Abstract
Mutations in the mitochondrial genome (mtDNA) are associated with different types of cancer, specifically colorectal cancer (CRC). However, few studies have been performed on precancerous lesions, such as ulcerative colitis (UC) lesions and adenomatous polyps (AP). The aim of this study was to identify mtDNA mutations in the cancerous and precancerous lesions of Egyptian patients. An analysis of the mutations found in six regions of the mtDNA genome (ND1, ND5, COI, tRNAser, D-loop 1, and 2) in 80 Egyptian patients (40 CRC, 20 UC, and 20 AP) was performed using polymerase chain reaction–single-strand conformational polymorphism techniques and followed up by direct sequencing. The overall incidence of mutations was 25%, 25%, and 35% in CRC, UC, and AP cases, respectively. Although there was no common mutation pattern within each group, a large number of mutations were detected in the D-loop region in all of the groups. Some mutations (e.g., T414G) were detected repeatedly in precancerous (UC and AP) and cancerous lesions. Mutations detected in patients with CRC were predominantly found in the ND1 gene (40%). Our preliminary study suggests that Egyptian patients with CRC have a large number of mtDNA mutations, especially in the D-loop region, which have not been previously reported. Mutations in the mtDNA of precancerous lesions (i.e., AP and UC) may contribute to transformation events that lead to CRC.
Introduction
Mitochondria are organelles that, according to the endosymbiosis theory, evolved from purple bacteria ∼1.5 billion years ago (Dyall et al., 2004; Cavalier-Smith, 2006). These organelles have their own genome that replicates and transcribes semiautonomously; this genome comprises 0.1%–2% of the total DNA in most mammalian cells. The mitochondrial genome controls many cellular functions that are critical for producing the cell's energy. The most important of these functions are as follows: (1) oxidative phosphorylation (OXPHOS), (2) production of most of the cell's reactive oxygen species (ROS), and (3) regulation of mitochondrial apoptosis (Wallace, 2005a). Expression of the entire complement of mitochondrial genes is required to maintain the proper function of the organelle, suggesting that even slight alterations in DNA sequences could have profound effects.
Mitochondrial DNA is thought to accumulate more mutations than nuclear DNA, in part, because mitochondria lack both protective histones and the highly efficient DNA repair mechanisms that are seen in the nucleus (Croteau and Bohr, 1997). Mutations in mtDNA may impact neoplastic transformation, because mitochondria play a central role in energy production, ROS production, and apoptosis. Indeed, certain tumors have been shown to result from mutations in nDNA-encoded mitochondrial proteins, which may result in increased ROS production (Wallace, 2005b). Since the G10398A mtDNA polymorphism was first associated with an increased risk of breast cancer in African-American women (Canter et al., 2005), mtDNA mutations have also been associated with other types of cancer. mtDNA mutations in cancers fall into two major classes: (1) those that affect OXPHOS, thus increasing ROS production and (2) those that alter the mitochondrial OXPHOS circuitry and permit tumor cells to adapt to new environmental constraints, such as alterations in the available nutrients and oxygen tension (Brandon et al., 2006). Several mtDNA mutations have been found specifically in human colorectal cancer (CRC) (Polyak et al., 1998).
CRC is the third most prevalent type of cancer in the world. Each year, CRC is diagnosed in >940,000 people, and 500,000 people die from the disease (Benson et al., 2008). Overall, the 5-year survival rate in the United States exceeds 60% but is <40% in less developed countries (Stewart and Kleihues, 2003). In Egypt, CRC accounts for 6.53% of all cancers according to the National Cancer Institute, Cairo University. According to Mokhtar et al. (2007), the incidence is 2.27% for colon carcinoma and 2.08% for rectal carcinoma. Adenomatous polyps (AP), which are slow growing overgrowths of the colonic mucosa, comprise ∼10% of colonic polyps. AP are usually small and have low (1%) malignant potential (Muto et al., 1975). Removal of these lesions reduces the risk of future CRC and advanced adenomas (Bertario et al., 2003; Winawer et al., 2006). Ulcerative colitis (UC) is a chronic inflammatory disease of the colon. Long-standing extensive UC is associated with the development of UC-related CRC (Ekbom et al., 1990). A meta-analysis reported the cumulative incidence of CRC in UC to be 2% at 10 years, 8% at 20 years, and 18% after 30 years of the disease (Eaden et al., 2001).
mtDNA mutations have been assumed to be associated with the development and progression of CRC, which is probably the most studied cancer type in the mitochondrial field (Polyak et al.,, 1998; Habano et al., 1999). In previous studies, several mutations were found in the D-loop region of the mtDNA of three CRC cell lines (Li et al., 2005). Moreover, similar nucleotide substitutions were also found in the D-loop region of 40 CRC tissues using polymerase chain reaction (PCR)–single-strand conformational polymorphism (SSCP) and direct sequencing techniques (Li et al., 2006). Several studies have shown an increased rate of D-loop mutation in association with CRC. However, the association between D-loop mutations and long-term survival has been controversial. A case series of 365 patients with resected CRC recorded in the Digestive Cancer Registry of Cote-d'Or (France; 1998–2000) suggests that D-loop mtDNA mutations are associated with decreased CRC survival (Lievre et al., 2005). However, a case series of 194 patients with CRC who underwent surgery in Taipei Veterans General Hospital (Taiwan; 1999–2000) showed no association between D-loop mutations and CRC patient prognosis (Chang et al., 2009). Recently, Theodoratou et al. (2010) conducted a more comprehensive study on 2838 Scottish patients with CRC. This study suggested an association between three mtDNA single-nucleotide polymorphisms and all-cause or CRC mortality. It is not yet clear whether the mtDNA mutations are the cause or the result of transformation, although Li et al. (2008) suggest that mutations in the D-loop may participate in the transformation process. They have shown that the D-loop from CRC cells promote the malignant phenotype in NIH3T3 mouse fibroblast cells, which suggests that these mutations might partially contribute to colorectal carcinogenesis.
In an attempt to understand the role of mtDNA mutations in CRC development, we performed a preliminary study in which we analyzed six regions of mtDNA in samples from precancerous lesions (AP and UC) and CRC in Egyptian patients. Our study showed that the D-loop regulatory region harbored the largest number of mutations, most previously unknown, in all examined stages.
Materials and Methods
Patient samples and DNA extraction
Endoscopic biopsies that included tissue samples from colorectal lesions and nearby endoscopically normal tissues were collected from 80 patients referred for colonoscopy at the Kasr Al-Aini Hospital, Faculty of Medicine, Cairo University. Blood samples were taken from the corresponding patients as a control. The study was approved by the local ethics committee, and consent was obtained from all the patients included in the study.
CRC was diagnosed in forty patients, 20 patients had AP, and 20 patients had UC. A portion of the biopsy was preserved in formalin for histopathological examination, whereas another portion was immediately snap-frozen in liquid nitrogen and kept at −80°C for DNA extraction. Data were collected from each patient, including age, grade, tumor type, size and location, pathological stages, and polyp size.
Genomic DNA was extracted from tissue and blood samples using the QiaAmp DNA mini kit (Qiagen) according to the manufacturer's instructions.
Primers and PCR-SSCP analysis of mitochondrial DNA
Six regions of the mtDNA were amplified: four genes NADH dehydrogenase 1 and 5 (NDI, ND5), cytochrome c oxidase subunit I (CO1), and one tRNA gene (tRNAser) and two regions of the displacement loop (D-loop 1, D-loop 2). PCR-SSCP analysis was conducted to look for mutations in these regions. Six sets of primers reported by Maximo et al. (2001) were used for the PCR amplification of mtDNA from the tissue and the control blood samples (Table 1). The PCR amplification protocol was as follows: denaturation at 95°C for 5 min, followed by 35 cycles of 94°C for 1 min, 50°C for 1 min, 72°C for 30 s, and then a final extension for 10 min at 72°C.
Table 1.
Nucleotide Position and Primer Sequence
| Gene | Nucleotide position | Primer sequence | Product size (bp) |
|---|---|---|---|
| ND1 | 3397–3617 | 5′-ATACAACTACGCAAAGGCCCCA-3′ 5′-AATAGGAGGCCTAGGTTGAGGT-3′ |
221 |
| ND5 | 12353–12622 | 5′-CTATAACCACCCTAACCCTGAC-3′ 5′-CGAACAATGCTACAGGGATGAA-3′ |
270 |
| CO1 | 6541–6730 | 5′-GCAACCTCAACACCACCTTCTT-3′ 5′- GCTCAGACCATACCTATGTATCC-3′ |
190 |
| tRNAser | 7359–7606 | 5′-GAAGAACCCTCCATAAACCTGG-3′ 5′-TACTTGCGCTGCATGTGCCATT-3′ |
248 |
| D-loop 1 | 458–637 | 5′-CCTCCCACTCCCATACTACTAA-3′ 5′-GTGATGTGAGCCCGTCTAAACA-3′ |
185 |
| D-loop 2 | 267–423 | 5′-TCCACACAGACATCATAACA-3′ 5′-AAAGTGCATACCGCCAAAAG-3′ |
157 |
SSCP and DNA sequencing
The amplified PCR products were subjected to SSCP analysis. Electrophoresis of the denatured PCR products was carried out on a 10% nondenaturing polyacrylamide gel and run at 20 W for 4–5 h. The gels were silver stained, dried, and digitally scanned using a computer. After the PCR-SSCP analysis, DNA bands that were abnormally shifted in the gel were compared with their corresponding normal control, extracted, reamplified with the original set of primers, and then reanalyzed with SSCP. Only the cases with reproducible band shifts in the tissue samples but not in the normal control samples were considered to harbor somatic mitochondrial mutations. Amplification products were purified using Axyprep PCR cleanup kit (Axygene Biosciences). Sequence analysis was then carried out using Big Dye Terminator cycle sequencing kit (Applied Biosystem) on an ABI Prism 3730 Genetic Analyzer automated sequencer. Sequences were compared against the human mtDNA sequence (GENBANK accession # NC-012920) and the comprehensive mitochondrial databank MITOMAP (www.mitomap.org/mitoseq.html) (MITOMAP, 2009).
Sequence variants that were both found at a particular location in the tumor and matched the corresponding mtDNA were classified as polymorphisms. If the DNA sequence at a particular location in the tumor mtDNA differed from the corresponding normal mtDNA, then it was defined as a somatic mutation.
Results
Detection of mutations in mitochondrial DNA samples by SSCP
Previous studies have examined mtDNA mutations in CRC, but few have examined these mutations in conditions that predispose to CRC, such as AP and UC. Six different regions of mtDNA were chosen for mutation analysis: four regions in genes NDI, ND5, CO1, and tRNAser and two regions in the D-loop regulatory region. These regions were chosen because mutations had been previously reported there (Maximo et al., 2001). Tissue samples (lesion and normal) from 80 Egyptian patients with different cancerous and precancerous colorectal conditions were collected at the same time as blood samples, which were used as controls (Tables 2–4). Twenty patients had AP, 20 had UC, and 40 had CRC.
Table 2.
Summary of Mitochondrial DNA Mutations in Adenomatous Polyps Samples
| Case number | Gender | Age | Sample site | Mutation position | Type | Gene/region | Amino acid change | Notes |
|---|---|---|---|---|---|---|---|---|
| 2 | F | 37 | Sigmoid | 357 | A to C | D-loop 2 | MITOMAP (Reeve et al., 2008; Birket et al., 2009) | |
| 376 | A to C | |||||||
| 379 | A to C | |||||||
| 383 | T to C | |||||||
| 391 | T to A | |||||||
| 411 | C to G | |||||||
| 414 | T to G | |||||||
| 5 | M | 45 | Sigmoid | 574 | A to C | D-loop 1 | Germline mutation | |
| 576 | CCC insertion | |||||||
| 587 | C to G | |||||||
| 598 | C insertion | |||||||
| 602 | C del | |||||||
| 618 | T to G | |||||||
| 622 | G to A | |||||||
| 624 | C to A | |||||||
| 628 | C to G | |||||||
| 6 | F | 45 | Ascending | 7424 | A to G | t-RNAser | MITOMAP (Yeh et al., 2000) | |
| 7596–7597 | CA to AG | |||||||
| 9 | M | 50 | Sigmoid | 523–524 | Deletion of AC | D-loop 1 | MITOMAP (Wanrooij et al., 2004) | |
| 532 | T Insertion | |||||||
| 532 | A to C | |||||||
| 540 | TN Insertion | |||||||
| 565 | T Insertion | |||||||
| 10 | M | 52 | Rectum | 12443 | G insertion | ND5 | FS | |
| 12454 | CN insertion | FS | ||||||
| 12501 | G insertion | FS | ||||||
| 12534 | C insertion | FS | ||||||
| 12611 | T to A | |||||||
| 13 | M | 56 | Sigmoid | 6633 | T deletion | CO1 | FS | |
| 6671 | T to C | NS | MITOMAP (Herrnstadt et al., 2002) | |||||
| 6680 | T to C | NS | MITOMAP (Herrnstadt et al., 2002) | |||||
| 6724 | T deletion | FS | ||||||
| 3617 | A insertion | ND1 | FS |
MITOMAP: a polymorphism reported in MITOMAP: A Human Mitochondrial Genome Database. 2009 (MITOMAP, 2009).
FS, frame shift; NS, nonsense mutation.
Table 4.
Summary of Mitochondrial DNA Mutations in Colorectal Cancer Samples
| Case number | Gender | Age | Site | Grade | Mutation position | Type | Gene/region | Amino acid change | Notes |
|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 13 | Rectum | II | 574 | A to C | D-loop 1 | ||
| 576 | CCCC insertion | Germline mutation | |||||||
| 584–585 | TA to GC | ||||||||
| 587 | C to T | ||||||||
| 591 | C to A | ||||||||
| 593 | T to C | MITOMAP (Mimaki et al., 2009; Gonzalez et al., 2007) | |||||||
| 596 | T to C | ||||||||
| 601–602 | GC to AA | ||||||||
| 605–607 | TAC to AAA | ||||||||
| 612 | N insertion | ||||||||
| 616 | T to G | ||||||||
| 618 | T del | ||||||||
| 622 | G to A | ||||||||
| 624 | C to A | ||||||||
| 628 | C to G | ||||||||
| 2 | M | 27 | Rectum | II | 489 | T to C | D-loop 1 | MITOMAP (Mimaki et al., 2009) | |
| 589 | T deletion | ||||||||
| 12403 | C to T | ND5 | L to F | MITOMAP (Gonzalez et al., 2007; Mimaki et al., 2009) | |||||
| 14 | F | 41 | Sigmoid | II | 6618 | C insertion | CO1 | FS | |
| 6724 | T deletion | FS | |||||||
| 16 | F | 43 | Ascending | II | 3594 | C to T | ND1 | V to V | MITOMAP (Mimaki et al., 2009) |
| 25 | M | 50 | Rectum | II | 3594 | C to T | ND1 | V to V | MITOMAP (Mimaki et al., 2009) |
| 29 | F | 52 | Ascending/poly | II | 3483 | A insertion | ND1 | FS | |
| 33 | M | 58 | Descending | III | 361 | A to C | D-loop 2 | MITOMAP (Mimaki et al., 2009; Jazin et al., 1996) | |
| 366 | G to A | ||||||||
| 372 | T to A | ||||||||
| 376 | A to C | ||||||||
| 379 | A to G | MITOMAP (Mimaki et al., 2009; Feder et al., 2008) | |||||||
| 383 | T to A | ||||||||
| 388–389 | AG to GA | ||||||||
| 392 | A del | ||||||||
| 404 | C to T | ||||||||
| 411 | C to G | ||||||||
| 414 | T to G | MITOMAP (Birket et al., 2009; Reeve et al., 2008) | |||||||
| 35 | M | 60 | Rectum | I | 3614 | C insertion | ND1 | FS | |
| 36 | F | 62 | Rectum | II | 392 | A to C | D-loop 2 |
MITMAP: a polymorphism reported in MITMAP: A Human Mitochondrial Genome Database. 2009 (MITOMAP, 2009).
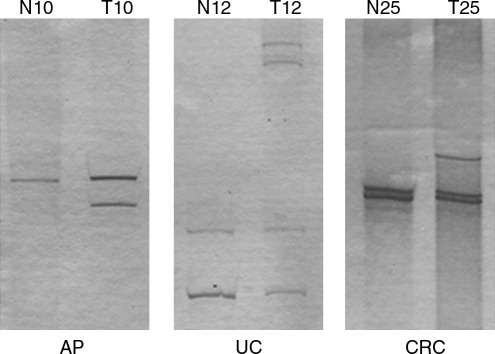
The PCR products of mtDNA from tissues and blood samples were analyzed by SSCP to identify DNA harboring mutations, which were identified as band shifts. Figure 1 shows a representation of several samples that exhibited an abnormal band migration relative to normal tissue control. The percentage AP samples in band shifts was 45% (9/20): sequencing revealed mutations in 7 samples, a mutation rate of 35% (Table 2). Among patients with UC, 35% of the samples (7/20) exhibited band shifts. Sequencing revealed mutations in 5 samples, a mutation rate of 25% (Table 3). In the CRC group, 35% of samples (14/40) exhibited abnormal band migration. Of the latter, 10 showed mutations on sequencing, for a mutation rate of 25%. It is important to note that four of the five confirmed mutations in the UC groups occurred in the D-loop region. Most of the mutations in the CRC group occurred in the ND1 gene and were detected by SSCP (6 out of 14) or by sequencing (5 out of 10 confirmed mutations). Samples in the AP group did not show a clear common pattern of mutations. A significant association between mtDNA mutations in patients with UC and both age and gender was observed. Of these, seven mutations occurred in patients below 50 years of age (p = 0.0358) who were men (p = 0.0317).
FIG. 1.
Representative gels of single-strand conformational polymorphism with band shift. This figure shows the single-strand conformational polymorphism results of the normal (N) and tumor biopsy (T) samples of cases 10 adenomatous polyps (AP), 12 ulcerative colitis (UC), and 25 colorectal cancer (CRC).
Table 3.
Summary of Mitochondrial DNA Mutations in Ulcerative Colitis Samples
| Case number | Gender | Age | Site | Dysplasia | Mutation position | Type | Gene/region | Amino acid change | Notes |
|---|---|---|---|---|---|---|---|---|---|
| 3 | F | 17 | Whole | Y | 352 | A to C | D- loop 2 | MITOMAP (Birket et al., 2009; Reeve et al., 2008) | |
| 362 | C to A | ||||||||
| 366 | G to A | ||||||||
| 372 | T to A | ||||||||
| 375–376 | CA to AC | ||||||||
| 379 | A to G | ||||||||
| 383 | T to A | ||||||||
| 388–389 | AG to GA | ||||||||
| 391 | T deletion | ||||||||
| 411 | C to G | ||||||||
| 414 | T to G | ||||||||
| 10 | M | 40 | Whole | Y | 6671 | T to C | CO1 | NS | MITOMAP (Herrnstadt et al., 2002) |
| 6680 | T to C | NS | MITOMAP (Herrnstadt et al., 2002) | ||||||
| 6724 | T deletion | FS | |||||||
| 11 | M | 43 | Left | N | 508 | T insertion | D-loop 1 | ||
| 12 | M | 45 | Recto-sigmoid | N | 523–52 | Deletion of AC | D-loop 1 | MITOMAP (Wanrooij et al., 2004) | |
| 13 | M | 50 | Whole | Y moderate | 523–524 | Deletion of AC | D-loop 1 | MITOMAP (Wanrooij et al., 2004) | |
| 532 | T insertion | ||||||||
| 539 | T insertion | ||||||||
| 546 | T insertion |
MITMAP: a polymorphism reported in MITMAP: A Human Mitochondrial Genome Database. 2009 (MITOMAP, 2009).
DNA sequencing
Sequencing was performed using the appropriate forward PCR primer. Due to the limited size of the tissue samples, some positive SSCP samples could not be sequenced. Tables 2–4 summarize the findings in our patients in the AP, UC, and CRCgroups. It is important to note the absence of a common mutation within each group. Nevertheless, some mutations are present in patients from different groups, such as most of the D-loop 2 mutations. One of these mutations (T414G) is a known polymorphism (Reeve et al., 2008; Birket et al., 2009; MITOMAP, 2009). Interestingly, all these mutations, including the T414G polymorphism, were not present in the normal tissue of the same patients, suggesting they are somatic mutations and may represent mutational hotspots in the D-loop 2 region (AP case # 2, UC case # 3, and CRC case # 33). CCC insertion was detected at position 576 in the D-loop 1 region of the DNA from both normal blood and tissue samples of a patient with AP (case # 5) and a patient with CRC (case # 1). Also, a somatic T deletion at position 6724 of the COI gene was detected in precancerous cases (AP case # 13, UC case # 10) and in cancerous cases (case # 14), as shown in Tables 2–4.
Discussion
Mitochondria control a number of critical cellular functions. As a result, mutations in the mtDNA have been associated with dozens of poorly understood disorders, as well as with the aging process and a variety of chronic degenerative diseases. Mitochondrial diseases range in severity from almost asymptomatic to fatal. These are a function of two types of mtDNA mutations: those inherited through the female germ line and those that accumulate with age in somatic cells. Given that the mtDNA genome is very compact, almost any mutation could be deleterious; In fact, by 2008, over 170 known mutations in the mtDNA were found to be responsible for a myriad of diseases (Crimi and Rigolio, 2008). Mutations in nDNA that encode mitochondrial proteins and other mtDNA have been associated with several cancers, including renal adenocarcinoma, head and neck tumors, astrocytic, thyroid, breast, prostate tumors, and CRC (Wallace, 2005b).
CRC is probably the most studied cancer type in the mitochondrial field (Polyak et al., 1998), but there have been very few studies carried out on patients with UC and none on patients with adenomatous polyposis. To date, there have been no analyses of mitochondrial DNA mutations in Egyptian patients with cancer. For these reasons, we decided to study mtDNA mutations in Egyptian patients with AP, UC, and CRC.
It has been previously reported that the incidence of CRC in young Jordanians is much higher than in other high-risk populations worldwide (Al-Jaberi et al., 2003). In Egypt, CRC seems to exhibit a trend similar to that seen in Jordan. Although CRC is considered an old age disease in the rest of the world, it seems to occur at younger ages in the Egyptian population. This suggest a different molecular etiology and compelled us to look for mtDNA mutation patterns different than those already published. Consequently, it was not surprising that none of the mutations found in the CRC group, other than the polymorphisms reported by MITOMAP, were previously reported (Tables 2–4). It is important to note that a somatic mutation is defined as a DNA variation present in the tumor samples and absent from the corresponding normal tissue sample, whereas mtDNA variations between different patients samples and rCRS are defined as inherited polymorphisms.
In an attempt to understand the early events leading to CRC, we performed a preliminary study to identify possible mtDNA mutations in precancerous lesions such as AP and UC. Although SSCP is not a very sensitive technique, it allows for a rapid screen of possible mutations. It is suitable for preliminary studies used to determine the possible involvement of mutations in a given disease. Our SSCP analysis results revealed a high incidence of band shifts: 45% in AP, 35% in UC, and 35% in CRC samples. The SSCP technique does not detect all mutations; under optimal conditions, ∼75%–95% of the potential base exchanges are detectable by SSCP (Scoggan and Bulman, 2003). This suggests that the rate of mtDNA mutations in the three groups analyzed herein may be even higher than reported. It is important to note that most of the mutations reported in this study have not been previously associated with cancers.
Most notable is the presence of numerous mutations in the regulatory none-coding regions (D-loop 1 and D-loop 2) compared with the normal tissue control, which is consistent with previous studies suggesting that D-loop mutations are common in colorectal tumors (Lievre et al., 2005; Chang et al., 2009). Although some of these mutations have been previously reported as polymorphisms in the MITOMAP, such as T414G and T593C, they were absent in the corresponding control samples in our study. This suggests that they are somatic mutations related to the associated lesion (AP, UC, or CRC). Only one mutation was found in the normal control tissue and in the cancer sample, which was the multiple C insertion at position 576 in AP case #5 and CRC case #1.
It has been previously suggested that there are no mutational hotspots in CRC (Fliss et al., 2000; Chatterjee et al., 2006), but the frequent incidences of mutations in the D-loop observed in our study suggest that it may be a mutational hotspot in Egyptian patients. The noncoding D-loop region has been reported to be a mutational hotspot in other tumors such as bladder, lung, head, and neck neoplasms (Zhu et al., 2005). Mutations in this region can be critical to the function of other mitochondrial genes, as the D-loop contains the main regulatory sequences for transcription and replication initiation (Basso et al., 2007). In addition, the transfection of a mutated D-loop into NIH3T3 cells induces transformation phenotypes (Li et al., 2008). In our study, it is not clear whether these multiple D-loop mutations are predisposing for transformation or whether they are the result of deregulation after transformation. A larger, more detailed study of the whole D-loop region is underway to clarify this point.
Cancer cells develop in a contiguous field of preneoplastic cells that are clonally related to the initial tumor cell. In this study, T deletion at position 6724 of the COI gene was detected in precancerous cases (AP case #13 and UC case #10) and in a CRC case (#14), which may suggest that this deletion has a role in the development of CRC. Mutations in the COI gene have been previously reported in preneoplastic lesions of the colon in several studies (Taylor et al., 2003; Greaves et al., 2006; Sui et al., 2006).
In this study, we noticed that a significant number of mutations detected in the CRC group occurred in the ND1 gene (40%), which was not the case in the AP or UC groups. Mutations in another NADH dehydrogenase subunit, ND6, have been associated with increased metastatic potential in mouse and human cancer cell lines, but not in nontransformed cells. This increased metastatic potential was related to the overproduction of ROS (Ishikawa et al., 2008). Other NADH dehydrogenase mutations have been linked to lung cancer (ND complex 3450, 4901) and urologic cancer (ND5 12477, 12714) (Jakupciak et al., 2008). The potential role of ND1 in the progression of CRC requires further investigation.
Our preliminary study suggests that Egyptian patients with CRC have a large number of mtDNA mutations which have not been previously reported and that mutations in the mtDNA of precancerous lesions (AP or UC) may contribute to the transformation events leading to CRC. Due to the limitations of the SSCP and our small sample size, we need to perform a larger scale study examining the entire D-loop region.
Disclosure Statement
No competing financial interests exist.
References
- Al-Jaberi T.M. Yaghan R.J. El-Heis H.A. Colorectal cancer in young patients under 40 years of age Comparison with old patients in a well defined Jordanian population. Saudi Med J. 2003;24:871–874. [PubMed] [Google Scholar]
- Basso D. Navaglia F. Fogar P. Zambon C.F. Greco E. Schiavon S., et al. DNA repair pathways and mitochondrial DNA mutations in gastrointestinal carcinogenesis. Clin Chim Acta. 2007;381:50–55. doi: 10.1016/j.cca.2007.02.020. [DOI] [PubMed] [Google Scholar]
- Benson V.S. Patnick J. Davies A.K. Nadel M.R. Smith R.A. Atkin W.S. Colorectal cancer screening: a comparison of 35 initiatives in 17 countries. Int J Cancer. 2008;122:1357–1367. doi: 10.1002/ijc.23273. [DOI] [PubMed] [Google Scholar]
- Bertario L. Russo A. Sala P. Pizzetti P. Ballardini G. Andreola S., et al. Predictors of metachronous colorectal neoplasms in sporadic adenoma patients. Int J Cancer. 2003;105:82–87. doi: 10.1002/ijc.11036. [DOI] [PubMed] [Google Scholar]
- Birket M.J. Passos J.F. von Zglinicki T. Birch-Machin M.A. The relationship between the aging- and photo-dependent T414G mitochondrial DNA mutation with cellular senescence and reactive oxygen species production in cultured skin fibroblasts. J Invest Dermatol. 2009;129:1361–1366. doi: 10.1038/jid.2008.373. [DOI] [PubMed] [Google Scholar]
- Brandon M. Baldi P. Wallace D.C. Mitochondrial mutations in cancer. Oncogene. 2006;25:4647–4662. doi: 10.1038/sj.onc.1209607. [DOI] [PubMed] [Google Scholar]
- Canter J.A. Kallianpur A.R. Parl F.F. Millikan R.C. Mitochondrial DNA G10398A polymorphism and invasive breast cancer in African-American women. Cancer Res. 2005;65:8028–8033. doi: 10.1158/0008-5472.CAN-05-1428. [DOI] [PubMed] [Google Scholar]
- Cavalier-Smith T. Origin of mitochondria by intracellular enslavement of a photosynthetic purple bacterium. Proc Biol Sci. 2006;273:1943–1952. doi: 10.1098/rspb.2006.3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S.C. Lin P.C. Yang S.H. Wang H.S. Liang W.Y. Lin J.K. Mitochondrial D-loop mutation is a common event in colorectal cancers with p53 mutations. Int J Colorectal Dis. 2009;24:623–628. doi: 10.1007/s00384-009-0663-9. [DOI] [PubMed] [Google Scholar]
- Chatterjee A. Mambo E. Sidransky D. Mitochondrial DNA mutations in human cancer. Oncogene. 2006;25:4663–4674. doi: 10.1038/sj.onc.1209604. [DOI] [PubMed] [Google Scholar]
- Crimi M. Rigolio R. The mitochondrial genome, a growing interest inside an organelle. Int J Nanomed. 2008;3:51–57. doi: 10.2147/ijn.s2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croteau D.L. Bohr V.A. Repair of oxidative damage to nuclear and mitochondrial DNA in mammalian cells. J Biol Chem. 1997;272:25409–25412. doi: 10.1074/jbc.272.41.25409. [DOI] [PubMed] [Google Scholar]
- Dyall S.D. Brown M.T. Johnson P.J. Ancient invasions: from endosymbionts to organelles. Science. 2004;304:253–257. doi: 10.1126/science.1094884. [DOI] [PubMed] [Google Scholar]
- Eaden J.A. Abrams K.R. Mayberry J.F. The risk of colorectal cancer in ulcerative colitis: a meta-analysis. Gut. 2001;48:526–535. doi: 10.1136/gut.48.4.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekbom A. Helmick C. Zack M. Adami H.O. Ulcerative colitis and colorectal cancer A population-based study. N Engl J Med. 1990;323:1228–1233. doi: 10.1056/NEJM199011013231802. [DOI] [PubMed] [Google Scholar]
- Feder J. Blech I. Ovadia O. Amar S. Wainstein J. Raz I., et al. Differences in mtDNA haplogroup distribution among 3 Jewish populations alter susceptibility to T2DM complications. BMC Genomics. 2008;9:198. doi: 10.1186/1471-2164-9-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fliss M.S. Usadel H. Caballero O.L. Wu L. Buta M.R. Eleff S.M., et al. Facile detection of mitochondrial DNA mutations in tumors and bodily fluids. Science. 2000;287:2017–2019. doi: 10.1126/science.287.5460.2017. [DOI] [PubMed] [Google Scholar]
- Gonzalez A.M. Larruga J.M. Abu-Amero K.K. Shi Y. Pestano J. Cabrera V.M. Mitochondrial lineage M1 traces an early human backflow to Africa. BMC Genomics. 2007;8:223. doi: 10.1186/1471-2164-8-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greaves L.C. Preston S.L. Tadrous P.J. Taylor R.W. Barron M.J. Oukrif D., et al. Mitochondrial DNA mutations are established in human colonic stem cells, and mutated clones expand by crypt fission. Proc Natl Acad Sci U S A. 2006;103:714–719. doi: 10.1073/pnas.0505903103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habano W. Sugai T. Yoshida T. Nakamura S. Mitochondrial gene mutation, but not large-scale deletion, is a feature of colorectal carcinomas with mitochondrial microsatellite instability. Int J Cancer. 1999;83:625–629. doi: 10.1002/(sici)1097-0215(19991126)83:5<625::aid-ijc10>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Herrnstadt C. Elson J.L. Fahy E. Preston G. Turnbull D.M. Anderson C., et al. Reduced-median-network analysis of complete mitochondrial DNA coding-region sequences for the major African, Asian, and European haplogroups. Am J Hum Genet. 2002;70:1152–1171. doi: 10.1086/339933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa K. Takenaga K. Akimoto M. Koshikawa N. Yamaguchi A. Imanishi H., et al. ROS-generating mitochondrial DNA mutations can regulate tumor cell metastasis. Science. 2008;320:661–664. doi: 10.1126/science.1156906. [DOI] [PubMed] [Google Scholar]
- Jakupciak J.P. Maragh S. Markowitz M.E. Greenberg A.K. Hoque M.O. Maitra A., et al. Performance of mitochondrial DNA mutations detecting early stage cancer. BMC Cancer. 2008;8:285. doi: 10.1186/1471-2407-8-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jazin E.E. Cavelier L. Eriksson I. Oreland L. Gyllensten U. Human brain contains high levels of heteroplasmy in the noncoding regions of mitochondrial DNA. Proc Natl Acad Sci U S A. 1996;93:12382–12387. doi: 10.1073/pnas.93.22.12382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y. Bing X. Zhuo S.L. Mutation of mitochondrial DNA in colorectal cancer cells. Chin J Dig Dis. 2005;25:376–377. [Google Scholar]
- Li Y. Bing X. Wei B.S. Yan G. Zhuo S.L. Mutation analysis of mitochondrial DNA in colorectal carcinoma tissues by silver-staining PCR-SSCP. J Furth Mil Med Univ. 2006;27:1188–1190. [Google Scholar]
- Li Y. Weibing S. Liu H. Hongli J. Zhuosheng L. Yadong W., et al. Mitochondrial DNA from colorectal cancer cells promotes the malignant phenotype of NIH3T3 cells. Cell Biol Int. 2008;32:979–983. doi: 10.1016/j.cellbi.2008.04.015. [DOI] [PubMed] [Google Scholar]
- Lievre A. Chapusot C. Bouvier A.M. Zinzindohoue F. Piard F. Roignot P., et al. Clinical value of mitochondrial mutations in colorectal cancer. J Clin Oncol. 2005;23:3517–3525. doi: 10.1200/JCO.2005.07.044. [DOI] [PubMed] [Google Scholar]
- Maximo V. Soares P. Seruca R. Rocha A.S. Castro P. Sobrinho-Simoes M. Microsatellite instability, mitochondrial DNA large deletions, and mitochondrial DNA mutations in gastric carcinoma. Genes Chromosomes Cancer. 2001;32:136–143. doi: 10.1002/gcc.1175. [DOI] [PubMed] [Google Scholar]
- Mimaki M. Hatakeyama H. Ichiyama T. Isumi H. Furukawa S. Akasaka M., et al. Different effects of novel mtDNA G3242A and G3244A base changes adjacent to a common A3243G mutation in patients with mitochondrial disorders. Mitochondrion. 2009;9:115–122. doi: 10.1016/j.mito.2009.01.005. [DOI] [PubMed] [Google Scholar]
- MITOMAP. A Human Mitochondrial Genome Database. 2009. www.mitomap.org www.mitomap.org
- Mokhtar N. Gouda I. Adel I. Cancer pathology registry 2003–2004 and time trend analysis. In: Mokhtar N., editor; Gouda I., editor; Adel I., editor. Malignant digestive system tumors. NCI, Elsheraa Press; Cairo: 2007. pp. 55–67. [Google Scholar]
- Muto T. Bussey H.J. Morson B.C. The evolution of cancer of the colon and rectum. Cancer. 1975;36:2251–2270. doi: 10.1002/cncr.2820360944. [DOI] [PubMed] [Google Scholar]
- Polyak K. Li Y. Zhu H. Lengauer C. Willson J.K. Markowitz S.D., et al. Somatic mutations of the mitochondrial genome in human colorectal tumours. Nat Genet. 1998;20:291–293. doi: 10.1038/3108. [DOI] [PubMed] [Google Scholar]
- Reeve A.K. Krishnan K.J. Turnbull D. Mitochondrial DNA mutations in disease, aging, and neurodegeneration. Ann N Y Acad Sci. 2008;1147:21–29. doi: 10.1196/annals.1427.016. [DOI] [PubMed] [Google Scholar]
- Scoggan K.A. Bulman D.E. Single-strand conformational polymorphism analysis (SSCP) and sequencing for ion channel gene mutations. Methods Mol Biol. 2003;217:143–151. doi: 10.1385/1-59259-330-5:143. [DOI] [PubMed] [Google Scholar]
- Stewart B.W. Kleihues P. In World Cancer Report. In: Stewart B.W., editor; Kleihues P., editor. Colorectal Cancer. IARC Press; Lyon: 2003. pp. 198–201. [Google Scholar]
- Sui G. Zhou S. Wang J. Canto M. Lee E.E. Eshleman J.R., et al. Mitochondrial DNA mutations in preneoplastic lesions of the gastrointestinal tract: a biomarker for the early detection of cancer. Mol Cancer. 2006;5:73. doi: 10.1186/1476-4598-5-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor R.W. Barron M.J. Borthwick G.M. Gospel A. Chinnery P.F. Samuels D.C., et al. Mitochondrial DNA mutations in human colonic crypt stem cells. J Clin Invest. 2003;112:1351–1360. doi: 10.1172/JCI19435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theodoratou E. Din F.V. Farrington S.M. Cetnarskyj R. Barnetson R.A. Porteous M.E., et al. Association between common mtDNA variants and all-cause or colorectal cancer mortality. Carcinogenesis. 2010;31:296–301. doi: 10.1093/carcin/bgp237. [DOI] [PubMed] [Google Scholar]
- Wallace D.C. The mitochondrial genome in human adaptive radiation and disease: on the road to therapeutics and performance enhancement. Gene. 2005a;354:169–180. doi: 10.1016/j.gene.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Wallace D.C. A mitochondrial paradigm of metabolic and degenerative diseases, aging, and cancer: a dawn for evolutionary medicine. Annu Rev Genet. 2005b;39:359–407. doi: 10.1146/annurev.genet.39.110304.095751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanrooij S. Luoma P. van Goethem G. van Broeckhoven C. Suomalainen A. Spelbrink J.N. Twinkle and POLG defects enhance age-dependent accumulation of mutations in the control region of mtDNA. Nucleic Acids Res. 2004;2:3053–3064. doi: 10.1093/nar/gkh634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winawer S.J. Zauber A.G. Fletcher R.H. Stillman J.S. O'Brien M.J. Levin B., et al. Guidelines for colonoscopy surveillance after polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer and the American Cancer Society. Gastroenterology. 2006;130:1872–1885. doi: 10.1053/j.gastro.2006.03.012. [DOI] [PubMed] [Google Scholar]
- Yeh J.J. Lunetta K.L. van Orsouw N.J. Moore F.D., Jr. Mutter G.L. Vijg J., et al. Somatic mitochondrial DNA (mtDNA) mutations in papillary thyroid carcinomas and differential mtDNA sequence variants in cases with thyroid tumours. Oncogene. 2000;19:2060–2066. doi: 10.1038/sj.onc.1203537. [DOI] [PubMed] [Google Scholar]
- Zhu W. Qin W. Bradley P. Wessel A. Puckett C.L. Sauter E.R. Mitochondrial DNA mutations in breast cancer tissue and in matched nipple aspirate fluid. Carcinogenesis. 2005;26:145–152. doi: 10.1093/carcin/bgh282. [DOI] [PubMed] [Google Scholar]