Abstract
Experimental as well as epidemiologic studies in human populations provide evidence that consumption of phytochemicals reduces the incidence of degenerative diseases. Green tea (GT) catechins are known for their antioxidative potential. Phytic acid (PA) also acts as a natural antioxidant and may have numerous health benefits. This experiment was designed to investigate the inhibitory effects of combinations of 1% and 2% GT, PA, and inositol (I) in reducing the incidence of azoxymethane-induced colon tumors in Fisher 344 male rats. After an acclimatization period of 1 week, nine groups of rats (15 rats per group) were initially assigned to consume AIN 93 G diet and later AIN 93 M diet after 20 weeks of age. Treatments were given in drinking water. All rats received azoxymethane injections (16 mg/kg of body weight) subcutaneously at 7 and 8 weeks of age. Rats were killed at 45 weeks of age by CO2 euthanasia. Tumor incidence (93.76%) and the number of tumors per tumor-bearing rat ratio (2.25) were significantly (P<.05) higher in the control group compared with treatment groups. Glutathione S-transferase activity was significantly (P<.05) higher in rats fed combinations of 2% GT+PA+I and GT+PA (33.25±1.23 and 29.83±1.10 μmol/mL, respectively) compared with other groups. These findings suggest that the synergistic effect of the 2% level of GT, PA, and I may reduce the incidence of colon tumors and therefore have potential as a chemopreventive agent.
Key Words: colon tumor, glutathione S-transferase, green tea, inositol, phytic acid
Introduction
Tea (Camellia sinensis) is the most popular beverage after water and is consumed by almost two-thirds of the world's population. There is increasing interest in the role of tea in maintaining good health and treating various chronic diseases. Consumption of tea has been associated with many health benefits, and tea's role and mechanism in cancer chemoprevention have been extensively reviewed. Green tea (GT) is now an acknowledged cancer preventative in Japan, and one of the key advantages of GT as a cancer preventative is its nontoxicity.1 Three types of tea available in the market are black tea, GT, and oolong tea. The type of tea depends on the process of drying and fermentation. GT is rich in polyphenolic compounds called catechins, which account for one-third of the dry weight of leaves.2 GT consists of four different types of catechins: (–)-epigallocatechin-3-gallate (EGCG), (–)-epigallocatechin, (–)-epicatechin-3-gallate, and (–)-epicatechin.2 EGCG has been considered to be a major constituent of GT, which may have an active anticancer property.3,4
GT consumption has been associated with anti-inflammatory,5 antihypertensive,6 antimutagenic,7 antioxidative,8,9 anticarcinogenic,3,10 antihypercholesterolemic,11 and antihyperglycemic12 effects. Among the various catechins, EGCG has the greatest antioxidant activity, and it is the most widely studied polyphenol for disease prevention.4,8 EGCG possesses cancer chemopreventive and therapeutic potentials against various types of cancer, due in part to its effects in vitro and in vivo on tumor cell signaling pathways regulating growth and apoptosis.13,14 Many health benefits of tea are presumed to be caused by its antioxidant effects. Antitumor activity of EGCG may be based on blocking of the epidermal growth factor receptor10 and platelet-derived growth factor.11 These receptors can inhibit the growth factor–induced activation of activator protein-1, a transcription factor involved in differentiation and proliferation of cancer cells.3 Ahmad et al.15 showed that polyphenols of GT modulate nuclear factor-κB in several cancer cells, making them more susceptible to apoptosis. Infusion of GT or EGCG in mice showed a reduced angiogenic response to vascular endothelial-derived growth factors, which regulate angiogenesis.16 Shenouda et al.17 reported that EGCG inhibits PC-3 cell proliferation via cell cycle arrest, resulting in increased apoptosis.
The inhibition of intestinal carcinogenesis by tea and tea polyphenols has been demonstrated in different animal models by several research groups.13,18 Ju et al.19 reported that administration of EGCG at doses of 0.08% or 0.16% in drinking fluid significantly decreased small intestinal tumor formation by 37% or 47%, respectively. GT administration (0.6% in drinking fluid) inhibited the formation of azoxymethane (AOM)-induced aberrant crypt foci (ACF) in CF-1 mice on a high-fat diet.20 Bettuzzi et al.21recently reported that development of prostate cancer in men with high-grade prostate intraepithelial neoplasia was significantly prevented by oral administration of GT catechins, 600 mg/day for 1 year. Yang et al.14 reported that regular intake of GT was shown to provide protection against the development of colorectal cancer in a large cohort of 69,710 Chinese women followed up for 2–3 years. Kakuta et al.22 demonstrated in a case-control study with habitual consumption of GT, which included 437 women, a marked reduction in the risk of developing endometrial endometriod adenocarcinoma. Many scientists have reported13,23,24 that EGCG, epicatechin gallate, and epigallocatechin are the main constituents of GT extract that have cancer preventive activities, including growth inhibition of human cancer cell lines in cultures, induction of apoptosis, inhibition of tumor promotion and carcinogenesis in animal experiments, antimutagenic and antioxidant activities, inhibition of tumor necrosis factor-α release from cells induced by a tumor promoter, and modulation of gene expression.
The major biochemical functions of phytic acid (PA) and lower inositol (I) phosphates in cell membranes are regulation of cellular responses to external stimuli as well as mediation of enzyme activity.25 PA also plays an important role in regulating vital cellular functions, such as signal transduction, cell proliferation, and cell differentiation.26 The anticarcinogenic effect of PA has been shown both in vivo and in vitro.27 Various mechanisms of action have been proposed for PA's antitumorigenic abilities (i.e., antioxidant properties, gene alteration, increased natural killer cell activity, and cell cycle inhibition). The exact mechanism by which PA exerts these effects has yet to be elucidated. PA affects the cell cycle by decreasing the S-phase of mitosis and arresting cells in the G0/G1 phase. Shamsuddin and Ullah28 reported that PA significantly lowered the mitosis rate in AOM-induced colon tumors in Fisher 344 rats. Saied and Shamsuddin29 reported that PA up-regulates expression of the tumor suppressor genes p53 and p21 waf1/Cip1. Nelson et al.30 reported the protective effects of PA on colon cancer. They reported that iron augmented the yield and incidence of 1,2-dimethylhydrazine-induced colonic tumors, which can be reversed by PA supplementation. They also proposed that dietary PA reduced colon cancer through chelation of iron and suppression of iron-related initiation and promotion of carcinogenesis. Reddy31 found oral administration of PA inhibited the colon carcinogenesis in rodents. In other experiments Pretlow et al.32 demonstrated that dietary PA decreased the incidence of ACF as a marker for preneoplastic lesions in rats.
Epidemiological findings indicate that incidence of colon cancer is higher in western countries. Various reports have shown a negative correlation between incidence of colon cancer and the intake of phytate-rich fiber foods.33–35 Jariwalla et al.36 reported that the most frequently studied anticarcinogenic property of PA is its effect on decreasing the iron-mediated colon cancer risk in an animal model; they proposed that PA may exert its antineoplastic effect by regulating cellular proliferation, even after carcinogenic stimulation. PA thus may be an important source for chemoprevention.37 Norazalina et al.38 reported that administration of PA extracted from rice bran and commercial PA at 0.2% (wt/vol) and 0.5% (wt/vol) levels significantly reduced the total number of ACF (P<.05) compared with AOM alone. Administration of PA extracted from rice bran at 0.2% (wt/vol) gave the greatest reduction (52%) of total number of ACF per colon. A reduction of 38% was seen in treatment with 0.5% (wt/vol) commercial PA, 35% in treatment with 0.2% (wt/vol) commercial PA, and 32% in treatment with 0.5% (wt/vol) extract PA. Challa et al.39 reported that feeding 1% and 2% levels of PA significantly reduced the number of ACF induced by AOM in Fisher 344 rats. They also reported that combinations of 2% PA and GT significantly reduced the incidence of total ACF in rat colon, compared with reductions with PA and GT when given singly at the 2% level. Thus increased efficacy was attributed to the synergistic effect of 2% PA and GT.
The glutathione S-transferases (GSTs) are a family of cytosolic enzymes involved in the detoxification of a range of xenobiotic compounds by conjugation to glutathione. GSTs are an important part of the cellular detoxification system and, perhaps, evolved to protect cells against reactive oxygen metabolites. These enzymes are found in all eukaryotic and prokaryotic systems, in the cytoplasm, in the microsomes, and in mitochondria.40,41 GSTs detoxify diverse electrophiles and carcinogens, mainly by conjugating them with glutathione. Carcinogens like heterocyclic amines have been implicated as a potential cause of colorectal cancer in humans. GST enzymes are also responsible for detoxifying the heterocyclic amines. In addition, foods that are known to induce the expression of GSTs are also thought to be protective against colorectal cancer. If we can confirm that GSTs are protective against colorectal cancer, it might be possible to identify individuals at high risk of this disease or to manipulate the expression of GSTs to prevent colorectal cancer by dietary or pharmacological means. Panza et al.42 reported that an open-labeled controlled study including 14 healthy men showed that the consumption of GT (6 g in 600 mL of water daily for 7 days) increased plasma glutathione, increased ferric reducing ability of plasma, and ameliorated the post-exercise increase in lipid hydroperoxidase.
Very few long-term rodent model studies have demonstrated the possible synergistic effects of dietary ingredients in suppressing the process of carcinogenesis. Thus, the objective of this study was to determine the combinational effect of oral administration of the 1% and 2% level of GT, PA, and I on AOM-induced colon tumors in Fisher 344 male rats.
Materials and Methods
Animals
Fisher 344 weanling rats (3 weeks age) were obtained from Harlan (Indianapolis, IN, USA) and housed in stainless steel wire cages with two rats per cage. The temperature and relative humidity were maintained at 21±1°C and 50%, respectively. Light and dark cycles were at 12 hours each. Feed and water were provided ad libitum. After 1 week of acclimatization, the animals were randomly divided into nine groups. GT, PA, and I were given in the drinking water throughout the experimental period (41 weeks). There were eight treatment groups with one control (15 rats per group). The rats in the control group were fed AIN 93 G (control) diet and water. The treatments consisted of combinations of 1% and 2% GT, PA, and I: GT+PA, GT+I, PA+I, and GT+PA+I. During the experimental period, weekly body weights and feed consumptions were recorded. Experimental protocols were approved by the Institutional Animal Care and Use Committee of Alabama A & M University, Normal, AL, USA.
Materials
GT was purchased from Frontier Herbs, Norway, IA, USA. GT at 1% and 2% concentrations was prepared daily in deionized water. The leaves were boiled in deionized water for 3 minutes and then decanted through a cheesecloth. The filtrate was placed in a cool place until it reached room temperature.
PA and I were purchased from Sigma Chemical Co. (St. Louis, MO, USA). PA and I at 1% and 2% concentrations were prepared fresh daily in deionized water. The solutions were heated to allow the PA and I to dissolve completely and cooled to room temperature, and the pH was adjusted to 7.0. A mixture of 1% and 2% GT, PA, and I was prepared daily by mixing the above prepared GT, PA, and I solutions. Dietary ingredients were obtained from ICN (Costa Mesa, CA, USA). Initially, experimental animals were given AIN 93 G diet, and after 20 weeks of age, diet was switched to AIN 93 M for the remaining period (until 45 weeks of age). The diets were mixed weekly and stored at 4°C until fed.
Carcinogen injection
AOM was purchased from Aldrich Chemicals (Milwaukee, WI, USA). All rats received a subcutaneous injection of AOM in saline at 16 mg/kg of body weight at 7 and 8 weeks of age. Another five rats received a saline injection and were fed control diet (negative control).
Sample collection
At 45 weeks of age, all rats were killed by CO2 euthanasia. The colons were removed and flushed with potassium phosphate buffer (0.1 M, pH 7.2). Liver samples were immediately frozen using liquid nitrogen and stored at −80°C until analyzed. The number and size of tumors present in distal and proximal part of colon were recorded.
Cecal weights and cecal pH
The cecums of all rats were weighed after removal of cecal contents and opened longitudinally. The cecal pH of the contents was recorded.
GST assay
GST activity in the liver was assayed by the procedure of Habig et al.43 Liver samples were homogenized in 10 volumes of potassium phosphate buffer (0.1 M, pH 7.0) using a Potter–Elvejem homogenizer (10 strokes) at 4°C. The homogenate was centrifuged at 10,000 g for 30 minutes. The clear supernatant was used for the assay. The assay mixture (1 mL) contained potassium phosphate buffer (0.1 M, pH 6.5), 1-chloro-2,4-dinitrobenzene (1 mM), and glutathione (1 mM). The reaction was started by addition of 100 μL of sample, and the change in absorbance at 340 nm as a function of time was monitored in a UV/VIS dual beam spectrophotometer (Cary 1/3; Varian) at 340 nm. The total enzyme activity was measured at the end of 5 minutes of reaction.
Statistical analysis
Percentage tumor incidence and number of tumors per tumor-bearing rat ratio of various treatment groups were calculated and compared with those of controls. Data were analyzed using analysis of variance, and values are expressed as mean±SEM. Means were separated by Tukey's Studentized range test with the 2009 SAS statistical package (SAS Institute, Cary, NC, USA), and differences were considered significant at P<.05.
Results
Feed intake and body weight
Oral administration of 1% and 2% combinations of GT, PA, and I in drinking water altered the feed intake and weight gain in Fisher 344 male rats. Simultaneously, it also changed the status of cecal pH and cecal weight in experimental animals. Table 1 shows the effect of various combinations of GT, PA, and I on different parameters. There were no significant (P<.05) differences in feed intake among the control and treatment groups except in the group fed the 1% combination of GT+PA+I. Rats consuming the 1% combination of GT+PA+I group showed significantly (P<.05) higher feed consumption (15.97±0.28 g/day) than the control and other treatment groups (Table 1). Oral administration of various combinations of GT, PA, and I at 1% and 2% had no significant effect on weight gain among treatment groups, but there was a significant difference (P<.05) between the control and other treatment groups. Rats in the control group had significantly (P<.05) lower weight gain (293.29±13.45 g) compared with treatment groups (ranging from 301.85±14.91 g to 332.00±5.49 g) (Table 1). Cecal weight and cecal pH were comparable among treatment and control groups (Table 1).
Table 1.
Effect of Oral Administration of 1% and 2% Green Tea, Phytic Acid, and Inositol in Combination on Feed Consumption, Body Weight Gain, and Cecal Weight and pH in Fisher 344 Male Rats
| Level, group | Feed intake (g/day) | Weight gain (g/41 weeks) | Cecal weight (g) | Cecal pH |
|---|---|---|---|---|
| Control | 13.58±0.79a | 293.29±13.45a | 0.795±0.04 | 7.60±0.07 |
| 2% | ||||
| GT+PA | 14.46±0.83a | 311.13±14.07b | 0.620±0.04 | 7.48±0.03 |
| GT+I | 14.43±0.88a | 309.24±14.14b | 0.628±0.05 | 7.48±0.03 |
| PA+I | 14.65±0.25a | 301.85±14.91b | 0.603±0.03 | 7.48±0.02 |
| GT+PA+I | 15.03±0.71a | 302.43±10.54b | 0.653±0.01 | 7.38±0.09 |
| 1% | ||||
| GT+PA | 15.49±0.50a | 311.60±7.75b | 0.645±0.03 | 7.50±0.05 |
| GT+I | 15.26±0.46a | 321.22±7.13b | 0.562±0.04 | 7.51±0.05 |
| PA+I | 15.41±0.25a | 328.89±15.0b | 0.595±0.01 | 7.52±0.05 |
| GT+PA+I | 15.97±0.28b | 332.53±5.49b | 0.595±0.01 | 7.51±0.09 |
Data are mean±SEM values.
Means in a column not sharing the same letter differ, P<.05.
GT, green tea; I, inositol; PA, phytic acid.
Tumor incidence and tumor-bearing rat ratio
Modulation of different parameters by oral administration of 1% and 2% combinations of GT, PA, and I in Fisher 344 male rats is given in Table 2. Analysis of the main effect showed tumor incidence was significantly higher (P<.05) in the control group and the 1% combinations of GT, PA, and I compared with the 2% combinations of GT, PA, and I. The percentage colon tumor incidence was significantly higher (P<.05) in the control group (94.12%) compared with the treatment groups (Table 2). The incidence of colon tumors was significantly (P<.05) higher in the distal compared with the proximal section of the colon in the control and treatment groups (Table 2). The total number of distal tumors was significantly (P<.05) higher (33) in the control group compared with the 1% and 2% combinations of GT, PA, and I (Table 2).
Table 2.
Oral Administration of 1% and 2% Green Tea, Phytic Acid, and Inositol in Combination Reduced the Tumor Incidences, Total Number of Colon Tumors, and Average Tumor Size in Fisher 344 Male Rats
| |
|
|
% incidence |
Total number of colon tumors |
|
||
|---|---|---|---|---|---|---|---|
| Level, group | N1/N2 | % colon tumor incidence | Distal | Proximal | Distal | Proximal | Average tumor size (mm) |
| Control | 16/17 | 94a | 92 | 8 | 33 | 3 | 15.95±0.98a |
| 2% | |||||||
| GT+PA | 5/14 | 36c | 100 | 5 | 2.40±0.19bc | ||
| GT+I | 5/11 | 45b | 83 | 17 | 5 | 1 | 2.60±0.21bc |
| PA+I | 5/13 | 38c | 100 | 6 | 2.80±0.46bc | ||
| GT+PA+I | 3/13 | 23c | 100 | 3 | 1.30±0.06c | ||
| 1% | |||||||
| GT+PA | 6/14 | 43b | 100 | 10 | 6.30±0.86b | ||
| GT+I | 7/14 | 50b | 100 | 13 | 6.20±0.85b | ||
| PA+I | 5/11 | 46b | 100 | 8 | 10.00±0.88a | ||
| GT+PA+I | 5/11 | 46b | 100 | 7 | 4.10±0.62b | ||
Data are mean±SEM values (n=11–17).
Means in a column not sharing the same letter differ, P<.05.
N1, number of rats having tumors; N2, number of rats at the end of the experiment.
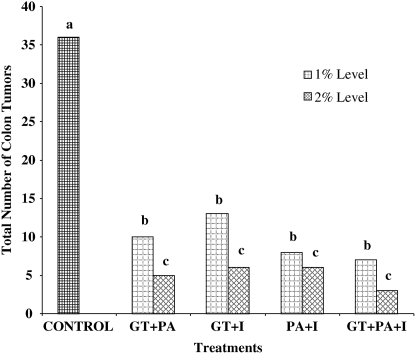
The total number of colon tumors was significantly (P<.05) lower in rats fed combinations of 2% GT, PA, and I compared with the 1% level of treatment and the control group (Fig. 1). The total number of colon tumors in the control group was 36, which was significantly (P<.05) higher than in the treatment groups (Fig. 1). Combinations of 2% GT, PA, and I significantly (P<.05) reduced the total number of colon tumors compared with the 1% level of the same treatment groups (Fig. 1). The number of colon tumors was significantly (P<.05) lower at the 2% level of GT+PA+I compared with the 1% level of the same combination (Fig. 1).
FIG. 1.
Oral administration of 1% and 2% GT, PA, and I in combination significantly (P<.05) reduced the total number of colon tumors induced by azoxymethane in Fisher 344 male rats. abcColumns not sharing the same letter differ, P<.05.
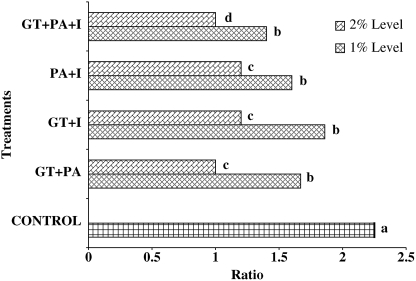
Oral administration of various combinations of 2% GT, PA, and I significantly reduced the average tumor size (in mm) in Fisher 344 male rats (Table 2). The average tumor size of control group was 15.95±0.98 mm, which was significantly (P<.05) higher than various combinations of 1% and 2% GT, PA, and I. Rats fed the combination of 2% GT+PA+I significantly (P<.05) reduced the average colon tumor size (1.30±0.06 mm) compared with the control and other treatment groups at 1% GT, PA, and I (Table 2). Figure 2 shows that oral administration of combinations of 2% GT+PA+I significantly reduced (P<.05) the tumor-bearing rat ratio (1.0) compared with the control and other treatment groups at the 1% level.
FIG. 2.
Combination of 1 and 2% GT, PA, and I reduced the tumors/tumor-bearing rat ratios in azoxymethane-induced colon tumors in Fisher 344 male rats. abcdColumns not sharing the same letter differ, P<.05.
GST activity
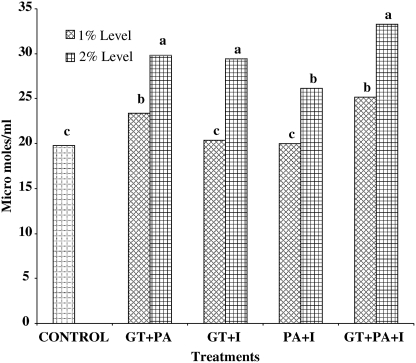
Oral administration of various combinations of GT, PA, and I modified the total GST activity (Fig. 3). Rats fed combinations of 2% GT+PA+I had significantly (P<.05) higher total GST activity in the liver compared with the control and 1% level of the same combinations. Rats in the control group had total GST activity of 19.75±6.81 μmol/mL, which was significantly (P<.05) lower than the combinations of 2% GT+PA+I, but not significantly different from various combinations of the 1% level of GT, PA, and I. Rats fed combinations of the 2% level of GT+PA, GT+I, PA+I, and GT+PA+I (29.83±1.10, 29.47±0.69, 26.13±0.76, and 33.25±1.23 μmol/mL, respectively) had significantly (P<.05) higher total GST activity compared with the control and combinations of the 1% level of GT, PA, and I groups.
FIG. 3.
Combinations of 1 and 2% GT, PA, and I increased the total glutathione S-transferase enzyme activity in the liver of Fisher 344 male rats. abcColumns not sharing the same letter differ, P<.05.
Discussion
Feed intake and body weight
Oral administration of 1% and 2% combinations of GT, PA, and I had no significant (P<.05) effects on feed consumption in Fisher 344 male rats, except in the rats fed 1% combination of GT+PA+I. Rats fed 1% combinations of GT+PA+I had significantly (P<.05) higher feed consumption, than the control and other treatment groups. Various reports showed that administration of GT or PA singly at the 2% level significantly reduced the feed consumption in experimental animals.39,40 Consumption of GT or PA for extended periods of time interferes with the absorption of protein, minerals, and vitamins; thus they may be considered as having antinutritive effects. The outcomes of this experiment clearly demonstrated that feeding 1% combinations of GT+PA+I significantly (P<.05) increased the feed consumption compared with the 2% level of the same treatment group in Fisher 344 male rats.
Oral administration of various combinations of 1% and 2% GT, PA, and I had no significant effect on weight gain among treatment groups, although numerically there was a difference in weight gain among the 1% and 2% level of treatment groups (ranging from 301.85±14.91 g to 332.53±5.49 g for 41 weeks of the experimental period). Challa et al.39 reported that feeding 2% GT and PA significantly (P<.05) reduced the weight of the experimental animals. The outcome of this experiment showed that rats fed combinations of 1% GT, PA, and I had higher weight gain (ranging from 311.60±7.75 g to 332.53±5.49 g) compared with the same combinations at the 2% level. These results also support the previous findings of Challa et al.39 Rats in the control group had significantly (P<.05) lower weight gain, even though their feed consumption was similar to those of the other treatment groups. This may be due to the higher tumor burden and the utilization of nutrients for nourishment of colon tumors rather than the physical development of rats. Uncontrolled growth and differentiation of colon tumors require more nutrients, which utilize most of the absorbed nutrients. In our opinion, it may be because the lower concentration (1%) was not sufficient to have an antinutritive effect compared with the 2% combinations of GT, PA, and I, or when the compounds interact together at lower concentration, their antinutritive effects may be lowered.
Tumor incidence and tumor-bearing rat ratio
The outcome of this experiments showed that oral administration of 2% GT+PA+I had the greatest effect in reducing the incidence of distal tumors in Fisher 344 male rats. These results are consistent with the findings that the distal colon shows a greater incidence of colon tumors than proximal in humans.45 Feeding the combination of 2% GT+PA+I resulted in significantly (P<.05) lower incidence of colon tumors compared with the same combination at the 1% level. These results clearly showed that the higher (2%) concentration of GT+PA+I was effective in reducing the total number of colon tumors in Fisher 344 male rats. Shamsuddin et al.25 reported that addition of 1% and 2% PA in drinking water suppresses colon tumors in Fisher 344 male rats and CD-1 mice either before or after carcinogen injection. Verghese et al.44 reported that oral supplementation of 2% GT and PA significantly (P<.05) reduced the ACF incidence in Fisher 344 male rats. Challa et al.39 reported that feeding 2% GT and PA in combination significantly reduced the incidence of ACF in Fisher 344 male rats. Pretlow et al.32 reported that feeding 2% GT and PA reduced the number of ACF in rat colon. Hirose et al.46 reported that PA did not significantly inhibit the incidence of colon tumors, when given in feed. The anticarcinogenic effect of PA was greater when administered in drinking water than when mixed in the feed.39 This may be because PA could form insoluble complexes with protein and other macromolecules and thus render them less available.
The average colon tumor size (in mm) was significantly (P<.05) higher in the control group of rats compared with the treatment groups. In long-term studies, the average tumor size is a reliable indicator and provides information about progression of colon tumors to colon carcinoma. The larger the tumor size, the greater the possibility of it developing into colon cancer. The results of this study clearly showed that combination of 2% GT+PA+I significantly (P<.05) reduced the average colon tumor size. It may be due to anti-angiogenic properties of GT16 or may be due to regulatory effect of PA on cell proliferation and differentiation.24 Results of this experiment showed that the number of tumors per tumor-bearing rat ratio was significantly (P<.05) higher in the control group compared with treatment groups. Oral administration of a combination of 2% GT+PA+I significantly (P<.05) reduced the tumor-bearing rat ratio, which is an effective indicator of chemopreventive effects of dietary components as it takes into account the number of tumors in rats that developed tumors.
GST activity
The hepatic bioactivation and detoxification system, which consists of phase I and phase II enzymes, plays a vital role in carcinogenesis.47 Phase II enzymes such as GST catalyze the conjugation of water-soluble molecules to xenobiotics, which facilitate their excretion.48 In vivo and in vitro studies have shown that various dietary compounds or their metabolites can induce the GST detoxification system (i.e., limonoids and flavonoids present in citrus fruits,49 glucosinolate metabolites and dithiolthiones present in Brassica vegetables,50 and GT and PA39). GST enzyme activity and GST protein level vary considerably among individuals.51 This may be related to the different susceptibility to colorectal cancer. The GST activity varies in individuals mainly because of differential exposure to bioactive compounds apart from inherited polymorphisms in GSTs.16 The outcome of this experiment clearly indicates that the 2% combination of GT+PA+I significantly increased the total GST activity compared with the control and the 1% level of various combinations. Challa et al.39 reported that feeding 2% PA significantly increased GST activity in the liver compared with the control group and animals receiving 1% PA. They also reported similar observations in animals receiving 2% GT. Results of this experiment also showed that there were no significant (P<.05) differences in GST activity among groups fed combinations of 1% GT, PA, and I and the control group, which also support the previous finding of Challa et al.39 They reported that there was no significant difference in enzyme activity between animals in the control group and those receiving 1% PA. Thus, the combination of 2% GT+PA+I was effective in increasing the total GST activity, showing the ability of GT, PA, and I to induce detoxification.
Several findings support the proposal that PA inhibits K-562 human erythroleukemia and HT-29 colon adenocarcinoma cell growth and differentiation.35,52 It was suggested that PA may exert its antineoplastic effect by regulating cell proliferation. This property of PA may be utilized for chemoprevention. In addition to being a chemopreventive agent, PA may have potential therapeutic use in cancer due to its property of enhancing the activity of natural killer cells associated with suppressed tumor incidence.37,52–54 Shamsuddin et al.53 reported that a lower level of PA (I triphosphate) is important in various cell functions (i.e., it acts as a second messenger for intracellular Ca2+ release and the resulting cell division). Wang et al.55 and Shamsuddin and Yang52 reported that PA plays an important role in quenching free radicals, signal transduction, and cell proliferation in reducing colon carcinogenesis and that GT helps to prevent 8-hydroxy-d-guanosine adduct formation.
GT and PA, the natural anticancer agents, have been found to be effective against various malignancies.35,39,56,57 There have been no studies conducted on long-term use of combinations of GT and PA in reducing the incidence of colon tumors. Plenty of epidemiological data support the proposal that consumption of GT and PA reduces the incidence of different cancer.39,58 In this experiment, oral administration of combinations of GT and PA at the 2% level reduced the tumor incidence and the number of tumors per tumor-bearing rat ratio and increased total GST activity and thus has the potential to inhibit colon carcinogenesis.
Acknowledgment
The authors gratefully acknowledge the expert editorial assistance of Cordella S. Rashid.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Kuzuhara T. Suganuma M. Fujiki H. Green tea catechin as a chemical chaperone in cancer prevention. Cancer Lett. 2008;261:12–20. doi: 10.1016/j.canlet.2007.10.037. [DOI] [PubMed] [Google Scholar]
- 2.Balentine DA. Wiseman SA. Bouwens LCM. The chemistry of tea flavonoids. Crit Rev Food Sci Nutr. 1997;37:693–704. doi: 10.1080/10408399709527797. [DOI] [PubMed] [Google Scholar]
- 3.Janakun J. Selman SH. Swiercz R. Why drinking green tea could prevent cancer. Nature. 1997;387:561. doi: 10.1038/42381. [DOI] [PubMed] [Google Scholar]
- 4.Nagle DG. Ferreira D. Zhou YD. Epigallocatechin-3-gallate (EGCG): chemical and biomedical perspectives. Phytochemistry. 2006;67:1849–1855. doi: 10.1016/j.phytochem.2006.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benelli R. Venè R. Bisacchi D. Garbisa S. Albini A. Anti-invasive effects of green tea polyphenol epigallocatechin-3-gallate (EGCG), a natural inhibitor of metallo and serine proteases. Biol Chem. 2002;383:101–105. doi: 10.1515/BC.2002.010. [DOI] [PubMed] [Google Scholar]
- 6.Hara Y. Tonooka F. Hypotensive effect of tea catechins on blood pressure of rats [in Japanese] Nippon Eiyo Shokuryo Gakkaishi. 1990;43:345–348. [Google Scholar]
- 7.Kada T. Kaneo K. Matsuzaki S. Matsuzaki T. Hara Y. Detection and chemical identification of natural bioantimutagens. Mutat Res. 1985;150:127–132. doi: 10.1016/0027-5107(85)90109-5. [DOI] [PubMed] [Google Scholar]
- 8.Matsuzaki T. Hara Y. Antioxidative activity of tea leaf catechins. J Agric Chem Soc Jpn. 1985;59:129–134. [Google Scholar]
- 9.Wang YC. Bachrach U. The specific anti-cancer activity of green tea (–)-epigallocatechin-3-gallate (EGCG) Amino Acids. 2002;22:131–143. doi: 10.1007/s007260200002. [DOI] [PubMed] [Google Scholar]
- 10.Hsu S. Green tea and the skin. J Am Acad Dermatol. 2005;52:1049–1059. doi: 10.1016/j.jaad.2004.12.044. [DOI] [PubMed] [Google Scholar]
- 11.Muramatsu K. Fukuyo M. Hara Y. Effect of green tea catechins on plasma cholesterol level in cholesterol-fed rats. J Nutr Sci Vitaminol. 1986;32:613–622. doi: 10.3177/jnsv.32.613. [DOI] [PubMed] [Google Scholar]
- 12.Shimizu M. Wada S. Hayashi T. Arisawa M. Ikegaya K. Ogaku S. Yano S. Morita N. Studies of hypoglycemic constituents of Japanese tea. Yakugaku Zasshi. 1988;108:964–970. [PubMed] [Google Scholar]
- 13.Yang CS. Lambert JD. Hou Z. Ju J. Lu G. Hao X. Molecular targets for the cancer preventive activity of tea polyphenols. Mol Carcinogen. 2006;45:431–435. doi: 10.1002/mc.20228. [DOI] [PubMed] [Google Scholar]
- 14.Yang CS. Lambert JD. Ju J. Lu G. Sang S. Tea and cancer prevention: molecular mechanisms and human relevance. Toxicol Appl Pharmacol. 2007;224:265–273. doi: 10.1016/j.taap.2006.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ahmad N. Gupta S. Mukhtar H. Green tea polyphenol epigallocatechin-3-gallate differentially modulates nuclear factor-κB in cancer cells versus normal cells. Arch Biochem Biophys. 2000;376:338–346. doi: 10.1006/abbi.2000.1742. [DOI] [PubMed] [Google Scholar]
- 16.Cao Y. Cao R. Angiogenesis inhibited by drinking tea. Nature. 1999;398:381. doi: 10.1038/18793. [DOI] [PubMed] [Google Scholar]
- 17.Shenouda NS. Zhou C. Browning JD. Ansell PJ. Sakla MS. Lubahn DB. Macdonald RS. Phytoestrogens in common herbs regulate prostate cancer cell growth in vitro. Nutr Cancer. 2004;49:200–208. doi: 10.1207/s15327914nc4902_12. [DOI] [PubMed] [Google Scholar]
- 18.Landau JM. Lambert JD. Lee MJ. Yang CS. Cancer Prevention by Tea and Tea Constituents. CRC Taylor and Francis; New York: 2005. [Google Scholar]
- 19.Ju J. Hong J. Zhou JN. Pan Z. Bose M. Liao J. Yang GY. Liu YY. Hou Z. Lin Y. Ma J. Shih WJ. Carothers AM. Yang CS. Inhibition of intestinal tumorigenesis in ApcMin/+ mice by (−) epigallocatechin-3-gallate, the major catechin in green tea. Cancer Res. 2005;65:10623–10631. doi: 10.1158/0008-5472.CAN-05-1949. [DOI] [PubMed] [Google Scholar]
- 20.Ju J. Liu Y. Hong J. Huang MT. Conney AH. Yang CS. Effects of green tea and high-fat diet on arachidonic acid metabolism and aberrant crypt foci formation in an azoxymethane-induced colon carcinogenesis mouse model. Nutr Cancer. 2003;46:172–178. doi: 10.1207/S15327914NC4602_10. [DOI] [PubMed] [Google Scholar]
- 21.Bettuzzi S. Brausi M. Rizzi F. Castagnetti G. Peracchia G. Corti A. Chemoprevention of human prostate cancer by oral administration of green tea catechins in volunteers with high-grade prostate intraepithelial neoplasia: a preliminary report from a one-year proof-of-principle study. Cancer Res. 2006;66:1234–1240. doi: 10.1158/0008-5472.CAN-05-1145. [DOI] [PubMed] [Google Scholar]
- 22.Kakuta Y. Nakaya N. Nagase S. Fujita M. Koizumi T. Okamura C. Niikura H. Ohmori K. Kuriyama S. Tase T. Ito K. Minami Y. Yaegashi N. Tsuji I. Case-control study of green tea consumption and the risk of endometrial endometroid adenocarcinoma. Cancer Causes Control. 2009;20:617–624. doi: 10.1007/s10552-008-9272-0. [DOI] [PubMed] [Google Scholar]
- 23.Clinical development plan: tea extracts. Green tea polyphenols. Epigallocatechin gallate. J Cell Biochem Suppl. 1996;26:236–257. [PubMed] [Google Scholar]
- 24.Fujiki H. Green tea: health benefits as cancer preventive for humans. Chem Rec. 2005;5:119–132. doi: 10.1002/tcr.20039. [DOI] [PubMed] [Google Scholar]
- 25.Shamsuddin AM. Vucenik I. Cole KE. IP6: a novel anti-cancer agent. Life Sci. 1997;614:343–354. doi: 10.1016/s0024-3205(97)00092-1. [DOI] [PubMed] [Google Scholar]
- 26.Huang C. Ma WY. Hecht SS. Dong Z. Inositol hexaphosphate inhibits cell transformation and activator protein 1 activation by targeting phosphatidylinositol-3' kinase. Cancer Res. 1997;57:2873–2878. Erratum in: Cancer Res 1997;57:5198. [PubMed] [Google Scholar]
- 27.Shamsuddin AM. Elsayed AM. Ullah A. Suppression of large intestinal cancer in F344 rats by inositol hexaphosphate. Carcinogenesis. 1988;94:577–580. doi: 10.1093/carcin/9.4.577. [DOI] [PubMed] [Google Scholar]
- 28.Shamsuddin AM. Ullah A. Inositol hexaphosphate inhibits large intestinal cancer in F344 rats 5 months after induction by azoxymethane. Carcinogenesis. 1989;10:625–626. doi: 10.1093/carcin/10.3.625. [DOI] [PubMed] [Google Scholar]
- 29.Saied IT. Shamsuddin AM. Up-regulation of the tumor suppressor gene p53 and WAF1 gene expression by IP6 in HT-29 human colon carcinoma cell line. Anticancer Res. 1998;18:1479–1484. [PubMed] [Google Scholar]
- 30.Nelson RL. Yoo SJ. Tanure JC. Andrianopoulos GM. The effect of iron on experimental colorectal carcinogenesis. Anticancer Res. 1989;9:1477–1482. [PubMed] [Google Scholar]
- 31.Reddy BS. Prevention of colon carcinogenesis by components of dietary fiber. Anticancer Res. 1999;19:3681–3683. [PubMed] [Google Scholar]
- 32.Pretlow TP. O'Riordan MA. Pretlow TG. Aberrant crypts correlated with tumor incidence in fisher 344 male rats treated with azoxymethane and phytate. Carcinogenesis. 1992;13:1509–1512. doi: 10.1093/carcin/13.9.1509. [DOI] [PubMed] [Google Scholar]
- 33.Graf E. Eaton JW. Dietary suppression of colonic cancer: fiber or phytate? Cancer. 1985;56:717. doi: 10.1002/1097-0142(19850815)56:4<717::aid-cncr2820560402>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 34.Steinmetz KA. Kushi LH. Bostick RM. Folsom AR. Potter JD. Vegetables, fruit and colon cancer in the Iowa Women's Health Study. Am J Epidemiol. 1994;139:1–15. doi: 10.1093/oxfordjournals.aje.a116921. [DOI] [PubMed] [Google Scholar]
- 35.Greenward P. Lanza E. Eddy GA. Dietary fiber in the reduction of colon cancer risk. J Am Diet Assoc. 1987;87:1178–1188. [PubMed] [Google Scholar]
- 36.Jariwalla RJ. Sabin R. Lawson S. Bloch DA. Prender M. Herman ZS. Effects of dietary phytic acid on the incidence and growth rate of tumors promoted in Fisher rats by a magnesium supplement. Nutr Res. 1977;8:813. [Google Scholar]
- 37.Baten A. Ullah VJ. Tomazic T. Shamsuddin AM. Inositol phosphate induced enhancement of natural killer cell activity correlates with tumor suppression. Carcinogenesis. 1989;10:1597–1598. doi: 10.1093/carcin/10.9.1595. [DOI] [PubMed] [Google Scholar]
- 38.Norazalina S. Norhaizan ME. Hairuszah I. Norashareena MS. Anticarcinogenic efficacy of phytic acid extracted from rice bran on azoxymethane-induced colon carcinogenesis in rats. Exp Toxicol Pathol. 2010;62:259–268. doi: 10.1016/j.etp.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 39.Challa A. Rao DR. Reddy B. Interactive suppression of aberrant crypt foci induced by azoxymethene in rat colon by phytic acid and green tea. Carcinogenesis. 1997;18:2023–2026. doi: 10.1093/carcin/18.10.2023. [DOI] [PubMed] [Google Scholar]
- 40.Laughlin LT. Bernat BA. Armstrong RN. Mechanistic imperative for the evolution of a metallo glutathione transferase of the vicinal oxygen chelate superfamily. Chem Biol Interact. 1998;111–112:41–50. doi: 10.1016/s0009-2797(97)00150-6. [DOI] [PubMed] [Google Scholar]
- 41.Pemble SE. Wardle AF. Taylor JB. Glutathione S-transferase class kappa: characterization by the cloning of rat mitochondrial GST and identification of a human homologue. Biochem J. 1996;319:749–754. doi: 10.1042/bj3190749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Panza VS. Wazlawik E. Ricardo Schütz G. Comin L. Hecht KC. da Silva EL. Consumption of green tea favorably affects oxidative stress markers in weighttrained men. Nutrition. 2008;24:433–442. doi: 10.1016/j.nut.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 43.Habig WH. Pabst MJ. Jakoby WB. Glutathione S transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem. 1974;249:7130–7139. [PubMed] [Google Scholar]
- 44.Verghese M. Rao DR. Chawan CB. Walker L.T. Shackelford L. Anticarcinogenic effect of phytic acid (IP6): apoptosis as a possible mechanism of action. Food Sci Technol. 2006;39:1093–1098. [Google Scholar]
- 45.Reddy BS. Diet and colon cancer: evidence from human and animal model studies. In: Reddy BS, editor; Cohen LA, editor. Diet, Nutrition and Cancer: A Critical Evaluation. Vol. 1. CRC Press Inc.; Boca Raton, FL: 1986. pp. 47–65. [Google Scholar]
- 46.Hirose M. Ozali K. Takabe K. Fukushima S. Shirai T. Ito N. Modifying effects of the naturally occurring γ-oryzanol, phytic acid, tannic acid and n-tritricontane-16,18-dione in rat—a wide spectrum organ carcinogenesis model. Carcinogenesis. 1991;12:1917–1921. doi: 10.1093/carcin/12.10.1917. [DOI] [PubMed] [Google Scholar]
- 47.Chen HW. Yang JJ. Tsai CW. Wu JJ. Sheen LY. Ou CC. Lii CK. Dietary fat and garlic oil independently regulate hepatic cytochrome P450 2B1 and the placental form of glutathione S-transferase expression in rats. J Nutr. 2001;131:1438–1443. doi: 10.1093/jn/131.5.1438. [DOI] [PubMed] [Google Scholar]
- 48.Talalay P. Fahey JW. Holtzclaw WD. Prestera T. Zhang Y. Chemoprotection against cancer by phase 2 enzyme induction. Toxicol Lett. 1995;82:173–179. doi: 10.1016/0378-4274(95)03553-2. [DOI] [PubMed] [Google Scholar]
- 49.Hasegawa S. Miyake M. Biochemistry and biological functions of citrus limonoids. Food Rev Int. 1996;12:413–435. [Google Scholar]
- 50.Hayes JD. Pulford D. The glutathione S-transferase supergene family: regulation of GST and the contribution of the isoenzymes to cancer chemoprotection and drug resistance. Crit Rev Biochem Mol Biol. 1995;30:445–600. doi: 10.3109/10409239509083491. [DOI] [PubMed] [Google Scholar]
- 51.Ebert MN. Klinder A. Peters WH. Schaferhenrich A. Sendt W. Scheele J. Pool Zobel BL. Expression of glutathione S-transferases (GSTs) in human colon cells and inducibility of GSTM2 by butyrate. Carcinogenesis. 2003;24:1637–1644. doi: 10.1093/carcin/bgg122. [DOI] [PubMed] [Google Scholar]
- 52.Shamsuddin AM. Yang GY. Inositol hexaphosphate inhibits growth and induces differentiation of PC-3 human prostate cancer cell. Carcinogenesis. 1995;16:1975–1979. doi: 10.1093/carcin/16.8.1975. [DOI] [PubMed] [Google Scholar]
- 53.Shamsuddin AM. Baten A. Lalwani ND. Effects of inositol hexaphosphate on growth and differentiation in K562 erythroleukemia cell line. Cancer Lett. 1992;64:195–202. doi: 10.1016/0304-3835(92)90043-u. [DOI] [PubMed] [Google Scholar]
- 54.Vucenik I. Tomazic VJ. Fabian D. Shamsuddin AM. Antitumor activity of phytic acid in murine transplanted and metastatic fibrosarcoma, a pilot study. Cancer Lett. 1992;65:9–13. doi: 10.1016/0304-3835(92)90206-b. [DOI] [PubMed] [Google Scholar]
- 55.Wang ZY. Khan WA. Bickers DR. Mukhtar H. Protection against polycyclic aromatic hydrocarbon-induced skin tumor initiation in mice by green tea polyphenols. Carcinogenesis. 1989;10:411–415. doi: 10.1093/carcin/10.2.411. [DOI] [PubMed] [Google Scholar]
- 56.Setiawan VW. Zhang ZF. Yu GP. Lu QY. Li YL. Protective effect of green tea on the risks of chronic gastritis and stomach cancer. Int J Cancer. 2001;92:600–604. doi: 10.1002/ijc.1231. [DOI] [PubMed] [Google Scholar]
- 57.Katiyar SK. Mukhtar H. Tea in chemoprevention of cancer: epidemiologic and experimental studies. Int J Oncol. 1996;8:221–238. doi: 10.3892/ijo.8.2.221. [DOI] [PubMed] [Google Scholar]
- 58.Kohlmeier L. Weterings KG. Steck S. Kok FJ. Tea and cancer prevention: an evaluation of the epidemiologic literature. Nutr Cancer. 1997;27:1–13. doi: 10.1080/01635589709514494. [DOI] [PubMed] [Google Scholar]