Abstract
Background
Fine needle aspiration (FNA), although very reliable for cytologically benign and malignant thyroid nodules, has much lower predictive value in the case of suspicious or indeterminate nodules. We aimed to identify clinical predictors of malignancy in the subset of patients with suspicious FNA cytology.
Methods
We reviewed the electronic medical records of 462 patients who had FNA of thyroid nodules at our institution with a suspicious cytological diagnosis, and underwent surgery at Mayo Clinic between January 2004 and September 2008. Demographic data including age, gender, history of exposure to radiation and use of thyroid hormone was collected. The presence of single versus multiple nodules by ultrasonography, nodule size, and serum thyroid-stimulating harmone (TSH) level before thyroid surgery were recorded. Analysis of the latter was limited to patients not taking thyroid hormone or antithyroid drugs at the time of FNA.
Results
Of the 462 patients, 327 had lesions suspicious for follicular neoplasm (S-FN) or Hürthle cell neoplasm (S-HCN), 125 had cytology suspicious for papillary carcinoma (S-PC) and 10 had a variety of other suspicious lesions (medullary cancer, lymphoma and atypical). Malignancy rate for suspicious neoplastic lesions (FN+HCN) was ∼15%, whereas malignancy rate for lesions S-PC was 77%. Neither age, serum TSH level, or history of radiation exposure were associated with increased malignancy risk. The presence of multiple nodules (41.1% vs. 26.4%, p=0.0014) or smaller nodule size (2.6±1.8 cm vs. 2.9±1.6 cm, p=0.008) was associated with higher malignancy risk. In patients with cytology suspicious for neoplasm (FN, HCN) malignancy risk was higher in those receiving thyroid hormone therapy than in nonthyroid hormone users (37.7% vs. 16.5%, p=0.0004; odds ratio: 3.1), although serum TSH values did not differ significantly between thyroid hormone users and nonusers.
Conclusion
In patients with cytologically suspicious thyroid nodules, the presence of multiple nodules or smaller nodule size was associated with increased risk of malignancy. In addition, our study demonstrates for the first time, an increased risk of malignancy in patients with nodules suspicious for neoplasm who are taking thyroid hormone therapy. The reason for this association is unknown.
Introduction
Thyroid nodules are very common in clinical practice, and although most are benign, approximately 5% may harbor malignancy. Fine needle aspiration biopsy (FNAB) is the most cost-effective method available to determine the need for further intervention. Nodules determined on FNAB to be benign in asymptomatic patients may be observed, whereas malignant nodules warrant surgery. However, a subset of patients with cytology suspicious for neoplasm (S-N), or indeterminate, whether follicular or Hürthle cell in origin, pose a more challenging problem for the clinician. On the one hand, the inability to distinguish benign from malignant follicular tumors on the basis of cytology alone has resulted in a general recommendation that patients with this cytological diagnosis undergo surgical excision for a definitive diagnosis (1). On the other hand, at the time of surgery, only approximately 20%–30% of such neoplasms will prove to be malignant (2–4). Hence, the inability to correctly identify preoperatively that small subset of patients harboring malignancy ultimately results in the majority of patients with this cytology undergoing potentially unnecessary surgery with its attendant morbidity and complications.
Many investigators have evaluated various markers in the attempt to better define this subset of nodules. However, no single molecular or immunohistochemical marker or set of markers has been found to have sufficient accuracy and negative predictive value to allow the clinician to comfortably recommend observation in a subset of S-N nodules, rather than surgery.
Many clinical parameters have long been recognized as predictors of malignancy in patients with thyroid nodules. Such parameters include age younger than 20 or older than 70 years (5), male gender (5,6), large nodule size (6,7), rapid growth, associated hoarseness, dysphagia or lymphadenopathy, history of exposure to radiation to head and neck (8), particularly in childhood, and type of goiter (single nodule versus multinodular goiter [MNG]) (9).
A recent prospective study by Boelaert et al. found that higher serum thyroid-stimulating harmone (TSH) levels at presentation, even within the normal range, was an independent predictor of malignancy (9). The authors provided a formula to predict malignancy using common clinical parameters, including serum TSH, age, gender, and clinical goiter type (9). Furthermore, a subsequent retrospective study by Haymart and colleagues found that higher serum TSH levels were associated with increased risk of differentiated thyroid carcinoma and advanced tumor stage in elderly patients (10).
Given the recent reports, we wished to determine whether we could identify clinical predictors of malignancy in this subset of patients presenting with thyroid nodules and suspicious FNA cytology. In addition, we sought to determine whether serum TSH is an independent predictor of malignancy in these patients and to characterize the accuracy of cytology in identifying malignancy in this subset of patients.
Subjects and Methods
The cytologic diagnoses for FNAB of the thyroid at the Mayo Clinic follow the recommended Bethesda nomenclature (11). The electronic medical record containing data from patients seen at Mayo Clinic, Rochester, MN, between January 1, 2004, and September 15, 2008, was queried to identify those who had undergone FNAB of thyroid nodules and whose cytological diagnosis was determined by the cytopathologist to be in the suspicious category (including categories III, IV, and V of the Bethesda system for cytopathology reporting) (11). Patients with previous thyroidectomy for thyroid cancer and cervical nodal biopsies were excluded. Demographic data, including age, gender, and previous history of exposure to radiation, were collected. The presence of single versus multiple nodules based on ultrasonography evaluation was recorded. The size (largest dimension measured in cm by high resolution ultrasonography) of the nodule that had undergone the FNAB in question was also recorded. The most recent serum-sensitive TSH level obtained within 3 months of the FNAB, but before thyroid surgery was documented.
Analysis of the influence of serum TSH measurement on outcome of surgery (malignancy) was limited to the subset of patients who were not taking thyroid hormone preparations (including levothyroxine, triiodothyronine, or combinations of both, Armour thyroid or other thyroid hormone-containing supplements) at the time of FNAB evaluation.
Cytological findings were sub-classified into one of four categories: (1) Suspicious for follicular neoplasm (S-FN); (2) Suspicious for Hürthle cell neoplasm (S-HCN); (3) Suspicious for papillary carcinoma (S-PC); and (4) Other (including cytological findings suspicious for lymphoma, medullary thyroid carcinoma, or others described simply as atypical).
The study was approved by the Mayo Clinic Institutional Review Board.
Statistical analysis
Final diagnostic outcome defined on the basis of the final pathology was recorded as the presence or absence of malignancy in the operative thyroid specimen. The influence of continuous factors such as age and nodule size on the final diagnostic outcome was assessed using a two-sample t-test. For categorical variables such as gender, exposure to radiation, and single versus MNG, an association with final diagnostic outcome was assessed using a Chi square test.
As one of the primary variables of interest, serum TSH level was analyzed both as a continuous as well as a categorical variable. Because TSH values did not follow a normal distribution, a log transformation was used to satisfy the assumptions of the t-test. For the purpose of statistical analysis, values below the lower end of detection in the TSH assay (<0.01 mIU/L) were recorded as 0.005 mIU/L. To examine the effect of serum TSH categories on the likelihood of a malignant diagnosis, only TSH values from subjects not taking thyroid hormone preparations were used in the analysis. Nonthyroid hormone users were classified according to their TSH values into four categories: (1) TSH values below the lower end of normal in our laboratory (<0.3 mIU/L); (2) TSH values between 0.3 mIU/L and the median TSH of the cohort (1.6 mIU/L); (3) TSH between 1.61 and 5.0 mIU/L (upper limit of normal range in our laboratory); (4) TSH >5 mIU/L. Similar analyses were performed on the subgroup of patients with cytological diagnosis S-N (includes S-FN and S-HCN).
Descriptive statistics were used to summarize the results of the accuracy of FNAB by cytological categories (1: S-FN; 2: S-HCN; 3: S-PC; 4: S-Other, including atypical, suspicious for lymphoma, suspicious for medullary thyroid carcinoma) with regard to outcome of malignancy. To evaluate the effect of age on likelihood of malignant diagnosis, patients were divided into one of four categories. (1) Age <30 years, (2) 30–49 years, (3) 50–69 years, (4) ≥70 years. All analyses were carried out using JMP statistical software (version 6.2; SAS Institute, Cary, NC). A p-value of <0.05 was considered statistically significant. Factors showing univariate association with the diagnostic outcome were evaluated for an independent association using multivariate logistic regression.
Results
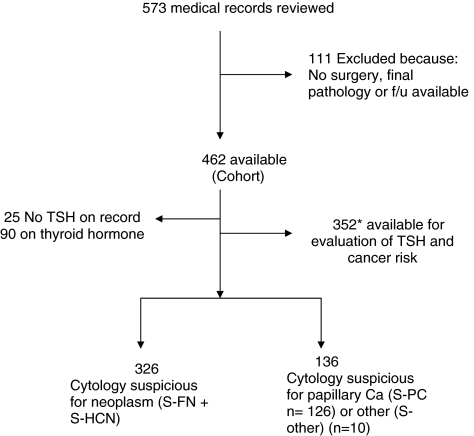
Of the 7039 FNAB of a thyroid nodule or nodules at Mayo Clinic between January of 2004 and September 2008, 573 (8%) were identified with a suspicious cytological diagnosis. One hundred eleven patients did not undergo surgery or have further follow up at Mayo, and therefore final pathology was not available. The remaining 462 patients who had surgery at Mayo Clinic and for whom a histological diagnosis was available constitute the cohort of this study (Fig. 1).
FIG. 1.
Study population. *Five patients without serum TSH values were on thyroid hormone replacement. TSH, thyroid-stimulating hormone.
The demographic characteristics of these patients (overall group) are summarized in Table 1. Sixty-nine percent were female, mean age 53.7 years±15.2. Twenty-five patients (5.4% of the cohort) had documented history of previous radiation exposure to head and neck, whereas 256 (55.6%) denied such exposure. No information was available in the remainder (39%). MNG was documented by ultrasonography in 65% of the cohort. Ninety patients (19.5%) in the study group were taking thyroid hormone preparations at time of evaluation.
Table 1.
Demographic Characteristics of Study Cohort, 462 Patients with Thyroid Nodules and Fine Needle Aspiration Biopsy Suspicious Cytological Diagnosis
| Female | 69% |
| Age (years, mean±standard deviation) | 53.7±15.2 |
| History of radiation exposure to head/neck | 5.4% |
| Patients with multiple nodules | 65% |
| Mean nodule size by ultrasonography (cm) | 2.8±1.7 |
| Proportion of patients on thyroid hormone therapy | 19.5% |
The cytopathologic diagnosis identified on FNAB in 326/462 (71%) was S-N. Of these, 100 biopsies indicated an S-HCN and 226 showed cytology S-FN. The remaining 126 biopsies were S-PC and 10 biopsies were miscellaneous suspicious, including 5 suggesting lymphoma, 2 suggesting medullary carcinoma, and 3 atypical without further characterization. (Fig. 2). Table 2 summarizes initial and final histopathological findings for all patients
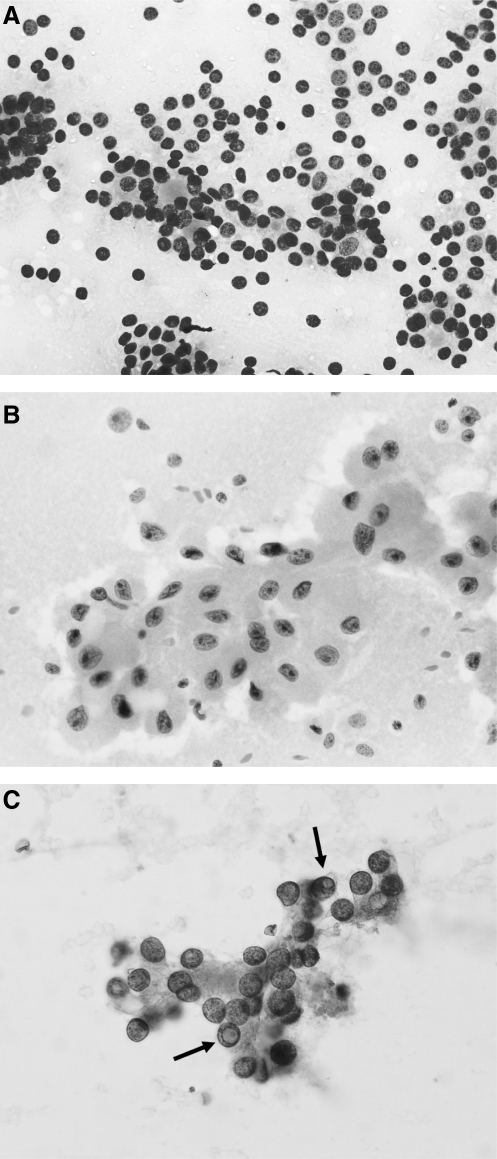
FIG. 2.
Representative samples of the following cytological categories. (A) Suspicious for follicular neoplasm: Clusters of follicular cells arranged in microfollicles. (B) Suspicious for Hürthle cell neoplasm: Cluster of Hürthle cells arranged in a flat sheet with larger nuclei with prominent nucleoli. (C) Suspicious for papillary carcinoma: Cluster of follicular cells with round nuclei and minimal size variation and nuclear grooving. Note the presence of two distinct intranuclear inclusions that raise the possibility of papillary carcinoma (marked with arrows).
Table 2.
Cytology Category with Incidence of Malignancy, and Final Histopathology
| Cytology category | No. of patients | Malignant, na(%) | Histopathology of malignant tumors in primary FNA target nodule [%] | Occurrence of IPC, n (%) | Histopathology of benign neoplasms in primary FNA target nodule |
|---|---|---|---|---|---|
| S-FN | 226 | 37 (16) | PC=18 (included 7 FV-PTC) [49%]; FC=15 [40%], HCC=3 [8%] Other (metastasis of RCC in a FA)=1 [3%] | 10b (4.4) | FA=134 FA-HCF=5 HCA=13 HPN=38 |
| S-HCN | 100 | 14 (14) | PC=2 [14%]; FC=2 [14%]; HCC=10 [72%] | 5 (5) | HCA=53 FA-HCF=14 FA=2 Other=16c |
| S-PC | 126 | 96 (76) | PC=93 (including 16 FV of PC) [97%]; FC=2; MC=1 | N/A | N/A |
| S-Other | 10 | 5 (50) | Lymphoma=3; MC=2 | N/A | N/A |
Does not include cases of IPC listed in final column.
Includes one patient with coexisting HCC and IPC.
Includes a variety of benign nonneoplastic findings.
N/A, not applicable; IPC, incidental papillary carcinoma (seen in nodule other than target of FNAB); S-FN, suspicious for follicular neoplasm; S-HCN, suspicious for Hürthle cell neoplasm; S-PC, suspicious for papillary carcinoma; S-Other, suspicious for other (includes lymphoma, MC, and atypical); FC, follicular carcinoma; HCC, Hürthle cell carcinoma; MC, medullary carcinoma, FV, follicular variant; RCC, renal cell carcinoma; HCA, Hürthle cell adenoma; FA, follicular adenoma. HCF, Hürthle cell features; HPN, Hyperplastic nodules; FNAB, fine-needle aspiration biopsy.
Predictors of malignancy
No significant association was seen in our cohort between gender and the risk of malignancy, or history of exposure to radiation although only a small number of patients in our cohort (n=25) reported such exposure, and in 181 records such information was unavailable.
Age
We did not find age to be a predictor of malignancy in the entire cohort (n=462; p=0.59) or in the subset of patients with cytology S-N (n=326; p=0.26).
Serum TSH level
Twenty-five patients did not have a serum TSH measurement on record. Ninety patients were taking thyroid hormone at the time of evaluation. Altogether, a total of 110 patients either did not have a TSH value documented or were on thyroid hormone replacement at the time of TSH measurement (5 patients who did not have serum TSH value on record were on thyroid hormone replacement). There were 352 nonthyroid hormone users with serum TSH levels available for evaluation. The mean (±standard deviation) TSH value in this group was 2.0 (±1.74) mIU/L. The median value was 1.6 mIU/L (interquartile range [IQR; 25%, 75%] 1.0–2.5).
We found no association between serum TSH values and risk of malignancy in the entire cohort (p=0.61) or in the subgroup with cytology S-N (S-FN and S-HCN combined) (p=0.397).
Mean serum TSH levels were not significantly different in patients on thyroid hormone replacement (median 1.6 mIU/L [IQR 0.8–3.1]) than in nonthyroid hormone users (median TSH 1.6 mIU/L [IQR 1.0–2.5; p=0.58]). When comparing serum TSH values in the entire cohort of patients with documented values (including users and nonusers, n=443) there was no significant difference between serum TSH values in patients with benign nodules (n=282) (median 1.7 [IQR 1.0–2.6]) and patients with malignant tumors (n=155) (median 1.4 [IQR 0.9–2.3]).
Thirteen nonthyroid hormone users had suppressed serum TSH values (<0.3mIU/L). Of these 13, 7 had a radioisotope scan before FNA and in 4 of them this study demonstrated increased uptake in the gland with a focal cold defect corresponding to the nodule, which was subsequently biopsied. Two of these four nodules were found to be malignant. In the other three, the scan showed a hyperfunctioning nodule; however, FNA was performed either because of large size (>4 cm) or the presence of other suspicious sonographic features. One of these three patients had a malignant nodule, whereas the other two had benign neoplastic lesions. The remaining 6/13 patients with suppressed TSH did not have a scan before FNA.
Nodule size
Smaller nodule size measured by ultrasonography preoperatively (2.6±1.8 vs. 2.9 cm±1.6; p=0.008) was associated with increased risk of malignancy.
However, we found that patients with cytological findings S-N had significantly larger nodule size (2.9±1.5 cm vs. 2.5±1.5 cm; p≤0.0001) than the other patients in the cohort (S-PC and S-Other), despite a much lower malignancy rate (15% vs. 76%). Of note is that 34% of all patients in the cohort with nodules ≥4 cm in size had documented malignancy.
Single nodule versus MNG
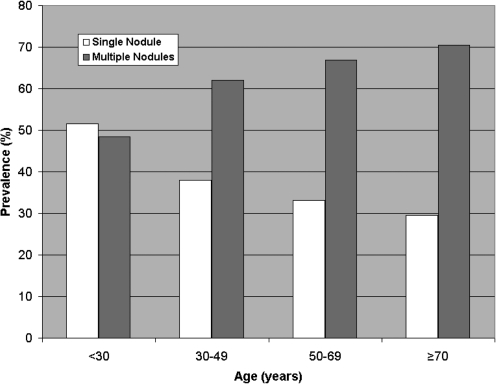
Patients with MNG (n=299) had a higher rate of malignancy in our cohort (41.1%) than patients with solitary nodules (n=163; 26.4%, p=0.0014). The proportion of patients with MNG increased with advancing age (48.5% for <30 years; 62% for age group 30–49 years; 66.8% for age group 50–69 years and 70.4% for age ≥70 (Fig. 3).
FIG. 3.
Prevalence of single nodules versus multinodular goiter by age group. Note increasing prevalence of multinodular goiter with advancing age.
Cytology category
A cytological finding of S-PC was strongly correlated with a diagnosis of malignancy in 76% of patients and correctly identified papillary carcinoma in 97% (93 of 96) of all malignant nodules in this group.
Thyroid hormone use
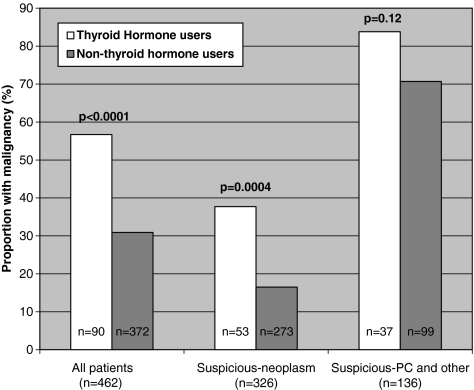
Overall, 19.5% of patients in our cohort (90/462) were taking thyroid hormone therapy. Of these, 51 had confirmed malignancy on final histopathology (56.7% vs. 30.9% malignancy rate for thyroid hormone users versus nonthyroid hormone users p<0.0001). Mean TSH values were not significantly different between thyroid hormone users and nonthyroid hormone users (2.84±5.05 vs. 2.0±1.74; p=0.58). The risk of malignancy was significantly higher in patients receiving thyroid hormone therapy than in nonthyroid hormone users (56.67% vs. 30.91%; p<0.0001) (odds ratio [OR]: 2.92; 95% confidence interval [CI]: 1.82–4.68). This was most obvious in patients with cytology S-N (FN and HCN) (37.7% vs. 16.5% p=0.0004) (OR: 3.1; 95% CI: 1.62–5.83). A similar, although nonstatistically significant trend (83.8% vs. 70.7%; p=0.12) (OR: 2.14; 95% CI: 0.81–5.68) was observed in the remainder of the cohort (includes patients in cytology categories 3: S-PC and 4: S-Other) (Fig. 4)
FIG. 4.
Percent of patients with malignancy among thyroid hormone users and nonusers. A total of 166 thyroid malignancies were identified in the entire cohort. Their distribution is shown for all cohort and by suspicious category. Suspicious-neoplasm includes cytology suspicious for follicular or Hürthle cell neoplasm.
FNAB predictive value
Final pathology confirmed the presence of malignancy in 166 patients (36% of the entire cohort). However, the proportion of malignant diagnoses varied depending on initial cytological category (Table 2). Overall, the predictive value of a cytological diagnosis S-FN subgroup to identify malignancy is 16%, with most of those malignant lesions comprising true follicular carcinomas (40%), or papillary carcinomas, including its follicular variant (49%). Of the nonmalignant neoplastic lesions in this subgroup, 61% (n=139) were follicular adenomas, and 5.7% (n=13) were Hürthle cell adenomas, with the remainder (n=37, 16%) being hyperplastic nodules.
Similarly, for lesions S-HCN the malignancy rate was 14%, with the majority of such tumors (72%) ultimately diagnosed as Hürthle cell carcinomas. The nonmalignant neoplastic lesions in this group included Hürthle cell adenomas (53%), and follicular adenomas (16%), most of which had Hürthle cell features. The remainder (16%) included a variety of nonneoplastic findings.
For patients with a cytological diagnosis S-PC the positive predictive value (76%) was significantly higher, with 97% of these malignant tumors comprising papillary thyroid cancers.
Approximately 5% of patients who underwent surgery for a suspicious neoplastic lesion (n=15) were found to have incidental papillary thyroid carcinoma (IPC) in the surgical specimen. In 14/15 IPC, the malignant foci were <1 cm (range 0.2–0.7 cm). Two additional patients had co-incidental PC (diagnosed preoperatively by FNA of a nodule other than the one yielding the S-N cytology).
Discussion
The present study confirms that FNAB is an effective tool in identifying patients with thyroid nodules who are surgical candidates because of increased risk for malignancy, particularly among the subset classified as cytologically S-PC. In our study, its positive predictive value overall was 76%. These results compare favorably to those reported in other series (12), including a previous report from our institution, in which 60% of nodules identified as S-PC were confirmed to be malignant on final histological diagnosis (2). Other studies have reported similar findings (13,14) and malignancy rates for this cytological category have been reported to range between 60% and 84% (15,16).
This study also highlights the ongoing difficulty in identifying preoperatively the subset of patients with malignancy among those undergoing FNAB of thyroid nodules classified as cytologically S-N (Hürthle cell or follicular type). The cohort of suspicious results included in our study represents approximately 10% of all patients typically undergoing FNAB and it is this group for which FNA cytology is least helpful in accurately identifying malignancy. It is clearly this subset of patients in which the greatest need exists for more reliable predictors of malignancy. This is especially problematic, given the low positive predictive value of such results (15%–20%) and the lack of other consistently reliable cytological, immunohistochemical, or molecular markers to identify such patients (17,18). Although certain clinical parameters (age, male gender, history of radiation exposure in childhood, and nodule size) have been variably associated in different studies with increased risk of malignancy, FNAB remains the most accurate available tool in the selection of patients with nodular thyroid disease that require surgery.
In our study 15/35 patients (43%) with an ultimate surgical finding of PC in the S-N group had only IPC identified, whereas the primary lesion for which surgery was performed was a nonmalignant follicular or Hürthle cell neoplasm or a hyperplastic nodule. In all except one of these patients, the size of the IPC was <1 cm, consistent with recent reports (19). All except two of these patients had multiple nodules identified by ultrasonography. Hence, the incidence of IPC in patients with cytology S-FN or S-HCN in our cohort was 4.6%, consistent with other studies showing the overall incidence of IPC in patients undergoing surgery for benign thyroid conditions to be between 5% and 10% (20–23). Recent reports have suggested that the increased incidence of thyroid cancer in the past three decades is largely due to increased detection of small papillary cancers (19). The wide availability and increased use of more sensitive technology such as high resolution ultrasonography have clearly contributed to this trend.
Contrary to the recent report by Boelaert et al. (9), in which the finding of clinical solitary nodule was associated with a higher risk of malignancy, our study found higher incidence of malignancy in patients with multiple nodules. A study by Kim et al. using high-resolution ultrasonography found a trend toward a lower rate of malignancy in patients with solitary nodules compared to patients with multiple nodules (24). This is in contrast to other studies showing a higher risk of malignancy in patients with clinically detectable solitary nodules (9,25). In our study, ultrasonography evaluation was used to determine the presence of additional nodules, many of which were small and nonpalpable. It has been shown that approximately 50% of patients with clinically single palpable nodules have evidence of MNG when evaluated sonographically (26), indicating that the prevalence of thyroid nodules is highly dependent on the methodology used for assessment (27). With the use of high-resolution ultrasonography the incidental finding of thyroid nodules has been reported to be as high as 67% (28). Other studies have shown the rate of malignancy to be similar in patients with single nodules to those with MNG (5,29).
Two clearly distinct subgroups of patients comprise our cytologically suspicious category. The largest group (n=326/462, 71% of the cohort) includes patients with cytological findings S-FN or S-HCN (S-N), in which the overall incidence of malignancy is 15%, and a smaller subgroup of patients with cytological finding S-PC (n=126) in which the overall rate of malignancy is significantly higher (76%). Patients with cytology S-N (S-FN and S-HCN) had significantly larger nodules (2.89±1.49 cm vs. 2.48±1.97 cm p=0.03) than patients with cytology S-PC, consistent with a report by Lubitz et al. in a similar patient population (7).
In our study, a serum TSH level obtained before surgery was not found to be a predictor of malignancy. However, to evaluate the effect of endogenous thyroid function and eliminate any potential effect of exogenous therapies on TSH values, our analysis excluded patients who were taking thyroid hormone therapy at the time of FNAB.
We also found, contrary to most other reports (6), that smaller nodule size was more frequently seen in patients with malignancy in this subset of patients. This is likely the result of the significantly higher malignancy rate in nodules with S-PC cytology, which in general were smaller than S-N nodules. A study by Sahin et al. did not find clinical features, sonographic appearance, or nodule size to be useful predictors of malignancy in a group of patients with cytologically indeterminate nodules (17). However, in our entire cohort, 34% of all nodules measuring ≥4 cm (n=88) were ultimately found to be malignant.
In our cohort, thyroid hormone use was associated with increased risk of malignancy, an association that was most obvious in the subgroup of patients with cytology S-N (S-FN and S-HCN) (OR: 3.1). Although a similar trend was seen in the remainder of the group (S-PC and S-Other), it did not reach statistical significance. This may be due to insufficient power given the much smaller sample size of this subgroup.
The underlying reason for this association is uncertain. Because of the retrospective nature of our study, only a small proportion of our cohort (18%) had thyroid peroxidase (TPO) antibody levels measured, and in this small group no association was seen between the presence of TPO antibodies and risk of malignancy. The small number of patients in this group precludes any valid conclusions on this topic.
The indications for thyroid hormone use in our cohort were not recorded. Use of thyroid hormone in patients with an intact thyroid is often due to the presence of autoimmune thyroid disease. Some studies have reported an increased frequency of autoimmune thyroiditis in patients with thyroid carcinoma (29), although such an association has not been consistently confirmed in other studies (30–33). The study by Boelaert et al. did not report the frequency of thyroid hormone use in their cohort or analyze the effect that such therapy (or underlying reason for it) could have on serum TSH values. One could speculate that thyroid hormone is most commonly used in patients with thyroid failure, which in turn is associated with increased serum TSH levels. The duration of the TSH elevation (even in patients with mild or subclinical hypothyroidism) before institution of thyroid hormone therapy could be one potential factor leading to increased risk of malignancy, as TSH is known to be a growth factor and thyroid hormone suppressive therapy has been shown in some studies to slow the growth of established nodules and possibly the development of new ones (30–32). Furthermore, some of these patients may have had very gradual thyroid failure over several years during which time serum TSH levels may have risen very slowly until hypothyroidism was finally detected either by the development of clinical symptoms or by routine laboratory testing. The duration of the hypothyroidism or TSH elevation before the institution of thyroid hormone therapy may also be a potential factor contributing to the increased risk of malignancy. Some of these patients may have been started on thyroid hormone suppressive therapy in an attempt to reduce growth of pre-existing thyroid nodules or of goiters. A study by D'Avanzo et al., from Northern Italy, found a clear association between pre-existing history of benign thyroid disease and increased risk of a diagnosis of thyroid cancer, especially for patients with adenomas (relative risk [RR]: 27; CI: 6.7–107.5) and goiters (RR: 8.5; CI: 3.5–20.7), conditions that, in their study, each accounted for approximately 8% of thyroid cancer cases (33). Goldman et al. found similar results with regard to a pre-existing history of thyroid adenomas (34). Another study in American women found a similar association, reporting that women with a history of goiter were more likely to develop follicular thyroid cancer (OR: 17) or papillary thyroid cancer (OR: 7) than women without such history (35). These studies, however, found no significant association with a history of hypothyroidism (35). We did not evaluate the suspicious sonographic features of nodules that underwent biopsy, or correlate such features with ultimate finding of malignancy. However, it has been confirmed in many studies that features such as hypoechogenicity, increased vascularity, and the presence of microcalcifications, and/or irregular borders, although not diagnostic of malignancy, are extremely useful in the proper selection of nodules that should undergo FNA biopsy, particularly in patients with multiple nodules (29,36,37).
Our study clearly has some limitations. Its retrospective nature makes it difficult to evaluate a number of factors. Data regarding serum thyroid hormone levels (free thyroxine [FT4] and total triiodothyronine [TT3]) were not available except in a small proportion of patients and the prevalence of autoimmunity as reflected by the presence of TPO antibodies was only available in less than 18% of our cohort. Furthermore, the duration of and indication for thyroid hormone replacement therapy as well as history of pre-existing thyroid disease, history of radiation exposure, and other important known risk factors for thyroid cancer were not routinely recorded and thus were unavailable for our review.
In conclusion, our study failed to demonstrate serum TSH level as a predictor of malignancy in this cohort of patients. We did however identify, for the first time a strong association between thyroid hormone use (possibly a surrogate indicator of pre-existing thyroid disease) and risk of cancer, particularly in patients with cytology suspicious for follicular and Hürthle cell neoplasms. Further well-designed prospective studies are needed to determine the potential clinical significance of this finding.
Acknowledgments
The authors wish to thank Ms. Desirae L. Howe-Clayton and Ms. Laura L. Kosok for their invaluable assistance with data collection. The project was supported by Grant Number 1 UL1 RR024150-01 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH) and the NIH Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH. The study was also supported by a Small grants program award from Mayo Foundation.
Disclosure Statement
The authors declare that no competing financial interests exist.
References
- 1.Cooper DS. Doherty GM. Haugen BR. Kloos RT. Lee SL. Mandel SJ. Mazzaferri EL. McIver B. Pacini F. Schlumberger M. Sherman SI. Steward DL. Tuttle RM. The ATA Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer 2009 Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 19:1167–1214. doi: 10.1089/thy.2009.0110. (Erratum appears in Thyroid 2010;20:674–675). [DOI] [PubMed] [Google Scholar]
- 2.Gharib H. Goellner JR. Johnson DA. Fine-needle aspiration cytology of the thyroid. A 12-year experience with 11,000 biopsies. Clin Lab Med. 1993;13:699–709. [PubMed] [Google Scholar]
- 3.Stang MT. Carty SE. Recent developments in predicting thyroid malignancy. Curr Opin Oncol. 2009;21:11–17. doi: 10.1097/CCO.0b013e32831db2af. [DOI] [PubMed] [Google Scholar]
- 4.Baloch ZW. Fleisher S. LiVolsi VA. Gupta PK. Diagnosis of “follicular neoplasm”: a gray zone in thyroid fine-needle aspiration cytology. Diagn Cytopathol. 2002;26:41–44. doi: 10.1002/dc.10043. [DOI] [PubMed] [Google Scholar]
- 5.Belfiore A. La Rosa GL. La Porta GA. Giuffrida D. Milazzo G. Lupo L. Regalbuto C. Vigneri R. Cancer risk in patients with cold thyroid nodules: relevance of iodine intake, sex, age and multinodularity. Am J Med. 1992;93:363–369. doi: 10.1016/0002-9343(92)90164-7. [DOI] [PubMed] [Google Scholar]
- 6.Raparia K. Min SK. Mody DR. Anton R. Amrikachi M. Clinical outcomes for “suspicious” category in thyroid fine-needle aspiration biopsy: patient's sex and nodule size are possible predictors of malignancy. Arch Pathol Lab Med. 2009;133:787–790. doi: 10.5858/133.5.787. [DOI] [PubMed] [Google Scholar]
- 7.Lubitz CC. Faquin WC. Yang J. Mekel M. Gaz RD. Parangi S. Randolph GW. Hodin RA. Stephen AE. Clinical and cytological features predictive of malignancy in thyroid follicular neoplasms. Thyroid. 2010;20:25–31. doi: 10.1089/thy.2009.0208. [DOI] [PubMed] [Google Scholar]
- 8.Ron E. Lubin JH. Shore RE. Mabuchi K. Modan B. Pottern LM. Schneider AB. Tucker MA. Boice JD., Jr Thyroid cancer after exposure to external radiation: a pooled analysis of seven studies. Radiat Res. 1995;141:259–277. [PubMed] [Google Scholar]
- 9.Boelaert K. Horacek J. Holder RL. Watkinson JC. Sheppard MC. Franklyn JA. Serum thyrotropin concentration as a novel predictor of malignancy in thyroid nodules investigated by fine-needle aspiration. J Clin Endocrinol Metab. 2006;91:4295–4301. doi: 10.1210/jc.2006-0527. [DOI] [PubMed] [Google Scholar]
- 10.Haymart MR. Repplinger DJ. Leverson GE. Elson DF. Sippel RS. Jaume JC. Chen H. Higher serum thyroid stimulating hormone level in thyroid nodule patients is associated with greater risks of differentiated thyroid cancer and advanced tumor stage. J Clin Endocrinol Metab. 2008;93:809–814. doi: 10.1210/jc.2007-2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cibas ES. Ali SZ. The Bethesda system for reporting thyroid cytopathology. Thyroid. 2009;19:1159–1165. doi: 10.1089/thy.2009.0274. [DOI] [PubMed] [Google Scholar]
- 12.Chen H. Zeiger MA. Clark DP. Westra WH. Udelsman R. Papillary carcinoma of the thyroid: can operative management be based solely on fine-needle aspiration? J Am Coll Surg. 1997;184:605–610. [PubMed] [Google Scholar]
- 13.Seningen JL. Nassar A. Henry MR. Correlation of thyroid nodule fine-needle aspiration cytology with corresponding histology at Mayo Clinic, 2001–2007: an institutional experience of 1,945 cases. Diagn Cytopathol. 2010 2010 Dec 03; doi: 10.1002/dc.21566. [Epub ahead of print]; [DOI] [PubMed] [Google Scholar]
- 14.Sclabas GM. Staerkel GA. Shapiro SE. Fornage BD. Sherman SI. Vassillopoulou-Sellin R. Lee JE. Evans DB. Fine-needle aspiration of the thyroid and correlation with histopathology in a contemporary series of 240 patients. Am J Surg. 2003;186:702–709. doi: 10.1016/j.amjsurg.2003.08.015. discussion 709–710. [DOI] [PubMed] [Google Scholar]
- 15.Cersosimo E. Gharib H. Suman VJ. Goellner JR. “Suspicious” thyroid cytologic findings: outcome in patients without immediate surgical treatment. Mayo Clin Proc. 1993;68:343–348. doi: 10.1016/s0025-6196(12)60128-1. [DOI] [PubMed] [Google Scholar]
- 16.Kwak JY. Kim E-K. Kim MJ. Hong SW. Choi SH. Son EJ. Oh KK. Park CS. Chung WY. Kim KW. The role of ultrasound in thyroid nodules with a cytology reading of “suspicious for papillary thyroid carcinoma”. Thyroid. 2008;18:517–522. doi: 10.1089/thy.2007.0271. [DOI] [PubMed] [Google Scholar]
- 17.Sahin M. Gursoy A. Tutuncu NB. Guvener DN. Prevalence and prediction of malignancy in cytologically indeterminate thyroid nodules. Clin Endocrinol (Oxf) 2006;65:514–518. doi: 10.1111/j.1365-2265.2006.02625.x. [DOI] [PubMed] [Google Scholar]
- 18.Kato MA. Fahey TJ., 3rd Molecular markers in thyroid cancer diagnostics. Surg Clin North Am. 2009;89:1139–1155. doi: 10.1016/j.suc.2009.06.012. [DOI] [PubMed] [Google Scholar]
- 19.Davies L. Welch HG. Increasing incidence of thyroid cancer in the United States, 1973–2002. JAMA. 2006;295:2164–2167. doi: 10.1001/jama.295.18.2164. [DOI] [PubMed] [Google Scholar]
- 20.Lin J-D. Kuo S-F. Chao T-C. Hsueh C. Incidental and nonincidental papillary thyroid microcarcinoma. Ann Surg Oncol. 2008;15:2287–2292. doi: 10.1245/s10434-008-9958-2. [DOI] [PubMed] [Google Scholar]
- 21.Bradly DP. Reddy V. Prinz RA. Gattuso P. Incidental papillary carcinoma in patients treated surgically for benign thyroid diseases. Surgery. 2009;146:1099–1104. doi: 10.1016/j.surg.2009.09.025. [DOI] [PubMed] [Google Scholar]
- 22.Ito Y. Higashiyama T. Takamura Y. Miya A. Kobayashi K. Matsuzuka F. Kuma K. Miyauchi A. Prognosis of patients with benign thyroid diseases accompanied by incidental papillary carcinoma undetectable on preoperative imaging tests. World J Surg. 2007;31:1672–1676. doi: 10.1007/s00268-007-9131-2. [DOI] [PubMed] [Google Scholar]
- 23.Wang C. Crapo L. The epidemiology of thyroid disease and implications for screening. Endocrinol Metab Clin North Am. 1997;26:189–218. doi: 10.1016/s0889-8529(05)70240-1. [DOI] [PubMed] [Google Scholar]
- 24.Kim ES. Lim DJ. Baek KH. Lee JM. Kim MK. Kwon HS. Song KH. Kang MI. Cha BY. Lee KW. Son HY. Thyroglobulin antibody is associated with increased cancer risk in thyroid nodules. Thyroid. 2010;20:885–891. doi: 10.1089/thy.2009.0384. [DOI] [PubMed] [Google Scholar]
- 25.Rago T. Fiore E. Scutari M. Santini F. Di Coscio G. Romani R. Piaggi P. Ugolini C. Basolo F. Miccoli P. Pinchera A. Vitti P. Male sex, single nodularity, and young age are associated with the risk of finding a papillary thyroid cancer on fine-needle aspiration cytology in a large series of patients with nodular thyroid disease. Eur J Endocrinol. 2010;162:763–770. doi: 10.1530/EJE-09-0895. [DOI] [PubMed] [Google Scholar]
- 26.Tan GH. Gharib H. Reading CC. Solitary thyroid nodule: comparison between palpation and ultrasonography. Arch Intern Med. 1995;155:2418–2423. doi: 10.1001/archinte.155.22.2418. [DOI] [PubMed] [Google Scholar]
- 27.Mazzaferri EL. Management of a solitary thyroid nodule. N Engl J Med. 1993;328:553–559. doi: 10.1056/NEJM199302253280807. [DOI] [PubMed] [Google Scholar]
- 28.Ezzat S. Sarti DA. Cain DR. Braunstein GD. Thyroid incidentalomas. Prevalence by palpation and ultrasonography. Arch Intern Med. 1994;154:1838–1840. doi: 10.1001/archinte.154.16.1838. [DOI] [PubMed] [Google Scholar]
- 29.Marqusee E. Benson CB. Frates MC. Doubilet PM. Larsen PR. Cibas ES. Mandel SJ. Usefulness of ultrasonography in the management of nodular thyroid disease. Ann Intern Med. 2000;133:696–700. doi: 10.7326/0003-4819-133-9-200011070-00011. [DOI] [PubMed] [Google Scholar]
- 30.Papini E. Petruci L. Guglielmi R. Panunzi C. Rinaldi R. Bacci V. Crescenzi A. Nardi F. Fabrini R. Pacella C. Long-term changes in nodular goiter: a 5-year prospective randomized trial of levothyroxine suppressive therapy for benign cold thyroid nodules. J Clin Endocrinol Metab. 1998;83:780–783. doi: 10.1210/jcem.83.3.4615. [DOI] [PubMed] [Google Scholar]
- 31.Zelmanovitz F. Genro S. Gross J. Suppressive therapy with levothyroxine for solitary thyroid nodules: a double-blind controlled clinical study and cumulative meta-analyses. J Clin Endocrinol Metab. 1998;83:3881–3885. doi: 10.1210/jcem.83.11.5215. [DOI] [PubMed] [Google Scholar]
- 32.Wemeau JL. Caron P. Schvartz C. Schlienger JL. Orgiazzi J. Cousty C. Vlaeminck-Guillem V. Effects of thyroid-stimulating hormone suppression with levothyroxine in reducing the volume of solitary thyroid nodules and improving extranodular nonpalpable changes: a randomized, double-blind, placebo-controlled trial by the French Thyroid Research Group. J Clin Endocrinol Metab. 2002;87:4928–4934. doi: 10.1210/jc.2002-020365. [DOI] [PubMed] [Google Scholar]
- 33.D'Avanzo B. La Vecchia C. Franceschi S. Negri E. Talamini R. History of thyroid diseases and subsequent thyroid cancer risk. Cancer Epidemiol Biomarkers Prev. 1995;4:193–199. [PubMed] [Google Scholar]
- 34.Goldman MB. Monson RR. Maloof F. Cancer mortality in women with thyroid disease. Cancer Res. 1990;50:2283–2289. [PubMed] [Google Scholar]
- 35.McTiernan AM. Weiss NS. Daling JR. Incidence of thyroid cancer in women in relation to previous exposure to radiation therapy and history of thyroid disease. J Natl Cancer Inst. 1984;73:575–581. [PubMed] [Google Scholar]
- 36.Fish SA. Langer JE. Mandel SJ. Sonographic imaging of thyroid nodules and cervical lymph nodes. Endocrinol Metab Clin North Am. 2008;37:401–417. doi: 10.1016/j.ecl.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 37.Papini E. Guglielmi R. Bianchini A. Crescenzi A. Taccogna S. Nardi F. Panunzi C. Rinaldi R. Toscano V. Pacella CM. Risk of malignancy in nonpalpable thyroid nodules: predictive value of ultrasound and color-Doppler features. J Clin Endocrinol Metab. 2002;87:1941–1946. doi: 10.1210/jcem.87.5.8504. [DOI] [PubMed] [Google Scholar]