Abstract
Vitamin A deficiency in the mouse results in an arrest in the progression of undifferentiated spermatogonia to differentiating spermatogonia. The supplement of retinol to vitamin-A-deficient mice reinitiates spermatogenesis in a synchronous manner throughout the testes. It is unclear whether the effects of retinoids are the result of a direct action on germ cells or are indirectly mediated through Sertoli cells. The expression of Stimulated by retinoic acid gene 8 (Stra8), which is required for spermatogenesis, is directly related to the availability of retinoic acid (RA). Analysis of gene expression by microarrays revealed moderate levels of Stra8 transcript in gonocytes and high levels in A and B spermatogonia. Stra8 mRNA levels were greatly reduced or absent in germ cells once they entered meiosis. This study examined the effect of retinoic acid on cultured neonatal testes and isolated gonocytes/spermatogonia in vitro. THY1+ and KIT+ germ cells were isolated by magnetic-activated cell sorting from the testes of mice of different ages. Isolated germ cells were cultured and treated with either vehicle (ethanol) or RA without feeder cells. We found that 1) Stra8 is predominantly expressed in premeiotic germ cells, 2) RA stimulates gonocyte DNA replication and differentiation in cultured neonatal testes, 3) in the absence of feeder cells, RA directly induces the transition of undifferentiated spermatogonia to differentiating spermatogonia by stimulating Stra8 and Kit gene expression, 4) RA dramatically stimulates Stra8 expression in undifferentiated spermatogonia but has a lesser impact in differentiating spermatogonia, 5) endogenous Stra8 gene expression is higher in differentiating spermatogonia than in undifferentiated spermatogonia and could mediate the RA effects on spermatogonial maturation, and 6) RA stimulates a group of genes involved in the metabolism, storage, transport, and signaling of retinoids.
Keywords: differentiation, gonocytes, in vitro, retinoic acid, spermatogenesis, spermatogonia, spermatogonial differentiation, Stra8
INTRODUCTION
In many adult male mammals, sperm production is a continuous process in which millions of sperm are generated each day, throughout life. Sperm production is truly one of the most dynamic cell-producing systems in the body, and it depends on the active proliferation and differentiation of spermatogonial stem cells (SSC) [1]. SSC originate from primordial germ cells, which, in turn, become gonocytes once they migrate to the genital ridges and become enclosed by differentiating Sertoli cells [2]. In rodents, gonocytes divide for a few days and then arrest in the G0/G1 phase of the cell cycle. The gonocytes arrested in mitosis migrate from the center of the cords to the peripheral region and reach the basement membrane. In mice, this migration is completed within the first few days after birth, and the gonocytes resume proliferation and gonocytes/prespermatogonia differentiate into SSC in the same period [1, 2].
In rodents, SSC are single cells located on the basement membrane of the seminiferous tubules and are called A-single (As) spermatogonia. The SSC divide either into two new single cells or into a pair of spermatogonia (Apr) that do not complete cytokinesis and stay connected by an intercellular bridge. The Apr spermatogonia divide further to form chains of 4, 8, and occasionally, up to 32 A-aligned (Aal) spermatogonia. The Aal spermatogonia go through a differentiation step and become A1 spermatogonia [3]. In rodents, there are five divisions following A1 formation, forming successively A2, A3, A4, In (intermediate), and B spermatogonia. The B spermatogonia divide into spermatocytes. Each step of differentiation except the transition of Aal to A1 is associated with a mitotic division. As spermatogonia are considered the only true SSC, even though As, Apr, and Aal are termed “undifferentiated spermatogonia,” whereas spermatogonia from A1 to B are termed “differentiating spermatogonia” [3].
The massive production of mature sperm is dependent on successive mitotic divisions and associated cell differentiation of spermatogonia. A few factors involved in the regulation of spermatogonial differentiation have been identified [4], including retinoic acid (RA) [5, 6], cyclin D2 (CCND2) [7], KIT/KITL [8, 9], and DAZL [10]. It has been known since 1925 that vitamin A is required for normal spermatogenesis. Spermatogenesis ceases and only A spermatogonia are found in vitamin-A-deficient (VAD) mouse testes. Supplementing vitamin A (retinol) to VAD mice reinitiates spermatogenesis in a synchronous fashion throughout the testis [11]. Haneji et al. [12] showed that both retinol and RA could induce differentiation of type A spermatogonia in cultured mouse cryptorchid testes. This conclusion was based on the morphological identification of In and type B spermatogonia after 9 days of culture with retinoids [12]. However, it is not known whether the action of RA in inducing spermatogonial differentiation is a direct effect on germ cells or an indirect effect mediated through Sertoli cells, because both spermatogonia and Sertoli cells possess nuclear receptors for retinoids [2, 13]. Moreover, the molecular mechanism of RA action in spermatogonia is obscure. In a recent study, RA was demonstrated to induce the expression of Stra8, a factor essential for the onset of meiosis in both male and female gonads [14]. The knockout of the Stra8 gene blocks entry into meiosis in both embryonic ovaries and pubertal testes [14, 15]. Thus, the expression of Stra8 appears to be a sensitive indicator of the availability of RA and entry of cells into meiosis.
Several different methods to collect spermatogonia enriched in SSC have been established, with one of the most effective ways being magnetic-activated cell sorting (MACS) directed against cell surface markers [16]. THY1 is likely a common surface marker for all undifferentiated spermatogonia, and THY1-positive selection using MACS results in significant enrichment of SSC [16]. This technique allows the examination of factors that potentially regulate the proliferation and differentiation of spermatogonia in a controlled, in vitro system [17]. Our study utilized short-term cultured neonatal mouse testes and specific MACS subpopulations of spermatogonia using Stra8 and Kit as endpoints to test two objectives: 1) determine if RA can induce the in vitro differentiation of early germ cells in the absence of somatic cells and 2) identify the subpopulation of spermatogonia in which RA induces Stra8 expression and spermatogonial differentiation.
MATERIALS AND METHODS
Animal Care and Treatment
All animal experiments were approved by Washington State University Animal Care and Use Committees and were conducted in accordance with the guiding principles for the care and use of research animals of the National Institutes of Health. A BL/6–129 mouse colony was maintained in a temperature- and humidity-controlled room with food and water provided ad libitum. To investigate the induction of STRA8 protein expression by RA in neonatal gonocytes, 2-day-postpartum (dpp) male mice were injected subcutaneously with all-trans-RA (90% oil, 10% ethanol) at a dose of 17.5 µg/mouse or injected with vehicle as control. Testes were fixed 24 h later in freshly prepared 4% (w/v) paraformaldehyde for immunohistochemical staining of STRA8 protein. Four mice were used in each treatment group.
Testicular Cell Types and RNA Preparation
Enriched germ cell isolates for microarray analysis were prepared via gravity sedimentation from CD1 mice as previously described [18]. These samples included type A and B spermatogonia, pachytene spermatocytes, and round spermatids from animals 8, 30, and 60 days of age, respectively. The purity of all the isolated and cultured cells was determined by morphology. The purity of type A and B spermatogonia was >85%, and the purity of all other germ cell types was >95%. The quality control of the total RNA samples was performed as previously described [19]. Two replicates were used in microarray analysis.
Microarray Processing
The following method was used for microarray processing of 2 dpp/5 dpp gonocytes, type A/type B spermatogonia, pachytene spermatocytes, round spermatids, and Kit-deficient (W/Wv) mutant testes. Ten micrograms of total RNA from each sample was used to create the target for the microarray. The labeled cRNA was hybridized to Mouse Genome 430 2.0 arrays (Affymetrix, Santa Clara, CA) and stained in accordance with the manufacturer’s standard protocol. The same procedure was performed on duplicate samples. The arrays were stained utilizing the Affymetrix GeneChip Fluidics Station 400 and scanned using a GeneArray Scanner 2500A (Agilent Technologies, Palo Alto, CA). The resulting data were viewed and analyzed using GeneSpring software (Agilent Technologies, Santa Clara, CA). All reactions and microarray hybridization procedures were performed in the Laboratory for Biotechnology and Bioanalysis I at Washington State University. The different cell type data was normalized within GeneSpring using the default/recommended normalization methods. These include setting of signal values below 0.01 to 0.01, total chip normalization to the 50th percentile. These normalizations allowed for the visualization of data based on relative abundance at any cell type or organ rather than compared to a specific control value.
A different method was used for microarray analysis of RA-treated 2 dpp THY1+ gonocytes. Approximately 75 ng total RNA from each sample was used to generate cDNA using the Ovation RNA Amplification System V2 (NuGEN, San Carlos, CA) according to the manufacturer’s instructions. Array quality was assessed, and images were quantified using GeneChip Operating Software v1.2 (Affymetrix). The data were viewed and analyzed using GeneSpring software. The criteria for RA-regulated genes included a raw signal value of no less than 50 in either control or treated samples, a 2-fold or higher change in signal between the control and treated samples, and the consistent changes in duplicates.
Neonatal Testis Organ Culture and Treatments
Neonatal testes from 2 dpp mice were detunicated, cut into four to six small pieces per testis, placed on Millicell CM filters (Millipore, Bedford, MA) floating on the surface of medium and covered with drops of medium (Dulbecco modified Eagle medium/F12/10% fetal bovine serum) and cultured for 24 h. The cultured testes were treated with all-trans-RA (ATRA) or vehicle (ethanol). The final concentration of ATRA, 9 cis-RA, 4-oxo-RA, and retinol acetate (ROL) in the culture medium was 0.7 µM [14], and the final concentration of ketoconazole was 0.94 µM. After 24 h, tissues were harvested, and RNA was isolated using the PicoPure system (Arcturus Bioscience, Mountain View, CA). For studies involving RNA, three vehicle-treated and three ATRA-treated cultured testes were used. For studies of 5-bromo-2′-deoxyuridine (BrdU) incorporation, the vehicle control group contained five testes, and the RA-treated group contained six cultured testes. The final concentration of BrdU in the culture medium was 10 µM. The testis tissues were fixed in Bouin solution after treatment.
Immunohistochemical Double Staining of BrdU and Germ Cell Nuclear Antigen 1
Tissues were fixed in Bouin solution and embedded in paraffin. Tissues were dewaxed and incubated in 2N HCl at 37°C for 30 min. The staining procedure was performed as previously described, with minor modifications [19]. Briefly, mouse BrdU antibody (1:200 dilution, Vision BioSystems, Norwell, MA) was applied first and incubated at 4°C overnight. After three washes in PBS, tissues were incubated with secondary antibody (tetramethylrhodamine-isothiocyanate-conjugated donkey anti-mouse IgG, 1:100; Jackson ImunoResearch Laboratory, West Grove, PA) at 37°C for 1 h. Tissues were blocked with 10% donkey serum and incubated with rat germ cell nuclear antigen 1 (GCNA1) antibody (1:50 dilution; gift from Dr. George Enders of the University of Kansas) at 37°C for 2 h, and then secondary antibody (1:100 dilution; fluorescein-isothiocyanate-conjugated donkey anti-rat IgG; Jackson ImunoResearch Laboratory, West Grove, PA) was applied at 37°C for 1 h. Sections incubated in PBS without primary antibody were used as negative controls for color development on the same slide. Images were captured with an Olympus OLY-200 digital camera (Olympus America, Inc., Melville, NY) using Olympus MagnaFire Camera Imaging and Control (version 1.0; Olympus America) and compiled using Adobe PhotoShop 7.0 (Adobe Systems, San Jose, CA). The number of GCNA1-positive cells in each testis section was counted first. The GCNA1 and BrdU staining images were overlapped in PhotoShop, and double positive cells were counted. Finally, the percentage of BrdU-positive germ cells per total germ cell number was calculated. The Student t-test was used for statistical analysis, and P < 0.05 was considered to be statistically significant.
Production of Anti-STRA8 Serum
To generate STRA8 antibody, a peptide termed P3 and corresponding to the C-terminus (C L N Q E P E P P D D) of mouse STRA8 protein was synthesized and conjugated with carrier protein maleimide activated keyhole limpet hemocyanin (mcKLH, Pierce, Rockford, IL) following manufacturer’s instructions. At Day 0, preimmune serum was collected from a New Zealand rabbit, then 1 mg of conjugated protein in 0.7 ml of PBS mixed with 0.7 ml of Freund complete adjuvant was injected into the rabbit subcutaneously. At Day 28, the rabbit was given a booster of 0.5 mg conjugated protein in Freund incomplete adjuvant. The STRA8 antiserum was harvested 2 wk after the booster injection.
Western Blot Analysis, Immunohistochemistry, and Antibody Competition
The specificity of the STRA8 antibody was tested in three ways: a Western blot analysis using P19 cells, immunohistochemistry performed on normal adult mouse testes and on Stra8-deficient mouse testes, and antibody competition with P3 peptide (corresponding to the C-terminus of STRA8 protein and used in STRA8 antibody generation) in Western blot and immunohistochemistry. Briefly, P3 peptide (300 µg/ml) was incubated together with STRA8 antibody overnight at 4°C, then the mixture was used as primary antibody as described later. Protein from the murine embryonic carcinoma cell line P19 cells (ATCC, Manassas, VA) was used for Western blot analysis because Stra8 was first identified in this cell line [20]. P19 cells were cultured based on the instructions of ATCC and were treated with either vehicle ethanol or 1 µM RA (final concentration in the culture medium) for 24 h. Protein was harvested in 2× Laemmli buffer and then separated by 12% SDS-PAGE and transferred to nitrocellulose membrane (0.45 µm). The membrane was blocked in PBS containing 5% nonfat milk and 0.025% Tween 20 for 1 h at room temperature and incubated with STRA8 antiserum (1:500) overnight at 4°C. Total proteins from MSC1 cells (a Sertoli cell line) were used as negative control. After incubation, membranes were rinsed with PBS with Tween 20 and incubated with horseradish peroxidase conjugated goat anti-rabbit IgG for 1 h at room temperature. Reactive proteins were visualized and exposed to films by Western lighting chemiluminescence reagent (Perkin Elmer, Boston, MA). For immunohistochemistry, serial sections of the mouse testis incubated with preimmune serum (1:500) were used as negative controls.
Immunohistochemistry of STRA8 Protein in Neonatal Testes
Neonatal testes were removed 24 h after RA injection and fixed in freshly prepared 4% (w/v) paraformaldehyde. Antigen retrieval in 0.01 M sodium citrate, pH 6.0, was done using microwave radiation and a trypsin (1 mg/ml) digestion for 7 min at 37°C. For immunohistochemical staining, ZYMED LAB-SA SYSTEM (Zymed Laboratory, South San Francisco, CA) was used, following the manufacturer’s instructions. Briefly, tissue sections were incubated with 10% goat serum followed by incubation with STRA8 antiserum (1:2000) or preim (1:2000) at 4°C overnight. The slides were incubated with diluted biotinylated secondary antibody for 45 min, followed by the application of streptavidin-horseradish peroxidase. The diaminobenzidine solution was applied until brown color developed. Staining was observed with a Nikon Microphot-FX (Meridian Instrument Company, Kent, WA) microscope. Photographs were taken with an Olympus OLY-200 digital camera using Olympus MagnaFire Camera Imaging and Control version 1.0 (Olympus America).
Real-Time RT-PCR
A two-step real-time RT-PCR was used to measure the expression of candidate genes as previously described [19]. Each RNA sample was analyzed in triplicate with primers for each gene. Stra8 primers amplify a 151-bp product (primers: 5′-GTTTCCTGCGTGTTCCACAAG-3′ and 5′-CACCCGAGGCTCAAGCTTC-3′); control Rps2 primers amplify a 112-bp product (primers: 5′-CTGACTCCCGACCTCTGGAAA-3′ and 5′-GAGCCTGGGTCCTCTGAACA-3′); Kit primers amplify a 100-bp product (primers: 5′-GATGGCGTTCCTCGCCT-3′ and 5′-GCCCGAAATCGCAAATCTTT-3′). Expression of Stra8 and Kit were normalized to Rps2 expression. The Student t-test was used to analyze the results from neonatal testes, and a pair-wise t-test was used to analyze the results of all experiments using isolated cells. Data represent mean ± SEM.
MACS of Gonocytes/Spermatogonia
Testes were collected from 2 dpp, 4 dpp, or 8 dpp mouse pups into Hanks balanced salt solution, and the tunica were removed. MicroBeads with mouse CD117 (KIT) and CD90 (THY1) antibodies and MS columns (Miltenyi Biotec, Auburn, CA) were used in the isolation according to manufacturer’s instructions. The culture was performed based on Kubota and Brinster’s method with modifications [16]. For experiments using 8 dpp testes, the cells were always subjected to THY1+ MACS first, then the THY1− cells were subjected to KIT+ MACS to obtain two separate cell populations. Isolated cells were cultured under feeder-cell-free, serum-free, and growth-factor-free conditions for 8 or 24 h with only vehicle (ethanol) or ATRA (0.7 µM final concentration) or ROL (0.7 µM final concentration). For each cell isolation, twelve to fifteen 2 dpp, ten to twelve 4 dpp, and six to ten 8 dpp mice were used to ensure a high enough number of cells for the subsequent experiments. Each treatment group contained at least three independent repeats.
STA-PUT Gonocyte Isolation
Testes from 2 dpp mice were enzymatically digested as described by O’Brien et al. [21] with modifications. Gonocytes or spermatogonia were identified by phase contrast microscopy and pooled. The identity of the cells was confirmed by GCNA1 immunocytochemistry, and 80%–90% of the cells obtained by STA-PUT were GCNA1 positive (data not shown).
Immunocytochemistry of POU5F1 (OCT4) and GCNA1
Spermatogonia isolated from 8 dpp testes by MACS (THY1 or KIT positive) were fixed in 4% paraformaldehyde (with 0.25% glutaraldehyde) for POU5F1 staining and in Bouin solution for GCNA1 staining. After a few PBS washes, cells were incubated with 3% H2O2 for 20 min at room temperature and then blocked with 10% normal goat serum (for POU5F1) or 10% normal rabbit serum (for GCNA1) for 30 min (goat anti-rabbit IgG for POU5F1 staining, Zymed; rabbit anti-rat IgM for GCNA1 staining, Cortex Biochem, San Leandro, CA). After PBS washes, primary antibodies, either rabbit polyclonal anti-POU5F1 (1:100; Abcam, Cambridge, MA) or GCNA1 (1:50), were added and incubated at 37°C for 2 h, followed by a PBS rinse and biotin-conjugated secondary antibody incubation at 37°C for 1 h. Diaminobenzidine was used as substrate for peroxidase after streptavidin conjugation. The cells subjected to GCNA1 staining were counterstained with hematoxylin (Sigma-Aldrich, St. Louis, MO), and cells stained for POU5F1 were counterstained with nuclear fast red (Vector, Burlingame, CA).
RESULTS
Stra8 Expression in Several Types of Premeiotic Germ Cells
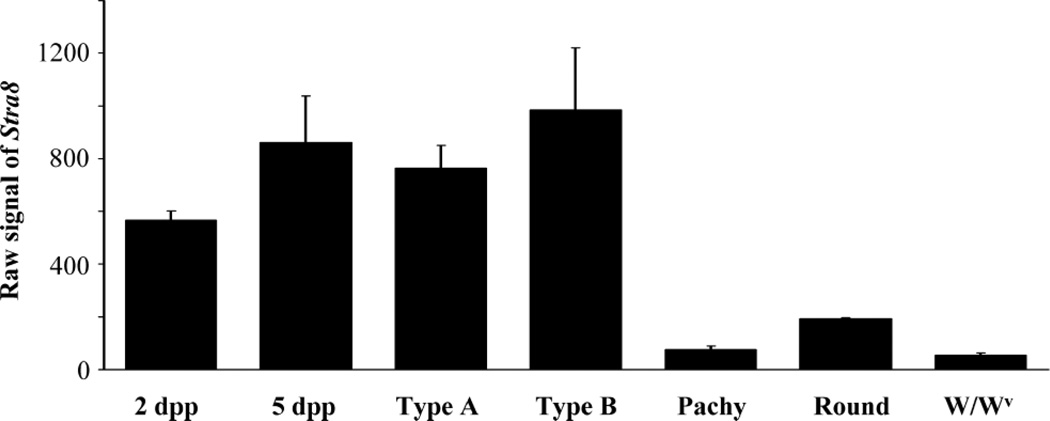
The gene expression profile of Stra8 mRNA was obtained by using microarray results from isolated germ cell samples (Fig. 1). Stra8 mRNA was expressed in 2 dpp and 5 dpp gonocytes, type A spermatogonia, and type B spermatogonia. The expression level of Stra8 mRNA decreased significantly in meiotic and postmeiotic germ cells with the lowest levels of expression observed in pachytene spermatocytes and round spermatids. In the adult W/Wv mutant testis, Stra8 expression was at background levels.
FIG. 1.
Stra8 mRNA expression in different germ cell types by microarray analysis. Values represent raw signal of Stra8 ± SEM. 2 dpp, 2 d post partum gonocytes; 5 dpp, 5 d post partum gonocytes; Type A, type A spermatogonia; Type B, type B spermatogonia; Pachy, pachytene spermatocytes; Round, round spermatids; W/Wv, adult W/Wv mutant testes.
RA Stimulation of DNA Replication in Vitamin-A-Sufficient Neonatal Gonocytes
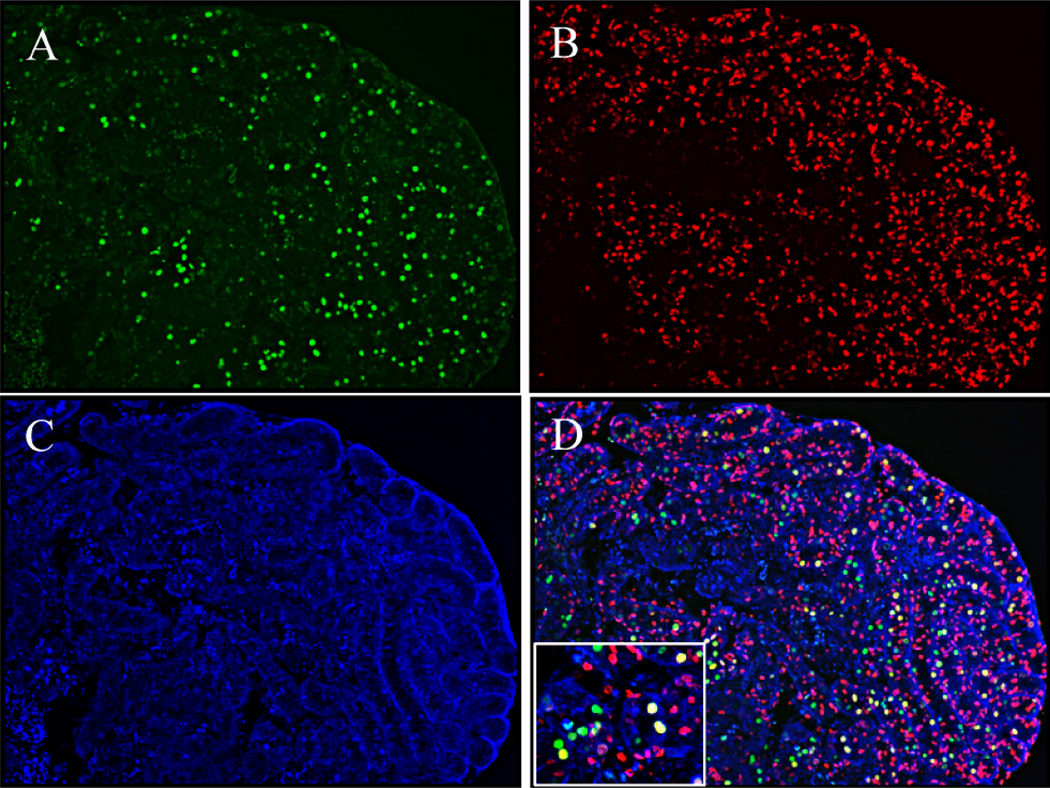
It has been shown that retinoids can induce DNA replication and proliferation in arrested A spermatogonia in VAD adult rats [5]. The effect of RA on gonocytes/prespermatogonia in vitamin-A-sufficient, normal neonatal testes was examined. Neonatal testes (2 dpp) were cultured for 24 h with or without RA, fixed in Bouin solution, and embedded in paraffin. Tissue sections were double-stained with GCNA1 and BrdU antibodies (Fig. 2). The BrdU-positive germ cells were counted and the percentage of BrdU-positive gonocytes/spermatogonia per total germ cell number calculated. The percentage of BrdU-positive gonocytes/spermatogonia per total germ cell number increased 17% (44% ± 6% vs. 61% ± 5%; P < 0.01) in the testes treated with RA, compared to vehicle-treated testes.
FIG. 2.
Immunohistochemical double staining of GCNA1 and BrdU in cultured neonatal testes. A) Immunostaining of GCNA1 (green). B) Immunostaining of BrdU (red). C) 4′,6-diamidino-2-phenylindole counterstaining of cell nuclei (blue). D) Overlay of images A, B, and C. Nuclei of proliferating germ cells appear yellow. Original magnification ×100 (A–D) and ×200 (inset).
RA Induction of Stra8 and Kit mRNA Expression in Cultured Vitamin-A-Sufficient Neonatal Testes
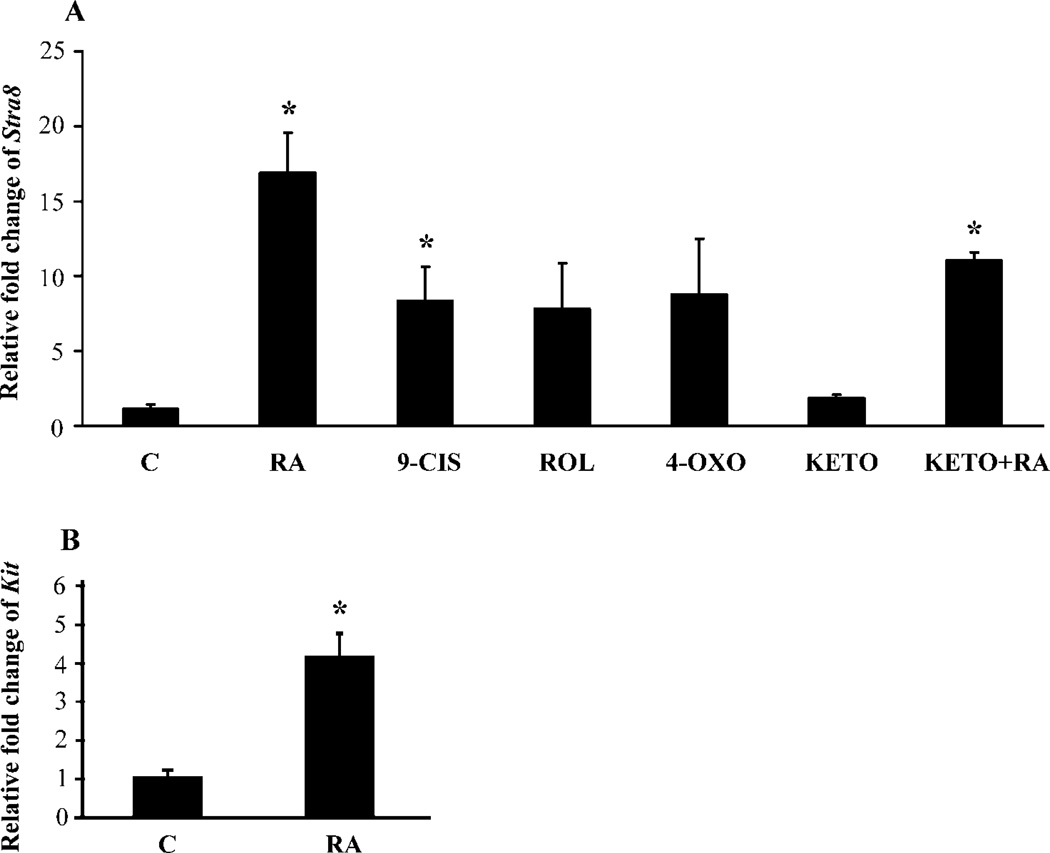
Previous studies demonstrated that males and females share an identical meiotic initiation pathway in which RA induces Stra8 expression in both embryonic ovaries and adult testes, and Stra8 is required for the transition into meiosis in both male and female germ cells [14, 15]. The action of RA on the expression of Stra8 mRNA in 2 dpp testes was examined in organ culture by real-time RT-PCR. ROL (0.7 µM), RA (0.7 µM), and 9-cis-RA (0.7 µM), one major retinoic acid metabolite 4-oxo-RA and a pan-P450 enzyme inhibitor ketoconazole (0.9 µM) were utilized in this study (Fig. 3A). In 2 dpp mouse testes cultured on floating membranes, RA significantly increased the expression of Stra8 17-fold 24 hr after treatment (P < 0.01), and 9-cis-RA treatment increased Stra8 expression 8-fold (P < 0.05). Retinol acetate and 4-oxo-RA increased Stra8 expression 7.8-fold and 8.7-fold, respectively. Ketoconazole interferes with the degradation of RA by inhibiting the enzymatic activity of CYP26B1 [14]. Ketoconazole alone did not make a significant change in Stra8 expression. However, ketoconazole seems to reduce the magnitude of the effect of RA. KIT has been demonstrated to be a marker for differentiating spermatogonia [8, 9]. We found that the expression of Kit transcript was increased 4-fold on average (P < 0.05) after RA treatment for 24 h (Fig. 3B).
FIG. 3.
Induction of Stra8/Kit expression by retinoids in cultured neonatal testes examined by real-time RT-PCR. Culture/treatment duration is 24 h. Values represent relative fold change of Stra8 or Kit ± SEM. A) Induction of Stra8 expression by different types of retinoids and pan-P450 inhibitor ketoconazole in cultured 2 dpp testes. B) Induction of Kit expression by RA in cultured 2 dpp testes. Asterisk (*) indicates a statistically significant difference (P < 0.05) between the labeled sample and control sample. C, vehicle control (ethanol); 9-CIS, 9-cis-RA; 4-OXO, 4-oxo-RA; KETO, ketoconazole.
RA Induction of STRA8 Protein Expression in Vitamin-A-Sufficient Neonatal Testes
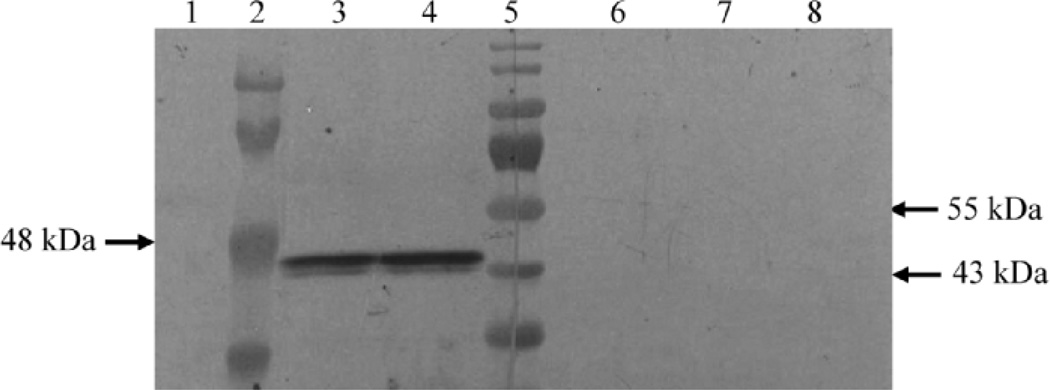
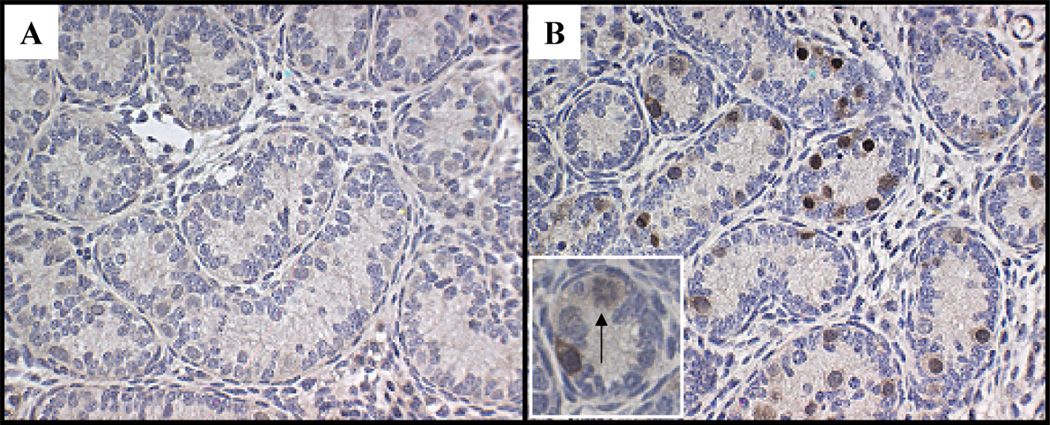
Western blot analysis confirmed that the STRA8 antibody recognized a 44–46 Kd protein product in P19 cells with or without RA treatment (Fig. 4, lanes 3 and 4) and did not detect any signal in MSC1 cells (Fig. 4, lane 1) as expected. The STRA8 band was completely abolished when antibody was incubated overnight with P3 peptide (Fig. 4, lanes 6 and 7), which was used to generate this antibody. STRA8 protein expression was mostly undetectable in the gonocytes of 2 dpp (starting age) testes treated by oil vehicle (for 24 h) by immunohistochemistry with only 0.03% of the tubules on average containing STRA8-positive germ cells (Fig. 5A). However, in 24 h RA-treated 2 dpp testes, STRA8 protein was induced in the cytoplasm of some, but not all, gonocytes, and the percentage of tubules containing STRA8-positive germ cells increased to 41% (P < 0.05; n =4; Fig. 5B). Preimmune serum was used as negative control and did not show any detectable positive staining. The positive immunostaining of STRA8 in spermatogonia in testes of 2 dpp mice was abolished by incubating antibody with P3 peptide overnight (data not shown). No immunohistochemical staining was detected using this antibody on testes from Stra8-deficient mice [15] (data not shown).
FIG. 4.
Western blot analyses and STRA8 antibody. Western blot results of STRA8 (against P3 peptide) antibody with total proteins from P19 cells and MSC1 cells before and after antibody competition with P3 peptide. Lanes 1, 2, 3, and 4 were exposed to STRA8 antibody only, and lanes 6, 7, and 8 were exposed to anti-STRA8 serum preincubated with P3 peptide. Lane 1: MSC1; lane 2: prestained molecular weight marker 1; lane 3: P19 + vehicle (ethanol); lane 4: P19 + RA; lane 5: prestained molecular weight marker 2; lane 6: P19 + RA; lane 7: P19 + vehicle; lane 8: MSC1.
FIG. 5.
Immunohistochemical detection of STRA8 protein induced by RA in cultured neonatal testes. A) Immunostaining of STRA8 after 24 h oil-treated (starting at age 2 dpp mouse testis). B) Immunostaining of STRA8 after 24 h RA treated (starting at age 2 dpp mouse testis). Inset shows a dividing STRA8-positive gonocyte (arrow). Original magnification ×200 (A, B) and ×400 (inset).
RA Induction of Stra8 Expression in Cultured Gonocytes/Prespermatogonia Without Feeder Cells
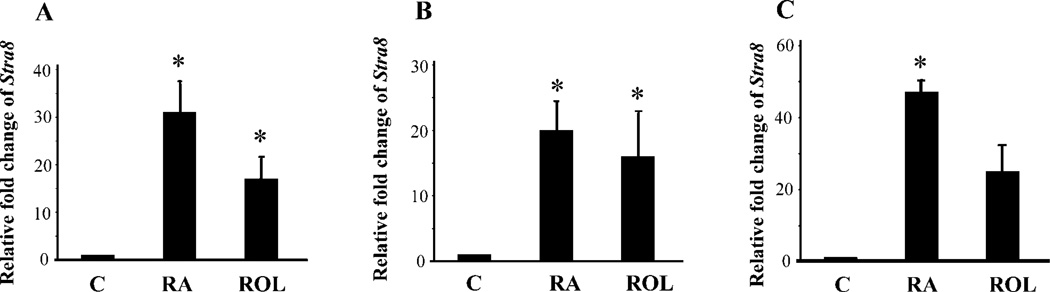
Previous studies have shown that RA can induce Stra8 expression in cultured embryonic testes and ovaries and also in VAD adult testes [14]. Whether this RA induction is a direct effect on germ cells or an indirect effect mediated through testicular somatic cells, in particular Sertoli cells, is still unknown. Neonatal gonocytes were isolated by THY1-positive selection from 2 dpp and 4 dpp testes. THY1 antigen is considered to be a surface marker for male germline stem cells, and THY1-positive selection was used extensively to isolate spermatogonial stem cells for germ cell transplantation experiments [16, 22]. The purity of the cell population was examined by immunocytochemical staining of GCNA1, and approximately 90% of the cells used in the following experiments were GCNA1 positive. The isolated THY1+ gonocytes/spermatogonia were cultured under feeder-cell-free, serum-free, and growth-factor-free conditions and treated with retinoids for 8 h. In THY1+ gonocytes isolated from 2 dpp testes, RA and ROL increased the Stra8 expression level 31-fold (P < 0.01) and 17-fold (P < 0.01), respectively (Fig. 6A). In THY1+ gonocytes/spermatogonia isolated from 4 dpp testes, RA and ROL stimulated Stra8 expression 20-fold (P < 0.01) and 16-fold (P < 0.01), respectively (Fig. 6B). Before establishment of THY1 sorting methods, STA-PUT sedimentation was used to separate different types of germ cells, based on their sizes and shapes [18]. To test whether gonocytes/prespermatogonia isolated by STA-PUT would confirm the RA induction of Stra8 expression observed in THY1-positive cells, isolated gonocytes (85%–90% purity as confirmed by GCNA1 staining) from 2 dpp testes were subjected to identical RA/ROL treatments. Stra8 mRNA expression increased 47-fold by RA (P < 0.05) and 25-fold by ROL in the 8-h treatment (Fig. 6C).
FIG. 6.
Induction of Stra8 expression by RA/ROL (8 h) in cultured neonatal gonocytes without feeder cells examined by real-time RT-PCR. Values represent relative fold change of Stra8 expression ± SEM. A) Induction of Stra8 expression by RA/ROL in THY1+ 2 dpp gonocytes cultured without feeder cells for 8 h. B) Induction of Stra8 expression by RA/ROL in THY1+ 4 dpp gonocytes cultured without feeder cells for 8 h. C) Induction of Stra8 expression by RA/ROL in 2 dpp gonocytes isolated by STA-PUT and cultured without feeder cells for 8 h. Culture medium did not contain serum or any growth factor. Asterisk (*) indicates a statistically significant difference (P < 0.05) between the labeled sample and control sample. C, vehicle control.
RA Induction of Stra8 Expression in Undifferentiated and Differentiating Spermatogonia
Premeiotic male germ cells include primordial germ cells, embryonic gonocytes, prespermatogonia in neonatal testes, spermatogonia, and preleptotene spermatocytes. In the spermatogonial population, As, Apr, Aal (undifferentiated spermatogonia), and differentiating spermatogonia, including A1–4, AIn, and B, have been described and studied [3]. We investigated whether RA could induce Stra8 expression in spermatogonia, and, if so, in which specific cell type does RA induce Stra8 expression? Eight-day-old mouse testes contain undifferentiated and differentiating spermatogonia. KIT is a surface protein preferentially expressed in differentiating spermatogonia [8]. THY1-positive sorting has been used to isolate cells enriched with SSC [22], which we believe is preferentially expressed in undifferentiated spermatogonia. Therefore, we isolated THY1+ and KIT+ spermatogonial cells from 8 dpp testes. The isolated cells were subjected to the THY1+ and KIT+ MACS sequentially to minimize potential variation. POU5F1 (previously known as OCT4) was used as a marker for undifferentiated spermatogonia [23]. Using immunocytochemical staining for GCNA1 and POU5F1, we found that 80%–90% of the spermatogonial cells isolated from 8 dpp testes by THY1+ selection were GCNA1+/POU5F1+, implying that the majority of these cells are undifferentiated spermatogonia. Meanwhile, 40%–50% of the KIT+ spermatogonial cells isolated by this means were also POU5F1 positive and classified as differentiating spermatogonia. The expression of Stra8 was shown to be 15-fold higher (P < 0.05) in differentiating (KIT+) spermatogonia than in undifferentiated (THY1+) spermatogonia. In order to determine if there was a difference between undifferentiated and differentiating spermatogonia with respect to their abilities to respond to RA stimulation, the isolated spermatogonia were cultured for 8 h in the absence of feeder cells with or without RA. Stra8 expression was increased 15.7-fold (P < 0.05) in THY1+ spermatogonia in 8 h, whereas there was only a 2.7-fold increase in Stra8 expression in KIT+ spermatogonia (Fig. 7).
FIG. 7.
RA induction of Stra8 expression in THY+ and KIT+ spermatogonia in 8 h without feeder cells. Values represent relative fold change of Stra8 expression ± SEM. Asterisk (*) indicates a statistically significant difference (P < 0.05) between the RA-treated sample and control sample. THY1+, THY1+ spermatogonia; KIT+, KIT+ spermatogonia.
RA Induction of Cell Differentiation in Cultured Undifferentiated Spermatogonia
It has been shown that retinoids induce differentiation of arrested A spermatogonia in VAD rat testes and cultured cryptorchid testes. It is unknown whether retinoids can induce the differentiation of murine As, Apr, or Aal spermatogonia in the absence of somatic cells in vitro. To this end, the expression of Kit after RA treatment under culture conditions without feeder cells was examined. Consistent, but not statistically significant, up-regulation of Kit after 8 h of RA treatment was observed in THY1+ spermatogonial cells (data not shown). Because the S phase of A spermatogonia lasts about 25 h, and differentiation (after a mitotic division) into A2 spermatogonia occurred only 48 h after retinoid stimulation in vivo under VAD conditions [5], the RA treatment duration was extended to 24 h, and 3.6-fold up-regulation (P < 0.05) of Kit expression in THY1+ spermatogonial cells was detected.
Altered Expression of Genes Involved in Vitamin A Metabolism and Signaling in RA-treated 2 dpp THY1+ Gonocytes Revealed by Microarray Profiling
Using the restrictions as described in Materials and Methods, a signal level of at least 50 in at least one of the samples and the expression levels being consistently up- or down-regulated by 2-fold in the two duplicates, 80 transcripts were up-regulated, and 97 transcripts were down-regulated by RA in 8 h in THY1+ gonocytes. A group of genes involved in the transport, storage, metabolism, and signaling of retinoids was significantly up-regulated by RA stimulation, including Stra6, Lrat, Cyp26a1, Cyp26b1, Por, Dhrs3, Rarb, and Stra8 (Table 1). A complete list of the transcripts will be available through the Griswold Lab home page (http://www.wsu.edu/griswold/microarray) and the National Center for Biotechnology Information Gene Expression Omnibus (http://www.ncbi.nlm.nih.gov/projects/geo/query/acc.cgi?acc=GSE9521).
TABLE 1.
Transcripts involved in retinoid metabolism, storage, transport and signaling that are up-regulated by RA greater than 2-fold in 2 dpp THY1+ gonocytes.
| Genes | GenBank accession no. | Fold change | Function |
|---|---|---|---|
| Lrat | BB518473 | 12 | Retinoids absorption and storage |
| Cyp26a1 | NM_007811 | 11 | Metabolism of RA |
| Stra8 | NM_009292 | 9 | Onset of meiosis |
| Rarb | BB266455 | 9 | Nuclear receptor of RA |
| Dhrs3 | NM_011303 | 6 | Metabolism of all-trans-retinal |
| Stra6 | NM_009291 | 5 | Membrane receptor for retinol binding protein |
| Cyp26b1 | AW049789 | 3 | Metabolism of RA |
| Por | NM_008898 | 2 | Metabolism of RA |
DISCUSSION
In this study we found that 1) Stra8 was predominantly, if not exclusively, expressed in premeiotic germ cells, 2) RA artificially induced Stra8 and Kit expression and increased gonocyte DNA replication in cultured 2 dpp testes, and 3) RA induced STRA8 protein in the cytoplasm of some, but not all of the 2 dpp gonocytes, 4) RA can directly stimulate Stra8 expression in vitro in premeiotic male germ cells without support from somatic cells, 5) RA induced expression of the spermatogonial differentiation marker gene, Kit, in the same feeder-cell-free culture environment, 6) RA dramatically stimulated Stra8 expression in undifferentiated spermatogonia but had a lesser impact on differentiating spermatogonia, 7) the endogenous level of Stra8 expression is higher in differentiating than in undifferentiated spermatogonia, and 8) RA significantly stimulated the expression of a group of genes involved in the metabolism, storage, transport, and signaling of retinoids.
Based on these findings, a detectable level of gonocyte/spermatogonia differentiation can be achieved in vitro by RA stimulation in the absence of somatic support. In addition, Stra8 is very likely a critical component mediating the RA regulation of spermatogonial differentiation. Previous studies suggest that, in the development of early spermatogonia, RA induces the transition of either Aal to A1 or A1 to A2 spermatogonia [5, 11]. RA induction of Stra8 expression is probably a vitally important trigger to the subsequent differentiation steps and commitment toward the formation of primary spermatocytes. It is worth noting that different types of prespermatogonia, including embryonic gonocytes and early neonatal gonocytes, were capable of responding to RA induction as well [14].
The recent findings from several studies revealed that RA is the signal triggering the onset of meiosis in embryonic ovaries [14, 24], and Stra8 is the downstream factor required for this commitment [14]. In the study of Koubova et al. [14], the same regulatory pathway was also confirmed in adult VAD testes. In the male germline, a series of developmental events occur within the first 10 days after birth. The male germ cells do not enter meiosis until 8–10 dpp, as evidenced by the first appearance of preleptotene spermatocytes. This current study demonstrates RA induction of Stra8 mRNA and STRA8 protein in cultured neonatal testes prior to meiotic initiation, which suggests that the first round of meiosis very likely shares the same mechanisms in embryonic ovaries and adult testes. Further, the strong induction of DNA replication by RA indicates that RA might promote the differentiation of these early gonocytes, because almost every step of spermatogonial development is associated with a mitotic division [3]. However, the possibility that RA also stimulates the self-renewal of these gonocytes cannot be ruled out. RA induction of Kit expression in these neonatal testes ultimately substantiates the hypothesis that RA triggers the differentiation of 2 dpp gonocytes, because KIT is proven to be a marker for differentiating spermatogonia.
Several lines of evidence suggest that neonatal gonocytes represent a heterogeneous population. In the early 1970s, Zamboni and Merchant [25] observed bridges connecting some of the gonocytes, indicating that these cells had already differentiated. Later, de Rooij [1, 2] proposed that at the beginning of spermatogenesis some gonocytes give rise to SSC (As), and others directly differentiate to A1 spermatogonia. A more recent result by Yoshida et al. [23], analyzing the unique expression of Ngn3, strongly indicates that the first round of spermatogenesis initiates directly from gonocytes, and they suggest that the state of undifferentiated spermatogonia is not an inevitable step but a developmental option. These recent data show that if an appropriate signal is given, the direct transition of gonocytes to A1 spermatogonia can be induced in an in vitro culture system. This report suggests that the signal inducing differentiation is RA. In this study, RA induced STRA8 protein in some of the 2 dpp gonocytes but not in all of them. This is a strong indication of heterogeneity of neonatal gonocytes. It is possible that some of these neonatal gonocytes could directly enter the phase of differentiating spermatogonia, and others would commit to becoming SSC. Koubova et al. [14] demonstrated that RA could artificially induce Stra8 in embryonic testes. However, the period of incubation of the embryonic testes was 2 days, and the possibility of serial, accelerated differentiation events, including the conversion of embryonic gonocytes to undifferentiated spermatogonia occurring after RA stimulation, could not be excluded.
The results from cultured neonatal testes generated two major observations. First, in culture systems with intact somatic support, RA induces Kit expression, a marker of differentiating spermatogonia. Second, it also induces Stra8, a marker for the onset of meiosis. These two observations question whether somatic cells are required for RA induction of gonocyte differentiation in vitro. Development of a method to induce in vitro differentiation of SSC has been widely pursued, and some studies show some progression of spermatogenesis in a culture system. However, the starting materials used in previous reports are mostly preleptotene or leptotene spermatocytes [26–28]. Our study is the first report of spermatogonial differentiation induced by one extrinsic factor in vitro. RA is a factor that can induce differentiation in many organs and cells. It has been demonstrated that RA could induce the differentiation of Aal to A1 in VAD animals and cultured cryptorchid adult testes [5, 12]. However, whether the RA effect on early germ cells is direct or indirect has not been determined, because receptors for retinoids are found in both germ cells and somatic cells. Retinoids play pivotal regulatory roles in Sertoli and Leydig cells and also interstitial macrophages. Our results obtained from cultured THY1+ gonocytes/spermatogonia in a feeder-cell-free condition demonstrate, for the first time, that in vitro differentiation of undifferentiated early germ cells could be initiated by RA without somatic cells and that the differentiation of these cells can be validated by the induction of expression of two markers, Kit and Stra8. Whether the differentiation triggered by RA in this somatic-free system can lead to formation of late differentiating spermatogonia (In and B spermatogonia) and primary spermatocytes is not clear, because germ cells under this feeder-cell-free and serum-free condition could survive only up to 48 to 72 hours (data not shown). It is noteworthy that in both cultured THY1+ gonocytes and neonatal testes, RA-stimulated germ cells showed a higher percentage of cell death after the first 24 h (data not shown). This strongly suggests that once RA triggers the differentiation of these germ cells, the somatic cells, particularly the mature Sertoli cells, become vital to their survival and are likely to be important for the continuing progression of the differentiation pathway.
If Stra8 is required for the onset of male meiosis, when does RA act during spermatogonial development and which subpopulation of spermatogonia is responsible for Stra8 expression? Analysis of Stra8-deficient testes demonstrated that Stra8 is essential for normal progression into meiotic prophase [14, 15]. The findings of robust induction of Stra8/Kit expression after 8 h using 2 dpp, 4 dpp, and 8 dpp THY1+ gonocytes/spermatogonia suggest that induction occurs during gonocyte/spermatogonial maturation. There is additional evidence supporting this conclusion: 1) previous studies from de Rooij and others [3] suggest that some gonocytes directly differentiate to A1 in the first round of spermatogenesis and 2) because the first appearance of KIT was found in late Aal to A1, and it was confirmed to be a marker of differentiating spermatogonia (A1-B), the magnitude and temporal sequences of induction of Stra8 vs. Kit expression in THY1+ gonocytes implies that RA induction of Stra8 expression might be earlier than induction of Kit expression. So far, data demonstrate that RA initially induces Stra8 expression, and gonocyte and spermatogonia differentiation occurs. However, some questions arise from this study that prevent the exclusion of the possibility of a second induction at B-preleptotene-leptotene. For instance, which subpopulation of differentiating spermatogonia (late Aal, A1, or B spermatogonia) contributes to the higher level of Stra8 in KIT+ cells?
Microarray profiling of RA-treated 2 dpp THY1+ gonocytes not only confirmed the induction of Stra8 expression demonstrated by real-time RT-PCR in this study, but also revealed that a group of genes involved in multiple aspects of RA action were significantly up-regulated by RA. The induction of Stra6, which was originally identified as an RA responsive gene in P19 carcinoma cells [29], further validates the effectiveness of RA treatment on these gonocytes. Recently, STRA6 was found to be a specific membrane receptor of retinol binding protein, representing a major physiological mediator of cellular vitamin A uptake [30]. So far, these gonocytes are the only known noncancer cells to stimulate Stra6 expression in response to RA. The increased Stra6 expression indicates a facilitated uptake of retinol into the cells, and the physiological relevance of this up-regulation in gonocytes needs to be studied. In the meantime, an accelerated RA degradation in these gonocytes upon the RA treatment was indicated by the dramatic increase of expression of three genes: Cyp26a1, Cyp26b1, and Por. CYP26A1 and CYP26B1 are two of the three P450 enzymes in the CYP26 subfamily to oxidize RA for further metabolism and inactivation [31]. Cytochrome P450 oxidoreductase (POR) coded by Por is an NADPH-dependent protein essential for transferring electron to microsomal cytochrome P450 [31]. Lack of POR would impair the activity of all three CYP26 enzymes [31]. In addition to the increased metabolism of RA, the synthesis of RA is likely attenuated, as indicated by the induction of Lrat and Dhrs3. Lecithin-retinol acyltransferase (LRAT) is the predominant enzyme in the body responsible for the physiologic esterification of retinol [32]. Increased Lrat indicates a potentially increased storage of retinyl ester and reduction of supply of retinol for the production of RA. Dehydrogenase/reductase member 3 (DHRS3) regenerates retinol from all-trans-retinal, which is an intermediate in the production of all-trans-RA [33]. The increased DHRS3 could mean a potential reduction of retinal for the synthesis of RA. Other RA-regulated genes in the gonocytes are being analyzed and studied. The data obtained from this microarray experiment will provide a fertile basis for the hypothesis-driven studies of RA-regulated gonocyte differentiation.
In conclusion, this study demonstrates that a limited level of in vitro differentiation of gonocytes/spermatogonia could be achieved after RA stimulation without somatic involvement. In addition, RA induction of Stra8 expression in the undifferentiated spermatogonia was demonstrated, implying its important role in spermatogonial differentiation.
Footnotes
Supported by NIH grants R01 HD 10808 and U54 HD 042454. The microarray data have been deposited in the National Center for Biotechnology Information Gene Expression Omnibus database (series no. GSE9521).
REFERENCES
- 1.Aponte PM, van Bragt MP, de Rooij DG, van Pelt AM. Spermatogonial stem cells: characteristics and experimental possibilities. Apmis. 2005;113:727–742. doi: 10.1111/j.1600-0463.2005.apm_302.x. [DOI] [PubMed] [Google Scholar]
- 2.de Rooij DG. Proliferation and differentiation of spermatogonial stem cells. Reproduction. 2001;121:347–354. doi: 10.1530/rep.0.1210347. [DOI] [PubMed] [Google Scholar]
- 3.de Rooij DG, Russell LD. All you wanted to know about spermatogonia but were afraid to ask. J Androl. 2000;21:776–798. [PubMed] [Google Scholar]
- 4.de Rooij DG, de Boer P. Specific arrests of spermatogenesis in genetically modified and mutant mice. Cytogenet Genome Res. 2003;103:267–276. doi: 10.1159/000076812. [DOI] [PubMed] [Google Scholar]
- 5.van Pelt AM, van Dissel-Emiliani FM, Gaemers IC, van der Burg MJ, Tanke HJ, de Rooij DG. Characteristics of A spermatogonia and preleptotene spermatocytes in the vitamin A-deficient rat testis. Biol Reprod. 1995;53:570–578. doi: 10.1095/biolreprod53.3.570. [DOI] [PubMed] [Google Scholar]
- 6.Gaemers IC, van Pelt AM, van der Saag PT, de Rooij DG. All-trans-4-oxo-retinoic acid: a potent inducer of in vivo proliferation of growth-arrested A spermatogonia in the vitamin A-deficient mouse testis. Endocrinology. 1996;137:479–485. doi: 10.1210/endo.137.2.8593792. [DOI] [PubMed] [Google Scholar]
- 7.Beumer TL, Roepers-Gajadien HL, Gademan IS, Kal HB, de Rooij DG. Involvement of the D-type cyclins in germ cell proliferation and differentiation in the mouse. Biol Reprod. 2000;63:1893–1898. doi: 10.1095/biolreprod63.6.1893. [DOI] [PubMed] [Google Scholar]
- 8.Schrans-Stassen BH, van de Kant HJ, de Rooij DG, van Pelt AM. Differential expression of c-kit in mouse undifferentiated and differentiating type A spermatogonia. Endocrinology. 1999;140:5894–5900. doi: 10.1210/endo.140.12.7172. [DOI] [PubMed] [Google Scholar]
- 9.de Rooij DG, Okabe M, Nishimune Y. Arrest of spermatogonial differentiation in jsd/jsd, Sl17H/Sl17H, and cryptorchid mice. Biol Reprod. 1999;61:842–847. doi: 10.1095/biolreprod61.3.842. [DOI] [PubMed] [Google Scholar]
- 10.Schrans-Stassen BH, Saunders PT, Cooke HJ, de Rooij DG. Nature of the spermatogenic arrest in Dazl −/− mice. Biol Reprod. 2001;65:771–776. doi: 10.1095/biolreprod65.3.771. [DOI] [PubMed] [Google Scholar]
- 11.Siiteri JE, Karl AF, Linder CC, Griswold MD. Testicular synchrony: evaluation and analysis of different protocols. Biol Reprod. 1992;46:284–289. doi: 10.1095/biolreprod46.2.284. [DOI] [PubMed] [Google Scholar]
- 12.Haneji T, Maekawa M, Nishimune Y. Retinoids induce differentiation of type A spermatogonia in vitro: organ culture of mouse cryptorchid testes. J Nutr. 1983;113:1119–1123. doi: 10.1093/jn/113.6.1119. [DOI] [PubMed] [Google Scholar]
- 13.Akmal KM, Dufour JM, Kim KH. Retinoic acid receptor alpha gene expression in the rat testis: potential role during the prophase of meiosis and in the transition from round to elongating spermatids. Biol Reprod. 1997;56:549–556. doi: 10.1095/biolreprod56.2.549. [DOI] [PubMed] [Google Scholar]
- 14.Koubova J, Menke DB, Zhou Q, Capel B, Griswold MD, Page DC. Retinoic acid regulates sex-specific timing of meiotic initiation in mice. Proc Natl Acad Sci U S A. 2006;103:2474–2479. doi: 10.1073/pnas.0510813103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baltus AE, Menke DB, Hu YC, Goodheart ML, Carpenter AE, de Rooij DG, Page DC. In germ cells of mouse embryonic ovaries, the decision to enter meiosis precedes premeiotic DNA replication. Nat Genet. 2006;38:1430–1434. doi: 10.1038/ng1919. [DOI] [PubMed] [Google Scholar]
- 16.Kubota H, Avarbock MR, Brinster RL. Growth factors essential for self-renewal and expansion of mouse spermatogonial stem cells. Proc Natl Acad Sci U S A. 2004;101:16489–16494. doi: 10.1073/pnas.0407063101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oatley JM, Avarbock MR, Telaranta AI, Fearon DT, Brinster RL. Identifying genes important for spermatogonial stem cell self-renewal and survival. Proc Natl Acad Sci U S A. 2006;103:9524–9529. doi: 10.1073/pnas.0603332103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shima JE, McLean DJ, McCarrey JR, Griswold MD. The murine testicular transcriptome: characterizing gene expression in the testis during the progression of spermatogenesis. Biol Reprod. 2004;71:319–330. doi: 10.1095/biolreprod.103.026880. [DOI] [PubMed] [Google Scholar]
- 19.Zhou Q, Shima JE, Nie R, Friel PJ, Griswold MD. Androgen-regulated transcripts in the neonatal mouse testis as determined through microarray analysis. Biol Reprod. 2005;72:1010–1019. doi: 10.1095/biolreprod.104.035915. [DOI] [PubMed] [Google Scholar]
- 20.OuladAbdelghani M, Bouillet P, Decimo D, Gansmuller A, Heyberger S, Dolle P, Bronner S, Lutz Y, Chambon P. Characterization of a premeiotic germ cell-specific cytoplasmic protein encoded by Stra8, a novel retinoic acid-responsive gene. J Cell Biol. 1996;135:469–477. doi: 10.1083/jcb.135.2.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O’Brien DA. Stage-specific protein synthesis by isolated spermatogenic cells throughout meiosis and early spermiogenesis in the mouse. Biol Reprod. 1987;37:147–157. doi: 10.1095/biolreprod37.1.147. [DOI] [PubMed] [Google Scholar]
- 22.Oatley JM, Brinster RL. Spermatogonial stem cells. Adult Stem Cells. 2006;419:259–282. doi: 10.1016/S0076-6879(06)19011-4. [DOI] [PubMed] [Google Scholar]
- 23.Yoshida S, Sukeno M, Nakagawa T, Ohbo K, Nagamatsu G, Suda T, Nabeshima Y. The first round of mouse spermatogenesis is a distinctive program that lacks the self-renewing spermatogonia stage. Development. 2006;133:1495–1505. doi: 10.1242/dev.02316. [DOI] [PubMed] [Google Scholar]
- 24.Bowles J, Knight D, Smith C, Wilhelm D, Richman J, Mamiya S, Yashiro K, Chawengsaksophak K, Wilson MJ, Rossant J, Hamada H, Koopman P. Retinoid signaling determines germ cell fate in mice. Science. 2006;312:596–600. doi: 10.1126/science.1125691. [DOI] [PubMed] [Google Scholar]
- 25.Zamboni L, Merchant H. The fine morphology of mouse primordial germ cells in extragonadal locations. Am J Anat. 1973;137:299–335. doi: 10.1002/aja.1001370305. [DOI] [PubMed] [Google Scholar]
- 26.Rassoulzadegan M, Paquis-Flucklinger V, Bertino B, Sage J, Jasin M, Miyagawa K, van Heyningen V, Besmer P, Cuzin F. Transmeiotic differentiation of male germ cells in culture. Cell. 1993;75:997–1006. doi: 10.1016/0092-8674(93)90543-y. [DOI] [PubMed] [Google Scholar]
- 27.Weiss M, Vigier M, Hue D, Perrard-Sapori MH, Marret C, Avallet O, Durand P. Pre- and postmeiotic expression of male germ cell-specific genes throughout 2-week cocultures of rat germinal and Sertoli cells. Biol Reprod. 1997;57:68–76. doi: 10.1095/biolreprod57.1.68. [DOI] [PubMed] [Google Scholar]
- 28.Staub C, Hue D, Nicolle JC, Perrard-Sapori MH, Segretain D, Durand P. The whole meiotic process can occur in vitro in untransformed rat spermatogenic cells. Exp Cell Res. 2000;260:85–95. doi: 10.1006/excr.2000.4998. [DOI] [PubMed] [Google Scholar]
- 29.Bouillet P, Sapin V, Chazaud C, Messaddeq N, Decimo D, Dolle P, Chambon P. Developmental expression pattern of Stra6, a retinoic acid-responsive gene encoding a new type of membrane protein. Mech Dev. 1997;63:173–186. doi: 10.1016/s0925-4773(97)00039-7. [DOI] [PubMed] [Google Scholar]
- 30.Kawaguchi R, Yu JM, Honda J, Hu J, Whitelegge J, Ping PP, Wiita P, Bok D, Sun H. A membrane receptor for retinol binding protein mediates cellular uptake of vitamin A. Science. 2007;315:820–825. doi: 10.1126/science.1136244. [DOI] [PubMed] [Google Scholar]
- 31.Ribes V, Otto DME, Dickmann L, Schmidt K, Schuhbaur B, Henderson C, Blomhoff R, Wolf CR, Tickle C, Dolle P. Rescue of cytochrome P450 oxidoreductase (Por) mouse mutants reveals functions in vasculogenesis, brain and limb patterning linked to retinoic acid homeostasis. Dev Biol. 2007;303:66–81. doi: 10.1016/j.ydbio.2006.10.032. [DOI] [PubMed] [Google Scholar]
- 32.O’Byrne SM, Wongsiriroj N, Libien J, Vogel S, Goldberg IJ, Baehr W, Palczewski K, Blaner WS. Retinoid absorption and storage is impaired in mice lacking lecithin: retinol acyltransferase (LRAT) J Biol Chem. 2005;280:35647–35657. doi: 10.1074/jbc.M507924200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haeseleer F, Huang J, Lebioda L, Saari JC, Palczewski K. Molecular characterization of a novel short-chain dehydrogenase/reductase that reduces all-trans-retinal. J Biol Chem. 1998;273:21790–21799. doi: 10.1074/jbc.273.34.21790. [DOI] [PubMed] [Google Scholar]