Non-technical summary
During exercise, skeletal muscle performance depends in great part on the use of aerobic metabolism to supply the energetic demand of contractions. Endurance training increases the muscle aerobic capacity, which is not only associated with enhanced exercise performance, but also with a decreased risk of cardiovascular and metabolic diseases. Recently, it has been shown that regular use of small doses of dark chocolate may result in similar health benefits to exercise training. We show here that mice fed for 15 days with (–)-epicatechin (present in dark chocolate) had improved exercise performance accompanied by: (1) an increased number of capillaries in the hindlimb muscle; and (2) an increased amount of muscle mitochondria as well as signalling for mitochondrial biogenesis. These results suggest that (–)-epicatechin increases the capacity for muscle aerobic metabolism, thereby delaying the onset of fatigue. These findings may have potential application for clinical populations experiencing muscle fatigue.
Abstract
Abstract
The flavanol (–)-epicatechin, a component of cacao (cocoa), has been shown to have multiple health benefits in humans. Using 1-year-old male mice, we examined the effects of 15 days of (–)-epicatechin treatment and regular exercise on: (1) exercise performance, (2) muscle fatigue, (3) capillarity, and (4) mitochondrial biogenesis in mouse hindlimb and heart muscles. Twenty-five male mice (C57BL/6N) were randomized into four groups: (1) water, (2) water–exercise (W-Ex), (3) (–)-epicatechin ((–)-Epi), and (4) (–)-epicatechin–exercise ((–)-Epi-Ex). Animals received 1 mg kg−1 of (–)-epicatechin or water (vehicle) via oral gavage (twice daily). Exercise groups underwent 15 days of treadmill exercise. Significant increases in treadmill performance (∼50%) and enhanced in situ muscle fatigue resistance (∼30%) were observed with (–)-epicatechin. Components of oxidative phosphorylation complexes, mitofilin, porin, nNOS, p-nNOS, and Tfam as well as mitochondrial volume and cristae abundance were significantly higher with (–)-epicatechin treatment for hindlimb and cardiac muscles than exercise alone. In addition, there were significant increases in skeletal muscle capillarity. The combination of (–)-epicatechin and exercise resulted in further increases in oxidative phosphorylation-complex proteins, mitofilin, porin and capillarity than (–)-epicatechin alone. These findings indicate that (–)-epicatechin alone or in combination with exercise induces an integrated response that includes structural and metabolic changes in skeletal and cardiac muscles resulting in greater endurance capacity. These results, therefore, warrant the further evaluation of the underlying mechanism of action of (–)-epicatechin and its potential clinical application as an exercise mimetic.
Introduction
Muscle aerobic capacity is a strong determinant of skeletal muscle function and exercise performance. Aerobic capacity is greatly enhanced by exercise training, generally due to increases in mitochondrial volume and capillarity in skeletal muscle (Holloszy & Coyle, 1984; Poole & Mathieu-Costello, 1996). In addition to the known benefits of increased mitochondrial volume on exercise performance, it is now becoming clear that exercise maintains mitochondrial viability throughout the human lifespan (Leick et al. 2010) and that reduced mitochondrial volume and mitochondrial degeneration are a strong determinant of the ageing process (Wallace, 1999). It has been recognized that skeletal muscle function and performance begin to deteriorate in middle aged humans, which can eventually lead to sarcopaenia (Nair, 2005), loss of normal activity levels, and thereby facilitate weight gain and the development of age-related pathological conditions such as diabetes and cardiovascular disease. As markers of reduced mitochondrial volume, oxidative phosphorylation proteins and mitochondrial content have been shown to decrease in skeletal muscle with ageing (Lanza & Nair, 2009).
It has been of great interest to identify natural products and nutritional supplements which may mimic and enhance the effects of exercise in order to improve muscle performance and possibly attenuate the effects of ageing-induced muscle wasting. Recent reports have linked the consumption of small amounts of dark chocolate (a product of cacao) with notable reductions in risk for the development of cardiovascular diseases (Buijsse et al. 2010). Using animal models, our group has focused on the study of cardiovascular effects of (–)-epicatechin (the main flavanoid present in dark chocolate) and have demonstrated that at low doses, there is increased nitric oxide production in endothelial cells (Ramirez-Sanchez et al. 2010) and reduced myocardial injury (Yamazaki et al. 2008, 2010). Furthermore, Ottaviani et al. (2011) recently reported that only (–)-epicatechin, and not the stereoisomers (+)-epicatechin, (–)-catechin or (+)-catechin, is capable of mediating in vivo vasodilatation. There is evidence that the production of physiological amounts of nitric oxide (via nitric oxide synthase) may play a role in skeletal muscle mitochondrial biogenesis (Wadley et al. 2007; Wadley & McConell, 2007) and capillarity (Lloyd et al. 2003), and enhance performance (Bailey et al. 2010). Interestingly, the potential effects of (–)-epicatechin have not been examined in relation to muscle aerobic capacity, fatigue development, mitochondrial volume and capillarity in skeletal and cardiac muscle. Recent studies have shown that some exercise mimetic compounds (e.g. GW1516) need a modest metabolic stimulus to enhance their effects (Narkar et al. 2008). We reasoned, therefore, that the use of (–)-epicatechin may also require such a stimulus. The combination of (–)-epicatechin plus exercise would allow us to compare and contrast the effects of this flavanol versus those of exercise alone or in combination with exercise.
Therefore, using middle aged mice (12 months old), the purposes of this present study were to examine the effects of low dose (–)-epicatechin administration (15 days) in the presence and absence of exercise on (1) exercise performance, (2) muscle fatigue, (3) muscle capillarity, and (4) mitochondrial biogenesis in mouse heart and hindlimb muscles. We hypothesized that (–)-epicatechin treated mice would demonstrate higher exercise capacity and exhibit a longer time to fatigue, which would occur secondarily to increases in skeletal muscle capillarity and oxidative capacity.
Methods
Animals and ethical approval
We studied 1-year-old, male C57BL/6N mice (n = 25; Harlan Laboratories, Inc., Indianapolis, IN, USA), which were randomized into four groups. Animals were placed four per cage and fed a standard diet without limitations. The room temperature was kept at 21°C with 12 h light–dark cycles. All animal care and experimental procedures were approved by the University of California, San Diego Animal Care and Use Committee and conform to NIH and American Physiological Society standards.
Experimental design and approach
A between-subjects design was used to determine the effects of (–)-epicatechin on the hindlimb muscles of 1-year-old mice. This age was selected because it has been shown that by 1 year there are decreases in exercise capacity when compared to young (4–6 months) mice (Leick et al. 2010). All animals performed an incremental treadmill test and were then subsequently randomized into four groups: (1) water, (2) water–exercise (W-Ex), (3) (–)-epicatechin ((–)-Epi), and (4) (–)-epicatechin–exercise ((–)-Epi-Ex). Groups 2 and 4 performed exercise on a rodent treadmill Monday through Friday during the study period. On the day after the final training session, all mice performed an incremental treadmill test. Forty-eight hours following the treadmill test, the mice were killed. The quadriceps femoris, extensor digitorum longus (EDL), and plantaris muscles for all groups were harvested and used for morphometric, biochemical, isolated-muscle preparation, and molecular analyses.
(–)-Epicatechin administration
Mice in the (–)-epicatechin groups 3 and 4 were given 1.0 mg (kg body mass)−1 twice a day (morning and evening) for 15 consecutive days, whereas animals in the control groups 1 and 2 received the vehicle (water). Both (–)-epicatechin (Sigma-Aldrich, St Louis, MO, USA) and vehicle were administered via oral gavage.
Incremental treadmill tests
On at least two occasions prior to the test all mice were familiarized with the treadmill (model CL-4, Omnitech, Columbus, OH, USA) at a slow speed (∼5 m min−1) at 10 deg incline for approximately 5–10 min. The incremental test consisted of warm-up at 4 m min−1 for 2 min followed by an increase of 2 m min−1 every minute thereafter. A shock grid (0.2 mA) and air jets at the back of the treadmill were used to discourage the mice from stopping while the treadmill belt was moving. Exhaustion was determined when the mouse was no longer able to maintain its normal running position on the treadmill and/or was unwilling to run as indicated by the frequent contact (i.e. touching the shock grid with each stride) or sitting on the shock grid consistent with previous studies from our laboratory (Malek & Olfert, 2009; Malek et al. 2010).
The running time was measured and running distance and power calculated. Distance is a function of time and speed of the treadmill, whereas power is the product of body weight (kg), gravity (9.81 m s–2), and vertical speed (m s–1× angle) (Handschin et al. 2007).
Exercise intervention
For 15 days, mice in groups 2 and 4 underwent treadmill training which began at approximately 14 m min−1 (50% of maximal treadmill speed) at 10 deg incline for 30 min five times per week. It should be noted, however, that the intent was not to provide a training stimulus, but rather to determine if (–)-epicatechin needed a metabolic stimulus to have an effect, as reported for GW1516 by Narkar et al. (2008).
Intact muscle contractility
Individual EDL muscles were rapidly dissected from each mouse. The EDL muscles (6 muscles for each group) were mounted horizontally in a muscle strip myograph system (800MS; Danish Myo Technology, Aahus, Denmark) and perfused with Tyrode solution (in mm: 121 NaCl, 5 KCl, 0.4 NaH2PO4, 1.8 CaCl2, 0.5 MgCl2, 24 NaHCO3, 5.5 glucose, 0.1 EGTA) bubbled continuously with 95% O2 and 5% CO2 (final pH 7.4) at 32–34°C during the time course of the experiment. Muscle contractions were evoked using electrical stimulation with an S48 stimulator (Grass Technologies, Quincy, MA, USA) at 1 ms pulse duration, 250 ms train duration, 40 V. After mounting the muscles, the optimal muscle length (L0) was determined at 100 Hz. After determination of L0 each muscle rested for 10 min. A force–frequency curve (1–200 Hz, 1 contraction each 100 s) was then conducted in order to determine the excitation–contraction coupling properties of the muscle. After resting again for 10 min, muscle performance was evaluated by a fatigue run in which repeated contractions were induced with increased stimulation frequency every 1 min (0.125, 0.25, 0.33 and 0.5 contractions per second; 150 Hz, 40 V, 1 ms pulse duration, 250 ms train duration) until the fatigue point, which was defined as a decrease in tension to 50% of the initial tension developed in the contractile run. After the experimental procedure, the muscles were blotted dry and weighed, and tension development was normalized with respect to the muscles cross-sectional area (N cm−2) according to Reid et al. (1987). Relative tension developed (P) was obtained and the force–frequency curves were computed from the maximum tetanic tension (P0) evoked at 150 or 200 Hz (%P/P0) and fitted by a sigmoid non-linear regression using the equation P = Pmin+ (P0XnH/F50nH+XnH), where Pmin is the minimum tension developed, F50 is the midpoint of the curve (in Hz) and nH is the Hill coefficient, which is correlated to the steepness of the curve.
Tissue preparation
All mice were anaesthetized with sodium pentobarbital (60 mg kg−1, i.p.) and both left and right gastrocnemius, plantaris and quadriceps femoris muscles were removed, along with the heart. For the plantaris muscle an entire transverse slice from the widest point of the middle belly portion of the muscles was excised and frozen in precooled isopentane (–140°C) and stored at –80°C until further processing. Transverse 10 μm serial sections were cut on a cryotome (Reichert Jung Cryocut 1800; Cambridge Instruments, Buffalo, NY, USA) at –20°C and mounted on slides for histochemical analysis of capillary number. Great care was taken to ensure that the widest part of the muscle was sectioned and that sectioning was perpendicular to the orientation of the fibres. The gastrocnemius muscle was prepared for enzyme/metabolite measurements, whereas the heart, contralateral plantaris, soleus, and quadriceps femoris muscles were processed and stored for subsequent use.
Capillary staining and indices
Sections from the plantaris muscle from all four groups were stained using the method of Rosenblatt et al. (1987). Briefly, the sections were first fixed for 5 min in a Guth and Samaha fixative (Guth & Samaha, 1970) and then incubated for 1 h at 36°C in a lead–adenosine triphosphatase staining medium to stain capillaries. It should be noted that there are no differences in the number of capillaries visualized with frozen biopsy samples using the Rosenblatt technique and the number visualized in tissue sections prepared from perfusion-fixed muscle (Hepple & Mathieu-Costello, 2001).
Muscle sections were viewed under a light microscope (40× magnification, Jenalumar, Zeiss, Jena, Germany), and digital images were taken of the sections (Sony Model DXC-960MD, Sony Corp., Tokyo, Japan). Capillaries were quantified manually from the digital image on individual fibres by a single individual. The following indices were measured: (1) the number of capillaries around a fibre (NCAF), (2) the capillary-to-fibre ratio on an individual-fibre basis (C/Fi), and (3) capillary density (CD), which was calculated by using the fibre area as the reference space (Hepple & Mathieu-Costello, 2001). Capillary-to-fibre perimeter exchange index (CFPE) was calculated as an estimate of the capillary-to-fibre surface area (Hepple, 1997). Quantification of the capillary supply was performed randomly selecting a fibre in an artifact-free region (no holes due to freeze fracture and fibres intact) (Wong et al. 2009). Fibre cross-sectional area (FCSA) and perimeter (FP) were measured with the image analysis system and commercial software (SigmaScan Pro v. 5.0; Systat Software, Inc., Point Richmond, CA, USA).
Muscle metabolic enzyme activity
The gastrocnemius muscle was separated into 10 mg pieces and homogenized in 100 volumes (w:v) of buffer (175 mm KCl, 2 mm EDTA; pH 7.4) for citrate synthase. In the buffer, frozen muscle sections were pulverized using a pestle for 60 s on ice. Homogenates were frozen in liquid nitrogen and underwent three freeze–thaw cycles using liquid nitrogen. Before use, the homogenates were thawed a final time and centrifuged at 8000 g for 1 min to remove particulate matter.
Using the supernatant, citrate synthase activity was measured via the technique of Srere (1969). The activity of citrate synthase was measured by the rate of production of the mercaptide ion based on conversion of acetyl-CoA and oxaloacetate into citrate synthase and CoA-SH. CoA-SH in the presence of 5,5′-disthiobis-2-nitrobenzoic acid (DTNB) produces mercaptide ion. Samples were analysed in a Beckman DU 730 spectrophotomer (Beckman, Fullerton, CA, USA) at 412 nm. All samples were tested in triplicate and measured at 37°C.
Western blotting
Approximately 50 mg of the heart and quadriceps femoris muscles were homogenized with a polytron in 500 μl lysis buffer (1% Triton X-100, 20 mm Tris, 140 mm NaCl, 2 mm EDTA, and 0.1% SDS) with protease and phosphatase inhibitor cocktails (P2714 and P2850, Sigma-Aldrich) supplemented with 0.15 mm PMSF, 5 mm Na3VO4 and 3 mm NaF. Homogenates were passed through an insulin syringe five times, sonicated for 30 min at 4°C and centrifuged (12,000 g) for 10 min. The total protein content was measured in the supernatant using the Bradford method. A total of 40 μg of protein was loaded onto a 4–15% precast TGX polyacrylamide gel (Bio-Rad), electrotransferred (12 V, 50 min), incubated for 1 h in blocking solution (5% non-fat dry milk in TBS plus 0.1% Tween 20 (TBS-T)), followed by a 3 h incubation at room temperature with primary mouse monoclonal antibodies: MitoProfile (complex I, 20 kDa; complex II, 30 kDa; complex III, 46 kDa; complex V, 55 kDa; Timmers et al. (2011); oxidative phosphorylation, Total OXPHOS from MitoSciences cat no. MS601), Porin (35 kDa; Huffman et al. (2008); MitoSciences cat no. MSA03), and Mitofilin (75 kDa; Iyer et al. (2009); MitoSciences cat no. MSM02). In addition, we used the following rabbit polyclonal antibodies against nNOS (∼155 kDa (Hayashi et al. 2009); Cell Signaling Cat#4234), anti-phospho-nNOS (∼155 kDa (Chen et al. 2000); Ser1417, Upstate Cat#07-544), anti-Tfam (30 kDa (Franko et al. 2008); Sigma-Aldrich Cat#SAB1401383-100UG) to examine potential mechanisms of mitochondrial biogenesis. All primary antibodies were diluted 1:1000 and GAPDH (37 kDa; Pansters et al. (2011); rabbit polyclonal, Cell Signaling cat no. 2118 14C10) primary antibody was diluted 1:2000 in TBS-T plus 5% non-fat dry milk. Membranes were washed (3× for 5 min) in TBS-T and incubated 1 h at room temperature in the presence of HRP-conjugated secondary antibodies (anti-mouse IgG, or anti-rabbit IgG HRP-linked antibodies, Cell Signaling cat nos 7076 and 7074, respectively) diluted 1:10,000 in blocking solution. Membranes were again washed 3 times in TBS-T, and the immunoblots were developed using an ECL Plus detection kit (Amersham-GE). The band intensities were digitally quantified using ImageJ software (http://www.nih.gov).
Electron microscopy
Pieces of the heart and plantaris muscles were fixed in 2% paraformaldehyde plus 2.5% glutaraldehyde (Ted Pella, Redding, CA, USA) in 0.1 m sodium cacodylate (pH 7.4) on ice for 24 h. The samples were washed three times with buffer consisting of 0.1 m sodium cacodylate plus 3 mm calcium chloride (pH 7.4) on ice and then post-fixed with 1% osmium tetroxide, 0.8% potassium ferrocyanide, 3 mm calcium chloride in 0.1 m sodium cacodylate (pH 7.4) for 3 h, washed 3 times with ice-cold distilled water, en bloc stained with 2% uranyl acetate at 4°C for 1 h, dehydrated through graded ethanol solutions, and embedded in Durcupan ACM resin (Fluka, St Louis, MO, USA). Ultrathin (80 nm) sections were post-stained with uranyl acetate and lead salts prior to imageing using a JEOL 1200FX transmission EM operated at 80 kV. The negatives were digitized at 1800 dpi using a Nikon CoolScan system, giving an image size of 4033 × 6010 pixels and a pixel resolution of 2.35 nm. A stereological analysis to ascertain the ratio of mitochondrial volume to cytoplasmic volume was performed with Adobe Photoshop. Point counting was used to determine the mitochondrial volume densities by overlaying a grid on each digitized image. Mitochondria and cytoplasm lying under intercepts were counted. The relative volume of mitochondria was expressed as the ratio of intercepts coinciding with this organelle to the intercepts coinciding with cytoplasm. Mitochondrial membrane surface areas were measured using ImageJ software (http://www.nih.gov).
Statistical analyses
All data are presented as means ± SEM. Separate one-way ANOVAs were performed to compare the relevant group means for each dependent variable. Separate 2 (time: pre-training and post-training) × 4 (group: water, W-Ex, (–)-Epi and (–)-Epi-Ex) mixed-factorial ANOVAs were performed for body mass as well as exercise indices. When appropriate, Tukey's HSD was used post hoc to determine which means were significantly different from each other (Keppel & Wickens, 2004). An α level at P ≤ 0.05 was selected for all statistical comparisons. The analyses were conducted using the Statistical Package for the Social Sciences software (SPSS, v.18.0, SPSS Inc., Chicago, IL, USA).
Results
Animals
As shown in Table 1, there were no statistically significant differences detected in body, heart and muscle masses between the four groups. In addition, there were no statistical differences detected when the data were normalized to body mass.
Table 1.
Body, heart and hindlimb muscle mass
| Groups | ||||||||
|---|---|---|---|---|---|---|---|---|
| Water only | W-Ex | (–)-Epi only | (–)-Epi-Ex | |||||
| n = 6 | n = 7 | n = 5 | n = 7 | |||||
| Day 0 | Day 15 | Day 0 | Day 15 | Day 0 | Day 15 | Day 0 | Day 15 | |
| Body mass (g) | 31.8 ± 0.8 | 32.3 ± 0.9 | 32.8 ± 0.7 | 32.4 ± 0.9 | 32.9 ± 0.9 | 32.3 ± 1.0 | 32.6 ± 0.8 | 33.1 ± 0.9 |
| Heart mass (mg) | — | 136.7 ± 6.6 | — | 151.3 ± 7.7 | — | 138.6 ± 3.8 | — | 142.2 ± 4.5 |
| Heart/body mass (mg g−1) | — | 4.30 ± 0.06 | — | 4.66 ± 0.21 | — | 4.29 ± 0.06 | — | 4.33 ± 0.21 |
| Quadriceps femoris mass (mg) | — | 169.4 ± 8.9 | — | 165.3 ± 8.5 | — | 168.3 ± 8.7 | — | 171.1 ± 5.2 |
| Quadriceps femoris/body mass (mg g−1) | — | 5.30 ± 0.39 | — | 5.09 ± 0.23 | — | 5.23 ± 0.31 | — | 5.20 ± 0.21 |
| Gastrocnemius mass | — | 109.9 ± 5.6 | — | 123.0 ± 3.4 | — | 111.7 ± 2.8 | — | 119.8 ± 3.2 |
| Gastrocnemius/body mass (mg g−1) | — | 3.42 ± 0.16 | — | 3.79 ± 0.05 | — | 3.46 ± 0.09 | — | 3.63 ± 0.09 |
| Plantaris (mg) | — | 16.4 ± 0.6 | — | 15.5 ± 1.3 | — | 17.9 ± 0.3 | — | 16.1 ± 1.1 |
| Plantaris/body mass (mg g−1) | — | 0.51 ± 0.03 | — | 0.48 ± 0.04 | — | 0.55 ± 0.02 | — | 0.49 ± 0.03 |
The data are means ± SEM. There was no significant difference (P > 0.05) between groups for any of the indices.
Incremental treadmill test
Table 2 shows the exercise indices for each group prior to and following the 15 day intervention. The two-way mixed factorial ANOVA resulted in a significant interaction. The statistical model was subsequently decomposed and these analyses indicated a statistically significant increase in all exercise indices for the (–)-epicatechin groups at Day 15 when compared to the water groups. There were no statistically significant differences detected, however, for the exercise indices within the two groups (i.e. water vs. W-Ex or (–)-Epi only vs. (–)-Epi-Ex).
Table 2.
Results of incremental treadmill test for all groups
| Groups | ||||||||
|---|---|---|---|---|---|---|---|---|
| Water only | W-Ex | (–)-Epi only | (–)-Epi-Ex | |||||
| n = 6 | n = 7 | n = 5 | n = 7 | |||||
| Day 0 | Day 15 | Day 0 | Day 15 | Day 0 | Day 15 | Day 0 | Day 15 | |
| Duration (s) | 772 ± 17 | 730 ± 29 | 806 ± 15 | 745 ± 27 | 771 ± 18 | 931 ± 32*,** | 804 ± 15 | 958 ± 27*,** |
| Speed (m min−1) | 27.3 ± 0.6 | 25.7 ± 1.0 | 28.0 ± 0.6 | 26.0 ± 0.9 | 26.8 ± 0.7 | 32.4 ± 1.1*,** | 28.3 ± 0.6 | 33.4 ± 0.9*,** |
| Distance (m) | 245 ± 9 | 222 ± 16 | 255 ± 8 | 226 ± 15 | 237 ± 10 | 323 ± 17*,** | 259 ± 8 | 343 ± 15*,** |
| Work (J) | 379 ± 16 | 343 ± 28 | 410 ± 15 | 351 ± 26 | 377 ± 18 | 533 ± 31*,** | 414 ± 15 | 580 ± 26*,** |
| Power (W) | 0.0142 ± 0.0004 | 0.0135 ± 0.0005 | 0.0150 ± 0.0004 | 0.0138 ± .0005 | 0.0144 ± 0.0004 | 0.0171 ± 0.0006*,** | 0.0150 ± 0.0004 | 0.0180 ± 0.0005*,** |
The data are means ± SEM.
Significantly (P < 0.05) different from Water-only group
significantly different (P < 0.05) from W-Ex group.
Hindlimb capillarity
In the plantaris muscle (Fig. 1) there were significant differences in capillary indices between the (–)-epicatechin and water groups. In all cases, the indices of muscle capillarity (NCAF, C/Fi, capillary density and CFPE) were greater in the (–)-epicatechin treated groups than in the water treated groups. As shown in Fig. 1, there were significant increases in capillarity for the W-Ex group when compared to the water only group. In addition, the (–)-Epi-Ex group had the highest amount of capillarity (P < 0.05) in the plantaris muscle than the other three groups. Also, there were significant mean differences between the (–)-epicatechin and water groups for FCSA and FP (Table 3). Lastly, the (–)-Epi-Ex group had greater FCSA than the (–)-Epi group (Table 3).
Figure 1. Plantaris muscle comparison of NCAF, C/Fi (B), capillary density (C) and capillary-to-fibre perimeter exchange (CFPE) (D) between the four groups.
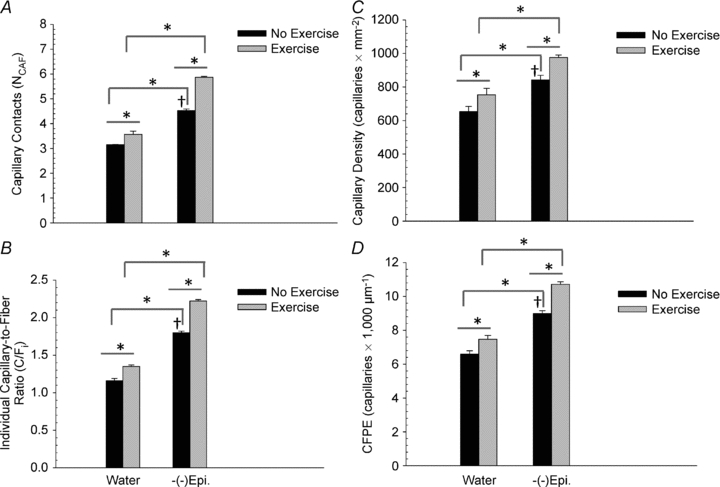
Data are means ± SEM. *P < 0.05; †significantly different from W-Ex group; n = 3 per group.
Table 3.
Muscle morphometry assessments for the plantaris muscle
| Groups | ||||||||
|---|---|---|---|---|---|---|---|---|
| Water only | W-Ex | (–)-Epi only | (–)-Epi-Ex | |||||
| Plantaris Muscle | Day 0 | Day 15 | Day 0 | Day 15 | Day 0 | Day 15 | Day 0 | Day 15 |
| Area (μm2) | — | 1794 ± 50 | — | 1939 ± 46 | — | 2205 ± 35*,** | — | 2428 ± 10*,**,† |
| Perimeter (μm) | — | 175.8 ± 2.5 | — | 184.6 ± 2.5 | — | 201.1 ± 2.5*,** | — | 210.8 ± 4.2*,** |
The data are means ± SEM.
Significantly (P < 0.05) different from Water-only group
significantly different from W-Ex group
significantly different from (–)-Epi only group.
Intact muscle contractility
Effects of exercise training on the contractile properties of EDL muscle
As shown in Fig. 2, the force–frequency curves from the four groups demonstrated a significant (P < 0.01) right-shift at the submaximal frequencies of stimulation in the two exercised trained groups (W-Ex and (–)-Epi-Ex), but no statistically significant differences were detected in the non-exercise groups. Table 4 summarizes all the contractile properties calculated in the four experimental groups. The midpoint of the curve (F50), was significantly increased in both exercised groups (W-Ex and (–)-Epi-Ex) when compared to the non-exercise groups (P < 0.05). There was no statistically significant difference detected on either the steepness of the curves (nH) or the absolute maximal tetanic tension (P0) between the four groups. Single twitch tension (Pt) and fraction of the maximal tension developed with a single twitch tension (Pt/P0) were significantly smaller in the (–)-Epi-Ex group compared to the (–)-Epi group (P < 0.05).
Figure 2. Relative isometric tension (P/P0) obtained at different frequencies of stimulation from EDL muscles from non-exercised mice (open and filled triangles) or exercised mice (open and filled circles) that were fed with (–)-epicatechin (open symbols) or in the control mice (filled symbols).
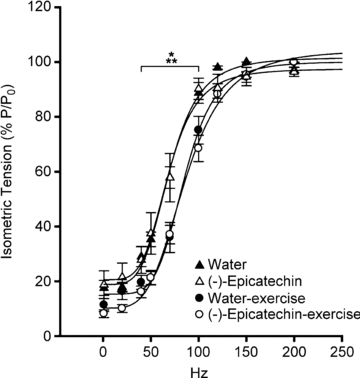
*P < 0.01, water vs. W-Ex; **P < 0.01, (–)-Epi vs. (–)-Epi-Ex. Data are means ± SEM, n = 6 muscles.
Table 4.
Contractile properties of the EDL for the group treated groups
| Water only | W-Ex | (–)-Epi Only | (–)-Epi-Ex | |
|---|---|---|---|---|
| Pt (N cm−2) | 3.6 ± 0.4 | 2.3 ± 0.8 | 3.3 ± 0.6 | 1.5 ± 0.6* |
| P0 (N cm−2) | 21 ± 2 | 21 ± 3 | 20 ± 4 | 17 ± 4 |
| Pt/P0 | 0.17 ± 0.03 | 0.10 ± 0.03 | 0.18 ± 0.05 | 0.07 ± 0.02** |
| F50 (Hz) | 72 ± 4 | 85 ± 5† | 68 ± 5 | 87 ± 4* |
| nH | 4.9 ± 0.2 | 5.2 ± 0.2 | 5.3 ± 0.2 | 4.3 ± 0.3* |
The data are means ± SEM. Pt, twitch tension; P0, maximal tetanic tension; F50, frequency of stimulation to evoke 50% of P0; nH, Hill coefficient.
P < 0.05, (–)-Epi vs. (–)-Epi-Ex
P < 0.05, water vs. (–)-Epi-Ex
P < 0.05, water vs. W-Ex.
Effects of (–)-epicatechin on the contractile properties of EDL muscle
When the animals were treated with (–)-epicatechin for 15 consecutive days, either in the presence or in the absence of exercise training, there was no statistically significant shift detected in the force–frequency relationship (Fig. 2) and no statistically significant differences were detected in P0, Pt, F50 and nH compared to the control group (Table 4). Thus, (–)-epicatechin did not alter contractile properties of the EDL muscle.
Effect of (–)-epicatechin treatment on the time to fatigue in in vitro EDL muscle
Figure 3A shows a representative recording of tetanic contractions during a fatigue run from the four groups of mice. The absolute initial tetanic force was not statistically different among the four groups; however, the muscles from animals treated with (–)-epicatechin were significantly more resistant to fatigue (130 ± 4 vs. 164 ± 10 s and 128 ± 5 vs. 179 ± 9 s for water vs. (–)-Epi and W-Ex vs (–)-Epi-Ex, respectively; P < 0.05, Fig. 3B) which corresponded to a difference of approximately 30–45 s. There were, however, no statistical differences detected in the time to fatigue within groups (water vs. W-Ex or (–)-Epi vs. (–)-Epi-Ex).
Figure 3. A, representative tension recordings, from the EDL muscle, during the fatigue run from the non-exercised group (left panel) and from the exercised group (right panel) normalized to the initial tension. B, results of fatigue run for all four groups.
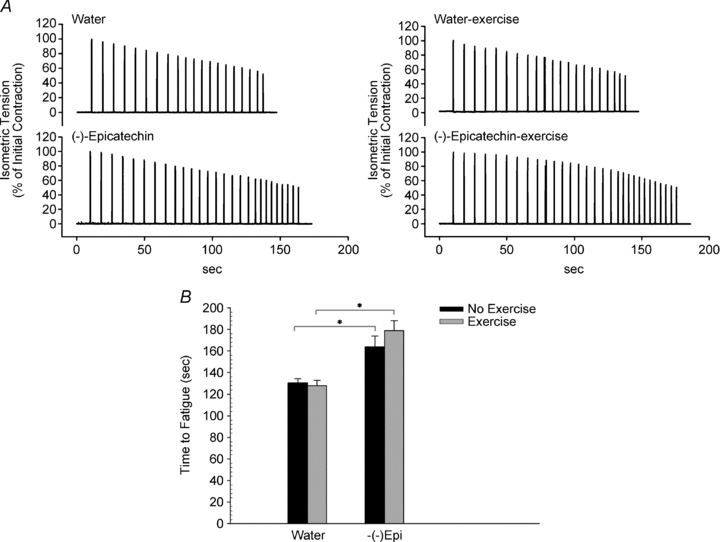
Data are means ± SEM, n = 6 muscles. *P < 0.05.
Enzyme activity
In order to determine the effects of (–)-epicatechin on citrate synthase concentration in the resting muscle we performed the assay on tissues from the non-exercise conditions (water vs. (–)-Epi). There was a significant difference in citrate synthase concentration between the two groups (water, 29.0 ± 0.7 mmol min−1 (kg wet muscle)−1versus (–)-Epi, 31.4 ± 0.3 mmol min−1 (kg wet muscle)−1; P < 0.05). Therefore, 15 days of (–)-Epi treatment increased citrate synthase concentration in the resting muscle of 1-year-old mice by ∼8.3%.
Mitochondrial oxidative phosphorylation protein complex in hindlimb and heart
As shown in Fig. 4A the protein content for components of mitochondrial complexes I (subunit NDUFB8; ∼20 kDa), II (FeS subunit; ∼30 kDa), III (Core 2 subunit; ∼47 kDa), and V (ATP synthase α-subunit; ∼53 kDa) was significantly higher in the W-Ex, (–)-Epi only, and (–)-Epi-Ex groups compared to the water only group. We were, however, unable to measure the band for complex IV (subunit II, ∼24 kDa), because of the close proximity to complex I despite multiple attempts to achieve separation between the two bands. Furthermore, the (–)-Epi and (–)-Epi-Ex groups had greater increases in these oxidative phosphorylation components when compared to the W-Ex group. In addition, the (–)-Epi-Ex had significantly higher levels of subunits of complex III and V than the (–)-Epi group (Fig. 4A). We also examined protein content of mitofilin and porin, which are components of the inner and outer membrane of the mitochondria, respectively. As shown in Fig. 4B, we found that levels of both proteins were significantly higher for the three groups when compared to the water only group. In addition, both mitofilin and porin were ∼50% higher in the (–)-Epi group than the W-Ex group. Lastly, we found that mitofilin and porin protein content were significantly higher in the (–)-Epi-Ex group than the (–)-Epi group.
Figure 4. A, mitochondrial protein complexes for the quadriceps femoris muscle.
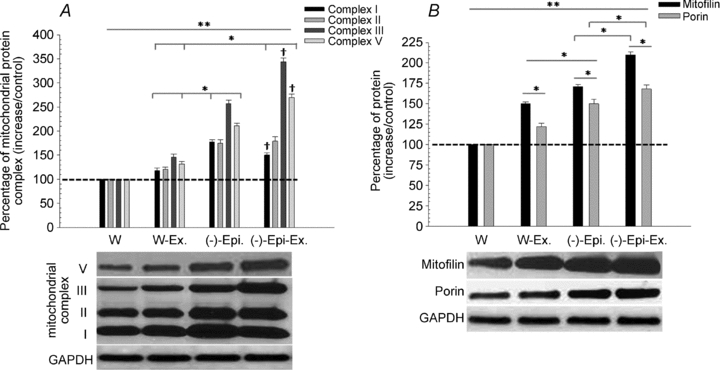
*Significant differences between groups; **significant differences between W-Ex, (–)-Epi, and (–)-Epi-Ex versus W group; †significantly different from corresponding complex for (–)-Epi group. B, mitofilin and porin proteins. **Significant differences between W-Ex, (–)-Epi, and (–)-Epi-Ex versus water group for mitofilin and porin. Data are means ± SEM, n = 3 per group.
We also examined the effects of (–)-epicatechin treatment on components of oxidative phosphorylation complexes in cardiac muscle. As shown in Fig. 5A, these mitochondrial proteins were significantly higher for the (–)-Epi-Ex group when compared to the water group; however, only in select components were significant differences detected between the water group versus the W-Ex and (–)-Epi groups. In addition, only the complex II subunit was significantly higher for the (–)-Epi-Ex when compared to the (–)-Epi group (see Fig. 5A). We also found that both mitofilin and porin protein content were significantly higher for the three groups (W-Ex, (–)-Epi, and (–)-Epi-Ex) when compared to the water only group; Fig. 5B). For mitofilin, we found that the (–)-Epi-Ex group had significantly higher protein content than the W-Ex and (–)-Epi groups. For porin, there were no statistical differences detected between the three groups (W-Ex, (–)-Epi, and (–)-Epi-Ex; Fig. 5B).
Figure 5. A, mitochondrial protein complexes for the cardiac muscle.
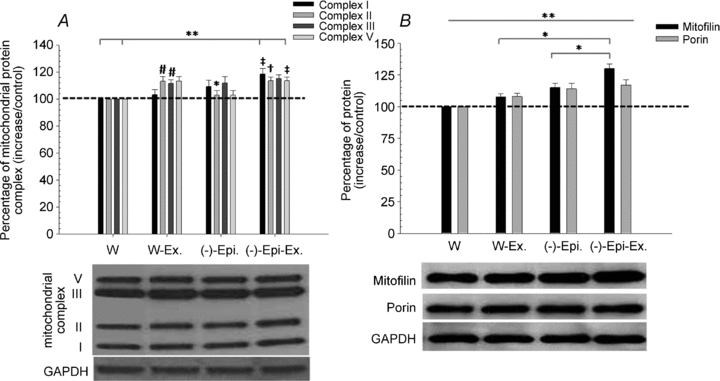
*Significantly different from corresponding complex for W group; #significantly different with corresponding complex for water group; ‡significantly different from corresponding complex for W-Ex group; **significant differences between W-Ex, (–)-Epi, and (–)-Epi-Ex versus W group; †significantly different from corresponding complex of (–)-Epi group. B, mitofilin and porin protein. **Significant differences between W-Ex, (–)-Epi, and (–)-Epi-Ex versus water group for mitofilin and porin. Data are means ± SEM, n = 3 per group.
To gain better insight into the underlying mechanisms of (–)-epicatechin's effect, we analysed changes in protein content of nNOS and the phosphorylation status of nNOS (p-nNOS) as well as for Tfam content in the quadriceps femoris muscles for each group. As shown in Fig. 6, both nNOS and p-nNOS, and the p-nNOS/nNOS ratio (Fig. A) as well as Tfam (Fig. B) were significantly increased in all three groups when compared to the water only group. However, there were no statistically significant differences detected in p-nNOS/nNOS ratio between the three groups (W-Ex, (–)-Epi and (–)-Epi-Ex). We were unable to detect eNOS in the skeletal muscle samples (data not shown). It should be noted, that the levels of Tfam were significantly higher in the (–)-Epi-Ex group compared to the W-Ex and (–)-Epi groups.
Figure 6. Representative Western blot results for nNOS, p-nNOS and p-nNOS/nNOS ratio (A) and Tfam (B) from quadriceps femoris muscle.
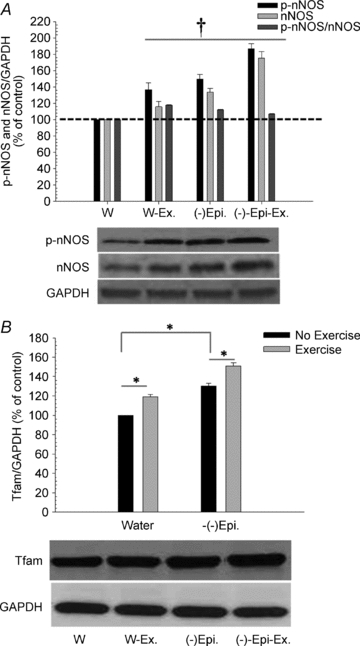
*P < 0.05; †significantly different from water only group for p-nNOS and nNOS.
Electron microscopy
Electron microscopy was used to investigate ultrastructural modifications to the plantaris muscle after (–)-epicatechin treatment. High-resolution images comprising large regions of muscle tissue were examined from three (–)-epicatechin only treated mice (group (3) and compared with images from three water only control mice (group (1). We observed that in (–)-epicatechin treated mouse plantaris muscle there were more abundant mitochondria and cristae than in the controls (Fig. 7A and B). The measured mean volume density of mitochondria, expressed as the percentage of the cytoplasm occupied by this organelle, was 10.0% after epicatechin treatment compared to 5.7% (P < 0.05) in the control. The cristae abundance in (–)-epicatechin treated mice was also significantly higher than in the control mitochondria (Fig. 7B). The mean cristae surface area, normalized to the mitochondrial outer membrane (and thus unitless), was 1.26 after (–)-epicatechin treatment compared to 0.77 in controls (P < 0.05). When the entire mitochondrial inner membrane surface area was measured and normalized to the outer membrane the mean inner membrane surface area after (–)-epicatechin treatment was 2.22 compared to 1.72 in controls (P < 0.05). In addition, we examined the mean cristae surface area for the cardiac muscle and found a statistically significant increase (∼25%) in (–)-Epi only group when compared to the water group. Treatment also significantly increased mitochondrial volume density by 11% in cardiac muscle (P < 0.05, Fig. 8).
Figure 7. Mitochondrial volume density for the plantaris muscle (A), and cristae membrane surface area normalized to the outer membrane surface area (B).
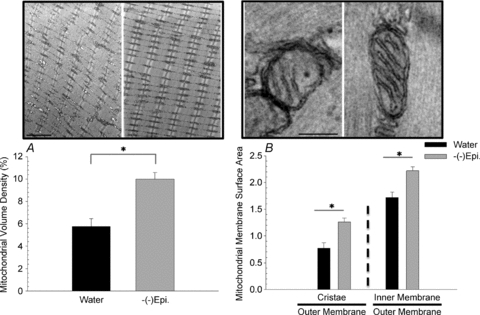
Data are means and SEM. The representative electron microscopy images of control (left side) and (–)-epicatechin (right side) treated muscle above each graph. For panel A, the scale bar represents 2 μm, whereas for panel B, the scale bar represents 200 nm. *P < 0.05.
Figure 8. Mitochondrial volume density for the heart muscle (A), and cristae membrane surface area normalized to the outer membrane surface area (B).
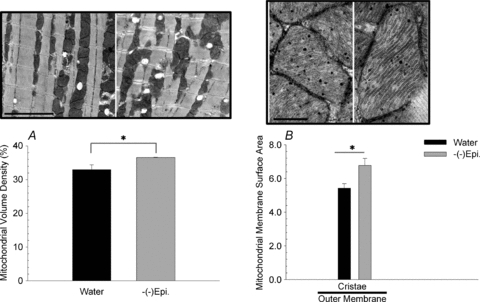
Data are means and SEM. The representative electron microscopy images of control (left side) and (–)-epicatechin (right side) treated muscle above each graph. For panel A, the scale bar represents 4 μm, whereas for panel B, the scale bar represents 400 nm. *P < 0.05.
Discussion
The principle and unique findings of the current investigation are that 15 consecutive days of (–)-epicatechin treatment resulted in (1) improved treadmill performance; (2) delayed onset of muscle fatigue; (3) increased muscle capillarity; (4) increased levels of proteins comprising the oxidative phosphorylation machinery as well as mitofilin and porin in skeletal and cardiac muscles; and (5) increased mitochondrial volume density and cristae abundance in hindlimb and heart muscles. In addition, we found increases in Tfam expression in (–)-epicatechin-treated mice. These effects were greater than those from exercise alone. Furthermore, the combination of exercise and (–)-epicatechin treatment resulted in greater improvements in selected endpoints. To our knowledge, this is the first study that has examined the effects of a low dose (–)-epicatechin treatment in mice. The nature of the observed changes indicate that the effects occur in multiple organs and tissues that as a whole can lead to a sustained increase in exercise performance.
Muscle capillarity and exercise performance in response to (–)-epicatechin treatment
In the current study, we did not detect statistical differences in body or muscle masses between the four groups, but mice in the (–)-Epi or (–)-Epi-Ex groups had higher treadmill running capacities than those in the control groups after 15 consecutive days of treatment (Table 2). The ability to deliver oxygen to the working muscle is a coordinated effort involving multiple factors. Capillaries are an integral part of this process since they provide an interface between the circulatory system and the muscle. For example, Waters et al. (2004) reported that 14 days of voluntary wheel running was sufficient to induce angiogenesis and increase the capillary-to-fibre ratio by ∼56% in the plantaris muscle of mice, whereas Olfert et al. (2009) reported that deletion of the vascular endothelial growth factor (VEGF) gene in cardiac and skeletal muscle resulted in profound reductions of exercise capacity when compared to littermate controls. In the present study, a number of established capillary indices (Hepple et al. 1997; Malek & Olfert, 2009; Malek et al. 2010) were used to characterize the effects of (–)-Epi and (–)-Epi-Ex on capillarization of the plantaris muscle. This muscle was selected because glycolytic fibres typically have lower capillary abundance relative to oxidative fibres (Malek & Olfert, 2009; Malek et al. 2010). When compared to the W-Ex group, the (–)-Epi group increased C/Fi by ∼33%, whereas the combination of (–)-Epi-Ex increased C/Fi by ∼64%. This pattern of increased capillarity was consistent for all other indices (Fig. 1). In addition, we also estimated CFPE, which provides a measure of the interface between the capillaries and muscle fibres (Hepple & Mathieu-Costello, 2001). The observed increase in the CFPE index for the (–)-Epi and (–)-Epi-Ex groups suggests an increased oxygen flux potential, which may explain the increase in endurance capacity (Table 2) when compared to the control groups. Although we did not observe any statistical differences in treadmill exercise indices (Table 2) between the (–)-Epi and (–)-Epi-Ex, this may, in part, be explained by the variability inherent with treadmill testing. Nevertheless, these data are consistent with well-established correlational studies between muscle capillarity, oxygen conductance and/or exercise performance (Richardson et al. 2000; Howlett et al. 2003) indicating that muscle capillarity plays a key role in limiting exercise capacity.
The ability to sustain exercise is an integrative response between central (cardiorespiratory) and peripheral (muscle) factors. Approaches for the evaluation of cardiac function during exercise in mice are limited and measurements of cardiac function during treadmill exercise are currently not feasible given the size of the mouse as well as practical issues such as equipment limitations (Malek & Olfert, 2009). Therefore, in the current study, it was not possible to separate the contribution of central and peripheral factors to endurance capacity with (–)-epicatechin treatment.
Intact whole muscle response to (–)-epicatechin treatment
In the current investigation, the effects of 15 consecutive days of (–)-epicatechin treatment with or without exercise were evaluated using isolated whole EDL muscle, which is composed mainly of fast-twitch, type IIA and IIB fibres in mice (Crow & Kushmerick, 1982). This approach allows a better evaluation of muscle fatigue properties in the absence of central factors (i.e. cardiorespiratory and microcirculatory). One advantage of using the EDL muscle compared to other thicker fast-twitch muscles, like gastrocnemius, is the relative small diameter of the EDL, allowing sufficient oxygenation of the core of the muscle during repetitive tetanic contractions (Barclay, 2005). In order to evaluate the contractile apparatus in the non-fatigued EDL muscle, tension was evoked electrically at different frequencies of stimulation. This resulted in the production of twitches (single pulse of depolarization) as well as unfused and fused tetanic contractions (Nogueira & Hogan, 2010).
As shown in Fig. 2 and by the increased F50, the force–frequency curves for the exercise groups were significantly shifted to the right when compared to the non-exercised group. Interestingly, the F50 in the non-exercised group (∼70 Hz) was smaller than the reported values in the literature for EDL muscle, which is around 80–90 Hz (Tang et al. 2004), whereas the exercised group had similar values (∼85 Hz) to those in the literature. It is important to note that the animals chosen for this work were 1 year old; therefore, it is possible that ageing per se affected the contractile parameters in these mice as reported previously using intact single mouse fibres (Gonzalez et al. 2000). Nevertheless, we did not detect significant statistical difference in the maximal tetanic tension developed in all four groups. One possible interpretation is that moderate exercise did not affect the maximal Ca2+ release and cross-bridge cycle during maximal activation (Tang et al. 2004; Ottenheijm et al. 2009). Several studies (Shindoh et al. 1990; Reid et al. 1993) have shown that inhibition of muscle submaximal tension in the non-fatigued state is related to the increased muscle antioxidant activity either by endogenous or exogenous antioxidant enzymes. It is possible that the exercise regimen used in the current study may have increased the antioxidant pool in the EDL muscle as indicated by the right-shift of the force–frequency curve. Chronic treatment with a flavanoid such as (–)-epicatechin with known antioxidant properties (Yamazaki et al. 2008) did not, however, affect maximal tension development nor promote any shift in the force–frequency relationship suggesting that (–)-epicatechin-induced effects are not mediated through changes on intracellular redox status.
After the force–frequency curves were determined, individual muscles from the four groups were repeatedly contracted at maximal tetanic tension up to a fatigue time point. Although the EDL muscles for the water groups were more fatigable than those for the (–)-epicatechin groups, there were no statistical differences within the two groups between the non-exercise and exercise conditions (Fig. 3B). These results are consistent with the in vivo treadmill test results (Table 2). Nevertheless, mice treated with (–)-epicatechin, on average, were 31% more fatigue resistant than mice treated with water (Fig. 3). Our findings seem to be unique since it has been reported that resveratrol, a flavonoid compound found in red grapes, did not improve muscle fatigability during in situ isometric muscle action in young (3–5 months) or old (26–28 months) rats (Ryan et al. 2010). The results of the current study indicates that (–)-epicatechin treatment may reduce muscle fatigue in fast-twitch muscle, independent of exercise training.
Changes in mitochondrial structure
Skeletal muscle
The increase in mitochondrial density is a typical adaptation to endurance training which is well documented in the literature (Holloszy, 1967; Wright et al. 2007). In the current investigation, we examined multiple components of the mitochondria including enzyme activities, ultrastructural parameters (i.e. cristae abundance), protein expression of oxidative phosphorylation machinery, and the inner (mitofilin) and outer (porin) membranes in the quadriceps femoris muscle. This muscle was selected because it predominantly contains the Type IIb myosin heavy chain isoform (Salerno et al. 2004; Kohn & Myburgh, 2007). As shown in Fig. 4A, there were significant increases in components for each of the probed complexes for the W-Ex, (–)-Epi, and (–)-Epi-Ex groups when compared to the water group. An interesting finding is that the W-Ex group had significantly higher amounts of protein for each complex component when compared to the water group even though we did not observe an increase in functional (i.e. treadmill) capacity (Table 2). Another unique finding was that the (–)-epicatechin group resulted in greater protein expression than the W-Ex group and this change was associated with a 25% improvement in treadmill performance.
Mitofilin is an inner membrane protein (Gieffers et al. 1997) that is enriched in the narrow space between the mitochondrial inner boundary and the outer membrane where it can assemble into a large multimeric protein complex. John et al. (2005) reported that down-regulation of mitofilin resulted in a disorganized mitochondrial inner membrane with a subsequent increase in reactive oxygen species production and membrane potential. Inner membranes failed to form tubular or vesicular cristae and appeared as closely packed stacks of membrane sheets that fused intermittently. In contrast, gross mitochondrial fission and fusion appeared normal. Thus, mitofilin appears to potentially play an important role in the generation and/or assembly of inner mitochondrial membrane. Porin, also known as a voltage-dependent anion channel (VDAC), is found predominantly in the outer membrane of the mitochondria and appears to facilitate ion exchange between the mitochondria and cytosol (Benz, 2004; Singha et al. 2009). Recently, Park et al. (2010) characterized the role of porin in Drosophila muscle and reported that porin mutants exhibited elongated mitochondria. This phenotype could be reversed by increased mitochondrial fission. Furthermore, increased fission by Drp1 expression suppressed flight defects in porin mutants. Collectively these results suggest that porin may play a critical role in mitochondrial remodelling.
Mitofilin and porin protein levels increased significantly in both W-Ex, (–)-Epi, and (–)-Epi-Ex when compared to the water group (Fig. 4B). Another unique finding was that the combination of (–)-epicatechin and exercise further increased mitofilin and porin levels when compared to (–)-epicatechin treatment alone (Fig. 4B). Thus, these results suggest that (–)-epicatechin treatment mimics the effects of exercise on these two proteins, which are important regulators of mitochondrial inner structure and remodelling.
We also examined mitochondrial volume density and cristae abundance in the plantaris muscle comparing only water and (–)-Epi groups (Fig. 7). This approach was taken to determine whether (–)-epicatechin treatment alone would result in mitochondrial biogenesis in a primarily glycolytic muscle independent of daily exercise. As shown in Fig. 7 (both panels), (–)-epicatechin treatment resulted in ∼75% greater mitochondrial volume density and ∼65% greater cristae membrane surface area when compared to the water group. It has been suggested that the cristae membranes are functionally distinct from the inner boundary membranes with the mitochondrial complexes enriched in the cristae (Frey et al. 2002; Gilkerson et al. 2003) Recently, Perkins & Ellisman (2010) reported that cristae abundance and shape are directly tied to energy production in nerve tissue. A predominance of lamellar cristae in the mitochondria of the plantaris muscle was found, which allows for a greater number of complexes. These data indicate that 15 consecutive days of (–)-epicatechin treatment resulted in ultrastructural changes in the mitochondria that potentially increase the capacity to produce energy. This assumption is supported by the observation that hummingbird muscles are known to possess mitochondria with very high cristae abundance and these features correlate with very high levels of oxidative capacity (Suarez et al. 1991).
One of the upstream signals that may be involved in mitochondrial biogenesis is nitric oxide (NO) (Nisoli et al. 2003; Wadley & McConell, 2007). Physiological increases in NO can result from the upregulation and/or activation of either endothelial or neuronal NOS isoforms (eNOS or nNOS, respectively). The precise role that NO plays in modulating mitochondrial biogenesis response with exercise is, however, unclear (Wadley & McConell, 2007). In the present investigation, we examined the effects of (–)-epicatechin on skeletal muscle eNOS and nNOS content and compared them with those induced in conjunction with exercise. No detectable levels of skeletal muscle eNOS were observed with any of the treatment groups, which agrees with the low activity of eNOS in skeletal muscle fibres (Kobzik et al. 1994). (–)-Epicatechin treatment increased the total amount of nNOS and phosphorylation of nNOS (p-nNOS/nNOS ratio) in skeletal muscle and the effects were comparable to the exercise groups. However, the apparent lack of additional increases in total nNOS and p-nNOS/nNOS ratio with (–)-Epi-Ex group prevents us from providing further evidence as to a possible stimulatory role for nNOS generated NO in skeletal muscle mitochondrial biogenesis as documented under these conditions.
The transcriptional factor Tfam has been recognized as an important downstream mediator of mitochondria biogenesis in response to exercise (Safdar et al. 2010). Increases in Tfam levels are typically associated with increases in mitochondrial density in muscle. In a study by Little et al. (2010), low-volume high interval training in humans was associated with increases in Tfam protein levels of ∼20% in skeletal muscle. In the present study, the results indicated that (–)-epicatechin stimulated Tfam protein levels and that the effects were comparable to those of exercise (Fig. 6). In addition, skeletal muscle Tfam protein levels increased further when (–)-epicatechin treatment was combined with exercise. Taken together, these results indicate that (–)-epicatechin may potentially exert exercise mimetic effects when taken alone, but also provides a synergist effect when combined with exercise.
Cardiac muscle
The heart relies exclusively on oxidative phosphorylation to generate energy and this is reflected by the abundant presence of mitochondria, which comprise ∼30% of the myocyte volume. Recent studies, however, have shown decreases in mitochondrial function of cardiac muscle with age (Hagen et al. 2002; Moreau et al. 2004). The effects of (–)-epicatechin on the myocardium are of great interest and therefore have been examined previously by our group (Yamazaki et al. 2008, 2010; Ramirez-Sanchez et al. 2010). For example, Yamazaki et al. (2010) reported that (–)-epicatechin, at the same dosage as in the current study, given for 10 consecutive days resulted in short term (∼52% reduction in infarction size after 48 h of permanent coronary occlusion (PCO)) as well as long term (33% after 3 weeks of PCO) myocardial protection. More recently, Panneerselvam et al. (2010) reported that treatment of mice with (–)-epicatechin (1 mg (kg body weight)−1) for 10 consecutive days resulted in myocardial protection from ischemia–reperfusion injury.
In the present study, we found increases in select mitochondrial complex proteins as well as mitofilin and porin (Fig. 5). Electron microscopy results show significant increases in cardiomyocyte mitochondria volume density. Most notably, there was increased cristae membrane surface area with (–)-epicatechin treatment. To our knowledge, this is the first study to report mitochondrial biogenesis in cardiac muscle using quantitative measures (Fig. 8). Findings from the present study may, in part, explain the cardioprotective properties of (–)-epicatechin as treatment likely results in increased oxidative capacity and energy reserves.
In summary, we report the novel finding that 15 consecutive days of (–)-epicatechin treatment in 1-year-old mice notably increased treadmill performance and time to fatigue. To our knowledge this is the first study with (–)-epicatechin to integrate functional and clinically relevant physiological animal data with structural and morphological data from electron microscopy. This improvement in exercise capacity is likely to be the result of the pleiotropic effects of (–)-epicatechin at multiple levels including capillarity as well as mitochondrial content, structure and function. The effects reported on mitochondrial cristae abundance appear quite unique and deserve further investigation. In addition, the combination of (–)-epicatechin treatment and physical activity was shown to increase components of oxidative phosphorylation and capillarity above and beyond (–)-epicatechin treatment alone. These results, therefore, warrant the evaluation of the underlying mechanisms of action of (–)-epicatechin and its potential clinical application as an exercise mimetic.
Acknowledgments
We are grateful to Maraliz Barraza-Hidalgo, Daniel Hogan, and Vincent Mendiola for their assistance with this project. This study was funded by Cardero Therapeutics, Inc. (M.H.M.). Part of the work was performed at the National Centre for Microscopy and Imageing Research funded by NIH grant RR004050.
Glossary
Abbreviations
- CFPE
capillary-to-fibre perimeter exchange index
- (–)-Epi
(–)-epicatechin group
- (–)-Epi-Ex
(–)-epicatechin–exercise group
- F50
frequency of stimulation to evoke 50% of P0
- FCSA
fibre cross-sectional area
- GTE
green tea extract
- nH
Hill coefficient
- Pt
twitch tension
- P0
maximal tetanic tension
- PCO
permanent coronary occlusion
- W-Ex
water and exercise group
Author contributions
Work was done at UCSD and WSU. Conception and design of the study were by M.H.M. All authors contributed to collection, analysis and interpretation of data, and to drafting the manuscript. All authors approved the final version of the manuscript for publication.
Disclosure
F. Villarreal and P. R. Taub are co-founders of Cardero Therapeutics, Inc.
References
- Bailey SJ, Winyard PG, Vanhatalo A, Blackwell JR, DiMenna FJ, Wilkerson DP, Jones AM. Acute L-arginine supplementation reduces the O2 cost of moderate-intensity exercise and enhances high-intensity exercise tolerance. J Appl Physiol. 2010;109:1394–1403. doi: 10.1152/japplphysiol.00503.2010. [DOI] [PubMed] [Google Scholar]
- Barclay CJ. Modelling diffusive O2 supply to isolated preparations of mammalian skeletal and cardiac muscle. J Muscle Res Cell Motil. 2005;26:225–235. doi: 10.1007/s10974-005-9013-x. [DOI] [PubMed] [Google Scholar]
- Benz R. Bacterial and Eukaryotic Porins: Structure, Function, Mechanism. Weinheim, Germany: Wiley-VCH; 2004. [Google Scholar]
- Buijsse B, Weikert C, Drogan D, Bergmann M, Boeing H. Chocolate consumption in relation to blood pressure and risk of cardiovascular disease in German adults. Eur Heart J. 2010;31:1616–1623. doi: 10.1093/eurheartj/ehq068. [DOI] [PubMed] [Google Scholar]
- Chen ZP, McConell GK, Michell BJ, Snow RJ, Canny BJ, Kemp BE. AMPK signaling in contracting human skeletal muscle: acetyl-CoA carboxylase and NO synthase phosphorylation. Am J Physiol Endocrinol Metab. 2000;279:E1202–1206. doi: 10.1152/ajpendo.2000.279.5.E1202. [DOI] [PubMed] [Google Scholar]
- Crow MT, Kushmerick MJ. Chemical energetics of slow- and fast-twitch muscles of the mouse. J Gen Physiol. 1982;79:147–166. doi: 10.1085/jgp.79.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franko A, Mayer S, Thiel G, Mercy L, Arnould T, Hornig-Do HT, Wiesner RJ, Goffart S. CREB-1α is recruited to and mediates upregulation of the cytochrome c promoter during enhanced mitochondrial biogenesis accompanying skeletal muscle differentiation. Mol Cell Biol. 2008;28:2446–2459. doi: 10.1128/MCB.00980-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey TG, Renken CW, Perkins GA. Insight into mitochondrial structure and function from electron tomography. Biochim Biophys Acta. 2002;1555:196–203. doi: 10.1016/s0005-2728(02)00278-5. [DOI] [PubMed] [Google Scholar]
- Gieffers C, Korioth F, Heimann P, Ungermann C, Frey J. Mitofilin is a transmembrane protein of the inner mitochondrial membrane expressed as two isoforms. Exp Cell Res. 1997;232:395–399. doi: 10.1006/excr.1997.3539. [DOI] [PubMed] [Google Scholar]
- Gilkerson RW, Selker JM, Capaldi RA. The cristal membrane of mitochondria is the principal site of oxidative phosphorylation. FEBS Lett. 2003;546:355–358. doi: 10.1016/s0014-5793(03)00633-1. [DOI] [PubMed] [Google Scholar]
- Gonzalez E, Messi ML, Delbono O. The specific force of single intact extensor digitorum longus and soleus mouse muscle fibers declines with aging. J Membr Biol. 2000;178:175–183. doi: 10.1007/s002320010025. [DOI] [PubMed] [Google Scholar]
- Guth L, Samaha FJ. Procedure for the histochemical demonstration of actomyosin ATPase. Exp Neurol. 1970;28:365–367. [PubMed] [Google Scholar]
- Hagen TM, Moreau R, Suh JH, Visioli F. Mitochondrial decay in the aging rat heart: evidence for improvement by dietary supplementation with acetyl-L-carnitine and/or lipoic acid. Ann N Y Acad Sci. 2002;959:491–507. doi: 10.1111/j.1749-6632.2002.tb02119.x. [DOI] [PubMed] [Google Scholar]
- Handschin C, Chin S, Li P, Liu F, Maratos-Flier E, Lebrasseur NK, Yan Z, Spiegelman BM. Skeletal muscle fiber-type switching, exercise intolerance, and myopathy in PGC-1α muscle-specific knock-out animals. J Biol Chem. 2007;282:30014–30021. doi: 10.1074/jbc.M704817200. [DOI] [PubMed] [Google Scholar]
- Hayashi YK, Matsuda C, Ogawa M, Goto K, Tominaga K, Mitsuhashi S, Park YE, Nonaka I, Hino-Fukuyo N, Haginoya K, Sugano H, Nishino I. Human PTRF mutations cause secondary deficiency of caveolins resulting in muscular dystrophy with generalized lipodystrophy. J Clin Invest. 2009;119:2623–2633. doi: 10.1172/JCI38660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hepple RT. A new measurement of tissue capillarity: the capillary-to-fibre perimeter exchange index. Can J Appl Physiol. 1997;22:11–22. doi: 10.1139/h97-002. [DOI] [PubMed] [Google Scholar]
- Hepple RT, Mackinnon SL, Thomas SG, Goodman JM, Plyley MJ. Quantitating the capillary supply and the response to resistance training in older men. Pflugers Arch. 1997;433:238–244. doi: 10.1007/s004240050273. [DOI] [PubMed] [Google Scholar]
- Hepple RT, Mathieu-Costello O. Estimating the size of the capillary-to-fiber interface in skeletal muscle: a comparison of methods. J Appl Physiol. 2001;91:2150–2156. doi: 10.1152/jappl.2001.91.5.2150. [DOI] [PubMed] [Google Scholar]
- Holloszy JO. Biochemical adaptations in muscle. Effects of exercise on mitochondrial oxygen uptake and respiratory enzyme activity in skeletal muscle. J Biol Chem. 1967;242:2278–2282. [PubMed] [Google Scholar]
- Holloszy JO, Coyle EF. Adaptations of skeletal muscle to endurance exercise and their metabolic consequences. J Appl Physiol. 1984;56:831–838. doi: 10.1152/jappl.1984.56.4.831. [DOI] [PubMed] [Google Scholar]
- Howlett RA, Gonzalez NC, Wagner HE, Fu Z, Britton SL, Koch LG, Wagner PD. Genetic models in applied physiology: Skeletal muscle capillarity and enzyme activity in rats selectively bred for running endurance. J Appl Physiol. 2003;94:1682–1688. doi: 10.1152/japplphysiol.00556.2002. [DOI] [PubMed] [Google Scholar]
- Huffman DM, Moellering DR, Grizzle WE, Stockard CR, Johnson MS, Nagy TR. Effect of exercise and calorie restriction on biomarkers of aging in mice. Am J Physiol Regul Integr Comp Physiol. 2008;294:R1618–1627. doi: 10.1152/ajpregu.00890.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer S, Thomas RR, Portell FR, Dunham LD, Quigley CK, Bennett JP., Jr Recombinant mitochondrial transcription factor A with N-terminal mitochondrial transduction domain increases respiration and mitochondrial gene expression. Mitochondrion. 2009;9:196–203. doi: 10.1016/j.mito.2009.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John GB, Shang Y, Li L, Renken C, Mannella CA, Selker JM, Rangell L, Bennett MJ, Zha J. The mitochondrial inner membrane protein mitofilin controls cristae morphology. Mol Biol Cell. 2005;16:1543–1554. doi: 10.1091/mbc.E04-08-0697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keppel G, Wickens TD. Design and Analysis: A Researcher's Handbook. Upper Saddle River, NJ: Pearson Prentice Hall; 2004. [Google Scholar]
- Kobzik L, Reid MB, Bredt DS, Stamler JS. Nitric oxide in skeletal muscle. Nature. 1994;372:546–548. doi: 10.1038/372546a0. [DOI] [PubMed] [Google Scholar]
- Kohn TA, Myburgh KH. Regional specialization of rat quadriceps myosin heavy chain isoforms occurring in distal to proximal parts of middle and deep regions is not mirrored by citrate synthase activity. J Anat. 2007;210:8–18. doi: 10.1111/j.1469-7580.2007.00661.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanza IR, Nair KS. Muscle mitochondrial changes with aging and exercise. Am J Clin Nutr. 2009;89:467S–471S. doi: 10.3945/ajcn.2008.26717D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leick L, Lyngby SS, Wojtasewski JF, Pilegaard H. PGC-1α is required for training-induced prevention of age-associated decline in mitochondrial enzymes in mouse skeletal muscle. Exp Gerontol. 2010;45:336–342. doi: 10.1016/j.exger.2010.01.011. [DOI] [PubMed] [Google Scholar]
- Little JP, Safdar A, Wilkin GP, Tarnopolsky MA, Gibala MJ. A practical model of low-volume high-intensity interval training induces mitochondrial biogenesis in human skeletal muscle: potential mechanisms. J Physiol. 2010;588:1011–1022. doi: 10.1113/jphysiol.2009.181743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd PG, Prior BM, Yang HT, Terjung RL. Angiogenic growth factor expression in rat skeletal muscle in response to exercise training. Am J Physiol Heart Circ Physiol. 2003;284:H1668–1678. doi: 10.1152/ajpheart.00743.2002. [DOI] [PubMed] [Google Scholar]
- Malek MH, Olfert IM. Global deletion of thrombospondin-1 increases cardiac and skeletal muscle capillarity and exercise capacity in mice. Exp Physiol. 2009;94:749–760. doi: 10.1113/expphysiol.2008.045989. [DOI] [PubMed] [Google Scholar]
- Malek MH, Olfert IM, Esposito F. Detraining losses of skeletal muscle capillarization are associated with vascular endothelial growth factor protein expression in rats. Exp Physiol. 2010;95:359–368. doi: 10.1113/expphysiol.2009.050369. [DOI] [PubMed] [Google Scholar]
- Moreau R, Heath SH, Doneanu CE, Harris RA, Hagen TM. Age-related compensatory activation of pyruvate dehydrogenase complex in rat heart. Biochem Biophys Res Commun. 2004;325:48–58. doi: 10.1016/j.bbrc.2004.10.011. [DOI] [PubMed] [Google Scholar]
- Nair KS. Aging muscle. Am J Clin Nutr. 2005;81:953–963. doi: 10.1093/ajcn/81.5.953. [DOI] [PubMed] [Google Scholar]
- Narkar VA, Downes M, Yu RT, Embler E, Wang YX, Banayo E, Mihaylova MM, Nelson MC, Zou Y, Juguilon H, Kang H, Shaw RJ, Evans RM. AMPK and PPARδ agonists are exercise mimetics. Cell. 2008;134:405–415. doi: 10.1016/j.cell.2008.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisoli E, Clementi E, Paolucci C, Cozzi V, Tonello C, Sciorati C, Bracale R, Valerio A, Francolini M, Moncada S, Carruba MO. Mitochondrial biogenesis in mammals: the role of endogenous nitric oxide. Science. 2003;299:896–899. doi: 10.1126/science.1079368. [DOI] [PubMed] [Google Scholar]
- Nogueira L, Hogan MC. Phenol increases intracellular [Ca2+] during twitch contractions in intact Xenopus skeletal myofibers. J Appl Physiol. 2010;109:1384–1393. doi: 10.1152/japplphysiol.00660.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olfert IM, Howlett RA, Tang K, Dalton ND, Gu Y, Peterson KL, Wagner PD, Breen EC. Muscle-specific VEGF deficiency greatly reduces exercise endurance in mice. J Physiol. 2009;587:1755–1767. doi: 10.1113/jphysiol.2008.164384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottaviani JI, Momma TY, Heiss C, Kwik-Uribe C, Schroeter H, Keen CL. The stereochemical configuration of flavanols influences the level and metabolism of flavanols in humans and their biological activity in vivo. Free Radic Biol Med. 2011;50:237–244. doi: 10.1016/j.freeradbiomed.2010.11.005. [DOI] [PubMed] [Google Scholar]
- Ottenheijm CA, Hidalgo C, Rost K, Gotthardt M, Granzier H. Altered contractility of skeletal muscle in mice deficient in titin's M-band region. J Mol Biol. 2009;393:10–26. doi: 10.1016/j.jmb.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panneerselvam M, Tsutsumi YM, Bonds JA, Horikawa YT, Saldana M, Dalton ND, Head BP, Patel PM, Roth DM, Patel HH. Dark chocolate receptors: epicatechin-induced cardiac protection is dependent on delta-opioid receptor stimulation. Am J Physiol Heart Circ Physiol. 2010;299:H1604–1609. doi: 10.1152/ajpheart.00073.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pansters NA, van der Velden JL, Kelders MC, Laeremans H, Schols AM, Langen RC. Segregation of myoblast fusion and muscle-specific gene expression by distinct ligand-dependent inactivation of GSK-3β. Cell Mol Life Sci. 2011;68:523–535. doi: 10.1007/s00018-010-0467-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J, Kim Y, Choi S, Koh H, Lee SH, Kim JM, Chung J. Drosophila porin/VDAC affects mitochondrial morphology. PloS One. 2010;5:e13151. doi: 10.1371/journal.pone.0013151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins GA, Ellisman MH. Mitochondrial configurations in peripheral nerve suggest differential ATP production. J Struct Biol. 2010;173:117–127. doi: 10.1016/j.jsb.2010.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole DC, Mathieu-Costello O. Relationship between fiber capillarization and mitochondrial volume density in control and trained rat soleus and plantaris muscles. Microcirculation. 1996;3:175–186. doi: 10.3109/10739689609148286. [DOI] [PubMed] [Google Scholar]
- Ramirez-Sanchez I, Maya L, Ceballos G, Villarreal F. (–)-Epicatechin activation of endothelial cell endothelial nitric oxide synthase, nitric oxide, and related signaling pathways. Hypertension. 2010;55:1398–1405. doi: 10.1161/HYPERTENSIONAHA.109.147892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid MB, Feldman HA, Miller MJ. Isometric contractile properties of diaphragm strips from alcoholic rats. J Appl Physiol. 1987;63:1156–1164. doi: 10.1152/jappl.1987.63.3.1156. [DOI] [PubMed] [Google Scholar]
- Reid MB, Khawli FA, Moody MR. Reactive oxygen in skeletal muscle. III. Contractility of unfatigued muscle. J Appl Physiol. 1993;75:1081–1087. doi: 10.1152/jappl.1993.75.3.1081. [DOI] [PubMed] [Google Scholar]
- Richardson RS, Wagner H, Mudaliar SR, Saucedo E, Henry R, Wagner PD. Exercise adaptation attenuates VEGF gene expression in human skeletal muscle. Am J Physiol Heart Circ Physiol. 2000;279:H772–H778. doi: 10.1152/ajpheart.2000.279.2.H772. [DOI] [PubMed] [Google Scholar]
- Rosenblatt JD, Kuzon WM, Plyley MJ, Pynn BR, McKee NH. A histochemical method for the simultaneous demonstration of capillaries and fiber type in skeletal muscle. Stain Technol. 1987;62:85–92. doi: 10.3109/10520298709107973. [DOI] [PubMed] [Google Scholar]
- Ryan MJ, Jackson JR, Hao Y, Williamson CL, Dabkowski ER, Hollander JM, Alway SE. Suppression of oxidative stress by resveratrol after isometric contractions in gastrocnemius muscles of aged mice. J Gerontol. 2010;65:815–831. doi: 10.1093/gerona/glq080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safdar A, Hamadeh MJ, Kaczor JJ, Raha S, Debeer J, Tarnopolsky MA. Aberrant mitochondrial homeostasis in the skeletal muscle of sedentary older adults. PloS One. 2010;5:e10778. doi: 10.1371/journal.pone.0010778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salerno MS, Thomas M, Forbes D, Watson T, Kambadur R, Sharma M. Molecular analysis of fiber type-specific expression of murine myostatin promoter. Am J Physiol Cell Physiol. 2004;287:C1031–1040. doi: 10.1152/ajpcell.00492.2003. [DOI] [PubMed] [Google Scholar]
- Shindoh C, DiMarco A, Thomas A, Manubay P, Supinski G. Effect of N-acetylcysteine on diaphragm fatigue. J Appl Physiol. 1990;68:2107–2113. doi: 10.1152/jappl.1990.68.5.2107. [DOI] [PubMed] [Google Scholar]
- Singha UK, Sharma S, Chaudhuri M. Downregulation of mitochondrial porin inhibits cell growth and alters respiratory phenotype in Trypanosoma brucei. Eukaryotic Cell. 2009;8:1418–1428. doi: 10.1128/EC.00132-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srere PA. Citrate synthase. Methods Enzymol. 1969;13:3–5. [Google Scholar]
- Suarez RK, Lighton JR, Brown GS, Mathieu-Costello O. Mitochondrial respiration in hummingbird flight muscles. Proc Natl Acad Sci U S A. 1991;88:4870–4873. doi: 10.1073/pnas.88.11.4870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang W, Ingalls CP, Durham WJ, Snider J, Reid MB, Wu G, Matzuk MM, Hamilton SL. Altered excitation-contraction coupling with skeletal muscle specific FKBP12 deficiency. FASEB J. 2004;18:1597–1599. doi: 10.1096/fj.04-1587fje. [DOI] [PubMed] [Google Scholar]
- Timmers S, de Vogel-van den Bosch J, Hesselink MK, van Beurden D, Schaart G, Ferraz MJ, Losen M, Martinez-Martinez P, De Baets MH, Aerts JM, Schrauwen P. Paradoxical increase in TAG and DAG content parallel the insulin sensitizing effect of unilateral DGAT1 overexpression in rat skeletal muscle. PLoS One. 2011;6:e14503. doi: 10.1371/journal.pone.0014503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadley GD, Choate J, McConell GK. NOS isoform-specific regulation of basal but not exercise-induced mitochondrial biogenesis in mouse skeletal muscle. J Physiol. 2007;585:253–262. doi: 10.1113/jphysiol.2007.141309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadley GD, McConell GK. Effect of nitric oxide synthase inhibition on mitochondrial biogenesis in rat skeletal muscle. J Appl Physiol. 2007;102:314–320. doi: 10.1152/japplphysiol.00549.2006. [DOI] [PubMed] [Google Scholar]
- Wallace DC. Mitochondrial diseases in man and mouse. Science. 1999;283:1482–1488. doi: 10.1126/science.283.5407.1482. [DOI] [PubMed] [Google Scholar]
- Waters RE, Rotevatn S, Li P, Annex BH, Yan Z. Voluntary running induces fiber type-specific angiogenesis in mouse skeletal muscle. Am J Physiol Cell Physiol. 2004;287:C1342–1348. doi: 10.1152/ajpcell.00247.2004. [DOI] [PubMed] [Google Scholar]
- Wong LE, Garland T, Jr, Rowan S, Hepple RT. Anatomic capillarization is elevated in medial gastrocnemius muscle of mighty mini mice. J Appl Physiol. 2009;106:1660–1667. doi: 10.1152/japplphysiol.91233.2008. [DOI] [PubMed] [Google Scholar]
- Wright DC, Han DH, Garcia-Roves PM, Geiger PC, Jones TE, Holloszy JO. Exercise-induced mitochondrial biogenesis begins before the increase in muscle PGC-1α expression. J Biol Chem. 2007;282:194–199. doi: 10.1074/jbc.M606116200. [DOI] [PubMed] [Google Scholar]
- Yamazaki KG, Romero-Perez D, Barraza-Hidalgo M, Cruz M, Rivas M, Cortez-Gomez B, Ceballos G, Villarreal F. Short- and long-term effects of (–)-epicatechin on myocardial ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol. 2008;295:H761–767. doi: 10.1152/ajpheart.00413.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki KG, Taub PR, Barraza-Hidalgo M, Rivas MM, Zambon AC, Ceballos G, Villarreal FJ. Effects of (–)-epicatechin on myocardial infarct size and left ventricular remodeling after permanent coronary occlusion. J Am Coll Cardiol. 2010;55:2869–2876. doi: 10.1016/j.jacc.2010.01.055. [DOI] [PMC free article] [PubMed] [Google Scholar]


