Abstract
Heart failure initiated by coronary artery disease and myocardial infarction (MI) is a widespread, debilitating condition for which there are a limited number of options to prevent disease progression. Intramyocardial biomaterial injection following MI theoretically provides a means to reduce the stresses experienced by the infarcted ventricular wall, which may alter the pathological remodeling process in a positive manner. Furthermore, biomaterial injection provides an opportunity to concurrently introduce cellular components and depots of bioactive agents. Biologically-derived, synthetic and hybrid materials have been applied, as well as designed expressly for this purpose, although optimal design parameters, including degradation rate and profile, injectability, elastic modulus, and various possible bioactivities largely remain to be elucidated. This review seeks to summarize the current body of growing literature where biomaterial injection, with and without concurrent pharmaceutical or cellular delivery, has been pursued to improve functional outcomes following MI. The literature to date generally demonstrates acute functional benefits associated with biomaterial injection therapy across a broad variety of animal models and material compositions. Further functional improvements have been reported when cellular or pharmaceutical agents have been incorporated into the delivery system. Despite these encouraging early results, the specific mechanisms behind the observed functional improvements remain to be fully explored and future studies employing hypothesis-driven material design and selection may increase the potential for this approach to alleviate the morbidity and mortality of heart failure.
Keywords: injectable biomaterial, myocardial infarction, cardiac tissue engineering, hydrogels, thermoresponsive polymer
1. Introduction
A diverse array of treatment methods and technologies has been developed in an attempt to alleviate the morbidity and mortality associated with cardiac failure.[1] With coronary artery disease contributing to 2 out of 3 cases of heart failure, interventional procedures that seek to revascularize the myocardium through coronary artery bypass grafting and coronary stent placement have become both common and highly effective following myocardial infarction (MI).[2, 3] Cardiac pacing in appropriate patients has been shown to increase the energy efficiency of the heart.[4] Pharmacological regimens are useful to improve systolic performance and decrease workload.[5, 6] The gold standard for those with end-stage heart failure remains cardiac transplantation, but donor hearts far outstrip the number of people who could benefit from transplantation.[7] To address this need, ventricular assist device implantation can take over the pumping function of the ventricle by independently circulating blood in late stage heart failure patients. [8, 9] Biomaterials development has played a critical role in the creation of medical devices that have advanced heart failure treatment, from membrane oxygenator microporous hollow fibers, to stents and stent coatings, prosthetic heart valves, pacing leads, and many other devices. An area of cardiovascular biomaterials research and development that has opened and grown over the past decade is the investigation of injectable biomaterials to treat cardiac failure.[10] This area of development has grown largely in parallel with cell injection therapy, but it has become clear that the mechanical effects that may be achieved with ventricular biomaterial injection may be beneficial independent of cellular delivery.
2. Mechanical Approaches to Heart Disease
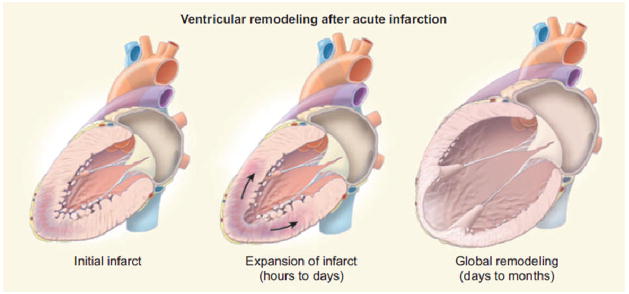
Myocardial infarction sets off a series of complicated processes, including cell death, scar formation, and ventricular dysfunction, that alter both the cellular, structural and mechanical properties of the heart.[11–13] Not only does the heart lose systolic capacity due to a decrease in the amount of functional myocardium after MI, simple geometric changes that take place in remodeling also play a role in heart failure progression. As pumping efficiency decreases, blood backs up in the left ventricle (LV) leading to increased intraventricular pressures contributing to LV dilation – Figure 1. While the initial dilation following infarction is believed to be a coping mechanism by which cardiac output can be maintained through the Frank-Starling mechanism, it has been shown that over time the cardiomyocytes lose the ability to respond with increased contractility when stretched, resulting in a dilated, under performing ventricle.[14] Ventricular dilation in terms of LV sphericity, and particularly LV volume is strongly linked to poor outcomes following MI.[15, 16]
Figure 1.
Ventricular dilation associated with progressive heart failure. After the initial insult, infarct expansion and ventricular wall thinning contribute to further ventricular remodeling, ultimately causing increased intraventricular pressure and decreased cardiac output.(From [1])
Pathological LV dilation is propagated through a positive feedback loop with LV wall stress.[13, 17] This stress is the preload against which the cardiomyocytes must contract during systole. As this stress increases due to stretching from dilation, the cardiomyocytes lose the capacity to shorten effectively, leading to further stretching and continued decreases in cardiac pumping efficiency, quantified as the ejection fraction (EF). The physics behind this unfortunate positive feedback system can be explained in part by the Law of Laplace, where the stress in the wall of a round chamber(T)is directly related to the pressure in the chamber(P), the radius of curvature(R), and the thickness of the wall (h), as shown in equation 1:
| (1) |
During the ventricular remodeling process following an MI, pressure and radius increase and the LV free wall thins. This increases the cardiac wall stress, which ultimately leads to more dilation, thinning, and stress. The heart can proceed down a pathway towards end-stage heart failure where transplantation, hospice care, or ventricular assist device implantation are the only options.
Many treatments exist that use mechanical approaches to discourage cardiac dilation, restore geometry, and reduce ventricular wall stress and thinning.[18] Cardiac restraint devices such as the CorCap (Acorn Cardiovascular Inc) and Heart Net (Paracor Medical Inc) employ Dacron and nitinol wraps, respectively, to provide physical support for the failing ventricular wall – Figure 2.[19–22] Experimental devices such as the Myosplint (Myocor Inc) and CardioClasp (CardioClasp Inc) force a dilated ventricle into two smaller lobes to reduce the intraventricular radius and thereby decrease wall stress.[23, 24] Advanced surgical procedures such as endoventricular patch plasty (the Dor procedure) and partial left ventriculectomy (the Batista procedure) physically restructure a spherical, dilated ventricle into a more natural elongated shape.[25, 26] While involving highly invasive procedures, these methods have demonstrated some clinical efficacy, even in chronic heart failure patients. An elastic cardiac patch based on degradable porous polyurethane was shown to halt LV dilation when sutured onto the epicardium. This material prevented further dilation and also was associated with the formation of a new smooth muscular layer in the infarction zone, possibly due to the stress shielding provided by the patch.[27]
Figure 2.
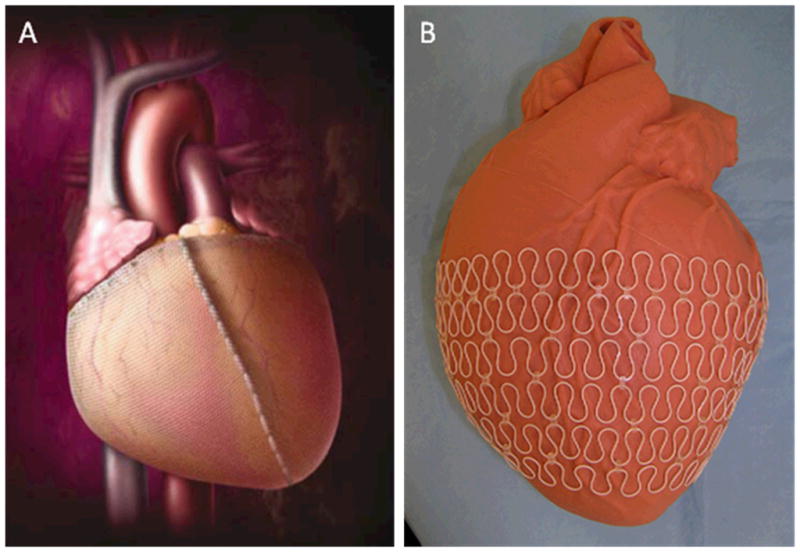
Cardiac restraint devices are used as a means to limit ventricular dilation following MI. The CorCap device (A) uses a Dacron woven mesh to maintain ventricular dimensions whereas the Heart Net (B) surrounds the ventricle in an elastic nitinol metal wrap. ((A) from [22], (B) from[20])
The use of biomaterials for cardiac repair has primarily focused on applications where the material is anchored to and interacts with the outer edges of the myocardium to provide support. Incorporating the material completely within the heart wall is an alternative approach where direct contact with cardiac cells occurs as the material acts as a bulking agent for the heart wall. Generally, materials are delivered through direct epicardial injection at distinct locations in and around the infarct leading to focal points of biomaterial presence – Figure 3. Current methods for direct injection have required sternotomy or thoractomy, though thorascopic or intravascular procedures could be feasible to decrease procedure-related morbidity. Not only may the injected material alter the mechanical environment upon injection, but appropriate biomaterials can have biological functions that encourage cardiac repair. Functions such as angiogenesis, cardiomyocyte protection, and stem cell recruitment may be another primary means to improve the efficacy of material injections for treating heart disease.
Figure 3.
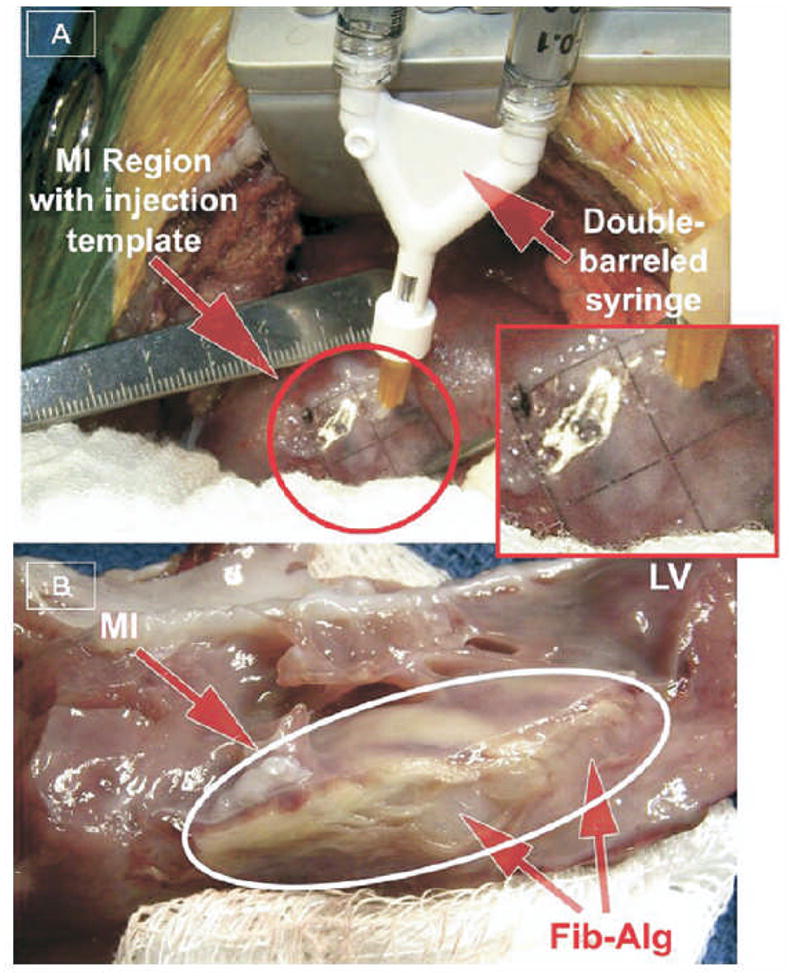
Direct epicardial injection of a fibrin-alginate composite into porcine myocardium.(A) Using a grid template, material was injected from a double-barreled syringe to distinct points across the infarcted area.(B) Myocardial cross section reveals the material present as amorphous compartments at the injection sites. (From [44])
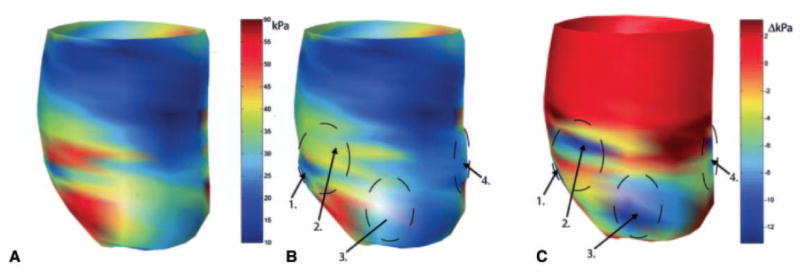
In 2006, a report by Wall et al. utilized a finite element model to evaluate the theoretical effect on cardiac wall stress of injecting a noncontractile biomaterial into the LV wall.[28] The injected volume changes the LV geometry by increasing the wall thickness, thus lowering local wall stress. The simulation showed that injection of a volume 4.5% of the total LV wall volume and with a stiffness 20% of the natural LV tissue into the infarct border zone could decrease the wall fiber stress in the border zone by 20% compared to a control simulation in which there was no injection – Figure 4. The mechanical simulation also showed that this attenuating effect on LV wall stress increased with the injection volume and modulus of the injected material. Importantly, while this model demonstrated a slight increase in EF following material injection, a more precise measure of global cardiac function, namely stroke volume divided by LV end-diastolic pressure, was unchanged. This disparity results from the fact that EF is computed from geometric factors and thus changes inventricular geometry from the presence of material can cause higher EF calculations without real functional improvement. Because EF is a common clinical metric of heart performance it is advisable to confirm perceived improvements to EF with other measures of function. Reductions in cardiac wall stress computed after material injection have been corroborated by recent finite element modeling where mean myofiber stress for the entire LV can be reduced by 15% and 5% at end-diastole and end-systole, respectively, depending on number and configuration of injection sites. [29] These finite element models, in conjunction with an understanding of the Law of Laplace, provide the theoretical basis for growing interest in injectable biomaterials to treat cardiac failure.
Figure 4.
Representation of mid wall ventricular fiber stress as a function of position in the heart. (A) Wall stress with an apical infarct (red is higher stress localized near the infarct). (B) Wall stress with injection of a theoretical gel material at 4 different numbered locations peripheral to the infarct region. (C) The difference in the previous two images. Marked reduction in wall stress is seen due to injection therapy (cooler colors representing reduced stress). (From [28]).
3. Biomaterial Injection
3.1 Biological Materials
An important subset of commonly utilized biomaterials is derivatives of natural materials produced by biological systems. Examples of biological materials that are amenable to injection delivery include collagen, gelatin, fibrin and alginate. These materials have been applied in a variety of physiological environments because they often have acceptable levels of biocompatibility without significant processing, and are readily accessible to clinicians and researchers. Thus, it is not surprising that early studies evaluating biomaterials for myocardial injection have employed these materials.
3.1.1 Alginate
Alginate, a linear block copolymer of (1–4)-linked β-D-mannuronate and α-L-guluronate residues, is bioinert and does not support cell attachment.[30] However, modifications to alginate, such as introduction of the cell binding sequence arginine-glycine-aspartate(RGD), can significantly improve attachment.[30, 31] The material properties of alginate, especially mechanical strength and degradation rate have been extensively studied leading to a good understanding of methods to adjust these properties[32]. Increasing alginate concentration in solution increases mechanical strength but also increases solution viscosity, which may be undesirable when material injection is desired. However, it has been demonstrated that a mixture of high and low molecular weight (MW)chains can lead to a relatively high elastic shear modulus of 10–24 kPa while still maintaining a low viscosity of less than 2 Pa s.[33] Variations in alginate composition and ionic makeup can vary the elastic modulus of the gel widely from 5 to 55 kPa.[34, 35] The same characteristics of alginate that influence mechanical properties, such as molecular weight, also influence the degradation time of the gel. To further tailor degradation time alginate polymer chains can be partially oxidized by reaction with sodium periodate, which ultimately provides hydrolysable bonds to speed degradation.[36] Alginate has been used in both drug delivery and tissue engineering applications with marked success.[30–32, 37, 38]
Landa et al. aimed to study the ability of alginate to improve LV remodeling and function when injected as a bulking agent into the myocardium. [39] They compared the results of injecting 150 μL of alginate (MW 30 kD) solution into both recent (7 days after) and old (8 weeks after) infarcts. Eight weeks after material injection into recent infarcts LV geometry was improved in the biomaterial group in the form of increased LV wall thickness and attenuated LV dilation compared to control hearts that received a saline injection. The improvements in old infarcts were less pronounced demonstrating the greater difficulty in rescuing a chronically infarcted heart. Using a biotin-labeled alginate, histological sections were able to accurately track material degradation in vivo. One hour after injection alginate occupied 45% of the infarct area, but this decreased to 6% after six weeks. Of particular note, material injection was compared to injection of 1×106 neonatal cardiomyoctyes in saline with similar or better results in all measures for material injection alone. In other reports alginate was able to show significantly improved cardiac function in terms of fractional area change (FAC)or fractional shortening(FS)compared to saline injection controls (The FAC and FS are common functional endpoints that are measured with echocardiography and frequently appear in cardiac biomaterial injection reports). The improvements in FAC and FS associated with alginate injection were demonstrated for as many as 5 to 8 weeks after injection, and even in a chronic infarct model where injections occur several weeks after infarction.[40, 41]
A mixture of alginate polymers with molecular weights of 125 kD and 5 kD at a ratio of 75:25 High MW: Low MW was injected in the peri-infarct region of rat hearts 7 days after coronary ligation.[42] The histological evaluation showed the inert properties of alginate, as the material was unable to increase capillary density or α-actin positive smooth muscle cell (SMC) density 28 days after injection. In this material 1% of the alginate sugar residues were oxidized by incubation with sodium periodate, to increase the material degradation rate. In vivo only a third of all hearts showed any material left at the 28 day endpoint – and then only in small fragments. The lack of angiogenesis in this study after alginate injection has not been universally observed. Other studies have reported that plain alginate injection into the myocardium can cause mild increases in capillary and arteriole density and myofibroblast infiltration. [39, 40, 43–45] The direct cause of the increased cell population is not completely understood but may be related to the alginate creating a healthier microenvironment through stress shielding. Alternatively, the inflammatory response to the foreign body injection may similarly have triggered increases in local cellular content. For cardiac biomaterials injection studies in general it is difficult to separate the underlying cause of the observed effects. While mechanical effects are most commonly hypothesized, the material-related inflammatory response is potentially contributing in concert or independently.
In order to improve upon results from cardiac injection of alginate alone by facilitating greater cellular infiltration into the material, the cell adhesion peptides RGD and tyrosine-isoleucine-glycine-serine-arginine (YIGSR) have been introduced into the polymer backbone.[40, 45] While in vitro studies confirmed that these peptide sequences allowed for improved cell attachment, functional and histological results with peptide-modified alginate have been mixed. For example, Yu et al. reported that 2 days after injection, both unmodified alginate and RGD-alginate showed mild improvements in FS and wall thickness but after 5 weeks no functional difference was seen between peptide-modified and plain alginate. However, there was a significant increase in arteriole density after 5 weeks in the RGD-alginate compared to plain alginate and saline injected control animals, indicating some local biological effect. While these results would suggest that the cell adhesion sequence may be beneficial for angiogenesis, a report by Tsur-Gang et al. showed that injection of alginate modified with both RGD and YIGSR peptides was not able to cause improved function or increase angiogenesis compared to unmodified alginate or alginate with a nonsense arginine-glycine-glutamate (RGE)peptide attached. Even though RGD/YIGSR-modified alginate showed the lowest blood vessel density compared to plain and RGE alginate, it was still able to maintain scar thickness. It is unknown why there was not a stronger cellular response in the RGD/YIGSR alginate group but the authors speculate that the modification, which involved 0.2% of uronic acid monomers, may have been inadequate or that the peptides were not well exposed to surrounding cells.
Another interesting approach to encourage cell infiltration into alginate was the blending of polypyrrole with alginate.[43] Polypyrrole, though mechanically brittle, may be particularly advantageous for cardiac injection because of its ability to conduct electrical signals and encourage endothelial cell growth.[46] Injection of 0.025% w/v polypyrrole with alginate led to significantly higher arteriole density than injection of alginate alone. The authors reasoned that increased cell attachment to polypyrrole, as was demonstrated in vitro, as well as improved electrical conductivity through the infarct region may have encouraged cell growth and arteriole formation.
While the most common delivery site for injectable biomaterials is through an epicardial approach, a recent report demonstrates that intracoronary injection of appropriate materials may be possible without detrimental side effects. An alginate-calcium solution was injected intravascularly into the left anterior descending artery of pigs post-MI through a coronary catheter.[47] The vasculature in the injured myocardium becomes leaky after infarction, allowing the alginate polymer to diffuse across the permeable endothelium into the extravascular space. While the alginate solution is unable to gel in the vasculature due to inadequate calcium ion concentration, within the myocardium the calcium concentration is adequate to allow ionic cross linking and gel formation throughout the myocardial wall – Figure 5. Different volumes of alginate solution were injected and results studied for 60 days. It was demonstrated that the volume injected was related to the measured benefit, as injection of 2 mL had better outcomes than 1 or 4 mL. It is not clear what volume is optimal for cardiac injection therapy but this report suggests that an optimal range may be desired wherein there is enough material to provide mechanical benefit but without impinging on diastolic expansion. In this study, even though the material was completely removed by the 2 month endpoint, improvements to FS and wall thickness remained. This method allows for diffuse delivery noninvasively but may lead to other concerns. For example many patients with heart failure also have co-morbidities such as diabetes and peripheral vascular disease.[48] Damaged blood vessels from these conditions may allow for alginate deposition and gelation in undesired locations, the effects of which are not known. It will be necessary for appropriate controls to prove efficacy, or appropriate patient selection to make this a clinically feasible approach.
Figure 5.
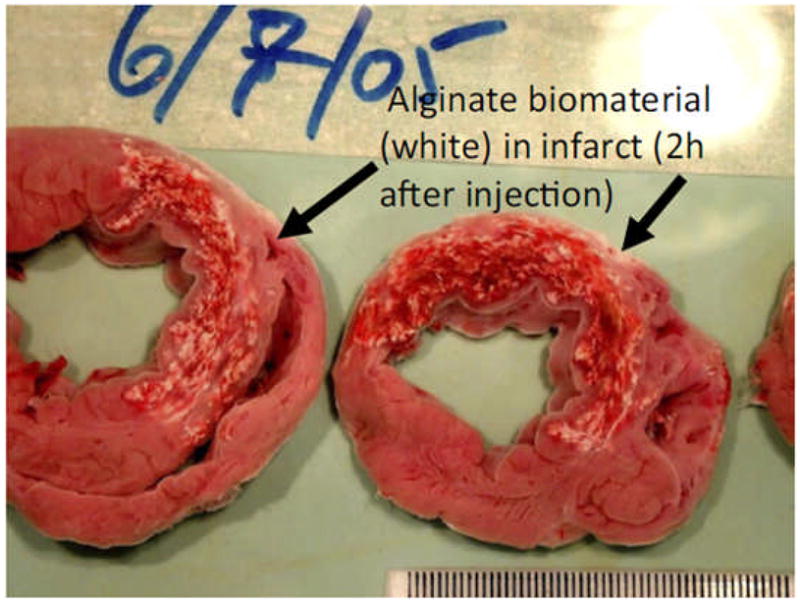
Two hours after intracoronary injection of alginate, the material has migrated from the vascular space and is found throughout the myocardial wall in a porcine model. The presence of this material will lead to increased wall thickness which persists after material has degraded six weeks later.(From [47])
3.1.2 Fibrin
Fibrin, which has been used as a biomaterial in a broad array of applications for decades, has been reviewed extensively.[49–51] A fibrin “glue” or fibrin “sealant” aims to reproduce the final steps of the coagulation cascade which forms a three dimensional cross-linked fibrin network. This occurs when fibrinogen is cleaved by thrombin into fibrin monomers that self-assemble and are subsequently cross-linked by factor XIIIa. In commercially available fibrin sealants such as Tisseel (Baxter Healthcare) or Crosseal (Ethicon Inc) the reactive components that form the fibrin clot come in contact only upon injection through a double-barreled syringe. One of the solutions consists of fibrinogen mixed with anti-fibrinolytic agent aprotinin, while the other solution contains thrombin, factor XIIIa and calcium ions. The deposited fibrin, which is digested by the enzyme plasmin, is usually completely degraded within days to weeks depending on implant location and the concentration of aprotinin. The material properties of these sealants depend on the starting solution compositions. For example, increasing the thrombin concentration increases clotting speed and also leads to stronger networks. A high fibrinogen concentration (70 mg/mL)can provide an elastic modulus up to 40 kPa compared to 10 kPa for a low concentration (30 mg/mL).[52] Importantly, fibrin sealants have been FDA approved in certain applications as a sealant or to aid hemostasis, allowing ready access in most operating rooms.
Fibrin injected into rat hearts following creation of an infarct by ischemia-reperfusion injury has been shown to yield positive results.[41, 53–56] Injection of 50 μL of fibrin into one site in week-old infarcts prevented wall thinning and maintained FS over 5 weeks after injection, even though the material is resorbed within 2 weeks.[53, 57] In contrast, injection into 5 week-old infarcts did not improve FS 5 weeks after fibrin injection.[41] After injection of fibrin infarct size, as measured by the ratio of infarcted LV area to total LV area, is consistently reduced compared to negative control studies. Arteriole density, but not capillary density, was increased after fibrin injection compared to bovine serum albumin injection, and importantly it was proven that the new arterioles formed were found within the infarct zone, were functional, and were connected to the existing vasculature. [55, 56] Since arteriole formation was consistently associated with fibrin injection, but functional improvement was not, improvements to the vasculature alone do not appear to be sufficient for improving long-term function.
A report of myocardial injection in a large animal model involved fibrin injection into infarcted porcine hearts.[44] Researchers injected 200 μL of a composite of fibrin with gelatin-grafted alginate into each of 25 points on a grid across the infarct area. While the report did not demonstrate functional improvement the researchers did show increased posterior wall thickness for one week following injection and infarct expansion was prevented for two weeks after injection. Likely due to the presence of alginate in the fibrin clot, material was still detected at three weeks. While limited conclusions can be drawn from this study, it is notable as one of the first reports of biomaterial injection therapy applied to a large animal model.
3.1.3 Chitosan
Chitosan, a cationic polymer derivative of chitin, has been used in biomedical, tissue engineering, and drug delivery applications because of its general biocompatibility, capacity to form porous structures and gels, and tunable degradation rate.[58, 59] While being soluble in acidic aqueous solutions through protonation of its many amine groups, neutralization of chitosan aqueous solutions to a pH exceeding 6.2 systematically leads to the formation of a hydrated gel-like precipitate.[60] Chitosan aqueous solutions can be transformed from purely pH-dependent solutions into temperature-controlled pH-dependent chitosan solutions by mixing with polyol salts bearing a single anionic head, such as glycerol-, sorbitol-, fructose-or glucose-phosphate. Conjugation of hydrophobic groups such as hydroxybutyl to the hydroxyl and amino reactive sites of chitosan can also confer temperature-responsive properties. [61] Thermal responsiveness allows chitosan to be optimized for injection, with achievable elastic moduli over 5 kPa reported after gelation.[60]
A thermally responsive chitosan formed by mixing chitosan with β-glycerol phosphate and hydoxyethyl cellulose has been injected into infarcted rat hearts one week after an ischemia-reperfusion injury. Injection of the material, which was almost completely degraded by 4 weeks, was shown to improve FS and decrease dilation to the same extent as injection of stem cells alone, and better than saline injections alone.[62] A new material developed by Yeo et al. combined an azidobenzoic acid-modified chitosan with an acryloyl-PEG-RGDS to make a photocrosslinkable gel for use in injection therapy.[63] The material was able to form a gel in situ after two minutes of UV irradiation and had a shear modulus up to 370 Pa. This material was shown to support cell growth at a level comparable to tissue culture polystyrene and was able to release vascular endothelial growth factor (VEGF) in vitro for more than 24 days. While not yet studied in vivo, this material does represent the growing interest in enhanced material properties through improved material design for the field of cardiac injection.
3.1.4 Extracellular Matrix and Derivatives
Extracellular matrix (ECM) derived materials are generally comprised of structural proteins such as collagen, laminin, fibronectin, and vitronectin, as well as an array of glycosaminoglycans, and are specific to their tissue source. ECM is naturally bioactive, is important in cell signaling and in the case of cardiac ECM, plays an important role in the inflammation and remodeling process following MI.[64, 65] Because cells are naturally inclined to interact well with ECM it is often used as scaffolding for tissue engineering applications.[66] Degradation speed of these materials is determined by the cellular milieu in vivo and factors such as concentration and extent of cross-linking. For example, gelatin, a denatured derivative of collagen, is much weaker and degrades quicker than its parent molecule.[67] The mechanical strength of injectable ECM derived materials is generally low. For example, the storage modulus, as determined by parallel plate rheometry, of injectable collagen is in the range of 20 to 80 Pa. Matrigel (Beckton & Dickenson), an ECM gel generated by mouse sarcoma cells, is comprised primarily of collagen and has a modulus of 30 to 120 Pa.[68, 69]
Gelatin has been injected into infarcted rat hearts but without improvement to function or histology after 4 weeks.[70] Gelatin was present in half the hearts after 2 weeks in vivo but was completely absent after 4 weeks of injection. Collagen has had slightly better results.[71] Injection of 100 μL of collagen at a single point in the infarct zone led to a higher EF than saline injection 6 weeks after treatment. Surprisingly the injected material did not allow for cell infiltration or angiogenesis, and thus seemed to behave similarly to the native collagen-rich infarcted tissue. The increased EF may have been a consequence of the increased wall thickness that was observed after injection. In contrast, Huang et al. were able to show improved myocardial angiogenesis and myofibroblast infiltration into injected collagen.[56] The reason for these disparate results could be related to the infarct model. No angiogenesis or cell infiltration was seen when injection occurred one week after infarction, whereas there was cell infiltration if injected after just 30 minutes of ischemia. This result may further indicate the difficulty in salvaging late-stage diseased tissue and reflect the importance of timing in therapeutic application.
Injection of Matrigel was able to form a collagen-fiber network that ran parallel to that of the host myocardium and integrated with native collagen.[72, 73] For ischemic hearts Matrigel injection was able to improve FS and wall thickening 4 weeks after treatment. While the application of Matrigel clinically is questionable due to its tumor cell source, ECM from a variety of animal tissues are routinely applied in a variety of settings and are of interest in cardiac applications. For injection therapy, a mechanically ground digest of ECM from small intestinal submucosa was hydrated and injected into infarcted rat hearts.[74] The ECM material was able to improve function and decrease remodeling in the 6 week follow-up compared to infarct controls. As was hypothesized, the ECM material increased cell infiltration into the infarct zone. There was a significant increase in myofibroblasts, macrophages, and importantly, c-kit positive cells. ECM-injected hearts had substantially more stem cell factor and VEGF present. This study demonstrates a relatively high level of regeneration in the form of stem cell recruitment and muscle repair that can be elicited by biologically active ECM, and sets the stage for future work using similar materials.
Under the belief that ECM of a certain tissue type is going to best facilitate regeneration of that tissue, many groups are interested in using tissue-specific ECM materials for tissue engineering. In a pilot study, Singelyn et al. injected a hydrogel made of digested porcine cardiac ECM into healthy rat hearts.[75] Animals were sacrificed 11 days after injection to demonstrate that the ECM was still present and in morphology similar to intact decellularized cardiac ECM. Both in vivo and in vitro results showed that SMCs and endothelial cells migrate to and infiltrate the ECM biomaterial. The ability to use pericardial ECM for cell injection has also been recently demonstrated.[76] This tissue source is important as it may allow for injection of autologous ECM. Both human and porcine pericardial ECM gels supported arteriole formation within the injected material in healthy rat hearts and a few c-kit+ precursor cells to be recruited around the material. These studies demonstrate the potential that ECM materials may have for this application, although without results from injection into injured hearts, more detailed conclusions cannot be made.
3.1.5 Comparative Studies
Unfortunately, only a few studies have directly compared different types of materials for cardiac injection.[41, 56] In these studies the specific differences in material properties can be more directly linked to outcomes since all other study parameters are held constant. In one report, fibrin, collagen, and Matrigel were each injected 1 week after an ischemia-reperfusion injury into separate groups of rats. While lacking data on cardiac function, this study did show that for all three materials there was a similar increase in myofibroblast infiltration, capillary density, and arteriole density compared to saline injection. In another report, by comparing alginate to fibrin injection the importance of material residence time may be demonstrated.[41] Two days after injection both of these materials caused similar increases to wall thickening, decreased intraventricular diameters, and increased FS – Figure 6. After five weeks however, the fibrin-injected hearts no longer maintained improved function despite largely preserving LV wall thickness. Alginate on the other hand improved FS, albeit at levels less than was shown at 2 days. An obvious difference between groups was that the alginate was still present in substantial amounts after 5 weeks, whereas fibrin was completely resorbed. The continued presence of a material that may provide physical support in this case appears to be more advantageous.
Figure 6.
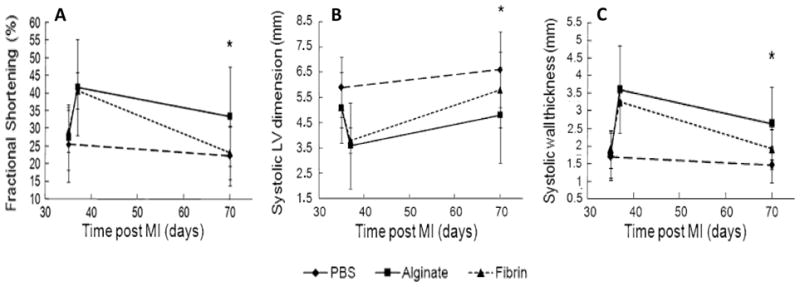
Comparison between injection of fibrin or alginate into 5-week old infarcts in rats. Two days after injection both materials demonstrated equivalent improvements to FS(A), LV dimension(B)and wall thickness (C). Five weeks after treatment the fibrin group lost much of the advantage seen at two days whereas alginate retained significant improvement.(* p<0.05, From [41])
A recent study aimed to evaluate the how mechanical strength of the injected material can influence outcomes following cardiac injection.[77] Methacrylated hyaluronic acid with 30% (MeHA Low) and 60% (MeHA High) methacrylate substitution were injected into ovine infarcts and crosslinked in situ. While the two materials demonstrated similar degradation rate, cellular response, and tissue distribution the storage modulus of the MeHA High group was over 3 times higher than that of the MeHA Low group. In vivo, the stronger material allowedless infarct expansion and LV dilation compared to the weaker material. Functionally, MeHA High was associated with better EF than MeHA Low after 8 weeks, especially under cardiac stress testing. By evaluating the functional effects of manipulating a specific material property, in this case mechanical strength, this comparative study provides further guidance for future material development.
3.2 Synthetic Materials
Materials that can be designed and synthesized in the laboratory from primary building blocks offer some advantages over those adapted from biological systems. In particular, every stage of material synthesis is under control of the engineer, allowing for material generation to meet specific design objectives, such as degradation, mechanical, and biocompatibility properties. A trade-off for this control is that synthetic materials are generally more likely to trigger inflammatory and foreign body responses that can be avoided for some minimally processed natural materials. Introducing biomolecules into the design process and utilizing labile synthetic materials that degrade into non-toxic components can potentially minimize the undesired effects associated with permanent foreign body implantation. In the field of cardiac injection therapy an array of synthetic materials have been explored to date.
3.2.1 Self-Assembling Peptides (SAPs)
Nature is replete with examples of materials that self-assemble to create larger structures. Materials ranging from lipids to proteins can form complex shapes with minimal outside influence so long as the appropriate complementarities exist between building blocks. Engineers have long sought to utilize self-assembling behavior to build materials on a small scale. [78, 79] For example, peptides with specifically placed amino acid sequences can self-assemble into macroscopic materials that have been shown to support cell growth and have been considered as injectables for cardiac wall therapy.[80, 81]
Peptides with the amino acid sequence AcN-RARADADARARADADA-CNH2 (RAD16-II), which form a nano-fibrous 3D gel, have been studied extensively for cardiac injection to repair the myocardium after MI. Injection of 10 μL of this gel into a non-infarcted LV free wall showed significant host cell infiltration including endothelial cells and cells that exhibit some cardiac-specific markers even though the peptide sequence did not possess any cell recruitment signals.[82] However, injection of 80 μL of the same material into sham animals in a similar study showed a temporary decline in FS and LVESD before returning to normal levels after 2 months.[83] This indicates that while this material may be advantageous on a cellular scale, the addition of the non-contractile material in a healthy heart may have a transient negative effect on systolic function in healthy animals. For the more relevant infarction model, RAD16-II nanofiber injection into a rat heart immediately after coronary ligation has not been able to improve cardiac function, geometry, or histology compared to MI control groups.[83–85] The lack of improvement has not been investigated but may be the result of too little material being injected and a material that is not mechanically robust. These results led to significant modifications of this material to act primarily as both a drug delivery and cell delivery vehicle with very promising results to be discussed later.
3.2.2 Synthetic Hydrogels
Because of their high water content and diffusivity, among other properties, hydrogels have been investigated as implantable biomaterials across a broad range of regenerative medicine applications.[86] One challenge for synthetic hydrogels for cardiac injection application is controlling the formation of hydrogel structure in situ following injection. While very weak hydrogels maybe forced through a needle, the material strength may be inadequate for such a mechanically taxing environment as the myocardium, and mechanical modeling discussed earlier suggests that a more mechanically robust material may be beneficial. Several material design strategies may be employed to confront this issue: in situ cross-linking [87], photo-induced polymerization[88], self-assembly[89] and thermally responsive hydrogels[90].
A solution of α-cyclodextrin and methoxy polyethylene glycol–poly (caprolactone)-(dodecanedioic acid)–poly(caprolactone)-methoxy polyethylene glycol (MPEG–PCL–MPEG) triblock polymer (α-cyclodextrin/MPEG–PCL–MPEG) has the ability to self assemble into a hydrogel.[91] This gelation is posited to occur by two mechanisms. First, the MPEG-PCL-MPEG polymer chain may be able to thread through the center of α-cyclodextrin rings to form microcrystal complexes. Second, micellization of the PCL blocks can further add physical crosslinks within the hydrogel network. This polymer solution can form a hydrogel within a few minutes at room temperature and then will degrade in accordance with PCL block hydrolysis. Injectability is obtained by separating the α-cyclodextrin solution from the MPEG-PCL-MPEG solution using a double barreled-syringe. Injection of this material into new or old infarcts in rabbits and rats has been able to show significant improvements in LVESD and LVEDD one month after infarction.[92, 93] FS was reported at 56% in the hydrogel injection group compared to only 37% in the saline injection group after 4 weeks in rabbits. Contrary to other studies, improvements in geometry and function were not present two days after injection but were so after one month. This indicates that presence of the material alone may not be the cause of enhancement. Rather, it appears the body’s longer-term reaction to this material generates the benefits. Indeed the LV wall thickness is increased after a month largely due to more collagen deposition. Interestingly, functional improvements could not be linked to increased blood flow to the infarcted area as there was no difference in blood vessel density between hydrogel and saline injection groups.
Because injected material acts as a mechanical support, it may be beneficial for the material to remain in situ indefinitely. To this end anon-degradable, in situ polymerized PEG hydrogel has been investigated for cardiac injection after MI.[94] Despite the often perceived inert nature of PEG hydrogels there was substantial macrophage response to the material throughout the 3 month follow-up. The benefits seen after injection – increased wall thickness and modestly attenuated dilation – only persisted during the first 4 weeks. By 3 months the hydrogel injection group had regressed to a cardiac geometry and function similar to the saline treated control group. In this case a long-remaining material did not provide long-term benefit, but rather appeared to only introduce a focal point for chronic inflammation. Indeed, hydrogel injection into sham operated hearts showed a significant reduction in FS at 3 months which may point to the damaging role that chronic inflammation can play. The authors also suggest that while PEG may be an adequate mechanical support initially, as the heart continues to dilate the wall stresses eventually become more than the PEG hydrogel can support and so remodeling continues unchecked.
Thermo responsive materials that transition from sol to gel near body temperature are frequently employed for tissue engineering and drug delivery applications because their transitional properties make them easy to deliver and manipulate.[95] N-isopropyl acrylamide (NIPAAm) is one of the most studied thermoresponsive molecules. Poly-NIPAAm (pNIPAAm) chains can be dissolved in water below their transition temperature of 32°C, but precipitate to form a hydrogel upon warming to body temperature. Disadvantages to using pNIPAAm are related to its inability to degrade in vivo as well as its apparent structural similarity to toxic acrylamides. However, copolymerization of NIPAAm with degradable polymers can create a material that is thermoresponsive, biodegradable, and lacking observed cytotoxicity.[96, 97] It is also feasible to incorporate protein-reactive mers in NIPAAm-based copolymers to allow for covalent attachment of ECM components and growth factors [96, 98] and to incorporate enzymatically labile peptide cross-links [99] and adhesive peptide sequences [100].
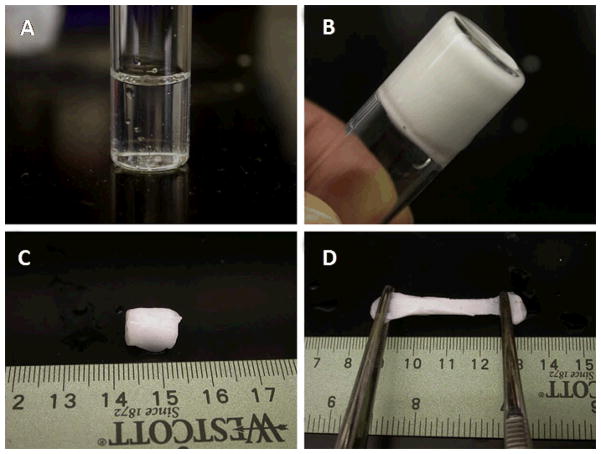
Fujimoto et al. developed a degradable, thermally responsive copolymer of NIPAAm, acrylic acid, and hydroxyl-ethyl methacrylate-poly(trimethylene carbonate) (HEMAPTMC).[101] The sol-gel transition of this polymer occurred around 33°C, forming a hydrogel with a mechanical stiffness of 20 kPa, and a degradation time greater than two months in vivo. The sol-gel transition of this material and subsequent elasticity is demonstrated in Figure 7. Using a chronic rat infarction model, injection of this material around the infarct prevented the progression of cardiac dilation and remodeling. In addition to cellular infiltration in the material, histological evaluation of the LV wall 8 weeks after injection also showed the formation of a layer of smooth muscle next to the remaining material. This muscle layer expressed proteins associated with a contractile phenotype, though the direct effect of the smooth muscle layer on overall LV contractility was not assessed. This phenomenon was similar to that found after alginate injection and may be the result of reduced ventricular wall stress altering the remodeling pathway away from a largely fibrotic result – Figure 8.
Figure 7.
Demonstration of the thermal properties of NIPAAm-co-AAc-co-HEMAPTMC hydrogel. (A) aqueous polymer solution at 4 °C, (B) gel formation after 30-second incubation in 37 °C water bath, (C) after 10 minutes the gel continues to shrink and stiffen, leading to (D)a mechanically robust material with some elastic behavior.(From [101])
Figure 8.
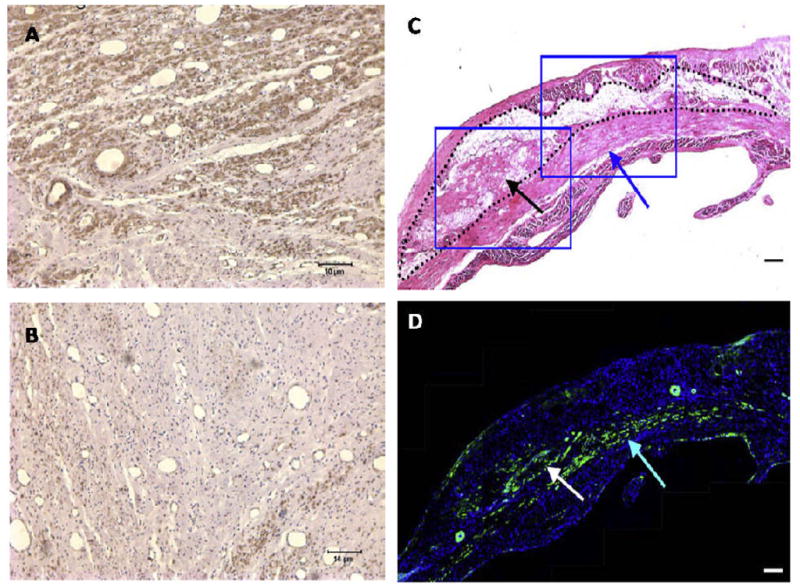
Histological evaluation of myocardium following material injection. Intracoronary injection of alginate (A) led to substantial growth of myofibroblasts that stain positive for α-smooth muscle actin (brown) into the infarct compared to saline injection (B). Direct epicardial injection of a thermally responsive NIPAAm copolymer leads to a smooth muscle layer (C, blue arrows) beneath the material that stains positive for α-smooth muscle actin (D, green stain). (Left panels from [47], scale bar = 10 μm; right panels from [101], scale bar = 100 μm)
A similar approach to the NIPAAm-based thermally responsive material above was investigated by Wu et al., who synthesized a hybrid biological-synthetic gel with PCL-grafted dextran chains that were subsequently copolymerized with NIPAAm monomer to form large branched copolymer chains.[102] Gel formation above 32°C led to a material with a storage modulus between 100 and 1500 Pa. Dextran cleavage in vivo led to breakdown and removal of the copolymer from the injection site within about 1 month. A volume of 200 μL of this material was injected into 4-day old infarcts in rabbits.[103] After 4 weeks, echocardiography showed that EF and LVEDD were significantly better in the material injection group compared to the saline injection group. While no material was evident in the histology at 4 weeks, the LV wall was substantially thicker. No analysis was done to determine if increased thickness was due to cell infiltration or more collagen deposition.
Adaptation of an off-the-shelf material for use in cardiac injection was seen when Radiesse (Bioform Medical Inc) was injected into ovine infarcts.[104] Radiesse is a soft tissue filler made up of calcium hydroxyapatite microspheres suspended in a gel-like solution of water, carboxymethyl cellulose, and glycerin. This material is typically used in cosmetic procedures and has been shown to increase local collagen production after injection.[105] Injection of a total of 1.3 mL of Radiesse at 20 uniformly distributed locations within the infarct area led to improved cardiac function within 15 minutes. The improvements were primarily the result of transforming the dyskinetic myocardium into akinetic tissue. Study of the ejection fraction in local regions of the myocardium demonstrated that apical and midventricular ejection fractions immediately improved after material injection contributing to the global improvement seen in EF. Four weeks after injection, improvements were seen compared to saline controls, although the benefits seen immediately after injection were attenuated and cardiac dilation continued.
4. Materials as Delivery Vehicles for Cardiac Injection
4.1 Drug Delivery
To build upon the results of material injections alone and to further encourage cardiac regeneration and functional improvements, many groups have sought to combine material injections with the controlled release of growth factors or other bioactive molecules. The injected material can serve as a depot for sustained biomolecule delivery, while also acting to protect these agents and thus extend their half-life in vivo.
It has been shown that VEGF and platelet derived growth factor (PDGF) play important roles in vessel formation.[106] While VEGF is important in the early stages of angiogenesis, PDGF is crucial to stabilize and mature the new vascular network. Thus it has been shown that sequential delivery of VEGF followed by PDGF can cause larger, more mature vasculature to develop in an ischemic region.[107] The differing natural affinity of alginate to VEGF and PDGF can lead to a level of sequential dual delivery of these growth factors from this material.[42] In vitro, about 50% of the VEGF is released from alginate during the first day compared to only 10% of the PDGF, though both show about 80% release by 4 weeks. In the infarcted myocardium, this sequential dual growth factor delivery translated to increased capillary density and α-actin SMC positive vessels compared to delivery of either factor individually. While EF and LVEDD were not changed after growth factor delivery, the researchers do show that the systolic velocity time integral – a measure of displacement of the myocardium during contraction – did improve significantly compared to saline injections and compared to single factor delivery.
Because it has been shown that high local doses of angiogenic factors such as VEGF can have deleterious effects Christman et al. aimed to deliver a plasmid coding for pleiotrophin with the fibrin glue injected into an infarcted rat heart.[55] While no studies were performed to demonstrate transfection efficiency or overall levels of pleiotrophin production in the infarct zone, it was shown that hearts which received 250 μg of pleiotrophin with fibrin glue had an 80% increase in arteriole density compared to those receiving pleiotrophin delivered in saline. Using a microbead perfusion assay it was shown that the neovasculature was functionally connected to the native vessel network. No data for cardiac function were presented in this study to determine if the increased vasculature translated to improved LV pumping capacity.
Similarly, basic fibroblast growth factor (bFGF) was delivered with gelatin injection into infarcted myocardium.[70] Delivery of 20 μg of bFGF either with saline or with gelatin lead to increases in arteriole density, capillary density, and fewer TUNEL-positive cells than injection of material without bFGF over 4 weeks. However, bFGF in gelatin consistently suggested a stronger effect than bFGF in saline. The only functional improvement was an increase in FS transiently at two weeks in the bFGF with gelatin which reverted back to a level similar to the saline control by 4 weeks. This again demonstrates that improvements in cardiac function cannot necessarily be directly associated with improvements on a cellular level though it may be a necessary beginning.
The hormone erythropoietin (EPO) has been found to inhibit cell apoptosis and increase neovasculature formation in ischemic tissue but may lead to increased risk of thromboembolic complications as well. Synthetic hydrogel α-cyclodextrin/MPEG-PCL-MPEG was used as delivery vehicle for EPO when injected into recent rat infarcts.[93] In vitro it had been shown that bioactive EPO is released from the biomaterial in a mostly linear fashion with 40% released after one week. In vivo, western blotting confirmed the presence of EPO both in and around the injected material after one week. There was a significant increase in the recruitment of CD 34+ cells and neovascularization, and decrease in apoptosis when EPO was delivered with the hydrogel compared to delivery of EPO with saline, injection of hydrogelalone, or injection of saline. Importantly in this study the EPO-containing hydrogel group also showed improvements to cardiac geometry, FS and left ventricular end-systolic pressure that mirrored the histological improvements.
The largest body of research related to protein delivery with a biomaterial for cardiac injection involves self assembled peptides (SAPs). Insulin-like growth factor 1 (IGF-1), PDGF, and stromal cell derived factor 1 (SDF-1) have all been incorporated into SAPs with promising results.[83–85, 108] PDGF has a natural affinity for SAPs at approximately 1 ng/μg material, leading to a steady in vivo release over 2 weeks. This is a large improvement over free PDGF in saline injections, which is all but removed by 3 days. Delivery of 8 ng of PDGF with nanofibers showed improved FS over 4 months, decreased caspase-3 activation, and decreased apoptosis in the infarct region but did not increase myocardial cell proliferation, neovascularization, or regional blood flow.[83, 84] IGF-1 within SAPs is also delivered over 2 weeks in vivo due to native peptide-growth factor affinity. However, in an attempt to increase the retention time of IGF-1, nanofibers and IGF-1 were both biotinylated and tethered together by streptavidin – Figure 9.[108] This binding kept IGF-1 present at the injection site for longer than 84 days. Because IGF-1 was associated with the nanofibers it was not free to diffuse out of the material. Thus, only cells that infiltrated the boundaries of the material showed the Akt signaling activation that IGF-1 elicits. While the IGF-1 was retained for a long duration and appeared to be bioactive it did nothing to improve FS, LVEDD, or wall thickness over a 3 week follow up period.
Figure 9.
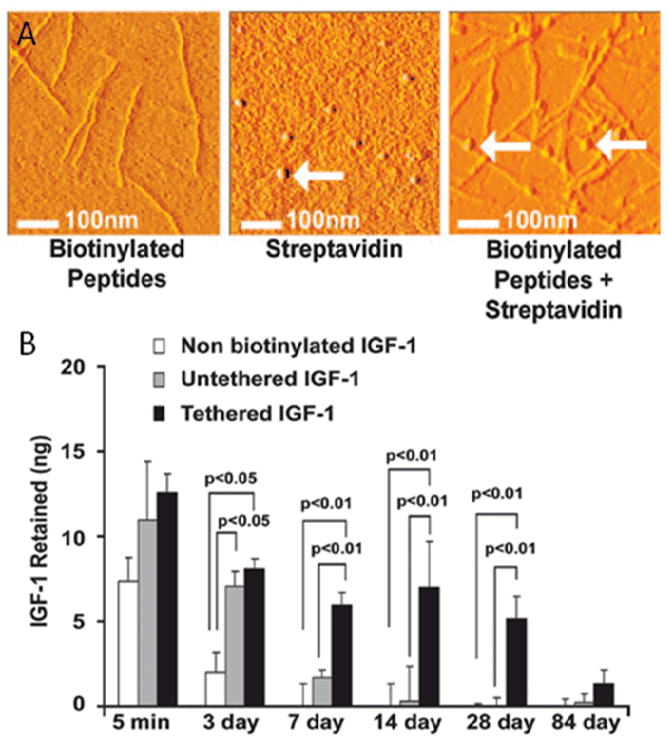
Atomic force microscopy (A) of biotinylated peptide nanofibers (left), streptavidin (center) and streptavidin-bound biotinylated peptides (right) creating a beads-on-a-string appearance. IGF-1 was biotinylated and bound to biotinylated peptides through streptavidin (B). This attachment dramatically slows the release of IGF-1 from the SAP environment compared to IGF-1 that is not biotinylated or not tethered by streptavidin. (From [108], image copyright 2006 National Academy of Sciences, U.S.A.)
Finally, specially designed SDF-1 was produced that was resistant to MMP cleavage to improve its half-life in vivo.[85] Fusion proteins of the resistant SDF-1 and the RAD16-II nanofiber sequence were synthesized, allowing direct SDF-1 incorporation into the SAPs. Using a cleavage sequence for MMP-2 as the spacer sequence between SDF-1 and RAD16-II allowed for release of SDF-I only in the presence of MMP-2, which is a method of stimuli-responsive drug release. SAPs containing SDF-1 fusion proteins delivered to injured myocardium elicited significant improvements in EF and left ventricular end systolic volume 28 days after injection. Stem cell recruitment, capillary density and arteriole density were also significantly improved after delivery of fusion protein nanofibers compared to nanofibers alone or nanofibers with free SDF-1.
Taken together, the results reported to date suggest that delivery of SAPs combined with growth factor delivery may have the ability to improve cardiac function. Furthermore, methods to increase drug retention time, such as using fusion proteins, appear to be worth while to improve therapeutic efficacy. It should also be noted that in all studies involving SAPs the material was injected within 1–2 minutes of coronary artery occlusion, which decreases the clinical relevance of the results. Further studies are necessary wherein more myocardial damage is allowed to occur before an intervention is performed as is generally the case in patients presenting with myocardial infarctions and ischemic cardiomyopathy.
4.2 Cell Delivery
A promising new area of research, cellular injection into the heart, has demonstrated modest improvements in cardiac function and repair.[109–112] While this field is currently under intense focus, there are certain limitations that need to be addressed before full clinical acceptance. First, it is not fully understood which cell types have the greatest potential to repair myocardium. Many cell types including mesenchymal stem cells, cardiac progenitor cells, bone marrow cells, and skeletal myoblasts have been injected with varying reported levels of success. The cell type chosen for injection becomes important because, as in the case of skeletal myoblasts, adverse events such as arrhythmias may occur.[113] Additionally, cell sourcing becomes a complicated regulatory and technical obstacle. Another significant problem with injection is the low cell engraftment that occurs. Injecting cells directly into the myocardium can result in more than 85% of cells being lost to the vasculature or squeezing back out of the injection site.[114, 115] Of those cells that do remain, engraftment with host myocardium and long-term survival is low. This fact further emphasizes that it is unclear if injected cells actually contribute to the contractile machinery of the infarct zone or act merely as a short term source of growth factors and cytokines for surviving host cells. Improvements to cell survival and engraftment after injection may make this treatment more clinically widespread. As techniques for delivering cells through catheters and other transcutaneous devices becomes more established, modifications may easily be made to accommodate biomaterials.[116, 117]
While it has been reported that cells injected into injured myocardium often relocate to the lungs, spleen, liver, kidneys, and non-infarcted cardiac muscle,[118, 119] many studies now demonstrate that this problem may be reduced by injecting cells concomitantly with a biomaterial matrix. At a fundamental level, mesenchymal stem cells that are injected into myocardium while still in the presence of small portions of their intact ECM, are able to more significantly improve cardiac function and cell engraftment than cells whose ECM was digested away before injection.[120] When collagen was injected with mesenchymal stem cells into infarcted myocardium, fewer cells were carried away to extracardiac organs.[121]
Christman et al. have reported on myoblast injection within fibrin glue into infarcted myocardium. While functional results were not significantly different between groups injected with cells, material, or cells with material, histological evaluation did show specific differences.[53, 54] Primarily it was shown that the fibrin allowed for cell survival within the infarct zone even 5 weeks after injection. By comparison, cells only survived in the healthier border zone when cells were injected with saline. In addition, cell injection increased arterioles within the infarct, and myoblasts were often found clumped around these vessels. It requires mention that fibrin alone was able to show a similarly high arteriole density within the infarct when injected without cells. Because no significant functional improvement was seen with cell injection, it is still to be determined what benefit myoblast cell injection with fibrin would ultimately have over material injection alone.
Other studies have been able to demonstrate the benefit that combined cell/material therapy can have over injection of either component alone. Injecting embryonic stem cells with chitosan in rats was able to double the graft size (defined as the % area occupied by injected cells)four weeks after implant to 13% of the infarct zone compared to 6% for injection of cells with saline.[62] The increased engraftment may have contributed to the reduced infarct size in the cell/material group, which was half that of the saline control group. The inclusion of cells increased EF, decreased intraventricular volumes, and increased microvessel density more than injection of cells or material independently.
Similarly, Kofidis et al. showed that injection of embryonic stem cells with Matrigel in rats improved cardiac parameters more than injection of material or cells alone.[72, 73] The cells were able to improve FS at least 4 weeks after injection compared to saline injection controls. The cells appeared to populate the pockets of space between aligned collagen fibers from the injected matrix. Further, the cells were shown over time to strongly express connexin 43 indicating connectivity to each other and host myocardium. This result suggests that materials that encourage cell attachment may be supportive of cell injection as they allow for natural cell spreading and connectivity toward establishing a functional graft tissue. Clearly the presence of Matrigel in this study allowed for a larger cell graft to survive than was able to without the biomaterial scaffold. It is also worth noting that concerns regarding embryonic stem cell injection and teratoma formation limit the direct applicability of the reported findings with these cells [122] although the results may generally be relevant for other pre-cursor cell types.
Synthetic materials have been able to elicit similar improvements to the natural materials discussed above when combined with cell therapy. For instance, rabbit bone marrow stem cells have been injected with α-cyclodextrin/MPEG-PCL-MPEG self assembling hydrogels.[123] This material, which was shown to support cell encapsulation and adhesion, allowed for 2.5 times more implanted cells to survive 5 weeks after injection compared to cells injected in growth medium, as shown in Figure 10. Importantly, the EF of rabbits injected with cells in the hydrogel was nearly back to pre-infarct levels (67% pre vs. 63% after injection)and was higher than injection of cells alone. Inclusion of cells was associated with increased vessel formation around the infarct, while injection of material alone in previous studies did not have this result.[92] Of note, this report did not include a material-only injection group and previous studies were also able to show a high level of EF recovery after injection of the material alone. It thus is not clear to what extent the cells directly contributed to improved cardiac function.
Figure 10.
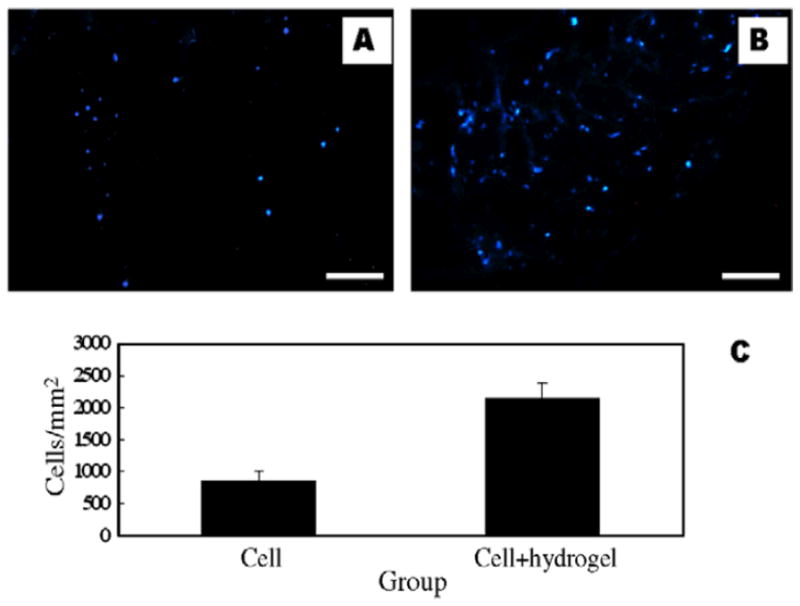
Fluorescently labeled bone marrow stem cells were injected into an infarct with or without α-CD/MPEG-PCL-MPEG hydrogel. Four weeks after injection cell engraftment in the group without the hydrogel (A) was less than half that of the hydrogel group (B) as determined both visually and quantitatively (C), thus demonstrating a benefit of material injection with cell therapy. (From [123], scale bar = 40 μm)
In conjunction with SAPs, both neonatal cardiomyocytes and embryonic stem cells have each been injected into healthy myocardium with the result of an increase in potential cardiac progenitor cells and spontaneous expression of α-myosin heavy chain, respectively.[82] Because of the cardioprotective role that IGF-1 has been shown to play, it has also been studied with SAPs for cell injection therapy. More importantly, in a rat infarct model, SAPs tethered to IGF-1 were able to significantly increase FS and decrease dilation and apoptosis when injected with neonatal cardiomyocytes.[108] Thus, IGF-1-linked SAPs, while unable to improve cardiac function independently as a drug-delivering material, seem to be beneficial when used in cell injection therapy. In vitro it has been demonstrated that IGF-1 bound SAPs were able to increase cardiac progenitor cell survival 2.5 fold when cells were under oxidative stress. Significant cardiac regeneration was demonstrated when cardiac progenitor cells were injected into an infarct with IGF-1 loaded SAPs.[124] It was shown that not only were the injected cells contributing to the improved myocyte number, size, and mitogenesis, they were also able to induce endogenous cardiac regeneration when in the presence of IGF-1 linked SAPs.
In reviewing the use of materials as a delivery vehicle for molecules or cells some notable general trends appear. First, injection of hydrogel materials can improve cardiac function and histology over injection of saline. Second, injection of material provides similar or better results than injection of only growth factors or cells in solution, when considering comparative studies. Third, combining material with cells, or material with growth factors, often improves upon the benefits seen when material is injected alone. It may be that delivery of appropriate material, cells, and growth factor together may ultimately prove to be the most effective method for restoring cardiac function. From a practical perspective, it may also be the case that the benefits that can be achieved by material injection alone may be more attractive for clinical translation in that they avoid the regulatory and implicit safety burden associated with cell-containing approaches, and to a lesser extent pharmaceutical-associated strategies. Ultimately, the risk-benefit of adding further complexity to intramyocardial biomaterial injections in the form of cellular or pharmaceutical agents will need to be evaluated as these technologies advance.
5. Limitations and Challenges
Reviewing the current literature it is difficult to determine which material properties and injection techniques are most advantageous for cardiac injection. For example, the volume of material injected into rat myocardium has varied between tens and hundreds of μL in different studies, which likely impacts the results regardless of material. Further, the number and location of these injections, whether a single injection directly in the middle of the infarct or multiple injections around the border zone, vary between studies. It is reasonable to assume, as was demonstrated in numerical modeling studies, that the volumes and locations will have an effect on outcomes.[28, 29] Delivery of material to the border zone may be better positioned to encourage regeneration from the healthier peripheral tissue than material injected in the center of the infarct. Looking at injection from another dimension, endocardial and epicardial injection approaches each have potential risks and benefits. The coupling of injection therapy with functional imaging is an area of promise, where the injection sites might be optimized and delivery of appropriate volumes achieved safely.
Another major limitation to drawing general conclusions from current data involves the myriad infarction models employed. Many different scenarios are represented wherein hearts are treated between minutes to weeks after infarction. While it is likely that injection of material soon after infarction could provide the most benefit, this is clinically problematic. Thus data on late stage infarction treatment are of particular value and relevance. The type of infarction, such as a sustained occlusion or an ischemia-reperfusion event, and the location and extent of the blockage may also influence recoverability. On top of this, underlying co-morbities, which would be encountered in the patient population (e.g. diabetes, valvular disease) would further confound conclusions drawn from current animal models. A further important variable between studies is that the materials used often have different mechanical properties and degradation times, even if they are from similar starting molecules. It was suggested by several studies that residence time in the tissue may have a role in maintaining improvements. Finally, with the longest follow-up period after treatment being just thirteen weeks, the long-term benefits of this treatment approach are not known.
As the field of intramyocardial biomaterial injection develops it will be necessary to conduct more comparative studies to elucidate which of the many material design variables provide functionally relevant cardiac improvements. It remains difficult to parse out what benefits are derived from changes exclusively in the mechanical environment of the LV and what benefits are a result of cardiac remodeling occurring concurrently, and likely dependently, in the ventricular wall. Furthermore, the potential positive and negative impact of the local inflammatory and phagocytic activity associated with the local degradable material injection site is not clear. While there is evidence to suggest that ongoing inflammation from a non-degradable hydogel injection is counter-productive[94], others have suggested that the inflammatory response may be beneficial, stimulating acute angiogenesis and potentially positively impacting the remodeling result[101, 125].
6. Conclusion
Intramyocardial biomaterial injection following MI clearly holds promise as a means to improve cardiac performance in the face of progressive ischemic cardiomyopathy. Biologically-derived, synthetic and hybrid materials can be designed with properties useful for this application. The optimal design parameters, including degradation rate and profile, injectability, elastic modulus, and various possible bioactivities largely remain to be elucidated and thus provide a fertile area for hypothesis-driven biomaterial design. The incorporation of specific signaling molecules such as growth factors and the inclusion of cellular components with injected materials provide an opportunity to further improve outcomes over material injection alone. Cells delivered in the context of hydrogels appear to survive longer and engraft more effectively than those injected in saline controls, demonstrating the value of coupling biomaterial and cellular approaches. The benefits of biomaterial injection alone, however, may require that incremental improvements when combined with cellular therapy must be justified with respect to increased complexity and safety concerns. Critical developments in the near future will be the demonstration of intramyocardial biomaterial injection therapy safety and efficacy in chronic large animal models and ultimately the transition of candidate technologies towards early stage clinical trials.
Acknowledgments
This work was supported by the National Institutes of Health (NIH), grant #HL069368 and the Commonwealth of Pennsylvania. Mr. Nelson was supported by NIH training grant #T32-HL076124.
Abbreviations
- bFGF
basic fibroblast growth factor
- ECM
extracellular matrix
- EF
ejection fraction
- EPO
erythropoietin
- FAC
fractional area change
- FS
fractional shortening
- IGF-1
insulin-like growth factor 1
- LV
left ventricle
- LVEDD
left ventricular end-diastolic diameter
- LVESD
left ventricular end-systolic diameter
- MI
myocardial infarction
- MeHA
methacrylated hyaluronic acid
- MPEG-PCL-MPEG
methoxy polyethylene glycol–poly (caprolactone)-(dodecanedioic acid)–poly(caprolactone)-methoxy polyethylene glycol
- MW
molecular weight
- NIPAAm
N-isopropyl acrylamide
- PDGF
platelet derived growth factor
- PEG
poly(ethylene glycol)
- RAD16-II
AcN-RARADADARARADADA-CNH2 (where R=arginine, A=alanine, D=aspartate)
- RGD
arginine-glycine-aspartate
- RGE
arginine-glycine-glutamate
- SAP
self-assembling peptide
- SDF-1
stromal cell derived factor 1
- SMC
smooth muscle cell
- VEGF
vascular endothelial growth factor
- YIGSR
tyrosine-isoleucine-glycine-serine-arginine
References
- 1.Jessup M, Brozena S. Heart failure. N Engl J Med. 2003;348:2007–18. doi: 10.1056/NEJMra021498. [DOI] [PubMed] [Google Scholar]
- 2.Hannan EL, et al. A comparison of three-year survival after coronary artery bypass graft surgery and percutaneous transluminal coronary angioplasty. J Am Coll Cardiol. 1999;33:63–72. doi: 10.1016/s0735-1097(98)00540-3. [DOI] [PubMed] [Google Scholar]
- 3.Hannan EL, et al. Long-term outcomes of coronary-artery bypass grafting versus stent implantation. N Engl J Med. 2005;352:2174–83. doi: 10.1056/NEJMoa040316. [DOI] [PubMed] [Google Scholar]
- 4.Nelson GS, et al. Left ventricular or biventricular pacing improves cardiac function at diminished energy cost in patients with dilated cardiomyopathy and left bundle-branch block. Circulation. 2000;102:3053–9. doi: 10.1161/01.cir.102.25.3053. [DOI] [PubMed] [Google Scholar]
- 5.Flather MD, et al. Long-term ACE-inhibitor therapy in patients with heart failure or left-ventricular dysfunction: a systematic overview of data from individual patients. ACE-Inhibitor Myocardial Infarction Collaborative Group. Lancet. 2000;355:1575–81. doi: 10.1016/s0140-6736(00)02212-1. [DOI] [PubMed] [Google Scholar]
- 6.van Zwieten PA. Current and newer approaches in the drug treatment of congestive heart failure. Cardiovasc Drugs Ther. 1997;10:693–702. doi: 10.1007/BF00053026. [DOI] [PubMed] [Google Scholar]
- 7.John R, et al. Long-term outcomes after cardiac transplantation: an experience based on different eras of immunosuppressive therapy. Ann Thorac Surg. 2001;72:440–9. doi: 10.1016/s0003-4975(01)02784-9. [DOI] [PubMed] [Google Scholar]
- 8.Goldstein DJ, Oz MC, Rose EA. Implantable left ventricular assist devices. N Engl J Med. 1998;339:1522–33. doi: 10.1056/NEJM199811193392107. [DOI] [PubMed] [Google Scholar]
- 9.Simon MA, Watson J, Baldwin JT, Wagner WR, Borovetz HS. Current and future considerations in the use of mechanical circulatory support devices. Annu Rev Biomed Eng. 2008;10:59–84. doi: 10.1146/annurev.bioeng.9.060906.151856. [DOI] [PubMed] [Google Scholar]
- 10.Christman KL, Lee RJ. Biomaterials for the treatment of myocardial infarction. J Am Coll Cardiol. 2006;48:907–13. doi: 10.1016/j.jacc.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 11.Holmes JW, Borg TK, Covell JW. Structure and mechanics of healing myocardial infarcts. Annu Rev Biomed Eng. 2005;7:223–53. doi: 10.1146/annurev.bioeng.7.060804.100453. [DOI] [PubMed] [Google Scholar]
- 12.Gupta KB, Ratcliffe MB, Fallert MA, Edmunds LH, Jr, Bogen DK. Changes in passive mechanical stiffness of myocardial tissue with aneurysm formation. Circulation. 1994;89:2315–26. doi: 10.1161/01.cir.89.5.2315. [DOI] [PubMed] [Google Scholar]
- 13.Pfeffer MA, Braunwald E. Ventricular remodeling after myocardial infarction. Experimental observations and clinical implications. Circulation. 1990;81:1161–72. doi: 10.1161/01.cir.81.4.1161. [DOI] [PubMed] [Google Scholar]
- 14.Schwinger RH, et al. The failing human heart is unable to use the Frank-Starling mechanism. Circ Res. 1994;74:959–69. doi: 10.1161/01.res.74.5.959. [DOI] [PubMed] [Google Scholar]
- 15.White HD, Norris RM, Brown MA, Brandt PW, Whitlock RM, Wild CJ. Left ventricular end-systolic volume as the major determinant of survival after recovery from myocardial infarction. Circulation. 1987;76:44–51. doi: 10.1161/01.cir.76.1.44. [DOI] [PubMed] [Google Scholar]
- 16.Tischler MD, Niggel J, Borowski DT, LeWinter MM. Relation between left ventricular shape and exercise capacity in patients with left ventricular dysfunction. J Am Coll Cardiol. 1993;22:751–7. doi: 10.1016/0735-1097(93)90187-6. [DOI] [PubMed] [Google Scholar]
- 17.Pfeffer MA, Pfeffer JM. Ventricular enlargement and reduced survival after myocardial infarction. Circulation. 1987;75:IV93–7. [PubMed] [Google Scholar]
- 18.Tonnessen T, Knudsen CW. Surgical left ventricular remodeling in heart failure. Eur J Heart Fail. 2005;7:704–9. doi: 10.1016/j.ejheart.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 19.Starling RC, et al. Sustained benefits of the CorCap Cardiac Support Device on left ventricular remodeling: three year follow-up results from the Acorn clinical trial. Ann Thorac Surg. 2007;84:1236–42. doi: 10.1016/j.athoracsur.2007.03.096. [DOI] [PubMed] [Google Scholar]
- 20.Klodell CT, Jr, et al. Worldwide surgical experience with the Paracor Heart Net cardiac restraint device. J Thorac Cardiovasc Surg. 2008;135:188–95. doi: 10.1016/j.jtcvs.2007.09.034. [DOI] [PubMed] [Google Scholar]
- 21.Gummert JF, Rahmel A, Bossert T, Mohr FW. Socks for the dilated heart. Does passive cardiomyoplasty have a role in long-term care for heart failure patients? Z Kardiol. 2004;93:849–54. doi: 10.1007/s00392-004-0160-7. [DOI] [PubMed] [Google Scholar]
- 22.Blom AS, et al. Infarct size reduction and attenuation of global left ventricular remodeling with the CorCap cardiac support device following acute myocardial infarction in sheep. Heart Fail Rev. 2005;10:125–39. doi: 10.1007/s10741-005-4640-2. [DOI] [PubMed] [Google Scholar]
- 23.Fukamachi K, McCarthy PM. Initial safety and feasibility clinical trial of the myosplint device. J Card Surg. 2005;20:S43–7. doi: 10.1111/j.1540-8191.2005.00157.x. [DOI] [PubMed] [Google Scholar]
- 24.Kashem A, et al. Early and late results of left ventricular reshaping by passive cardiac-support device in canine heart failure. J Heart Lung Transplant. 2003;22:1046–53. doi: 10.1016/s1053-2498(02)01162-2. [DOI] [PubMed] [Google Scholar]
- 25.Sartipy U, Albage A, Lindblom D. The Dor procedure for left ventricular reconstruction. Ten-year clinical experience. Eur J Cardiothorac Surg. 2005;27:1005–10. doi: 10.1016/j.ejcts.2005.01.055. [DOI] [PubMed] [Google Scholar]
- 26.Batista R. Partial left ventriculectomy--the Batista procedure. Eur J Cardiothorac Surg. 1999;15(Suppl 1):S12-9. discussion S39–43. [PubMed] [Google Scholar]
- 27.Fujimoto KL, et al. An elastic, biodegradable cardiac patch induces contractile smooth muscle and improves cardiac remodeling and function in subacute myocardial infarction. J Am Coll Cardiol. 2007;49:2292–300. doi: 10.1016/j.jacc.2007.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wall ST, Walker JC, Healy KE, Ratcliffe MB, Guccione JM. Theoretical impact of the injection of material into the myocardium: a finite element model simulation. Circulation. 2006;114:2627–35. doi: 10.1161/CIRCULATIONAHA.106.657270. [DOI] [PubMed] [Google Scholar]
- 29.Wenk JF, et al. A method for automatically optimizing medical devices for treating heart failure: designing polymeric injection patterns. J Biomech Eng. 2009;131:10111–10117. doi: 10.1115/1.4000165. [DOI] [PubMed] [Google Scholar]
- 30.Rowley JA, Madlambayan G, Mooney DJ. Alginate hydrogels as synthetic extracellular matrix materials. Biomaterials. 1999;20:45–53. doi: 10.1016/s0142-9612(98)00107-0. [DOI] [PubMed] [Google Scholar]
- 31.Alsberg E, Anderson KW, Albeiruti A, Franceschi RT, Mooney DJ. Cell-interactive alginate hydrogels for bone tissue engineering. J Dent Res. 2001;80:2025–9. doi: 10.1177/00220345010800111501. [DOI] [PubMed] [Google Scholar]
- 32.Augst AD, Kong HJ, Mooney DJ. Alginate hydrogels as biomaterials. Macromol Biosci. 2006;6:623–33. doi: 10.1002/mabi.200600069. [DOI] [PubMed] [Google Scholar]
- 33.Kong HJ, Lee KY, Mooney DJ. Decoupling the dependence of rheological/mechanical properties of hydrogels from solids concentration. Polymer. 2002;43:6239–6246. [Google Scholar]
- 34.Drury JL, Dennis RG, Mooney DJ. The tensile properties of alginate hydrogels. Biomaterials. 2004;25:3187–99. doi: 10.1016/j.biomaterials.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 35.Stokke BJ, Draget KI, Smidsrod O, Yuguchi Y, Urakawa H, Kajiwara K. Small-angle x-ray scattering and rheological characterization of alginate gels. 1. Ca-alginate gels. Macromolecules. 2000;33:1853–1863. doi: 10.1021/bm034105g. [DOI] [PubMed] [Google Scholar]
- 36.Bouhadir KH, Lee KY, Alsberg E, Damm KL, Anderson KW, Mooney DJ. Degradation of partially oxidized alginate and its potential application for tissue engineering. Biotechnol Prog. 2001;17:945–50. doi: 10.1021/bp010070p. [DOI] [PubMed] [Google Scholar]
- 37.Tonnesen HH, Karlsen J. Alginate in drug delivery systems. Drug Dev Ind Pharm. 2002;28:621–30. doi: 10.1081/ddc-120003853. [DOI] [PubMed] [Google Scholar]
- 38.Simmons CA, Alsberg E, Hsiong S, Kim WJ, Mooney DJ. Dual growth factor delivery and controlled scaffold degradation enhance in vivo bone formation by transplanted bone marrow stromal cells. Bone. 2004;35:562–9. doi: 10.1016/j.bone.2004.02.027. [DOI] [PubMed] [Google Scholar]
- 39.Landa N, et al. Effect of injectable alginate implant on cardiac remodeling and function after recent and old infarcts in rat. Circulation. 2008;117:1388–96. doi: 10.1161/CIRCULATIONAHA.107.727420. [DOI] [PubMed] [Google Scholar]
- 40.Tsur-Gang O, et al. The effects of peptide-based modification of alginate on left ventricular remodeling and function after myocardial infarction. Biomaterials. 2009;30:189–95. doi: 10.1016/j.biomaterials.2008.09.018. [DOI] [PubMed] [Google Scholar]
- 41.Yu J, Christman KL, Chin E, Sievers RE, Saeed M, Lee RJ. Restoration of left ventricular geometry and improvement of left ventricular function in a rodent model of chronic ischemic cardiomyopathy. J Thorac Cardiovasc Surg. 2009;137:180–7. doi: 10.1016/j.jtcvs.2008.08.036. [DOI] [PubMed] [Google Scholar]
- 42.Hao X, et al. Angiogenic effects of sequential release of VEGF-A165 and PDGF-BB with alginate hydrogels after myocardial infarction. Cardiovasc Res. 2007;75:178–85. doi: 10.1016/j.cardiores.2007.03.028. [DOI] [PubMed] [Google Scholar]
- 43.Mihardja SS, Sievers RE, Lee RJ. The effect of polypyrrole on arteriogenesis in an acute rat infarct model. Biomaterials. 2008;29:4205–10. doi: 10.1016/j.biomaterials.2008.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mukherjee R, et al. Targeted myocardial microinjections of a biocomposite material reduces infarct expansion in pigs. Ann Thorac Surg. 2008;86:1268–76. doi: 10.1016/j.athoracsur.2008.04.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yu J, Gu Y, Du KT, Mihardja S, Sievers RE, Lee RJ. The effect of injected RGD modified alginate on angiogenesis and left ventricular function in a chronic rat infarct model. Biomaterials. 2009;30:751–6. doi: 10.1016/j.biomaterials.2008.09.059. [DOI] [PubMed] [Google Scholar]
- 46.Garner B, Georgevich A, Hodgson AJ, Liu L, Wallace GG. Polypyrrole-heparin composites as stimulus-responsive substrates for endothelial cell growth. J Biomed Mater Res. 1999;44:121–9. doi: 10.1002/(sici)1097-4636(199902)44:2<121::aid-jbm1>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 47.Leor J, et al. Intracoronary injection of in situ forming alginate hydrogel reverses left ventricular remodeling after myocardial infarction in Swine. J Am Coll Cardiol. 2009;54:1014–23. doi: 10.1016/j.jacc.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 48.Lee DS, Austin PC, Rouleau JL, Liu PP, Naimark D, Tu JV. Predicting mortality among patients hospitalized for heart failure: derivation and validation of a clinical model. JAMA. 2003;290:2581–7. doi: 10.1001/jama.290.19.2581. [DOI] [PubMed] [Google Scholar]
- 49.Breen A, O’Brien T, Pandit A. Fibrin as a Delivery System for Therapeutic Drugs and Biomolecules. Tissue Eng Part B Rev. 2009 doi: 10.1089/ten.TEB.2008.0527. [DOI] [PubMed] [Google Scholar]
- 50.Jackson MR. Fibrin sealants in surgical practice: An overview. Am J Surg. 2001;182:1S–7S. doi: 10.1016/s0002-9610(01)00770-x. [DOI] [PubMed] [Google Scholar]
- 51.Sierra DH. Fibrin sealant adhesive systems: a review of their chemistry, material properties and clinical applications. J Biomater Appl. 1993;7:309–52. doi: 10.1177/088532829300700402. [DOI] [PubMed] [Google Scholar]
- 52.Sierra DH, Eberhardt AW, Lemons JE. Failure characteristics of multiple-component fibrin-based adhesives. J Biomed Mater Res. 2002;59:1–11. doi: 10.1002/jbm.1210. [DOI] [PubMed] [Google Scholar]
- 53.Christman KL, Fok HH, Sievers RE, Fang Q, Lee RJ. Fibrin glue alone and skeletal myoblasts in a fibrin scaffold preserve cardiac function after myocardial infarction. Tissue Eng. 2004;10:403–9. doi: 10.1089/107632704323061762. [DOI] [PubMed] [Google Scholar]
- 54.Christman KL, Vardanian AJ, Fang Q, Sievers RE, Fok HH, Lee RJ. Injectable fibrin scaffold improves cell transplant survival, reduces infarct expansion, and induces neovasculature formation in ischemic myocardium. J Am Coll Cardiol. 2004;44:654–60. doi: 10.1016/j.jacc.2004.04.040. [DOI] [PubMed] [Google Scholar]
- 55.Christman KL, Fang Q, Yee MS, Johnson KR, Sievers RE, Lee RJ. Enhanced neovasculature formation in ischemic myocardium following delivery of pleiotrophin plasmid in a biopolymer. Biomaterials. 2005;26:1139–44. doi: 10.1016/j.biomaterials.2004.04.025. [DOI] [PubMed] [Google Scholar]
- 56.Huang NF, Yu J, Sievers R, Li S, Lee RJ. Injectable biopolymers enhance angiogenesis after myocardial infarction. Tissue Eng. 2005;11:1860–6. doi: 10.1089/ten.2005.11.1860. [DOI] [PubMed] [Google Scholar]
- 57.Auger FA, Guignard R, López Valle CA, Germain L. Role and innocuity of Tisseel®, a tissue glue, in the grafting process and in vivo evolution of human cultured epidermis. British Journal of Plastic Surgery. 1993;46:136–142. doi: 10.1016/0007-1226(93)90145-2. [DOI] [PubMed] [Google Scholar]
- 58.Kim IY, et al. Chitosan and its derivatives for tissue engineering applications. Biotechnol Adv. 2008;26:1–21. doi: 10.1016/j.biotechadv.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 59.Khor E, Lim LY. Implantable applications of chitin and chitosan. Biomaterials. 2003;24:2339–49. doi: 10.1016/s0142-9612(03)00026-7. [DOI] [PubMed] [Google Scholar]
- 60.Chenite A, et al. Novel injectable neutral solutions of chitosan form biodegradable gels in situ. Biomaterials. 2000;21:2155–61. doi: 10.1016/s0142-9612(00)00116-2. [DOI] [PubMed] [Google Scholar]
- 61.Dang JM, Sun DD, Shin-Ya Y, Sieber AN, Kostuik JP, Leong KW. Temperature-responsive hydroxybutyl chitosan for the culture of mesenchymal stem cells and intervertebral disk cells. Biomaterials. 2006;27:406–18. doi: 10.1016/j.biomaterials.2005.07.033. [DOI] [PubMed] [Google Scholar]
- 62.Lu WN, et al. Functional Improvement of Infarcted Heart by Co-Injection of Embryonic Stem Cells with Temperature-Responsive Chitosan Hydrogel. Tissue Eng Part A. 2008 doi: 10.1089/ten.tea.2008.0143. [DOI] [PubMed] [Google Scholar]
- 63.Yeo Y, Geng W, Ito T, Kohane DS, Burdick JA, Radisic M. Photocrosslinkable hydrogel for myocyte cell culture and injection. J Biomed Mater Res B Appl Biomater. 2007;81:312–22. doi: 10.1002/jbm.b.30667. [DOI] [PubMed] [Google Scholar]
- 64.Dobaczewski M, Gonzalez-Quesada C, Frangogiannis NG. The extracellular matrix as a modulator of the inflammatory and reparative response following myocardial infarction. J Mol Cell Cardiol. 2009 doi: 10.1016/j.yjmcc.2009.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Juliano RL, Haskill S. Signal transduction from the extracellular matrix. J Cell Biol. 1993;120:577–85. doi: 10.1083/jcb.120.3.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Badylak SF, Freytes DO, Gilbert TW. Extracellular matrix as a biological scaffold material: Structure and function. Acta Biomaterialia. 2009;5:1–13. doi: 10.1016/j.actbio.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 67.Tomihata K, Burczak K, Shiraki K, Ikada Y. Polymers of Biological and Biomedical Significance. American Chemical Society; Washington, DC: 1993. Cross-Linking and Biodegradation of Native and Denatured Collagen; pp. 275–286. [Google Scholar]
- 68.Barocas VH, Moon AG, Tranquillo RT. The fibroblast-populated collagen microsphere assay of cell traction force--Part 2: Measurement of the cell traction parameter. J Biomech Eng. 1995;117:161–70. doi: 10.1115/1.2795998. [DOI] [PubMed] [Google Scholar]
- 69.Semler EJ, Ranucci CS, Moghe PV. Mechanochemical manipulation of hepatocyte aggregation can selectively induce or repress liver-specific function. Biotechnol Bioeng. 2000;69:359–69. doi: 10.1002/1097-0290(20000820)69:4<359::aid-bit2>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 70.Shao ZQ, et al. Effects of intramyocardial administration of slow-release basic fibroblast growth factor on angiogenesis and ventricular remodeling in a rat infarct model. Circ J. 2006;70:471–7. doi: 10.1253/circj.70.471. [DOI] [PubMed] [Google Scholar]
- 71.Dai W, Wold LE, Dow JS, Kloner RA. Thickening of the infarcted wall by collagen injection improves left ventricular function in rats: a novel approach to preserve cardiac function after myocardial infarction. J Am Coll Cardiol. 2005;46:714–9. doi: 10.1016/j.jacc.2005.04.056. [DOI] [PubMed] [Google Scholar]
- 72.Kofidis T, et al. Injectable bioartificial myocardial tissue for large-scale intramural cell transfer and functional recovery of injured heart muscle. J Thorac Cardiovasc Surg. 2004;128:571–8. doi: 10.1016/j.jtcvs.2004.05.021. [DOI] [PubMed] [Google Scholar]
- 73.Kofidis T, Lebl DR, Martinez EC, Hoyt G, Tanaka M, Robbins RC. Novel injectable bioartificial tissue facilitates targeted, less invasive, large-scale tissue restoration on the beating heart after myocardial injury. Circulation. 2005;112:I173–7. doi: 10.1161/CIRCULATIONAHA.104.526178. [DOI] [PubMed] [Google Scholar]
- 74.Zhao ZQ, et al. Improvement in cardiac function with small intestine extracellular matrix is associated with recruitment of C-kit cells, myofibroblasts, and macrophages after myocardial infarction. J Am Coll Cardiol. 2010;55:1250–61. doi: 10.1016/j.jacc.2009.10.049. [DOI] [PubMed] [Google Scholar]
- 75.Singelyn JM, Dequach JA, Seif-Naraghi SB, Littlefield RB, Schup-Magoffin PJ, Christman KL. Naturally derived myocardial matrix as an injectable scaffold for cardiac tissue engineering. Biomaterials. 2009 doi: 10.1016/j.biomaterials.2009.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Seif-Naraghi SB, Salvatore MA, Schup-Magoffin PJ, Hu DP, Christman KL. Design and Characterization of an Injectable Pericardial Matrix Gel: A Potentially Autologous Scaffold for Cardiac Tissue Engineering. Tissue Eng Part A. doi: 10.1089/ten.tea.2009.0768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ifkovits JL, et al. Injectable hydrogel properties influence infarct expansion and extent of postinfarction left ventricular remodeling in an ovine model. Proc Natl Acad Sci U S A. doi: 10.1073/pnas.1004097107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhang S. Fabrication of novel biomaterials through molecular self-assembly. Nat Biotechnol. 2003;21:1171–8. doi: 10.1038/nbt874. [DOI] [PubMed] [Google Scholar]
- 79.Zhao X, Zhang S. Molecular designer self-assembling peptides. Chem Soc Rev. 2006;35:1105–10. doi: 10.1039/b511336a. [DOI] [PubMed] [Google Scholar]
- 80.Narmoneva DA, Vukmirovic R, Davis ME, Kamm RD, Lee RT. Endothelial cells promote cardiac myocyte survival and spatial reorganization: implications for cardiac regeneration. Circulation. 2004;110:962–8. doi: 10.1161/01.CIR.0000140667.37070.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Holmes TC. Novel peptide-based biomaterial scaffolds for tissue engineering. Trends Biotechnol. 2002;20:16–21. doi: 10.1016/s0167-7799(01)01840-6. [DOI] [PubMed] [Google Scholar]
- 82.Davis ME, et al. Injectable self-assembling peptide nanofibers create intramyocardial microenvironments for endothelial cells. Circulation. 2005;111:442–50. doi: 10.1161/01.CIR.0000153847.47301.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hsieh PC, MacGillivray C, Gannon J, Cruz FU, Lee RT. Local controlled intramyocardial delivery of platelet-derived growth factor improves postinfarction ventricular function without pulmonary toxicity. Circulation. 2006;114:637–44. doi: 10.1161/CIRCULATIONAHA.106.639831. [DOI] [PubMed] [Google Scholar]
- 84.Hsieh PC, Davis ME, Gannon J, MacGillivray C, Lee RT. Controlled delivery of PDGF-BB for myocardial protection using injectable self-assembling peptide nanofibers. J Clin Invest. 2006;116:237–48. doi: 10.1172/JCI25878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Segers VF, Tokunou T, Higgins LJ, MacGillivray C, Gannon J, Lee RT. Local delivery of protease-resistant stromal cell derived factor-1 for stem cell recruitment after myocardial infarction. Circulation. 2007;116:1683–92. doi: 10.1161/CIRCULATIONAHA.107.718718. [DOI] [PubMed] [Google Scholar]
- 86.Hoffman AS. Hydrogels for biomedical applications. Adv Drug Deliv Rev. 2002;54:3–12. doi: 10.1016/s0169-409x(01)00239-3. [DOI] [PubMed] [Google Scholar]
- 87.Zheng Shu X, Liu Y, Palumbo FS, Luo Y, Prestwich GD. In situ crosslinkable hyaluronan hydrogels for tissue engineering. Biomaterials. 25:1339–1348. doi: 10.1016/j.biomaterials.2003.08.014. [DOI] [PubMed] [Google Scholar]
- 88.Wang D-a, Williams CG, Li Q, Sharma B, Elisseeff JH. Synthesis and characterization of a novel degradable phosphate-containing hydrogel. Biomaterials. 2003;24:3969–3980. doi: 10.1016/s0142-9612(03)00280-1. [DOI] [PubMed] [Google Scholar]
- 89.Li J, Ni X, Leong KW. Injectable drug-delivery systems based on supramolecular hydrogels formed by poly(ethylene oxide)s and alpha-cyclodextrin. Journal of Biomedical Materials Research Part A. 2003;65A:196–202. doi: 10.1002/jbm.a.10444. [DOI] [PubMed] [Google Scholar]
- 90.Lee BH, Vernon B. In situ-gelling, erodible N-isopropylacrylamide copolymers. Macromol Biosci. 2005;5:629–35. doi: 10.1002/mabi.200500029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wu DQ, et al. Fabrication of supramolecular hydrogels for drug delivery and stem cell encapsulation. Langmuir. 2008;24:10306–12. doi: 10.1021/la8006876. [DOI] [PubMed] [Google Scholar]
- 92.Jiang XJ, et al. Injection of a novel synthetic hydrogel preserves left ventricle function after myocardial infarction. J Biomed Mater Res A. 2009;90:472–7. doi: 10.1002/jbm.a.32118. [DOI] [PubMed] [Google Scholar]
- 93.Wang T, et al. The inhibition of postinfarct ventricle remodeling without polycythaemia following local sustained intramyocardial delivery of erythropoietin within a supramolecular hydrogel. Biomaterials. 2009;30:4161–7. doi: 10.1016/j.biomaterials.2009.04.033. [DOI] [PubMed] [Google Scholar]
- 94.Dobner S, Bezuidenhout D, Govender P, Zilla P, Davies N. A Synthetic Non-degradable Polyethylene Glycol Hydrogel Retards Adverse Post-infarct Left Ventricular Remodeling. Journal of Cardiac Failure. 2009;15:629–636. doi: 10.1016/j.cardfail.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 95.Jeong B, Kim SW, Bae YH. Thermosensitive sol-gel reversible hydrogels. Adv Drug Deliv Rev. 2002;54:37–51. doi: 10.1016/s0169-409x(01)00242-3. [DOI] [PubMed] [Google Scholar]
- 96.Guan J, Hong Y, Ma Z, Wagner WR. Protein-reactive, thermoresponsive copolymers with high flexibility and biodegradability. Biomacromolecules. 2008;9:1283–92. doi: 10.1021/bm701265j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ma Z, Nelson DM, Hong Y, Wagner WR. Thermally Responsive Injectable Hydrogel Incorporating Methacrylate-Polylactide for Hydrolytic Lability. Biomacromolecules. doi: 10.1021/bm1004299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wang F, et al. Injectable, rapid gelling and highly flexible hydrogel composites as growth factor and cell carriers. Acta Biomater. 6:1978–91. doi: 10.1016/j.actbio.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 99.Kim S, Healy KE. Synthesis and Characterization of Injectable Poly(N-isopropylacrylamide-co-acrylic acid) Hydrogels with Proteolytically Degradable Cross-Links. Biomacromolecules. 2003;4:1214–1223. doi: 10.1021/bm0340467. [DOI] [PubMed] [Google Scholar]
- 100.Stile RA, Healy KE. Thermo-Responsive Peptide-Modified Hydrogels for Tissue Regeneration. Biomacromolecules. 2001;2:185–194. doi: 10.1021/bm0000945. [DOI] [PubMed] [Google Scholar]
- 101.Fujimoto KL, et al. Synthesis, characterization and therapeutic efficacy of a biodegradable, thermoresponsive hydrogel designed for application in chronic infarcted myocardium. Biomaterials. 2009;30:4357–68. doi: 10.1016/j.biomaterials.2009.04.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wu DQ, Qiu F, Wang T, Jiang XJ, Zhang XZ, Zhuo RX. Toward the development of partially biodegradable and injectable thermoresponsive hydrogels for potential biomedical applications. ACS Appl Mater Interfaces. 2009;1:319–327. doi: 10.1021/am8000456. [DOI] [PubMed] [Google Scholar]
- 103.Wang T, et al. Novel thermosensitive hydrogel injection inhibits post-infarct ventricle remodelling. Eur J Heart Fail. 2009;11:14–9. doi: 10.1093/eurjhf/hfn009. [DOI] [PubMed] [Google Scholar]
- 104.Ryan LP, et al. Dermal filler injection: a novel approach for limiting infarct expansion. Ann Thorac Surg. 2009;87:148–55. doi: 10.1016/j.athoracsur.2008.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Jacovella PF. Use of calcium hydroxylapatite (Radiesse) for facial augmentation. Clin Interv Aging. 2008;3:161–74. doi: 10.2147/cia.s2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Richardson TP, Peters MC, Ennett AB, Mooney DJ. Polymeric system for dual growth factor delivery. Nat Biotech. 2001;19:1029–1034. doi: 10.1038/nbt1101-1029. [DOI] [PubMed] [Google Scholar]
- 107.Chen RR, Silva EA, Yuen WW, Mooney DJ. Spatio-temporal VEGF and PDGF delivery patterns blood vessel formation and maturation. Pharm Res. 2007;24:258–64. doi: 10.1007/s11095-006-9173-4. [DOI] [PubMed] [Google Scholar]
- 108.Davis ME, et al. Local myocardial insulin-like growth factor 1 (IGF-1) delivery with biotinylated peptide nanofibers improves cell therapy for myocardial infarction. Proc Natl Acad Sci U S A. 2006;103:8155–60. doi: 10.1073/pnas.0602877103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Segers VF, Lee RT. Stem-cell therapy for cardiac disease. Nature. 2008;451:937–42. doi: 10.1038/nature06800. [DOI] [PubMed] [Google Scholar]
- 110.Wollert KC, Drexler H. Clinical applications of stem cells for the heart. Circ Res. 2005;96:151–63. doi: 10.1161/01.RES.0000155333.69009.63. [DOI] [PubMed] [Google Scholar]
- 111.Laflamme MA, Murry CE. Regenerating the heart. Nat Biotechnol. 2005;23:845–56. doi: 10.1038/nbt1117. [DOI] [PubMed] [Google Scholar]
- 112.Menasche P. Skeletal myoblasts as a therapeutic agent. Prog Cardiovasc Dis. 2007;50:7–17. doi: 10.1016/j.pcad.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 113.Menasche P. Cellular transplantation: hurdles remaining before widespread clinical use. Curr Opin Cardiol. 2004;19:154–61. doi: 10.1097/00001573-200403000-00016. [DOI] [PubMed] [Google Scholar]
- 114.Muller-Ehmsen J, et al. Survival and development of neonatal rat cardiomyocytes transplanted into adult myocardium. J Mol Cell Cardiol. 2002;34:107–16. doi: 10.1006/jmcc.2001.1491. [DOI] [PubMed] [Google Scholar]
- 115.Muller-Ehmsen J, et al. Effective engraftment but poor mid-term persistence of mononuclear and mesenchymal bone marrow cells in acute and chronic rat myocardial infarction. J Mol Cell Cardiol. 2006;41:876–84. doi: 10.1016/j.yjmcc.2006.07.023. [DOI] [PubMed] [Google Scholar]
- 116.Dib N, et al. Recommendations for successful training on methods of delivery of biologics for cardiac regeneration: a report of the International Society for Cardiovascular Translational Research. JACC Cardiovasc Interv. 3:265–75. doi: 10.1016/j.jcin.2009.12.013. [DOI] [PubMed] [Google Scholar]
- 117.Ota T, Patronik NA, Schwartzman D, Riviere CN, Zenati MA. Minimally invasive epicardial injections using a novel semiautonomous robotic device. Circulation. 2008;118:S115–20. doi: 10.1161/CIRCULATIONAHA.107.756049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zhang H, et al. Injection of bone marrow mesenchymal stem cells in the borderline area of infarcted myocardium: heart status and cell distribution. J Thorac Cardiovasc Surg. 2007;134:1234–40. doi: 10.1016/j.jtcvs.2007.07.019. [DOI] [PubMed] [Google Scholar]
- 119.Hale SL, Dai W, Dow JS, Kloner RA. Mesenchymal stem cell administration at coronary artery reperfusion in the rat by two delivery routes: a quantitative assessment. Life Sci. 2008;83:511–5. doi: 10.1016/j.lfs.2008.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wang CC, et al. Direct intramyocardial injection of mesenchymal stem cell sheet fragments improves cardiac functions after infarction. Cardiovasc Res. 2008;77:515–24. doi: 10.1093/cvr/cvm046. [DOI] [PubMed] [Google Scholar]
- 121.Dai W, Hale SL, Kay GL, Jyrala AJ, Kloner RA. Delivering stem cells to the heart in a collagen matrix reduces relocation of cells to other organs as assessed by nanoparticle technology. Regen Med. 2009;4:387–95. doi: 10.2217/RME.09.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Nussbaum J, et al. Transplantation of undifferentiated murine embryonic stem cells in the heart: teratoma formation and immune response. FASEB J. 2007;21:1345–57. doi: 10.1096/fj.06-6769com. [DOI] [PubMed] [Google Scholar]
- 123.Wang T, et al. Bone marrow stem cells implantation with alpha-cyclodextrin/MPEG-PCL-MPEG hydrogel improves cardiac function after myocardial infarction. Acta Biomater. 2009;5:2939–44. doi: 10.1016/j.actbio.2009.04.040. [DOI] [PubMed] [Google Scholar]
- 124.Padin-Iruegas ME, et al. Cardiac progenitor cells and biotinylated insulin-like growth factor-1 nanofibers improve endogenous and exogenous myocardial regeneration after infarction. Circulation. 2009;120:876–87. doi: 10.1161/CIRCULATIONAHA.109.852285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hayakawa K, et al. Inhibition of Granulation Tissue Cell Apoptosis During the Subacute Stage of Myocardial Infarction Improves Cardiac Remodeling and Dysfunction at the Chronic Stage. Circulation. 2003;108:104–109. doi: 10.1161/01.CIR.0000074225.62168.68. [DOI] [PubMed] [Google Scholar]