, Deacon JC, Warrick HM, Spudich JA, Leinwand LA. Functional diversity among a family of human skeletal muscle myosin motors. Proc Natl Acad Sci U S A. 2010;107:1053–1058. doi: 10.1073/pnas.0913527107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi AC, Mammucari C, Argentini C, Reggiani C, Schiaffino S. Two novel/ancient myosins in mammalian skeletal muscles: MYH14/7b and MYH15 are expressed in extraocular muscles and muscle spindles. J Physiol. 2010;588:353–364. doi: 10.1113/jphysiol.2009.181008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinstein NA, Hoh JF. The distribution of myosin heavy chain isoforms among rat extraocular muscle fiber types. Invest Ophthalmol Vis Sci. 2000;41:3391–3398. [PubMed] [Google Scholar]
- Rubinstein NA, Porter JD, Hoh JF. The development of longitudinal variation of myosin isoforms in the orbital fibers of extraocular muscles of rats. Invest Ophthalmol Vis Sci. 2004;45:3067–3072. doi: 10.1167/iovs.04-0106. [DOI] [PubMed] [Google Scholar]
- Ruff R, Kaminski H, Maas E, Spiegel P. Ocular muscles: physiology and structure-function correlations. Bull Soc Belge Ophtalmol. 1989;237:321–352. [PubMed] [Google Scholar]
- Ryan AK, Blumberg B, Rodriguez-Esteban C, Yonei-Tamura S, Tamura K, Tsukui T, de la Pena J, Sabbagh W, Greenwald J, Choe S, Norris DP, Robertson EJ, Evans RM, Rosenfeld MG, Izpisua Belmonte JC. Pitx2 determines left-right asymmetry of internal organs in vertebrates. Nature. 1998;394:545–551. doi: 10.1038/29004. [DOI] [PubMed] [Google Scholar]
- Sartore S, Mascarello F, Rowlerson A, Gorza L, Ausoni S, Vianello M, Schiaffino S. Fibre types in extraocular muscles: a new myosin isoform in the fast fibres. J Muscle Res Cell Motil. 1987;8:161–172. doi: 10.1007/BF01753992. [DOI] [PubMed] [Google Scholar]
- Schachat F, Briggs MM. Phylogenetic implications of the superfast myosin in extraocular muscles. J Exp Biol. 2002;205:2189–2201. doi: 10.1242/jeb.205.15.2189. [DOI] [PubMed] [Google Scholar]
- Spencer RF, Porter JD. Biological organization of the extraocular muscles. Prog Brain Res. 2006;151:43–80. doi: 10.1016/S0079-6123(05)51002-1. [DOI] [PubMed] [Google Scholar]
- Wieczorek DF, Periasamy M, Butler-Browne GS, Whalen RG, Nadal-Ginard B. Co-expression of multiple myosin heavy chain genes, in addition to a tissue-specific one, in extraocular musculature. J Cell Biol. 1985;101:618–629. doi: 10.1083/jcb.101.2.618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Cheng G, Dieter L, Hjalt TA, Andrade FH, Stahl JS, Kaminski HJ. An altered phenotype in a conditional knockout of Pitx2 in extraocular muscle. Invest Ophthalmol Vis Sci. 2009;50:4531–4541. doi: 10.1167/iovs.08-2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Liu D, Kaminski HJ. Myosin heavy chain expression in mouse extraocular muscle: more complex than expected. Invest Ophthalmol Vis Sci. 2010;51:6355–6363. doi: 10.1167/iovs.10-5937. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


