Abstract
In murine models, the adoptive transfer of CD4+/CD25+ regulatory T cells (Tregs) inhibited graft-versus-host disease (GvHD). Previous work has indicated a critical role for the adhesion molecule L-selectin (CD62L) in the function of Tregs in preventing GvHD. Here we examined the capacity of naive wild-type (WT), CD62L−/− and ex vivo expanded CD62LLo Tregs to inhibit acute GvHD. Surprisingly, we found that CD62L−/− Tregs were potent suppressors of GvHD, whereas CD62LLo Tregs were unable to inhibit disease despite being functionally competent to suppress allo T cell responses in vitro. Concomitant with improved outcomes, WT and CD62L−/− Tregs significantly reduced liver pathology and systemic pro-inflammatory cytokine production, although CD62L−/− Tregs were less effective in reducing lung pathology. While accumulation of CD62L−/− Tregs in GvHD target organs was equivalent to WT Tregs, CD62L−/− Tregs did not migrate as well as WT Tregs to peripheral lymph nodes (PLNs) over the first 2 weeks posttransplantation. This work demonstrated that CD62L was dispensable for Treg-mediated protection from GvHD.
Keywords: Allogeneic stem cell transplantation, CD62L, GvHD, regulatory T cells
Introduction
Allogeneic hematopoeitic stem cell transplantation (allo-HSCT) is a potentially curative therapy for numerous blood born-malignant and nonmalignant disorders (1,2). Although allo-HSCT holds much promise, the prevalence of graft-versus-host disease (GvHD) limits its widespread use (3). CD4+/CD25+ naturally occurring T regulatory (Treg) cells offer a potential solution to the prevention of GvHD. Importantly, Tregs can suppress allo-reactive T cell responses, including those involved in solid organ and skin allograft rejection (4). Multiple groups, including our own, have demonstrated that Tregs are capable of inhibiting GvHD without impacting the GvL response (5,6).
L-selectin (CD62L) is a member of the selectin family that is involved in leukocyte homing (7). CD62L is constitutively expressed by myeloid cells, nave lymphocytes and central memory T cells (7,8). We, and others, have shown the importance of Treg homing molecule expression in preventing GvHD (5,9,10). These studies provided evidence that the phenotype of the CD62LHi Treg population was responsible for inhibition of GvHD; however, they did not directly assess the role of CD62L in this process. Decreased expression of CD62L may be indicative of a Treg subset that is biologically distinct from CD62Lhi Tregs independent of the function of CD62L.
Using a clinically relevant model of allo-HSCT we now show that CD62L expression by Tregs was not required for the inhibition of GvHD as CD62L−/− Tregs provided similar protection from lethal acute GvHD compared to WT Tregs. In addition, CD62L expression was not critical for Treg migration to GvHD target organs. However, the expression of CD62L was important for the prompt migration of Tregs to PLNs.
Methods
Mice
Donor mice consisted of male C57BL/6J (B6), (H-2b; The Jackson Laboratory, Bar Harbor, ME), Thy1.1+ mice (H-2b; The Jackson Laboratory) and CD62L−/− mice, which have been described previously (11). CD62L−/− mice were crossed with B6 mice expressing the enhanced GFP (eGFP) protein to generate eGFP-expressing CD62L−/− mice. The generation of B6-eGFP mice has been described (12). In some experiments Treg cells were isolated from FIR mice (expressing red fluorescent protein (RFP) under the FoxP3 promoter) as described (13). Recipient mice were male (C57BL/6JXDBA/2) FI mice, (B6D2) (H-2bxd; The Jackson Laboratory).Within each experiment, all recipient and donor mice were male mice ranging from 9 to 14 weeks. All animal experiments were performed in accordance with protocols approved by the University of North Carolina Institutional Animal Care and Use Committee.
Antibodies and flow cytometry
Antibodies with the following specificities were purchased from eBiosciences (San Diego, CA): anti-CD4 (RM4.5), CD62L (Mel-14), CD25 (PC61), CD8 (53-6-7), Thy1.1 (HIS51) and FoxP3 (FJK-16s). Acquisition was performed on a FACSCalibur using CellQuest software (BD Biosciences; San Jose, CA). Analysis was performed using FlowJo (Treestar Inc., Ashland, OR) software.
Preparation of cells for transplant and bone marrow transplants
T cell-depleted bone marrow (TCD BM) cells, Teff cells and Tregs were isolated and infused as described (14).
Treg cell expansion
CD4+/CD25+/RFP+/CD62Lhigh cells were sorted on a MoFlo cell sorter (Dako A/S, Glostrup, Denmark) from the spleens of RFP-FoxP3 mice. Sorted cells were expanded with plate-bound anti-CD3 (145-2C11, 15µg/mL; eBioscience) and CD28 (37.51, 10µg/mL; eBioscience) supplemented with IL-2 (500 units/mL; Peprotech; Rocky Hill, NJ) for 12 days. After 12 days, cells were recovered, stained for CD62L and sorted. Sorted cells were always >95% CD4+/mRFP+/CD62LLo.
In vitro suppression assay
In vitro suppression assays were performed as previously described (10).
GvHD grading
Mice were observed twice weekly for signs of GvHD using the previously described clinical scoring system (15).
Fluorescence microscopy
Animals were anesthetized with avertin and organs were imaged with a Zeiss SteREO Lumar V12 microscope with eGFP bandpass filter (Carl Zeiss MicroImaging, Inc., Thornwood, NY) as described (14).
Competitive Treg migration assay
Competitive migration of WT versus CD62L −/− Treg cells was done as described (14).
Histopathology
The sections were scored by one of us (A.P.-M.) who was blinded to the treatment given using a previously described method (14).
Quantitation of chemokine receptor transcripts
RNA was isolated from sort-purified Tregs using the Qiagen RNeasy Kit (Qiagen; Valencia, CA). Quantitative RT-PCR for chemokine receptor transcripts was performed using primers and probes to CCR1, CCR2, CCR4, CCR5, CCR7, CCR8, CCR9, CCR10, CXCR3 and CXCR4 (Applied Biosystems; Corvalis, OR). The ΔCt method was used to normalize transcripts to 18S RNA and to calculate fold induction.
Measurement of serum IFN-γ
Serum samples were obtained from mice receiving whole naïve T cells, with or without WT Tregs, CD62L−/− Tregs, CD62LLo Tregs or BM only. The samples were recovered when animals reached a clinical GvHD score of 4. IFN-γ concentrations were determined according to the manufacturer’s instructions using ELISA (Biolegend San Diego CA).
Statistical analysis
For GvHD scoring, we used Student’s t-test; for overall survival we used Fisher’s exact test, and for median survival we used the Mann–Whitney log rank test. p values ≤ 0.05 were considered significant.
Results
CD62L−/− Tregs mediate protection against lethal GvHD
To determine the precise requirement for CD62L expression in Treg-mediated protection during GvHD, we isolated fresh CD4+/CD25+ cells from WT or CD62L deficient animals (CD62L−/−). Unexpectedly, we did not observe a significant difference in the overall survival (p = 1.0) or median survival time (p = 0.86) in recipient mice given WT compared to CD62L−/− Tregs (Figure 1A). Both WT and CD62L−/− Tregs recipients had significantly improved overall survival (p < 0.001) compared to recipients of WT T cells alone. Our previous work has demonstrated that in vitro expanded CD62LLo Tregs were unable to ameliorate GvHD pathology (5); however, our subsequent analysis of expanded CD4+/CD25+ cells has revealed considerable contamination by FoxP3− cells in the CD62LLo fraction (M. Carlson, J. Serody; unpublished observation). We therefore isolated cells from FIR mice in which the red fluorescent protein is expressed under control of the FoxP3 promoter (13), and thus, Tregs can be identified from the CD4+/CD25+ fraction by their expression of mRFP. Recipients of ex vivo expanded mRFP+/CD62LLo Tregs displayed only a very modest improved overall (p = 0.09) and median survival time (p = 0.12) relative to animals receiving T cells alone (Figure 1A). These results demonstrated that CD62L−/− Tregs were capable of providing protection from lethal acute and GvHD. These data also demonstrate that contamination of CD62Lo Treg cells with effector cells was not an explanation for the lack of activity of CD62LLo Tregs in the current study. The paucity of CD62LLo Tregs present in FIR mice precluded the evaluation of this population of cells without ex vivo expansion.
Figure 1. CD62L−/− Tregs protect from lethal acute GvHD and are potent suppressers in vitro.
1.25 × 106 WT (of which approximately 80% expressed CD62L and 67% had high levels of expression of CD62L), CD62LLo Tregs, or CD62L−/− Tregs were transferred with 3 × 106 TCD BM cells into lethally irradiated B6D2 recipients on day 0. 4 × 106 whole splenic T cells from WT mice were then transferred on day +2 (n = 9 WT Tregs, n = 11 CD62LLo Tregs, n = 12 CD62L−/− Tregs, n = 10 Effectors alone, n = 4 BM only). Animals were monitored for (A) survival and (B) signs of GvHD. Data represent mean score ± SEM at each time point. (C) Suppression of WT responder cell (CD4+/CD25−) proliferation in response to B6D2 alloantigen by WT (■) or CD62L−/− Tregs ( ) was determined as described in the Methods section.
) was determined as described in the Methods section.
Next, we determined disease severity using a defined clinical scoring system (15). Although the survival outcomes were not significantly different, WT Tregs did afford reduced clinical GvHD scores compared to CD62L−/− Tregs during the first 21 days posttransplant (p < 0.04 for days 7–21) (Figure 1B). Starting on day 24, and for the duration of the experiment, GvHD scores were not significantly different (p > 0.05) in recipients given either WT or CD62L−/− Tregs. Consistent with no improvement in overall or median survival, CD62LLo Tregs did not reduce clinical manifestations of GvHD as compared to T cells alone (Figure 1B). Collectively, these data demonstrated that CD62L−/− Tregs were able to protect animals from lethal GvHD, albeit they did not suppress clinical GvHD manifestations as well as WT Treg cells in the first 3 weeks posttransplant. In addition, CD62L−/− Tregs functioned more efficiently to prevent GvHD than mRFP+/CD62LLo Tregs posttransplantation.
CD62L−/− Tregs function normally to suppress T cell responses to allo-antigen in vitro
Because we observed significant differences early posttransplant in the clinical appearance of GvHD between recipients of WT and CD62L−/− Tregs, we sought to determine the ability of CD62L−/− Tregs to inhibit effector T cell responses to allo-antigen. To address this question, freshly isolated CD4+/CD25+ cells from WT and CD62L−/− mice were co-cultured with WT CD4+/CD25− responder cells stimulated with irradiated B6D2 splenocytes. CD62L−/− and WT Tregs displayed equivalent suppression of WT effector T cells up to a 1:8 Treg:Effector cell ratio (Figure 1C). Therefore, the early elevated GvHD scores of animals given CD62L−/− Tregs was not due to an intrinsic defect in their suppressive function. As described, CD62LLo Tregs were potent suppressors of allo-reactive T cells in vitro up to a ratio of 1:32 Tregs: Effector cells (Figure S1).
GvHD target organ histopathology
Given the differences observed in clinical GvHD scores, we were interested in determining the impact that phenotypically different Tregs had on individual organ pathology. Histopathology scores in the colon were not statistically different between any of the groups (Figure 2A). Recipients of WT Tregs demonstrated less pathological damage in the lung as compared to recipients of CD62L−/− Tregs (p = 0.05) (Figure 2B). Examination of the liver demonstrated that both WT and CD62L−/− Tregs significantly inhibited GvHD pathology (p < 0.03) compared to recipients of effector T cells alone (Figure 2C). Interestingly, despite the modest difference in tissue pathology, there were significant differences in serum IFN-γ levels in mice given effector T cells alone compared to WT or CD62L−/− Tregs (p < 0.01) (Figure 2D). Overall, these results demonstrated that with the exception of worsened lung pathology, CD62L−/− Tregs functioned as well as WT Tregs to prevent GvHD, while both were potent in their ability to inhibit systemic IFN-γ production.
Figure 2. CD62L−/− and WT Tregs suppress liver pathology.
1.25 × 106 WT or CD62L−/− Tregs were transferred with 3 × 106 TCD BM cells into lethally irradiated B6D2 recipients on day 0. 4 × 106 whole splenic T cells from WT mice were then transferred on day +2 (n = 6 WT Tregs (■), n = 7 CD62L−/− Tregs, ( ), n = 8 Effectors alone (
), n = 8 Effectors alone ( ), n = 3 BM only (□). Animals were recovered when clinical scores reached a total of >4. Animals that did not reach a score of 4 were recovered on days 25–27 posttransplant. Histopathological assessment of the (A) colon, (B) lung, (C) liver. (D) Serum was recovered from animals at the time of histopathology assessment and analyzed by ELISA for levels of IFN-γ.
), n = 3 BM only (□). Animals were recovered when clinical scores reached a total of >4. Animals that did not reach a score of 4 were recovered on days 25–27 posttransplant. Histopathological assessment of the (A) colon, (B) lung, (C) liver. (D) Serum was recovered from animals at the time of histopathology assessment and analyzed by ELISA for levels of IFN-γ.
CD62L−/− Tregs traffic to secondary lymphoid tissues and GvHD target organs
Because we observed differences early on in the clinical manifestation of GvHD between recipients of WT and CD62L−/− Tregs, we were interested in determining the trafficking pattern of these Tregs. To evaluate in vivo Treg trafficking, we used a competitive lymphocyte migration assay (16). As illustrated in Figure 3A, 6 days after transfer, CD62L−/− Tregs were found at a similar frequency as WT Tregs in the liver, lung, spleen, bone marrow and mesenteric lymph node (MLN), although as expected, there were substantially fewer CD62L−/− Tregs in the PLNs of recipient animals. Further analysis 16 days post-Treg transfer showed no difference between WT and CD62L−/− Treg migration to liver, spleen, bone marrow or MLN (Figure 3B). However, although not statistically different, there were fewer CD62L−/− Tregs detected in the lung and PLN on day 16 compared to WT Tregs, which correlated with the enhanced GvHD in the lung of recipient animals receiving CD62L−/− Tregs (Figure 3B).
Figure 3. CD62L−/− and WT Treg trafficking.
5 × 105 WT-GFP (■) or CD62L−/−-GFP ( ) Tregs were transferred with 5 × 105 Thy1.1+ Tregs along with 3 × 106 TCD BM cells into lethally irradiated B6D2 recipients on day 0. 2 × 106 whole splenic T cells from WT mice were then transferred on day +2. On days 6 (A) and 16 (B) post-Treg transfer, lymphocytes were isolated from GvHD target organs and secondary lymphoid tissues as described in the Methods section. The ratio of eGFP+/FoxP3+:Thy1.1+/FoxP3+ cells are shown (n = 4 for each group). N = 4 animals/time point (MLNs were pooled for each group, and PLNs were pooled for each group). 1.0 × 106 WT-GFP (top) or CD62L−/−-GFP (bottom) Tregs were transferred along with 3 × 106 TCD BM cells into lethally irradiated B6D2 recipients on day 0. 2 × 106 whole splenic T cells from WT mice were then transferred on day +2. 16 days post-Treg transfer animals were anesthetized with avertin and organs were imaged with a Zeiss SteREO Lumar.V12 microscope with eGFP bandpass filter. Brightfield images (left), and GFP images (middle) were taken for each organ. GFP intensities (right) were determined by software analysis. (C) MLN (D) spleen (E) lung (F) peripheral lymph node. Original magnification: lung = 25X, spleen = 40X, MLN = 45X, ILN = 45X. Data represent mean score ± SEM for each organ.
) Tregs were transferred with 5 × 105 Thy1.1+ Tregs along with 3 × 106 TCD BM cells into lethally irradiated B6D2 recipients on day 0. 2 × 106 whole splenic T cells from WT mice were then transferred on day +2. On days 6 (A) and 16 (B) post-Treg transfer, lymphocytes were isolated from GvHD target organs and secondary lymphoid tissues as described in the Methods section. The ratio of eGFP+/FoxP3+:Thy1.1+/FoxP3+ cells are shown (n = 4 for each group). N = 4 animals/time point (MLNs were pooled for each group, and PLNs were pooled for each group). 1.0 × 106 WT-GFP (top) or CD62L−/−-GFP (bottom) Tregs were transferred along with 3 × 106 TCD BM cells into lethally irradiated B6D2 recipients on day 0. 2 × 106 whole splenic T cells from WT mice were then transferred on day +2. 16 days post-Treg transfer animals were anesthetized with avertin and organs were imaged with a Zeiss SteREO Lumar.V12 microscope with eGFP bandpass filter. Brightfield images (left), and GFP images (middle) were taken for each organ. GFP intensities (right) were determined by software analysis. (C) MLN (D) spleen (E) lung (F) peripheral lymph node. Original magnification: lung = 25X, spleen = 40X, MLN = 45X, ILN = 45X. Data represent mean score ± SEM for each organ.
To confirm our findings regarding the function of CD62L in the migration of Tregs in a lymphopenic environment, we performed in vivo imaging using fluorescence stereomicroscopy. In the MLN(Figure 3C) and spleen (Figure 3D)we found similar distribution and GFP signal intensity by WT and CD62L−/− Tregs, indicating that the migration and accumulation of CD62L−/− Tregs was indistinguishable from WT Tregs in these organs 16 days posttransplantation. Interestingly, we observed fewer GFP+ CD62L−/− Tregs in the lung (Figure 3E) and PLN (Figure 3F) at this time point. Taken together, these observations illustrated that CD62L−/− Tregs home to GvHD target organs, similar to WT Tregs, with the exception of a modest impairment in migration to the lung. Differences in the migration of WT compared to CD62L−/− Tregs to PLNs were found in the first week posttransplantation demonstrating the importance of CD62L in the initial migration of Tregs to PLNs. However, at day 16 these differences were minimized indicating that CD62L was not absolutely required for the eventual migration of Tregs to PLNs.
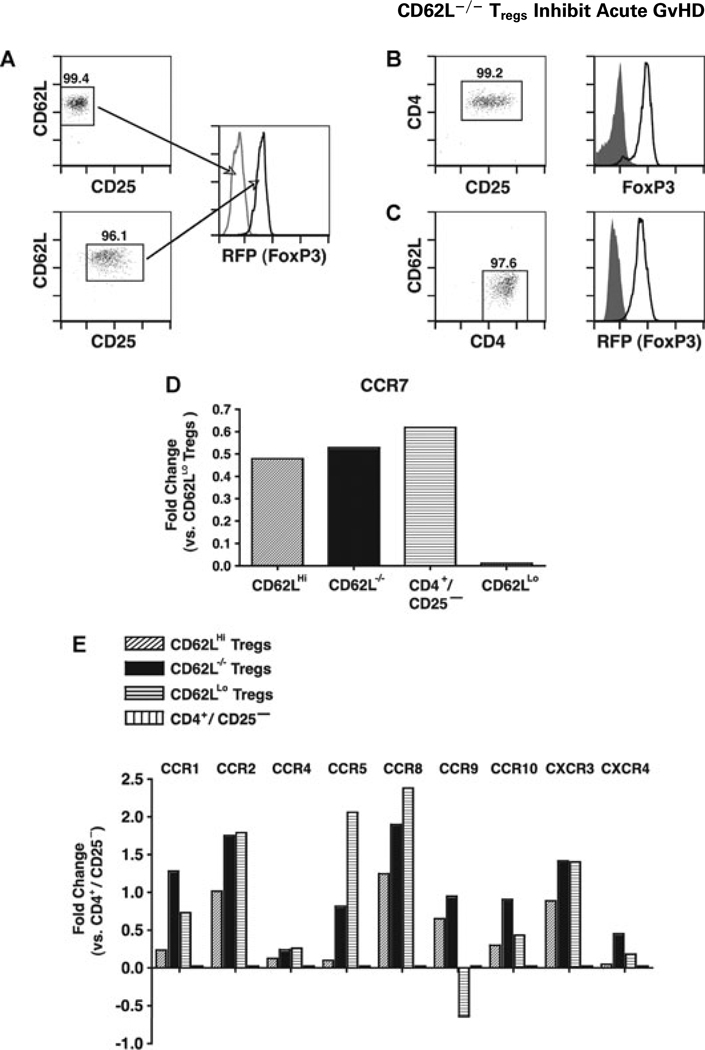
CD62LHi, CD62L−/− and CD62LLo Tregs display differential expression of chemokine receptors
The finding of CD62L−/− Tregs in the PLNs of irradiated recipients was somewhat surprising given the role that CD62L plays in T cell rolling and homing to lymph nodes. This observation suggests that in the absence of CD62L other proteins important for T cell migration may serve a similar function. To this end we examined the phenotypic profile of Tregs based on CD62L expression (Figures 4A–C). As shown in Figure 4D, CD62LHi and CD62L−/− Tregs have increased expression of CCR7 mRNA relative to CD62LLo Tregs. We then compared the three Treg types to naïve CD4+/mRFP− cells in their mRNA expression of other chemokine receptors. CD62L−/− Tregs resembled CD62LLo Tregs in the expression of CCR2, CCR4 and CXCR3, and resembled CD62LHi Tregs in the expression of CCR9. CD62L−/− Tregs had intermediate levels of CCR5 and CCR8, with distinctive expression of CCR1 and CCR10. Collectively, these data demonstrated that the migratory profile of CD62L−/− Tregs was that of an intermediate activated phenotype with higher expression of pro-inflammatory chemokine receptors compared to CD62LHi Tregs and much greater expression of CCR7 compared to CD62LLo Tregs.
Figure 4. Treg chemokine receptor expression based on CD62L expression.
Sort purification of cells for quantitative real-time PCR analysis. (A) CD4+/CD25+/mRFP+/CD62LHi Tregs (bottom) and CD4+/CD25−/mRFP−/CD62LHi naïve T cells (top) were sort purified from mRFP-FoxP3 mice. (B) CD4+/CD25+ Tregs were sort purified from CD62L−/− mice. (C) CD4+/mRFP+/CD62LLo Tregs were sort purified following in vitro expansion. RNA was extracted and real-time PCR performed as described in the Methods section. (D) CCR7 expression on mRFP+/CD62LHi Tregs ( ), CD4+/CD25+/CD62L−/− Tregs (■), naïve CD4+/CD25− T cells (
), CD4+/CD25+/CD62L−/− Tregs (■), naïve CD4+/CD25− T cells ( ) and mRFP+/CD62LLo Tregs (
) and mRFP+/CD62LLo Tregs ( ). Data are shown as relative change in expression (logarithmic scale) compared to CD4+/mRFP+/CD62LLo Tregs. (E) Chemokine receptor expression on mRFP+/CD62LHi Tregs (
). Data are shown as relative change in expression (logarithmic scale) compared to CD4+/mRFP+/CD62LLo Tregs. (E) Chemokine receptor expression on mRFP+/CD62LHi Tregs ( ), CD4+/CD25+/CD62L−/− Tregs (■), mRFP+/CD62LLo Tregs (
), CD4+/CD25+/CD62L−/− Tregs (■), mRFP+/CD62LLo Tregs ( ) and naïve CD4+/CD25− T cells (
) and naïve CD4+/CD25− T cells ( ). Data are shown as relative change in expression (logarithmic scale) compared to naïve CD4+/CD25− T cells. Data are representative of three independent experiments.
). Data are shown as relative change in expression (logarithmic scale) compared to naïve CD4+/CD25− T cells. Data are representative of three independent experiments.
Discussion
In the current work, we were interested in determining whether CD62L itself was critical for Treg function and migration into lymphoid tissue. We demonstrated that CD62L was not critical for Treg function to prevent GvHD lethality as CD62L−/− Tregs afforded substantial protection from lethal acute GvHD in the clinically relevant model employed. WT Tregs yielded reduced clinical scores compared to CD62L−/− Tregs during the first 3 weeks posttransplant, which correlated with delayed migration of CD62L−/− Tregs to PLNs. Histopathological analysis of GvHD target organs correlated with the clinical scores, as recipients of WT Tregs showed improved pathology in the lung and similar pathology in the colon and liver compared to CD62L−/− Tregs. Lastly, we demonstrated differential chemokine receptor expression of Tregs based on CD62L expression, where the CD62L−/− Tregs displayed a phenotype that appeared to be an intermediate between the naïve CD62LHi and activated CD62LLo.
Previous reports examining the role of CD62L in Treg-mediated inhibition of GvHD suggested either that (1) CD62L itself was critically important in the function of Tregs or that (2) the CD62LHi phenotype functioned differently than CD62LLo Tregs but that CD62L itself was not critical (5,9). Our data demonstrated that CD62L itself was not critically required for the prevention of GvHD lethality or for the ability to migrate into LN post transplantation. While there was no difference in either overall or median survival time, our data indicated that CD62L did serve as an accessory molecule given the statistical difference in clinical scores between WT and CD62L−/− Tregs during the first 3 weeks posttransplantation. It is of interest that we also observed no statistical difference in clinical scores between CD62L−/− and CD62LLo Tregs for the first 2 weeks posttransplantation suggesting CD62L serves early on to promote Treg inhibition of GvHD most likely by enhancing the migration of Tregs into lymphoid tissue.
One concern in our previous studies in which we expanded CD4+/CD25+ T cells to obtain a CD62LLo population was the difficulty in eliminating CD25+ effector cells from the Treg infusion (5). Here, we have circumvented this concern by using Tregs from FIR mice in which mRFP is under control of the FoxP3 promoter and thus cells expressing FoxP3 can be detected using flow cytometry (13). Our data confirm previous observations that CD62LLo Tregs were not sufficient to prevent GvHD in the overwhelming majority of transplanted recipients. The possibility of impaired suppressive function of these cells was ruled out by in vitro analysis in which CD62LLo Tregs were more proficient suppressors of T cell responses to allo-antigen, consistent with the previously published data (17). Therefore, the inability of CD62LLo Tregs to provide protection against GvHD could not be explained by impaired function but may be due to impaired homing to lymphoid tissue or diminished survival after infusion.
Examination of the pathology in individual organs revealed that WT and CD62L−/− Tregs ameliorated disease in the liver, whereas WT Treg recipients displayed reduced pathology in the lung as compared to CD62L−/− Treg recipients. The increased lung pathology correlated with modestly impaired CD62L−/− Treg migration to the lung. The accumulation of IFN-γ in the serum has been shown to be a predictor of GvHD mortality (18). We also documented a substantial reduction in the level of IFN-γ in the serum of animals receiving either WT or CD62L−/− Tregs an effect not seen in recipients of mRFP+/CD62LLo Tregs (data not shown). Here again, a functional distinction was made between CD62L−/− and CD62LLo Tregs.
While it is clear that Tregs do inhibit effector T cell expansion and suppress effector functions, it is less clear as to whether the inhibition is in lymphoid tissues or GvHD target organs. In the current report, we demonstrated that CD62L−/− Tregs migrate to GvHD target organs with similar efficiency as WT Tregs; however, their accumulation within the PLNs was delayed. It is interesting to note that this delay corresponded with increased clinical GvHD scores, thus supporting the hypothesis that entry into lymph nodes by Tregs was important in inhibiting the initial expansion of donor T cells. The inability of CD62LLo Tregs to inhibit GvHD has been attributed to ineffective trafficking to secondary lymphoid tissues (9). Normal trafficking seen in CD62L−/− Tregs provides another distinction between CD62LLo and CD62L−/− phenotypes.
Other studies have examined chemokine receptor expression on Treg subsets, including the CD62LHi and CD62LLo populations (19,20). Our data are in agreement that the CD62LHi fraction expressed high levels of the lymph node homing chemokine receptor CCR7. Of interest, the CD62L−/− population also expressed high levels of CCR7, providing a plausible mechanism for their migration to secondary lymphoid tissues. In keeping with an activated status the CD62LLo Tregs expressed high levels of CCR5 and CCR8 while the CD62L−/− Tregs displayed intermediate expression.
In summary, our data demonstrate that posttransplant, CD62L was dispensable for Treg inhibition of GvHD lethality.
Supplementary Material
Acknowledgments
This researchwas supported by grants from the National Institutes of Health R01 CA102052, R01 AI064363 to JSS; RO1 AI034495, R37 HL56067, P01 065299 to B.R.B. and T32 HL007149 to M.J.C. This work was also supported by the Mary Elizabeth Thomas Endowment fund (J.S.S).
Abbreviations
- Allo-HSCT
allogeneic hematopoietic stem cell transplantation
- APC
antigen presenting cell
- eGFP
enhanced green fluorescent protein
- GvHD
graft-versus-host disease
- GvL
graft-versus-leukemia
- PLN
peripheral lymph nodes
- MLN
mesenteric lymph node
- TCD BM
T cell depleted bone marrow
- Tregs
regulatory T cells
- WT
wild type.
Footnotes
Disclosure
The authors of this manuscript have no conflict of interest to disclose as described by the American Journal of Transplantation.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Figure S1: CD62LLo Tregs are potent suppressers in vitro
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- 1.Appelbaum FR. Haematopoietic cell transplantation as immunotherapy. Nature. 2001;411:385–389. doi: 10.1038/35077251. [DOI] [PubMed] [Google Scholar]
- 2.Armitage JO. Bone marrow transplantation. N Engl J Med. 1994;330:827–838. doi: 10.1056/NEJM199403243301206. [DOI] [PubMed] [Google Scholar]
- 3.Ferrara JL, Cooke KR, Pan L, Krenger W. The immunopathophysiology of acute graft-versus-host-disease. Stem Cells. 1996;14:473–489. doi: 10.1002/stem.140473. [DOI] [PubMed] [Google Scholar]
- 4.Kingsley CI, Karim M, Bushell AR, Wood KJ. CD25+CD4+ regulatory T cells prevent graft rejection: CTLA-4- and IL-10-dependent immunoregulation of alloresponses. J Immunol. 2002;168:1080–1086. doi: 10.4049/jimmunol.168.3.1080. [DOI] [PubMed] [Google Scholar]
- 5.Taylor PA, Panoskaltsis-Mortari A, Swedin JM, et al. L-Selectin(hi) but not the L-selectin(lo) CD4+25+ T-regulatory cells are potent inhibitors of GVHD and BMgraft rejection. Blood. 2004;104:3804–3812. doi: 10.1182/blood-2004-05-1850. [DOI] [PubMed] [Google Scholar]
- 6.Trenado A, Charlotte F, Fisson S, et al. Recipient-type specific CD4+CD25+ regulatory T cells favor immune reconstitution and control graft-versus-host disease while maintaining graft-versus-leukemia. J Clin Invest. 2003;112:1688–1696. doi: 10.1172/JCI17702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kansas GS. Selectins and their ligands: current concepts and controversies. Blood. 1996;88:3259–3287. [PubMed] [Google Scholar]
- 8.Boyman O, Letourneau S, Krieg C, Sprent J. Homeostatic proliferation and survival of naive and memory T cells. Eur J Immunol. 2009;39:2088–2094. doi: 10.1002/eji.200939444. [DOI] [PubMed] [Google Scholar]
- 9.Ermann J, Hoffmann P, Edinger M, et al. Only the CD62L+ subpopulation of CD4+CD25+ regulatory T cells protects from lethal acute GVHD. Blood. 2005;105:2220–2226. doi: 10.1182/blood-2004-05-2044. [DOI] [PubMed] [Google Scholar]
- 10.Wysocki CA, Jiang Q, Panoskaltsis-Mortari A, et al. Critical role for CCR5 in the function of donor CD4+CD25+ regulatory T cells during acute graft-versus-host disease. Blood. 2005;106:3300–3307. doi: 10.1182/blood-2005-04-1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arbones ML, Ord DC, Ley K, et al. Lymphocyte homing and leukocyte rolling and migration are impaired in L-selectin-deficient mice. Immunity. 1994;1:247–260. doi: 10.1016/1074-7613(94)90076-0. [DOI] [PubMed] [Google Scholar]
- 12.Serody JS, Burkett SE, Panoskaltsis-Mortari A, et al. T-lymphocyte production of macrophage inflammatory protein-1alpha is critical to the recruitment of CD8(+) T cells to the liver, lung, and spleen during graft-versus-host disease. Blood. 2000;96:2973–2980. [PubMed] [Google Scholar]
- 13.Wan YY, Flavell RA. Identifying Foxp3-expressing suppressor T cells with a bicistronic reporter. Proc Natl Acad Sci USA. 2005;102:5126–5131. doi: 10.1073/pnas.0501701102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coghill JM, Carlson MJ, Panoskaltsis-Mortari A, et al. Separation of graft-versus-host disease from graft-versus-leukemia responses by targeting CC-chemokine receptor 7 on donor T cells. Blood. 2010;115:4914–4922. doi: 10.1182/blood-2009-08-239848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Van Den Brink MR, Moore E, Horndasch KJ, et al. FAS-deficient LPR mice are more susceptible to graft-versus-host disease. J Immunol. 2000;164:469–480. doi: 10.4049/jimmunol.164.1.469. [DOI] [PubMed] [Google Scholar]
- 16.Sheng-Tanner X, McKerlie C, Spaner D. Characterization of graft-versus-host disease in SCID mice and prevention by physicochemical stressors. Transplantation. 2000;70:1683–1693. doi: 10.1097/00007890-200012270-00004. [DOI] [PubMed] [Google Scholar]
- 17.Chai JG, Coe D, Chen D, Simpson E, Dyson J, Scott D. In vitro expansion improves in vivo regulation by CD4+CD25+ regulatory T cells. J Immunol. 2008;180:858–869. doi: 10.4049/jimmunol.180.2.858. [DOI] [PubMed] [Google Scholar]
- 18.Krenger W, Hill GR, Ferrara JL. Cytokine cascades in acute graft-versus-host disease. Transplantation. 1997;64:553–558. doi: 10.1097/00007890-199708270-00001. [DOI] [PubMed] [Google Scholar]
- 19.Huehn J, Siegmund K, Lehmann JC, et al. Developmental stage, phenotype, and migration distinguish naive- and effector/memory-like CD4+ regulatory T cells. J Exp Med. 2004;199:303–313. doi: 10.1084/jem.20031562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Szanya V, Ermann J, Taylor C, Holness C, Fathman CG. The subpopulation of CD4+CD25+ splenocytes that delays adoptive transfer of diabetes expresses L-selectin and high levels of CCR7. J Immunol. 2002;169:2461–2465. doi: 10.4049/jimmunol.169.5.2461. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.