Abstract
Human rhinovirus is responsible for the majority of virus-induced asthma exacerbations. To determine the immunologic mechanisms underlying rhinovirus-induced asthma exacerbations, we combined mouse models of allergic airways disease and human rhinovirus infection. We inoculated ovalbumin-sensitized and challenged BALB/c mice with rhinovirus serotype 1B, a minor group strain capable of infecting mouse cells. Compared to sham-infected, ovalbumin-treated mice, virus-infected mice showed increased lung infiltration with neutrophils, eosinophils and macrophages, airway cholinergic hyperresponsiveness, and increased lung expression of cytokines including eotaxin-1/CCL11, IL-4, IL-13 and IFN-γ. Administration of anti-eotaxin-1 attenuated rhinovirus-induced airway eosinophilia and responsiveness. Immunohistochemistry showed eotaxin-1 in the lung macrophages of virus-infected, ovalbumin-treated mice, and confocal fluorescence microscopy revealed co-localization of rhinovirus, eotaxin-1 and IL-4 in CD68-positive cells. RV inoculation of lung macrophages from ovalbumin-treated, but not PBS-treated, mice induced expression of eotaxin-1, IL-4, and IL-13 ex vivo. Macrophages from ovalbumin-treated mice showed increased expression of arginase-1, Ym-1, Mgl-2 and IL-10, indicating a shift in macrophage activation status. Depletion of macrophages from ovalbumin-sensitized and -challenged mice reduced eosinophilic inflammation and airway hyperreactivity following RV infection. We conclude that augmented airway eosinophilic inflammation and hyperresponsiveness in RV-infected mice with allergic airways disease is directed in part by eotaxin-1. Airway macrophages from mice with allergic airways disease demonstrate a change in activation state characterized in part by altered eotaxin and IL-4 production in response to RV infection. These data provide a new paradigm to explain RV-induced asthma exacerbations.
INTRODUCTION
Viral infections trigger 80% of asthma exacerbations in children and nearly 50% in adults (1, 2), with human rhinovirus (RV) being the most common virus identified. While RV infections were once thought to be restricted to upper airway tissues (3), it is now clear that infections of the upper respiratory tract are accompanied by the entry of virus into lower respiratory tract cells (4-7), though the quantity of viral replication is not known.
In normal subjects, RV causes airway narrowing in response to methacholine as well as increased airway neutrophils and submucosal CD3+ cells (8, 9). In theory, RV infection of airway cells elicits the production of chemokines, subsequently inducing recruitment of inflammatory cells to the airways. Inflammatory cells, in turn, elaborate cytokines and mediators capable of increasing airways responsiveness. This paradigm, however, does not explain why asthmatic subjects experience exacerbations of lower airways disease following respiratory tract infection while control subjects do not.
Numerous clinical studies suggest a role for interleukin (IL)-8/CXCL8 in the pathogenesis of RV-induced asthma exacerbations. IL-8 and neutrophils are found in the nasal secretions, sputum or bronchoalveolar lavage fluid of allergic subjects undergoing experimental RV infection (9-14). After RV16 infection, asthmatic patients show increased levels of IL-8 in their nasal lavage which correlates with the level of airways responsiveness (11), in contrast to unaffected individuals in whom IL-8 does not increase (15). Eosinophils and eosinophil cationic protein have also been detected in the airways following experimental RV infection (10, 14, 16). Asthmatics undergoing experimental RV infection demonstrate greater eosinophilic inflammation than RV-infected control subjects (14). Together, these data suggest that patients with asthma experience a different response to viral infection than controls.
We recently showed that inoculation of C57BL/6 mice with RV1B, a minor group virus which binds to proteins of the highly conserved low-density lipoprotein receptor family, induces airway neutrophilic inflammation and methacholine hyperresponsiveness (17). In contrast, replication-deficient UV-irradiated virus did not cause lasting hyperresponsiveness. We also found positive and negative-strand viral RNA in the lungs up to 4 days after infection, suggesting replication of RV in vivo. It was recently shown that RV infection of BALB/c mice induces similar airway changes (18). Infection of ovalbumin (OVA)-sensitized and -challenged mice increased bronchoalveolar neutrophils, eosinophils and lymphocytes compared with allergen-challenged mice treated with UV-inactivated virus. However, the mechanism by which eosinophils are attracted to the airways following RV infection, and the requirement of eosinophils for the development of RV-induced airway hyperresponsiveness, were not examined.
In the present study, we show that, in OVA-sensitized and challenged BALB/c mice, RV1B infection increased production of pro-inflammatory cytokines including eotaxin-1/CCL11, Th-2 cytokines IL-4, IL-13. Bronchoalveolar and lung neutrophils, eosinophils, and macrophages, as well as airways responsiveness, were elevated in the RV-infected, OVA-treated mice. Neutralization of eotaxin-1/CCL11 reduced both airway eosinophilic inflammation and hyperresponsiveness. Eotaxin-1 and IL-4 were localized to RV-infected airway macrophages. Finally, macrophages from OVA-treated, but not PBS-treated, mice expressed eotaxin-1, IL-4, IL-13 in response to RV infection ex vivo, as well as the alternative activation markers arginase-1, Ym-1, MGL-2, and IL-10. Finally, depletion of macrophages from OVA/RV treated mice significantly decreased eosinophil infiltration and airway responses compared to non-depleted controls. These results suggest that allergen sensitization and challenge skews a predominantly neutrophilic RV response in naïve mice to a Th-2-dominant eosinophil response that is augmented, at least in part, by alternatively activated macrophages.
METHODS
Generation of RV
RV1B (ATCC, Manassas, VA) was concentrated, purified and titered as described previously (19, 20). Fifty percent tissue culture infectivity doses (TCID50) were determined by the Spearman-Karber method. RV1B was UV-irradiated using a CL-1000 crosslinker (UVP, Upland, CA).
OVA sensitization/challenge and RV exposure
This study was approved by the Institutional Animal Care and Use Committee. Animal usage followed guidelines set forth in the “Principles of Laboratory Animal Care” (National Society for Medical Research). Female 8 wk-old BALB/c mice (Jackson Laboratories, Bar Harbor, MA) were injected intraperitoneally with 200 μl of a 5 mg/ml solution of alum and endotoxin-free OVA or PBS (Sigma-Aldrich, St. Louis, MO) on days 1 and 7 and treated intranasally with 50 μl of a 20 mg/ml solution of OVA or PBS on days 14, 15 and 16. Immediately following the last OVA or PBS treatment, mice were inoculated intranasally with 45 μl of 1×108 TCID50/ml RV1B, UV-irradiated RV or an equal volume sham HeLa cell lysate (17).
Bronchoalveolar inflammatory cells and macrophage culture
Bronchoalveolar lavage (BAL) was performed using 1 ml PBS aliquots. Cytospins were stained with Diff-Quick (Dade Behring, Newark, DE) and differential counts determined from 200 cells. BAL fluid from PBS- and OVA-treated mice was seeded in 12 well plates. To partially purify macrophages, cells were allowed to adhere for 90 min and non-adherent cells removed by suction. Diff-Quick staining showed adherent cells to consist of >90% macrophages, with the rest of the cells being neutrophils. Remaining cells were resuspended in RPMI (Invitrogen, Carlsbad, CA), stimulated for 2 h with sham or RV1B (multiplicity of infection, 5.0), and RNA harvested 8 h after infection. In selected experiments, cells were pre-treated with 30 ng/ml IL-4 and IL-13 (Peprotech, Rocky Hill, NJ).
Lung inflammation
To quantify inflammatory cells, lung digests were obtained by mincing the tissue, proteolysis in collagenase type IV (Gibco Invitrogen, Carlsbad, CA) and straining through a 70 μm nylon mesh (BD Falcon, San Jose, CA), as described (21). The resulting pellet was treated with red blood cell lysis buffer (BD Pharmingen, San Diego, CA) and leukocytes were enriched by spinning the cells through 40% Percoll (Sigma-Aldrich). Lung leukocyte cytospins were stained and counted as described above.
Cytokine/chemokine expression
Lung RNA was extracted with Trizol (Sigma-Aldrich) and analyzed for cytokine and mucin gene expression by quantitative real time PCR using specific primers and probes. Signals were normalized to GAPDH and expressed as fold-increase. BAL fluid was spun for 15 min at 1500 g, and the supernatants were analyzed for cytokine protein by multiplex immune assay (Biorad, Hercules, CA).
Respiratory system resistance
Airway responsiveness was assessed by measuring changes in respiratory system resistance in response to increasing doses of nebulized methacholine (17).
Macrophage depletion
Depletion of alveolar macrophages was accomplished by intratracheal instillation of liposomes containing clodronate (dichloromethylenediphosphonic acid, disodium salt), as previously described (22). PBS-containing liposomes were used for control experiments. Briefly, 8 mg cholesterol and 86 mg phosphatidylcholine (Avanti Polar Lipids, Alabaster, AL) were dissolved in chloroform and slowly evaporated. The filmy layer was resuspended in 10 ml of PBS or 0.6 M clodronate. The mixture was exposed to N2 gas and incubated for 2 h at room temperature with gentle shaking. The mixture was then sonicated and incubated for another 2 h to allow liposome swelling. The solution was centrifuged at 10,000 × g for 15 min, and the liposomes were collected and washed twice with sterile PBS. Liposomes were kept at 4°C under N2 until use. Depletion was performed 24 h after the last OVA challenge by introducing 60 μl of clodronate- or PBS-containing liposomes intratracheally. Twenty-four h later, mice were infected with RV1B, as described above. Differential cell counts were performed on lung digests and respiratory resistance to methacholine was measured.
Immunohistochemistry and confocal fluorescence microscopy
Lungs were fixed with 10% formaldehyde overnight and paraffin embedded. Blocks were sectioned at 500 μm intervals at a thickness of 5 μm and each section was deparaffinized, hydrated and stained with goat anti-mouse eotaxin-1 (Santa Cruz Biotechnology, Santa Cruz, CA). For immunohistochemistry, sections were incubated with biotinylated secondary goat-IgG, ABC reagent (Vector Laboratories, Burlingame, CA), diaminobenzidine (DAB, Sigma-Aldrich) and Gill’s hematoxylin (Fisher Scientific, Kalamazoo, MI). For fluorescence microscopy, slides were incubated with Alexa Fluor (AF)-conjugated donkey anti-goat IgG (Molecular Probes, Portland, OR) and rat anti-mouse CD68 (AbD Serotec, Raleigh, NC) or isotype control IgG. In selected experiments, sections were co-stained with antiserum against RV1B (ATCC). Antiserum was partially purified by incubation with nitrocellulose-bound HeLa cell proteins and passing through an affinity resin containing non-denatured mouse lung protein. Repurified antibody was directly conjugated to AF. Nuclei were stained with Hoescht 33258. Images were visualized using a Zeiss LSM 510 confocal microscope and Axiovert 100M inverted microscope. CD68-, eotaxin-1/CCL11-positive cells were counted at 500 μm intervals and expressed as the number per field.
Data analysis
Data are represented as mean±SEM. Statistical significance assessed by one- or two-way analysis of variance (ANOVA), as appropriate. Differences were pinpointed by Student Newman-Keuls’ multiple range test.
RESULTS
RV infection of OVA-sensitized and -challenged mice further increases airway inflammation
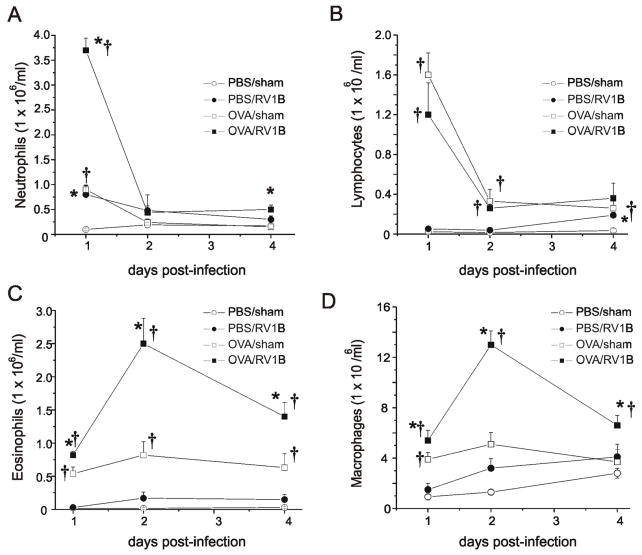
We previously showed that RV1B infection of naïve C57BL/6 mice induces a state of modest airways hyperresponsiveness which lasts at least four days after viral inoculation (17). Hyperresponsiveness was associated with a short-lived increase in bronchoalveolar neutrophils. In the present study, we infected OVA-sensitized and -challenged BALB/c mice. Tissue eosinophils, macrophages and neutrophils were elevated in OVA-treated mice up to four days after RV inoculation (Figure 1), with maximal recruitment of macrophages and eosinophils occurring two days post-infection. In terms of absolute numbers, macrophages were the cell type most heavily recruited to the tissues. Lung neutrophil recruitment was a relatively brisk event, with a significant increase in OVA/RV mice on day 1 after infection and a dramatic decrease on days 2 and 4. Although OVA treatment significantly increased lung lymphocytes compared to naïve mice, there was no significant difference in lymphocyte counts between OVA/RV and OVA/sham mice. In the BAL, RV infection increased neutrophils, eosinophils and lymphocytes in both naïve and OVA-sensitized and -challenged mice (see Figure S1 in the Online Supplement). As in the lung tissue, the largest absolute increase in BAL inflammatory cells following RV infection of OVA-sensitized and -challenged mice was observed in the macrophage line.
Figure 1. OVA/RV treated mice show increased tissue eosinophils and macrophages in response to RV infection.
Wild type BALB/c mice were sensitized intraperitoneally with endotoxin-free OVA and alum on days 1 and 7, and challenged intranasally on days 14, 15, and 16 with OVA. Controls were treated with PBS. Mice were inoculated with RV1B or sham (HeLa cell supernatant) on day 16. Mouse lungs were harvested 1, 2 and 4 after infection. Lungs were digested for 1 h in Type IV collagenase in serum free RPMI. Strained cells were treated with RBC lysis buffer, spun and enriched for leukocytes with 40% Percoll. Resulting pellets were resuspended in PBS and total cell count determined. Cytospins of leukocytes were stained with Diff-Quik and differential cell count determined for 200 cells. Time courses for tissue neutrophils (A), lymphocytes (B), eosinophils (C) and macrophages (D) are shown. (N=4-5 mice per group, bars represent mean±SEM, *different from respective sham group, †different from respective PBS group, P<0.05, one-way ANOVA.)
Effects of RV infection on lung pro-inflammatory cytokines
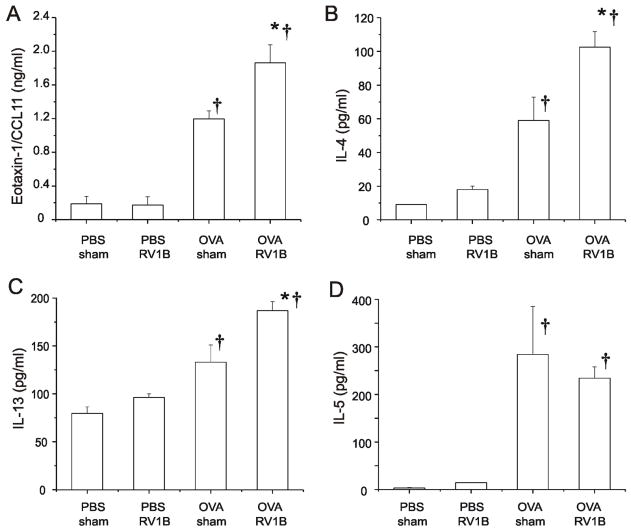
In order to determine changes in pro-inflammatory cytokines that might be responsible for the observed increase in eosinophilic inflammation, we measured lung cytokine levels by multiplex immunoassay. Compared to OVA-treated mice, OVA/RV mice demonstrated significantly higher levels of eotaxin-1/CCL11, IL-4 and IL-13 one day after infection (Figure 2). In contrast, there was no effect of RV infection on lung IL-5 levels. On day 4, lungs from RV-infected OVA-treated mice showed a sustained increase in eotaxin-1 and IL-4 levels (Figure 3). Eotaxin mRNA was elevated in the OVA/RV treated groups on day 1, 2, and 4 post infection.
Figure 2. RV infection of OVA-sensitized and -challenged mice increases cytokine production.
Twenty-four h after sham or RV infection, lung BAL fluid was centrifuged at 1500g and the resulting supernatant subjected to multiplex immunoassay. Results are shown for eotaxin-1/CCL-11 (A), IL-4 (B), IL-13 (C) and IL-5 (D). (N=5 mice per group, bars represent mean ± SEM, *different from respective sham group, p<0.05; †different from respective PBS group, P<0.05 one-way ANOVA.)
Figure 3. RV infection of OVA-sensitized and -challenged mice increases cytokine production four days after infection.
Lung BAL fluid was centrifuged at 1500g and the resulting supernatant subjected to multiplex immunoassay. Results are shown for eotaxin-1/CCL11 (A and D), IL-4 (B) and IL-13 (C). cDNA for eotaxin-1/CCL-11 was synthesized using reverse transcriptase and subjected to quantitative real time PCR employing a Taqman probe. (N=5 mice per group, bars represent mean ± SEM, *different from respective sham group, p<0.05; †different from respective PBS group, P<0.05 one-way ANOVA.)
RV infection increases the airways responsiveness of OVA-sensitized and -challenged mice
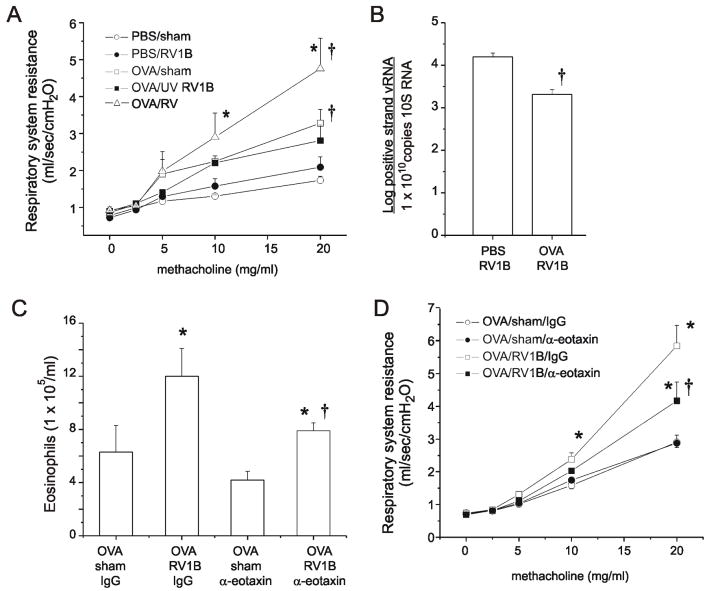
In order to determine whether the observed airway inflammation was functionally significant, all groups were tested for responsiveness to a bronchoconstrictor agonist four days after sham or RV1B treatment. Methacholine (0-20 mg/ml) was administered by nebulization and total respiratory system resistance values recorded. As expected, OVA treatment increased airway cholinergic responsiveness (Figure 4A). However, RV-infected OVA mice demonstrated significantly higher airways responses, with significant differences noted at methacholine doses of 10 and 20 mg/ml (p < 0.05, two-way ANOVA). In contrast, UV-irradiated, replication-deficient RV had no effect on airways responses in OVA-sensitized and -challenged mice. To determine whether the observed elevated airway responses to methacholine were due to a higher viral load, we measured lung positive-strand RV RNA. Surprisingly, vRNA levels were significantly lower in the OVA/RV treatment group (Figure 4B), suggesting that allergen sensitization and challenge increases viral clearance. As shown previously (17), viral copy numbers were negligible by four days after infection, consistent with the notion that airways hyperresponsiveness persists after viral clearance in this model.
Figure 4. RV infection of OVA-sensitized and -challenged mice induces eotaxin-1-mediated airways cholinergic responsiveness.
A. Mice were anesthetized and endotracheally intubated, and changes in respiratory system resistance to nebulized methacholine measured using the FlexiVent system (Scireq, Montreal, CA). Four days after infection, RV-infected OVA mice demonstrated significantly higher airways responses than all other groups at methacholine doses of 10 and 20 mg/ml. B. Measurement of viral copy number from lungs of PBS/RV and OVA/RV treated mice 1 day post infection. OVA/RV treatment significantly reduced viral copy number by 1 log. (N= 5 mice per group, bars represent mean ± SEM, *different from respective sham group, p<0.05; †different from respective PBS group, P<0.05 one-way ANOVA.) C. Selected RV-infected, OVA-sensitized and -challenged mice were given two systemic injections of rabbit anti-mouse eotaxin-1. Additional mice were treated with the isotype control antibody. Mice given anti-eotaxin displayed reduced tissue eosinophils 4 days after infection. D. Neutralizing antibody and isotype control-treated mice were administered increasing doses of aerosolized methacholine. Treatment with anti-eotaxin-1 significantly reduced airway cholinergic responsiveness compared to the IgG-treated group. (Bars represent mean ± SEM, *different from respective sham group, †different from IgG group, P<0.05, one-way ANOVA.)
Eotaxin-1 is required for maximal RV-induced eosinophilic airway inflammation and hyperresponsiveness in OVA mice
In OVA-treated mice, RV infection increased lung eosinophils (Figure 1) and the protein level of eotaxin-1/CCL11 (Figures 2 and 3), an eosinophil-specific chemokine. We therefore sought to examine the contribution of eosinophils to RV-induced airway responsiveness by administering neutralizing antibody to mouse eotaxin-1. To ensure the suppression of augmented eosinophilic inflammation in RV-infected mice, a subset of OVA-treated mice was given two systemic injections of rabbit antiserum, the first on the day of RV inoculation and the second two days later. Control mice were treated with the isotype control. We found that, compared to IgG, anti-eotaxin treatment significantly reduced lung eosinophils in OVA-treated, RV-infection mice, but not OVA-treated, sham-inoculated mice (Figure 4C). Anti-eotaxin-1 neutralizing antibody did not reduce the infiltration of neutrophils, macrophages or lymphocytes (data not shown), suggesting that the antibody specifically targeted eosinophils. Further, administration of anti-eotaxin to OVA/RV mice significantly reduced responsiveness to methacholine compared to IgG (Figure 4D), suggesting that eotaxin-1 and eosinophils are required for maximal airway responses in RV-infected allergen-sensitized and -challenged mice. Anti-eotaxin had no effect on lung vRNA one day post infection (data not shown).
Eotaxin-1 is mainly localized to CD68-positive macrophages
We examined lungs of PBS-and OVA treated mice for eotaxin-1 localization (Figure 5, panels A-C). Immunohistochemical analysis of lungs from RV-infected OVA-treated mice showed abundant eotaxin-1-staining which appeared to be localized to airway and submucosal macrophages. There also appeared to be a small number of eotaxin-positive eosinophils as well as limited staining in the airway epithelium. Fluorescence confocal microscopy with anti-eotaxin-1 and anti-CD68 showed intense yellow-orange staining, consistent with colocalization of CD68 and eotaxin-1 in lung macrophages (see Figure S2 in the Online Supplement). When CD68+/eotaxin-1/CCL11 positive cells were counted, OVA/RV mice showed a significantly higher number of cells per field compared to the other groups. We also found colocalization of RV1B in CD68-, eotaxin-1/CCL11-positive cells (Figure 5, panels B-F), suggesting that RV infection initiates cytokine expression and/or secretion in airway macrophages. Most RV-infected macrophages were located in the submucosa of large airways, but others were found in the airways and epithelium (Figure 5, panel G). A minor amount of RV1B and eotaxin staining was also found in airway epithelial cells. Finally, we found co-localization of RV1B, IL-4 and CD68 in the lungs of OVA-treated (Figure 5, panels I-M) but not IgG antibody treated sections (panel H) or PBS-treated mice (data not shown), indicating that, following exposure to an allergic environment, lung macrophages produce Th2 cytokines in response to RV infection in vivo.
Figure 5. RV colocalizes with CD68+ macrophages, eotaxin-1, and IL-4 in OVA-sensitized and -challenged mice.
A. Lungs were formaldehyde fixed overnight, paraffin embedded, sectioned at 5 μm and incubated with a 1:2500 dilution of donkey-anti-mouse eotaxin-1 (Santa Cruz Biotechnology, CA) or isotype control IgG. Eotaxin was identified by DAB staining. Following OVA/RV treatment, eotaxin-1 localization is noted in macrophages (arrows) and eosinophils (arrowheads) but not in the airway epithelium (line segment = 50 μm). B-E. OVA/RV lung sections were co-stained with antiserum against RV1B which was directly conjugated to AF-594 (red), while CD68 was conjugated to AF-633 (far red, shown in blue). Secondary antibody to eotaxin-1 was conjugated to AF-488 (green). Cells with colocalization (white) are designated by arrows and a high magnification view is shown in panel F. Original magnification, 600X. G. RV infection of CD68-positive cells in the airway lumen and epithelial layer. In this panel, RV anti-serum was directly conjugated to AF-594 (red), while CD68 was conjugated to AF488 (green). Colocalization is yellow. Colocalization in the epithelium suggests infiltration by a macrophage. H. Sections incubated with secondary antibodies alone showed no staining. I-M. RV co-localizes with IL-4 in CD68-positive cells. RV and CD68 were conjugated with AF-594 (red), and AF-633 (shown in blue) respectively. There is some blue background staining of elastin in the epithelial basement membrane. IL-4 was directly conjugated to AF-488 (green). M. Co-localization of RV, IL-4, and CD68 is white. Original magnification, 400X.
Macrophages are required for RV-induced eosinophil infiltration and airway hyperresponsiveness in OVA-sensitized and -challenged mice
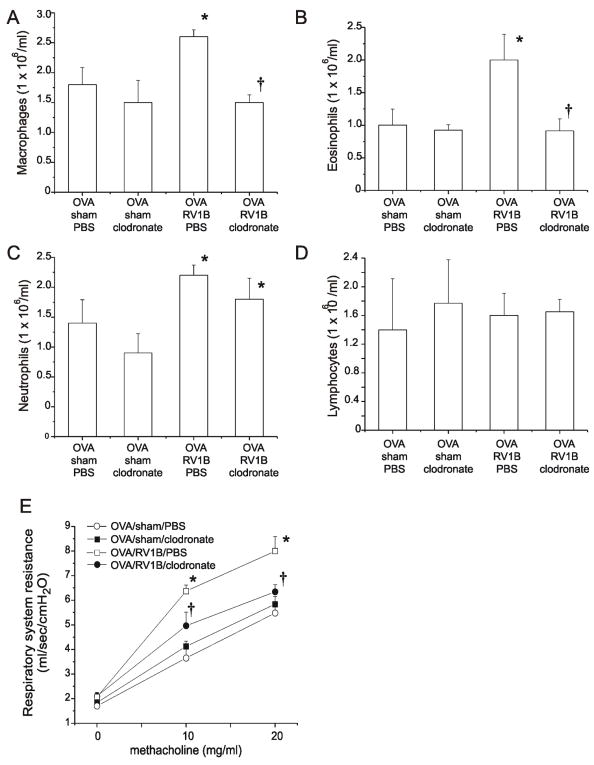
We delivered clodronate- or PBS-containing liposomes to OVA-treated mice intratracheally. Twenty-four h later, mice were inoculated with sham or RV1B. As expected, clodronate treatment depleted total lung macrophages (Figure 6A). Differential cell counts revealed a markedly lower eosinophil influx in clodronate treated mice (Figure 6B). Compared to PBS-containing liposomes, no significant differences in neutrophil or lymphocyte accumulation were observed (Figure 6, panels C and D). OVA/RV mice receiving clodronate liposomes demonstrated a significantly reduced response to methacholine (Figure 6E). We also observed significant reductions in the mRNA expression of eotaxin-1 and IL-13 (Figure 7, panels A and B) but not IL-4 (data not shown) in OVA/RV mice treated with clodronate, suggesting that depletion of macrophages decreases eosinophil influx by attenuating macrophage production of eotaxin-1. Together, these data suggest that macrophages play an essential role in eosinophil infiltration and airway hyperreactivity in RV-infected mice with allergic airways disease.
Figure 6.
Macrophage-depleted OVA-treated mice show reduced airway eosinophils and hyperresponsiveness following RV infection. Clodronate or PBS-containing liposomes were instilled into the trachea 24 h after the last OVA challenge. Twenty-four h following macrophage depletion, mice were inoculated with sham or RV and harvested 24 h after infection. Lung digests were performed as described in Figure 1. Differential counts were determined. Cytospins of leukocytes were stained with Diff-Quik and differential cell count determined for 200 cells. Macrophages (A), eosinophils (B), neutrophils (C) and lymphocytes (D) are shown. E. Airway resistance for each group was measured following treatment with 0, 10, and 20 mg/ml methacholine. (N=4 mice per group, bars represent mean±SEM, *different from respective sham group, †different from OVA/RV/ PBS liposome group, P<0.05, one-way ANOVA.)
Figure 7.
Clodronate-mediated depletion of macrophages reduces OVA/RV induced cytokine expression. Lung mRNA was extracted and corresponding cDNA subjected to quantitative real time PCR. (N=4 mice per group, bars represent mean±SEM, *different from sham group, †different from OVA/RV/ PBS liposome group, P<0.05, one-way ANOVA.)
OVA sensitization and challenge alters macrophage pro-inflammatory cytokine expression in response to RV and upregulates markers of alternative activation
In order to determine the combined effects of allergen sensitization and RV on macrophage responses, adherent BAL cells were studied. Cells from PBS- or OVA-sensitized and -challenged mice were then stimulated with HeLa cell lysate (sham) or RV1B ex vivo. Eight h after sham or RV exposure, cells were harvested for total RNA. Cytokine expression was determined by quantitative real time PCR. Macrophages from control mice produced no eotaxin-1 ex vivo, either at baseline or in response to RV (Figure 8). However, macrophages exposed to an allergic environment in vivo expressed eotaxin-1 mRNA, and this level was significantly increased following RV1B stimulation. In addition, RV treatment of macrophages from OVA sensitized mice induced expression of IL-4, IL-13, IL-10 and IFN-γ. UV-irradiation of RV abrogated the eotaxin, IL-10 and IFN responses, indicating that expression is dependent on viral replication. Because UV-irradiated virus also increased IL-4 and IL-13 mRNA levels, expression of these cytokines appeared to replication-independent. Finally, in contrast to the above cytokines, the TNF-α response to RV infection was significantly decreased in macrophages isolated from OVA-sensitized and -challenged mice.
Figure 8.
Macrophages from OVA-sensitized and -challenged mice show increased cytokine mRNA expression after RV stimulation ex vivo. BAL fluid was extracted from PBS-treated and OVA-sensitized and -challenged mice and seeded in 12-well plates. Cells were allowed to adhere to plates for 90 minutes. Adherent cells were subsequently infected with RV1B, or sham or media (controls). A. Eotaxin-1 expression was observed in adherent BAL cells from OVA-treated but not PBS-treated mice. Eotaxin-1 significantly increased following RV stimulation. B. IL-13. C. TNF-α. D. IL-4. E. IL-10. F. IFN-γ. (N=3-4, bars represent mean±SEM. Because some treatment conditions yielded no detectable mRNA expression, data were normalized to the condition with the lowest detectable mRNA signal. *different from respective sham group, †different from respective UV RV group, §different from respective PBS group, P<0.05, one-way ANOVA.)
We also measured the production of selected cytokines in cell supernatants following ex vivo RV stimulation (see Figure S3 in the Online Supplement). IL-4 production was significantly increased in RV-stimulated macrophages from OVA-treated mice. We detected a small but significant increase in eotaxin-1/CCL11 production in macrophages from OVA mice exposed to RV 8 h post-infection. It is conceivable that, in contrast to eotaxin mRNA expression, the release of eotaxin-1 requires the coordinated action of other mediators which may not be present in vitro. In contrast, macrophages from OVA-sensitized and -challenged mice showed reduced levels of TNF-α and p70 IL-12 production after RV stimulation.
Based on the pattern of increased Th2 cytokine expression, we hypothesized that allergic sensitization induces macrophages to deviate from their classical pattern of activation, and instead exhibit a functionally polarized phenotype. To test this hypothesis, we measured markers of macrophage activation in cells isolated from PBS- and OVA-treated mice. We also found significant upregulation of the M2 polarization markers Arg-1, MGL-2, Ym-1 (Figure 9A) and, as noted above, IL-10.
Figure 9.
Effect of Th2 environment on macrophage polarization. A. OVA sensitization and challenge alters the mRNA expression of macrophage activation markers. Data are fold-increase compared to macrophages from PBS-treated mice (N=3-4, bars represent mean±SEM). B. Effect of IL-4/IL-13 incubation on the eotaxin response to RV infection in macrophages from PBS-treated naïve mice. (N=3, bars represent mean±SEM.of fold increase in mRNA expression compared to control cells, mean±SEM, *P<0.05, one-way ANOVA).
IL-4 and IL-13 treatment has been shown to shift classically activated M1 macrophages to an M2 alternative activation phenotype (23-25). We therefore tested the effect of these cytokines on eotaxin mRNA expression in macrophages from naïve mice. In vitro exposure of macrophages from PBS-treated mice to the Th2 cytokines IL-4 and IL-13 significantly increased RV-induced eotaxin mRNA expression (Figure 9B). Taken together, these data suggest that allergen sensitization and challenge alters the activation state and augments the cytokine response of lung macrophages to RV infection, contributing to enhanced recruitment of eosinophils to the airways.
DISCUSSION
RV is responsible for majority of the common colds and approximately 50% of asthma exacerbations (1, 2). Previous studies have demonstrated that neutrophils are the predominant inflammatory cell in the airways of patients with acute asthma exacerbation (26-28). Experimental RV infection has been shown to increase airway neutrophilic inflammation in normal and asthmatic subjects (9-14). Eosinophils and cationic protein have also been detected in the airways following experimental RV infection (10, 14, 16). However the precise mechanism of RV-induced asthma exacerbations is not well understood. After experimental RV16 infection, asthmatic patients show increased levels of IL-8 in their nasal lavage which correlates with the level of airways responsiveness (11), in contrast to unaffected individuals in whom IL-8 does not increase (15). In a recent study, asthmatics undergoing experimental RV infection demonstrated greater neutrophilic, lymphocytic and eosinophilic inflammation than RV-infected control subjects, though only the number of eosinophils achieved statistical significance (14). Together, these data suggest that patients with asthma experience a different response to viral infection than control subjects.
Previously, we showed that RV infection of naïve mice induces airway inflammation marked predominantly by neutrophils and lymphocytes (20). RV infection also induced moderate airways hyperresponsiveness to methacholine. In the present study, we delineate the response to RV in the context of allergic inflammation. We found that, following RV infection of allergen-sensitized and -challenged mice, the largest populations of cells elicited in the BAL fluid were, in fact, eosinophils and macrophages. The increase in eosinophils was associated with a concomitant rise in expression of the eosinophil chemoattractant eotaxin-1/CCL11, which was significantly greater in OVA/RV mice in comparison to all other groups. Eosinophil infiltration was also accompanied by a synergistic increase in the Th-2 cytokines IL-4 and IL-13, each of which were significantly higher in the OVA/RV-treated mice compared to all other groups. It is worth noting that RV infection alone failed to significantly increase airway eosinophils, eotaxin-1, IL-4 and IL-13. RV infection also enhanced airways responsiveness in allergen-sensitized and -challenged mice, with hyperresponsiveness persisting at least 4 days after infection. These data confirm and extend a recent report (18), and are consistent with the notion that the allergic environment qualitatively alters the response to RV.
We measured viral copy number in the lungs of infected PBS- and OVA-treated mice. Viral load was not increased in allergen-sensitized and -challenged mice, demonstrating that the augmented airway inflammation and responsiveness was not due to an increase in the susceptibility to RV. Indeed, RV copy number was unexpectedly decreased in mice with allergic airways disease. These data are consistent with a previous report examining parainfluenza infection of OVA-treated animals (29), and suggest that inflammatory cells play a role in viral clearance. More importantly, these data demonstrate an uncoupling of viral load and airway inflammation. While this may seem surprising, viral infection may set off a pro-inflammatory cascade that outlasts the presence of live virus. Consistent with this, we previously found that RV-infected, naïve mice demonstrate airways hyperresponsiveness four days after RV infection, when viral copy number is decreasing (20). We also found that replication-deficient virus is sufficient to induce moderate neutrophilic inflammation and airways responsiveness one day after inoculation.
In OVA-treated mice, RV infection increased lung eosinophils and expression of eotaxin-1/CCL11. Eosinophils and the eotaxin/CCR3 axis are known to play a critical role in chronic experimental allergic airway inflammation (30-32). To test for the requirement of eotaxin-1 for enhanced eosinophilic inflammation and airways hyperresponsiveness in allergic, RV-infected mice, we targeted eotaxin-1 production by administering an anti-mouse eotaxin-1 neutralizing antibody following the last OVA challenge and RV infection. Anti-eotaxin-1 significantly reduced the number of airway eosinophils, but not the neutrophils or lymphocytes, demonstrating that eotaxin-1 is required for homing of eosinophils to the airway following RV infection. Further, administration of anti-eotaxin-1 decreased RV-induced airways hyperresponsiveness in allergen-sensitized and -challenged mice. While we did not determine the precise mechanism by which eosinophils increase airways responses, eosinophils are a known source of bronchoconstrictor agonists including major basic protein (MBP) and cysteinyl leukotrienes. When guinea pigs are sensitized to OVA and subsequently infected with parainfluenza, virus-induced hyperresponsiveness and M2 receptor dysfunction are blocked by depletion of eosinophils with antibody to IL-5 or antibody to MBP (29). RV infection has also been shown to increase 5-lipoxygenase and cyclooxygenase-2 in bronchial biopsy specimens from nonatopic subjects (33). Further, it should be noted that administration of anti-eotaxin-1 did not completely abolish RV1B-induced airways eosinophilic inflammation and hyperresponsiveness. It is conceivable that the neutralizing antibody was not fully effective in eliminating eotaxin function. It is also possible that other cytokines also contribute to airway narrowing. For example, our unpublished observations (D. Schneider, M. Hershenson) indicate that RV1B infection also increases lung expression of macrophage chemotactic protein (MCP)-1/CCL2, and that MCP-1/CCL2 partially contributes to RV-induced airways inflammation and hyperresponsiveness in OVA-sensitized and -challenged mice.
To determine the cell(s) responsible for the observed increase in eotaxin-1 expression in response to RV, we performed immunohistochemistry on OVA/RV mice. Though previous reports demonstrated production of eotaxin-1/CCL11 by RV-infected, cultured airway epithelial cells (34-38), eotaxin-1 was only minimally localized to the airway epithelium. Instead, eotaxin-1 protein abundance was readily apparent in alveolar macrophages. These data are consistent with previous reports showing that, in airway inflammatory cells from asthmatic patients, eotaxin-1 immunoreactivity is colocalized predominantly to macrophages, with a lesser contribution from eosinophils (39-41). Further, macrophages isolated from allergen-sensitized and -challenged mice demonstrated a significant eotaxin-1 response to RV stimulation ex vivo, in contrast to cells from naïve mice, which showed no response. RV has previously been shown to induce cytokine responses in alveolar macrophages in vitro. Production of monocyte chemoattractant protein (MCP)-1/CCL2 and IP-10/CXCL10 is replication-independent (42-45), whereas production of tumor necrosis factor-α may be replication-dependent (44). In the present study, we show for the first time that ex vivo macrophage responses to RV are augmented following allergen-sensitization and -challenge, and that macrophages produce cytokines in response to airway RV infection in vivo. However, we cannot tell from our images whether colocalization represents true replicative infection, endocytosis of virus, or phagocytosis of RV1B by airway macrophages.
Eotaxin production in response to RV infection has not been previously demonstrated in vivo. In the one study of which we aware examining eotaxin-1 expression in response to natural or experimental RV infection in asthmatic subjects, mRNA transcripts for eotaxin-1 were not expressed at consistently detectable levels in induced sputum (46). However, our preliminary studies examining nasal washes from asthmatic children show that natural viral infection, as detected by PCR, is associated with a 6-fold increase in eotaxin-1 protein abundance compared to virus-negative weeks (T. Lewis, T. Henderson, M. Hershenson, unpublished data).
In addition to eotaxin-1, we found that the combination of OVA treatment and RV infection increased production of IL-4 from alveolar macrophages, both in vivo and ex vivo. Macrophages from OVA-treated mice also expressed higher levels of IL-13 in response to RV ex vivo. The notion that a non-T cell source of Th2 cytokines may also act to enhance allergic inflammation by secreting IL-4 or IL-13 has not been well-studied. IL-13 production has been noted in lung macrophages from Sendai virus-infected C57BL/6J mice (47). The role of macrophages in the pathogenesis of asthma and allergic inflammation is unresolved. Macrophage subsets are recruited into the lung following OVA sensitization and challenge of Balb/cJ mice, and transfer to naïve mice increased airways responsiveness, eosinophilic inflammation and Th-2 cytokine secretion (48). On the other hand, transfer of alveolar macrophages from OVA-exposed Sprague-Dawley rats protects against the development of airways hyperresponsiveness in macrophage-depleted OVA-treated Brown-Norway rats (49). In our study, depletion of macrophages resulted in a significant amelioration of eosinophil infiltration and airway responsiveness. Macrophage-depleted OVA/RV mice also showed significantly reduced eotaxin-1 expression, suggesting for the first time that RV-induced asthma exacerbations may be directed by eotaxin-producing lung macrophages. There are other possible explanations, however. It is conceivable that clodronate attenuated airways eosinophilic inflammation and hyperresponsiveness by directly killing phagocytic eosinophils. However, to our knowledge this effect of clodronate has not been reported previously. Also, we should note that, while clodronate treatment decreased macrophage infiltration in RV-infected OVA mice, there was no effect in sham-inoculated OVA mice. It is possible that administration of a fourth OVA challenge after clodronate treatment restored the macrophage response. Alternatively, the phagocytic response of macrophages from sham-inoculated OVA-sensitized and -challenged mice might have been poor, owing to their strict M2 polarization. In RV-infected OVA mice, this effect might have been partially mitigated by viral infection, which led to the submaximal induction of TNF-α, a pro-phagocytic M1 signal (50).
Our data demonstrating increased production of eotaxin-1 and IL-4 from alveolar macrophages, both in vivo and ex vivo, suggests an alteration in the phenotype of tissue macrophages in response to allergen sensitization and RV infection. In addition, RV treatment of macrophages from OVA sensitized mice, but not PBS-treated mice, induced expression of IL-13, IL-10 and IFN-γ. In contrast, TNF-α and p70 IL-12 were significantly decreased. Shift of classically activated M1 macrophages to an M2 alternative activation phenotype, under the influence of the Th2 cytokines IL-4 and IL-13, has been associated with an altered secretory repertoire and pattern of phagocytic receptors (reviewed in (25)). IL-4 and IL-13 have been shown to induce alternative macrophage activation in vitro (23) and in vivo (24). In the latter study, IL-13-overexpressing transgenic mice infected with C. neoformans showed the presence of alternatively-activated macrophages expressing Arg-1, macrophage mannose receptor and Ym-1, as well as lung eosinophilia, goblet cell metaplasia, elevated mucus production and enhanced airway hyperreactivity. Consistent with this, we found significant up-regulation of M2 markers in OVA sensitized and challenged mice including Arg-1, MGL-2, Ym-1, Fizz-1 and IL-10. Modulation of Arg-1, Ym-1, Fizz-1, MGL-1 and MGL-2 expression was previously noted following OVA sensitization and challenge (51). Upregulation of Arg-1 may be of particular physiologic importance. In patients with asthma, Arg-1 mRNA expression is increased in submucosal inflammatory cells (52). Arginase expression is increased in the lungs of allergen-sensitized and challenged mice, and inhibition attenuates methacholine responsiveness in OVA-sensitized and challenged mice (53).
In addition to changes in macrophage receptor and cytokine expression typically associated with M2 polarization including Arg-1, MGL-2, Ym-1 and IL-10, we also found that OVA treatment increased expression of the classical activation marker IFN-γ. Patterns of macrophage gene expression may not display a strict dichotomy between type 1 and type 2 responses. For example, it has been reported that exposure of macrophages to IL-4 prior to LPS stimulation strongly enhances inflammatory activity (TNF-α, IL-12 production) as well as Arg-1 expression (54). These data suggest the possibility that exposure to a Th2 environment induces a functional phenotypic change in airway macrophages which does not strictly fit the M1/M2 model, leading to increased secretion of both type I and type II cytokines in response to RV stimulation.
In cultured macrophages, UV-irradiation of RV abrogated the eotaxin, IL-10 and IFN responses. The reduced cytokine expression following treatment with UV-irradiated virus is consistent with the notion that RV causes a replicative infection in macrophages. In vitro studies have noted attachment of HRV to peripheral blood monocytes and airway macrophages, with subsequent secretion of numerous pro-inflammatory cytokines, chemokines and IFNs (42-45, 55, 56). A small amount of viral replication has been noted in HRV-infected peripheral blood monocyte-derived macrophages, but not in bronchoalveolar lavage (BAL)-derived macrophages (42, 44).
Finally, we would like to add a few comments about our mouse combined model of asthma and RV infection. First, while experimental RV infection increased airways responsiveness of mice with allergic airways disease as it does in human asthmatics (11, 14), OVA sensitization and challenge of BALB/c mice may not recapitulate the severity and genetic background of many human patients with asthma. With regard to the RV infection model, we (20) and others (18) have found that a much higher viral titer is required to infect mice compared to humans. This is to be expected, as differences in the homology of viral receptors and intracellular signaling mechanisms are likely to restrict viral infection and replication in mice. Nevertheless, we have clearly shown that human RV1B replicates in mouse lungs, as evidenced by: 1) the presence of negative-strand viral RNA in the lungs of inoculated mice; 2) transmissibility of RV infection from the lung homogenates of inoculated mice to cultured HeLa cells; and 3) the induction of a robust lung interferon response (20). Replication-deficient UV-irradiated virus has none of these effects. We therefore believe that our mouse model of human RV infection holds promise for the study of RV-induced exacerbations of chronic airways diseases such as asthma.
In conclusion, we have shown in allergen-sensitized and -challenged mice that lung macrophages participate in RV-enhanced airway eosinophilic inflammation and hyperreactivity. Macrophages from allergen-sensitized and -challenged mice, but not control animals, produce eotaxin-1 and IL-4 in response to RV infection, both in vivo and ex vivo. The altered response to RV infection is driven by a functional change in macrophage polarization state, likely a response to Th2 cytokines in the allergic environment. These data provide a new paradigm to explain RV-induced asthma exacerbations, and identify the macrophage as a potential therapeutic target for the treatment of viral-induced exacerbations of chronic airways disease.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health grants HL81420 and 82550 (M.B.H.)
Footnotes
The online version of the article contains supplemental material.
References
- 1.Johnston SL, Pattemore PK, Sanderson G, Smith S, Lampe F, Josephs L, Symington P, O’Toole S, Myint SH, Tyrrell DA, Holgate ST. Community study of role of viral infections in exacerbations of asthma in 9-11 year old children. Brit Med J. 1995;310:1225–1229. doi: 10.1136/bmj.310.6989.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nicholson KG, Kent J, Ireland DC. Respiratory viruses and exacerbations of asthma in adults. Brit Med J. 1993;307:982–986. doi: 10.1136/bmj.307.6910.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Halperin SA, Eggleston PA, Hendley JO, Suratt PM, Groschel DH, Gwaltney JM. Pathogenesis of lower respiratory tract symptoms in experimental rhinovirus infection. Am Rev Respir Dis. 1983;128:806–810. doi: 10.1164/arrd.1983.128.5.806. [DOI] [PubMed] [Google Scholar]
- 4.Gern JE, Galagan DM, Jarjour NN, Dick EC, Busse WW. Detection of rhinovirus RNA in lower airway cells during experimentally induced infection. Am J Respir Crit Care Med. 1997;155:1159–1161. doi: 10.1164/ajrccm.155.3.9117003. [DOI] [PubMed] [Google Scholar]
- 5.Papadopoulos NG, Bates PJ, Bardin PG, Papi A, Leir SH, Fraenkel DJ, Meyer J, Lackie PM, Sanderson G, Holgate ST, Johnston SL. Rhinoviruses infect the lower airways. J Infect Dis. 2000;181:1875–1884. doi: 10.1086/315513. [DOI] [PubMed] [Google Scholar]
- 6.Mosser AG, Vrtis R, Burchell L, Lee W-M, Dick CR, Weisshaar E, Bock D, Swenson CA, Cornwell RD, Meyer KC, Jarjour NN, Busse WW, Gern JE. Quantitative and qualitative analysis of rhinovirus infection in bronchial tissues. Am J Respir Crit Care Med. 2005;171:645–651. doi: 10.1164/rccm.200407-970OC. [DOI] [PubMed] [Google Scholar]
- 7.Kaiser L, Aubert J-D, Pache J-C, Deffernez C, Rochat T, Garbino J, Wunderli W, Meylan P, Yerly S, Perrin L, Letovanec I, Nicod L, Tapparel C, Soccal PM. Chronic rhinoviral infection in lung transplant recipients. Am J Respir Crit Care Med. 2006;174:1392–1399. doi: 10.1164/rccm.200604-489OC. [DOI] [PubMed] [Google Scholar]
- 8.de Kluijver J, Grunberg K, Sont JK, Hoogeveen M, van Schadewijk WA, de Klerk EP, Dick CR, van Krieken JH, Sterk PJ. Rhinovirus infection in nonasthmatic subjects: effects on intrapulmonary airways. Eur Respir J. 2002;20:274–279. doi: 10.1183/09031936.02.00247202. [DOI] [PubMed] [Google Scholar]
- 9.Fleming H, Little F, Schnurr D, Avila P, Wong H, Liu J, Yagi S, Boushey H. Rhinovirus-16 colds in healthy and in asthmatic subjects: similar chages in upper and lower airways. Am J Respir Crit Care Med. 1999;160:100–108. doi: 10.1164/ajrccm.160.1.9808074. [DOI] [PubMed] [Google Scholar]
- 10.Grunberg K, Smits HH, Timmers MC, De Klerk EP, Dolhain RJ, Dick EC, Hiemstra PS, Sterk PJ. Experimental rhinovirus 16 infection. effects on cell differentials and soluble markers in sputum in asthmatic subjects. Am J Respir Crit Care Med. 1997;156:609–616. doi: 10.1164/ajrccm.156.2.9610079. [DOI] [PubMed] [Google Scholar]
- 11.Grunberg K, Timmers MC, Smits HH, de Klerk EP, Dick EC, Spaan WJ, Hiemstra PS, Sterk PJ. Effect of experimental rhinovirus 16 colds on airway hyperresponsiveness to histamine and interleukin-8 in nasal lavage in asthmatic subjects in vivo. Clin Exp Allergy. 1997;27:36–45. doi: 10.1111/j.1365-2222.1997.tb00670.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pizzichini MM, Pizzichini E, Efthimiadis A, Chauhan AJ, Johnston SL, Hussack P, Mahony J, Dolovich J, Hargreave FE. Asthma and natural colds. Inflammatory indices in induced sputum: a feasibility study. Am J Respir Crit Care Med. 1998;158:1178–1184. doi: 10.1164/ajrccm.158.4.9712082. [DOI] [PubMed] [Google Scholar]
- 13.Gern JE, Vrtis R, Grindle KA, Swenson C, Busse WW. Relationship of upper and lower airway cytokines to outcome of experimental rhinovirus infection. Am J Respir Crit Care Med. 2000;162:2226–2231. doi: 10.1164/ajrccm.162.6.2003019. [DOI] [PubMed] [Google Scholar]
- 14.Message SD, Laza-Stanca V, Mallia P, Parker HL, Zhu J, Kebadze T, Contoli M, Sanderson G, Kon OM, Papi A, Jeffery PK, Stanciu LA, Johnston SL. Rhinovirus-induced lower respiratory illness is increased in asthma and related to virus load and Th1/2 cytokine and IL-10 production. Proc Natl Acad Sci USA. 2008;105:13562–13567. doi: 10.1073/pnas.0804181105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Kluijver J, Grunberg K, Pons D, de Klerk EP, Dick CR, Sterk PJ, Hiemstra PS. Interleukin-1beta and interleukin-1ra levels in nasal lavages during experimental rhinovirus infection in asthmatic and non-asthmatic subjects. Clin Exp Allergy. 2003;33:1415–1418. doi: 10.1046/j.1365-2222.2003.01770.x. [DOI] [PubMed] [Google Scholar]
- 16.Fraenkel DJ, Bardin PG, Sanderson G, Lampe F, Johnston SL, Holgate ST. Lower airways inflammation during rhinovirus colds in normal and in asthmatic subjects. Am J Respir Crit Care Med. 1995;151:879–886. doi: 10.1164/ajrccm/151.3_Pt_1.879. [DOI] [PubMed] [Google Scholar]
- 17.Newcomb DC, Sajjan US, Nagarkar DR, Wang Q, Nanua S, Zhou Y, McHenry CL, Hennrick KT, Tsai WC, Bentley JK, Lukacs NW, Johnston SL, Hershenson MB. Human rhinovirus 1B exposure induces phosphatidylinositol 3-kinase-dependent airway inflammation in mice. Am J Respir Crit Care Med. 2008;177:1111–1121. doi: 10.1164/rccm.200708-1243OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bartlett NW, Walton RP, Edwards MR, Aniscenko J, Caramori G, Zhu J, Glanville N, Choy KJ, Jourdan P, Burnet J, Tuthill TJ, Pedrick MS, Hurle MJ, Plumpton C, Sharp NA, Bussell JN, Swallow DM, Schwarze J, Guy B, Almond JW, Jeffery PK, Lloyd CM, Papi A, Killington RA, Rowlands DJ, Blair ED, Clarke NJ, Johnston SL. Mouse models of rhinovirus-induced disease and exacerbation of allergic airway inflammation. Nat Med. 2008;14:199–204. doi: 10.1038/nm1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Papi A, Johnston SL. Rhinovirus infection induces expression of its own receptor intercellular adhesion molecule 1 via increased NF-kappaB-mediated transcription. J Biol Chem. 1999;274:9707–9720. doi: 10.1074/jbc.274.14.9707. [DOI] [PubMed] [Google Scholar]
- 20.Newcomb DC, Sajjan U, Nanua S, Jia Y, Goldsmith AM, Bentley JK, Hershenson MB. Phosphatidylinositol 3-kinase is required for rhinovirus-induced airway epithelial cell interleukin-8 expression. J Biol Chem. 2005;280:36952–36961. doi: 10.1074/jbc.M502449200. [DOI] [PubMed] [Google Scholar]
- 21.Ojielo CI, Cooke K, Mancuso P, Standiford TJ, Olkiewicz KM, Clouthier S, Corrion L, Ballinger MN, Toews GB, Paine R, III, Moore BB. Defective phagocytosis and clearance of Pseudomonas aeruginosa in the lung following bone marrow transplantation. J Immunol. 2003;171:4416–4424. doi: 10.4049/jimmunol.171.8.4416. [DOI] [PubMed] [Google Scholar]
- 22.Kechichian TB, Shea J, Del Poeta M. Depletion of alveolar macrophages decreases the dissemination of a glucosylceramide-deficient mutant of Cryptococcus neoformans in immunodeficient mice. Infect Immun. 2007;75:4792–4798. doi: 10.1128/IAI.00587-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stein M, Keshav S, Harris N, Gordon S. Interleukin 4 potently enhances murine macrophage mannose receptor activity: a marker of alternative immunologic macrophage activation. J Exp Med. 1992;176:287–292. doi: 10.1084/jem.176.1.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Muller U, Stenzel W, Kohler G, Werner C, Polte T, Hansen G, Schutze N, Straubinger RK, Blessing M, McKenzie AN, Brombacher F, Alber G. IL-13 induces disease-promoting type 2 cytokines, alternatively activated macrophages and allergic inflammation during pulmonary infection of mice with Cryptococcus neoformans. J Immunol. 2007;179:5367–5377. doi: 10.4049/jimmunol.179.8.5367. [DOI] [PubMed] [Google Scholar]
- 25.Martinez FO, Helming L, Gordon S. Alternative activation of macrophages: An immunologic functional perspective. Ann Rev Immunol. 2009;27:451–483. doi: 10.1146/annurev.immunol.021908.132532. [DOI] [PubMed] [Google Scholar]
- 26.Fahy JV, Figueroa DJ, Wong HH, Liu JT, Abrams JS. Similar RANTES levels in healthy and asthmatic airways by immunoassay and in situ hybridization. Am J Respir Crit Care Med. 1997;155:1095–1100. doi: 10.1164/ajrccm.155.3.9116993. [DOI] [PubMed] [Google Scholar]
- 27.Norzila MZ, Fakes K, Henry RL, Simpson J, Gibson PG. Interleukin-8 secretion and neutrophil recruitment accompanies induced sputum eosinophil activation in children with acute asthma. Am J Respir Crit Care Med. 2000;161:769–774. doi: 10.1164/ajrccm.161.3.9809071. [DOI] [PubMed] [Google Scholar]
- 28.Ordonez CL, Shaughnessy TE, Matthay MA, Fahy JV. Increased neutrophil numbers and IL-8 levels in airway secretions in acute severe asthma . clinical and biologic significance. Am J Respir Crit Care Med. 2000;161:1185–1190. doi: 10.1164/ajrccm.161.4.9812061. [DOI] [PubMed] [Google Scholar]
- 29.Adamko DJ, Yost BL, Gleich GJ, Fryer AD, Jacoby DB. Ovalbumin Sensitization changes the inflammatory response to subsequent parainfluenza infection: eosinophils mediate airway hyperresponsiveness, M2 muscarinic receptor dysfunction, and antiviral effects. J Exp Med. 1999;190:1465–1478. doi: 10.1084/jem.190.10.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Campbell EM, Kunkel SL, Strieter RM, Lukacs NW. Temporal role of chemokines in a murine model of cockroach allergen-induced airway hyperreactivity and eosinophilia. J Immunol. 1998;161:7047–7053. [PubMed] [Google Scholar]
- 31.Pope SM, Brandt EB, Mishra A, Hogan SP, Zimmermann N, Matthaei KI, Foster PS, Rothenberg ME. IL-13 induces eosinophil recruitment into the lung by an IL-5- and eotaxin-dependent mechanism. J Allergy Clin Immunol. 2001;108:594–601. doi: 10.1067/mai.2001.118600. [DOI] [PubMed] [Google Scholar]
- 32.Fulkerson PC, Fischetti CA, McBride ML, Hassman LM, Hogan SP, Rothenberg ME. A central regulatory role for eosinophils and the eotaxin/CCR3 axis in chronic experimental allergic airway inflammation. Proc Natl Acad Sci U S A. 2006;103:16418–16423. doi: 10.1073/pnas.0607863103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seymour ML, Gilby N, Bardin PG, Fraenkel DJ, Sanderson G, Penrose JF, Holgate ST, Johnston SL, Sampson AP. Rhinovirus infection increases 5-lipoxygenase and cyclooxygenase-2 in bronchial biopsy specimens from nonatopic subjects. J Infect Dis. 2002;185:540–544. doi: 10.1086/338570. [DOI] [PubMed] [Google Scholar]
- 34.Subauste MC, Jacoby DB, Richards SM, Proud D. Infection of a human respiratory epithelial cell line with rhinovirus. Induction of cytokine release and modulation of susceptibility to infection by cytokine exposure. J Clin Invest. 1995;96:549–557. doi: 10.1172/JCI118067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schroth MK, Grimm E, Frindt P, Galagan DM, Konno S-I, Love R, Gern JE. Rhinovirus replication causes RANTES production in primary bronchial epithelial cells. Am J Respir Cell Mol Biol. 1999;20:1220–1228. doi: 10.1165/ajrcmb.20.6.3261. [DOI] [PubMed] [Google Scholar]
- 36.Papadopoulos NG, Papi A, Meyer J, Stanciu LA, Salvi S, Holgate ST, Johnston SL. Rhinovirus infection up-regulates eotaxin and eotaxin-2 expression in bronchial epithelial cells. Clin Exp Allergy. 2001;31:1060–1066. doi: 10.1046/j.1365-2222.2001.01112.x. [DOI] [PubMed] [Google Scholar]
- 37.Konno S, Grindle KA, Lee WM, Schroth MK, Mosser AG, Brockman-Schneider RA, Busse WW, Gern JE. Interferon-gamma enhances rhinovirus-induced RANTES secretion by airway epithelial cells. Am J Respir Cell Mol Biol. 2002;26:594–601. doi: 10.1165/ajrcmb.26.5.4438. [DOI] [PubMed] [Google Scholar]
- 38.Gern JE, French DA, Grindle KA, Brockman-Schneider RA, Konno S-I, Busse WW. Double-stranded RNA induces the synthesis of specific chemokines by bronchial epithelial cells. Am J Respir Cell Mol Biol. 2003;28:731–737. doi: 10.1165/rcmb.2002-0055OC. [DOI] [PubMed] [Google Scholar]
- 39.Lamkhioued B, Renzi PM, Abi-Younes S, Garcia-Zepada EA, Allakhverdi Z, Ghaffar O, Rothenberg MD, Luster AD, Hamid Q. Increased expression of eotaxin in bronchoalveolar lavage and airways of asthmatics contributes to the chemotaxis of eosinophils to the site of inflammation. J Immunol. 1997;159:4593–4601. [PubMed] [Google Scholar]
- 40.Ying S, Meng Q, Zeibecoglou K, Robinson DS, Macfarlane A, Humbert M, Kay AB. Eosinophil chemotactic chemokines (eotaxin, eotaxin-2, RANTES, monocyte chemoattractant protein-3 (MCP-3), and MCP-4), and C-C chemokine receptor 3 expression in bronchial biopsies from atopic and nonatopic (intrinsic) asthmatics. J Immunol. 1999;163:6321–6329. [PubMed] [Google Scholar]
- 41.Lilly CM, Nakamura H, Belostotsky OI, Haley KJ, Garcia-Zepeda EA, Luster AD, Israel E. Eotaxin expression after segmental allergen challenge in subjects with atopic asthma. Am J Respir Crit Care Med. 2001;163:1669–1675. doi: 10.1164/ajrccm.163.7.9812044. [DOI] [PubMed] [Google Scholar]
- 42.Gern JE, Dick EC, Lee WM, Murray S, Meyer K, Handzel ZT, Busse WW. Rhinovirus enters but does not replicate inside monocytes and airway macrophages. J Immunol. 1996;156:621–627. [PubMed] [Google Scholar]
- 43.Hall DJ, Bates ME, Guar L, Cronan M, Korpi N, Bertics PJ. The role of p38 MAPK in rhinovirus-induced monocyte chemoattractant protein-1 production by monocytic-lineage cells. J Immunol. 2005;174:8056–8063. doi: 10.4049/jimmunol.174.12.8056. [DOI] [PubMed] [Google Scholar]
- 44.Laza-Stanca V, Stanciu LA, Message SD, Edwards MR, Gern JE, Johnston SL. Rhinovirus replication in human macrophages induces NF-κB-dependent tumor necrosis factor alpha production. J Virol. 2006;80:8248–8258. doi: 10.1128/JVI.00162-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Korpi-Steiner NL, Bates ME, Lee W-M, Hall DJ, Bertics PJ. Human rhinovirus induces robust IP-10 release by monocytic cells, which is independent of viral replication but linked to type I interferon receptor ligation and STAT1 activation. J Leukoc Biol. 2006;80:1364–1374. doi: 10.1189/jlb.0606412. [DOI] [PubMed] [Google Scholar]
- 46.Grissell TV, Powell H, Shafren DR, Boyle MJ, Hensley MJ, Jones PD, Whitehead BF, Gibson PG. IL-10 gene expression in acute virus-induced asthma. Am J Respir Crit Care Med. 2005:200412–201621OC. doi: 10.1164/rccm.200412-1621OC. [DOI] [PubMed] [Google Scholar]
- 47.Kim EY, Battaile JT, Patel AC, You Y, Agapov E, Grayson MH, Benoit LA, Byers DE, Alevy Y, Tucker J, Swanson S, Tidwell R, Tyner JW, Morton JD, Castro M, Polineni D, Patterson GA, Schwendener RA, Allard JD, Peltz G, Holtzman MJ. Persistent activation of an innate immune response translates respiratory viral infection into chronic lung disease. Nat Med. 2008;14:633–640. doi: 10.1038/nm1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moon K-A, Kim SY, Kim T-B, Yun ES, Park C-S, Cho YS, Moon H-B, Lee K-Y. Allergen-induced CD11b+ CD11cint CCR3+ macrophages in the lung promote eosinophilic airway inflammation in a mouse asthma model. Int Immunol. 2007;19:1371–1381. doi: 10.1093/intimm/dxm108. [DOI] [PubMed] [Google Scholar]
- 49.Careau E, Bissonnette EY. Adoptive transfer of alveolar macrophages abrogates bronchial hyperresponsiveness. Am J Respir Cell Mol Biol. 2004;31:22–27. doi: 10.1165/rcmb.2003-0229OC. [DOI] [PubMed] [Google Scholar]
- 50.Varin A, Mukhopadhyay S, Herbein G, Gordon S. Alternative activation of macrophages by IL-4 impairs phagocytosis of pathogens but potentiates microbial-induced signalling and cytokine secretion. Blood. 115:353–362. doi: 10.1182/blood-2009-08-236711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Raes G, Brys L, Dahal BK, Brandt J, Grooten J, Brombacher F, Vanham G, Noel W, Bogaert P, Boonefaes T, Kindt A, Van den Bergh R, Leenen PJM, De Baetselier P, Ghassabeh GH. Macrophage galactose-type C-type lectins as novel markers for alternatively activated macrophages elicited by parasitic infections and allergic airway inflammation. J Leukoc Biol. 2005;77:321–327. doi: 10.1189/jlb.0304212. [DOI] [PubMed] [Google Scholar]
- 52.Zimmermann N, King NE, Laporte J, Yang M, Mishra A, Pope SM, Muntel EE, Witte DP, Pegg AA, Foster PS, Hamid Q, Rothenberg ME. Dissection of experimental asthma with DNA microarray analysis identifies arginase in asthma pathogenesis. J Clin Invest. 2003;111:1863–1874. doi: 10.1172/JCI17912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.North ML, Khanna N, Marsden PA, Grasemann H, Scott JA. Functionally important role for arginase 1 in the airway hyperresponsiveness of asthma. Am J Physiol Lung Cell Mol Physiol. 2009;296:L911–920. doi: 10.1152/ajplung.00025.2009. [DOI] [PubMed] [Google Scholar]
- 54.Stout RD, Jiang C, Matta B, Tietzel I, Watkins SK, Suttles J. Macrophages sequentially change their functional phenotype in response to changes in microenvironmental influences. J Immunol. 2005;175:342–349. doi: 10.4049/jimmunol.175.1.342. [DOI] [PubMed] [Google Scholar]
- 55.Johnston SL, Papi A, Monick MM, Hunninghake GW. Rhinoviruses induce interleukin-8 mRNA and protein production in human monocytes. J Infect Dis. 1997;175:323–329. doi: 10.1093/infdis/175.2.323. [DOI] [PubMed] [Google Scholar]
- 56.Khaitov MR, Laza-Stanca V, Edwards MR, Walton RP, Rohde G, Contoli M, Papi A, Stanciu LA, Kotenko SV, Johnston SL. Respiratory virus induction of alpha-, beta- and lambda-interferons in bronchial epithelial cells and peripheral blood mononuclear cells. Allergy. 2009;64:375–386. doi: 10.1111/j.1398-9995.2008.01826.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.