Abstract
Clinical trials have demonstrated that rituximab improves overall survival in non-Hodgkin lymphoma (NHL), except in mantle cell lymphoma (MCL). We used Surveillance Epidemiology and End Results (SEER)–Medicare data to compare survival in older MCL patients who began chemotherapy with or without rituximab within 180 days of diagnosis. Patients were followed from diagnosis (January 1999 to December 2005) until death or the end of observation (December 2007). Medicare administrative and claims data were used to identify the date and cause of death and the immunochemotherapy regimen. Of 638 patients, the mean age at diagnosis was 75 years, 75% had stage III/IV disease, 67% had extranodal involvement, and 64% received rituximab. The average length of first-line treatment was 21 weeks, with no difference between the 2 groups (P = .76). Median survival was 27 months for chemotherapy alone, compared with 37 months for chemotherapy plus rituximab (P < .001). In multivariate analysis of 2-year survival, rituximab plus chemotherapy was associated with lower all-cause (hazard ratio [HR] 0.58; 95% confidence interval [CI] 0.41-0.82; P < .01), and cancer-specific (HR 0.56; 95% CI 0.37-0.84; P < .01) mortality. Results were similar when using the entire observation period, propensity score analysis, and limiting chemotherapy to CHOP/CHOP-like. We conclude that first-line chemotherapy including rituximab is associated with significantly improved survival in older patients diagnosed with MCL.
Introduction
The clinical course of mantle cell lymphoma (MCL) is characterized by an initially high response rate but a constant relapse pattern, resulting in poor long-term outcome.1 However, recent studies suggest significant heterogeneity based on clinical and biologic risk factors.2,3 Because the median age at MCL diagnosis is > 65 years, the majority of patients do not qualify for dose-intensified regimens, which represent the current standard of care in younger patients.4
The addition of rituximab to first-line chemotherapy has been shown in randomized trials to improve overall survival in both aggressive5–8 and indolent9–12 subtypes of non-Hodgkin lymphoma (NHL) and chronic lymphocytic leukemia (CLL).13 However, this benefit has not been confirmed in MCL.14 In MCL, the benefit of rituximab on overall survival has been suggested by the results of a recent meta-analysis15 that included data from 3 separate trials,14,16,17 with a total of 260 MCL patients. In the meta-analysis, the calculated hazard ratio (HR) for death was 0.60 (95% confidence interval [CI] 0.37-0.98), indicating a significant advantage for patients receiving rituximab plus chemotherapy compared with chemotherapy alone. However, there was significant heterogeneity among these trials, and when 48 patients from one trial of rituximab in relapsed or refractory disease16 were removed, the HR for death, while still suggestive (HR = 0.78), was no longer statistically significant.
Additional indirect evidence of a survival benefit for rituximab in MCL comes from a historical comparison of patients treated by the German Low Grade Lymphoma Study Group (GLSG; 1996-2004) with patients treated by the Kiel Lymphoma Study Group (KLSG; 1975-1986).18 KLSG patients were treated with cyclophosphamide, vincristine, and prednisone (COP, also known as the Bagley regime) or cyclophosphamide, vincristine, doxorubicin, and prednisone (CHOP), whereas patients from GLSG were treated with mitoxantrone, chlorambucil, and prednisone (MCP), CHOP, or rituximab plus CHOP (R-CHOP). Patients from the 2 groups were matched, and overall survival was compared. The median overall survival rate was 2.7 years in the KLSG study compared with 4.8 years in the GLSG study (P < .0001), and the 5-year survival rates were 22% and 47%, respectively (P < .05), suggesting that use of immunochemotherapy in the GLSG patients contributed to the observed differences in overall survival. These studies notwithstanding, the only published randomized trial of rituximab added to chemotherapy in previously untreated patients with MCL showed no difference in overall survival, with a 2-year probability of 76.6% overall (P = .93 for the difference between the 2 groups).14
Whereas they are not a substitute for randomized trials, observational data can be used to study treatment effects: (1) in routine clinical practice, where treatment patterns may differ from those in trials; (2) in populations that are underrepresented in trials, such as the elderly, those with comorbidities, and those with early or advanced stage disease; and (3) where for epidemiologic or logistical reasons it may be difficult to conduct a trial. For example, according to the Surveillance Epidemiology and End Results (SEER) database of the National Cancer Institute (NCI), among those diagnosed with NHL in the United States from 2000-2007, 44% of diffuse large B-cell lymphoma, 48% of follicular lymphoma (FL), 63% of MCL, and 69% of CLL patients were ≥ 65 years of age at diagnosis.19 In contrast to the underlying age distribution of the NHL population as a whole, patients in many of the randomized trials of front-line immunochemotherapy in NHL were considerably younger.9,11–13 Because older age is among the risk factors for overall survival in the International Prognostic Index (IPI),20 the Follicular Lymphoma International Prognostic Index (FLIPI),21 and the MCL International Prognostic Index (MIPI),22 this raises the question of whether survival outcomes in younger, healthier patients enrolled in clinical trials might accurately reflect outcomes in the general patient population. The purpose of the present study was to examine the survival impact of adding rituximab to first-line chemotherapy in a cohort of older MCL patients treated in routine clinical practice.
Methods
Data source
The source of data for this study was the NCI's SEER cancer registry linked to Medicare enrollment and claims data. This database has been described in detail elsewhere.23 Briefly, as of 2010, SEER collects and publishes cancer incidence and survival data from 18 population-based cancer registries throughout the United States covering ∼ 26% of the US population.24 SEER coverage includes 23% of African Americans, 40% of Hispanics, 42% of Native Americans, 53% of Asians, and 70% of Hawaiian/Pacific Islanders.
The registries routinely collect data on patient demographics, primary tumor site, tumor morphology and stage at diagnosis, first course of treatment, and follow-up for vital status. In the SEER-Medicare data, cancer registry data are linked to Medicare enrollment and claims data. For persons 65 years of age or older, 97% are eligible for Medicare, and 93% of patients in the SEER files are matched to the Medicare enrollment file.25 Almost all Medicare beneficiaries have Part A coverage, which includes hospital, skilled nursing facility, hospice, and some home health care, and 96% of Part A beneficiaries choose to enroll in Part B of Medicare, which covers physician and outpatient services. At the time this study was performed, the SEER-Medicare linkage included all Medicare-eligible persons appearing in the SEER data through 2005 and their Medicare claims through 2007.
Medicare claims files linked to SEER consist of the following: Medicare Provider Analysis and Review (MEDPAR), which includes all hospital (Part A) short-stay, long-stay, and skilled nursing facility bills; National Claims History (NCH), which includes all physician/supplier (Part B) bills; Outpatient, which includes all bills from institutional outpatient (Part B) providers; Home Health Agency (HHA), which includes all claims for home health services; Hospice; and Durable Medical Equipment (DME). The Medicare benefit for oral drugs (Part D) began on January 1, 2006, and claims for oral drugs without an intravenous equivalent were not available for our study.
Inclusion and exclusion criteria
Patients were included in this study if they were diagnosed with MCL between January 1, 1999 and December 31, 2005, MCL was the first primary cancer diagnosed, and they began infused chemotherapy within 180 days following diagnosis. Identification of MCL was made using the World Health Organization (WHO) International Classification of Diseases for Oncology, 3rd Edition (ICD-O-3) histology code 9673 (MCL).26 The NCI provides a comprehensive list, the International Classification of Diseases (9th revision), Clinical Modification (ICD-9-CM) diagnosis and procedure codes, and Healthcare Common Procedure Coding System (HCPCS) “J” codes, which are used to identify claims for immunochemotherapy.27–29 The HCPCS codes are for specific drugs, whereas the ICD-9-CM codes indicate only that chemotherapy was provided but do not identify the types of drugs used. We searched the Medicare NCH and Outpatient files for HCPCS codes to identify patients who began infused chemotherapy with or without rituximab within 180 days after MCL diagnosis. In addition, to ensure complete claims history, patients had to have been enrolled in both Medicare Parts A and B, with no health maintenance organization (HMO) coverage for 12 months before MCL diagnosis. Patients were excluded for the following reasons: diagnosis before age 65, diagnosis made by death certificate or autopsy, death within the first month after diagnosis, or Medicare enrollment < 12 months before diagnosis. Furthermore, we excluded patients who had only ICD-9-CM codes for chemotherapy because we were unable to classify the type of chemotherapy regimen these patients received, and we also excluded those who received rituximab monotherapy.
Patients and variables
Patients were described according to their demographic, clinical, and socioeconomic characteristics. Patient age at diagnosis was stratified into 4 groups: 66-70, 71-75, 76-80, and > 80 years. Requiring eligible patients to have at least one year of Medicare enrollment before diagnosis ensured that the minimum age in the cohort was 66 years. Race/ethnicity was defined using the SEER recoded race variable as follows: white, code 01; black, code 02; Hispanic, code 11; and other, which in SEER consists predominantly of Native American, Native Hawaiian or Other Pacific Islander, and Asian.30
Summary staging is the approach SEER uses to categorize how far a cancer has spread from its point of origin.31 It uses all information available in the medical record, and is a combination of the most precise clinical and pathologic documentation of the extent of disease. All patients were classified according to whether their disease was confined to one or more lymph node regions or involved an extralymphatic organ or site (extranodal).
Medicare claims do not contain the results of laboratory tests. Therefore, it was not possible to obtain directly information on elevated leukocyte count and lactate dehydrogenase (LDH) level, 2 independent prognostic factors for overall survival in the MIPI.22 In lieu of laboratory data on elevated leukocyte count, we searched Medicare claims, excluding claims for laboratory tests, to identify patients diagnosed with leukocytosis (ICD-9-CM codes 288.3 or 288.8)32 from 12 months before MCL diagnosis until the day before first-line immunochemotherapy began. Medicare nonlaboratory claims also were used to identify anemia, as described previously.33 Another prognostic factor for overall survival in MIPI22 that is not included in SEER-Medicare, is Eastern Cooperative Oncology Group (ECOG) performance status.34 In the absence of performance status, we used Medicare claims to identify several claims-based predictors of poor performance status,35 including the use of oxygen and related respiratory therapy supplies, wheelchairs and supplies, home health agency use, and skilled nursing facility use, all from 12 months before until 30 days after MCL diagnosis. Individual services were combined into a score of 0 (none) or 1 (use of any service).
We used the Medicare inpatient (Part A), outpatient, and physician (Part B) records to calculate an NCI Comorbidity Index score for each patient.36,37 This approach38,39 entails first removing claims that are considered to have unreliable diagnosis coding, such as those for testing procedures used to rule out conditions. Then, remaining diagnosis and procedure codes are used to identify the 15 noncancer comorbidities in the Charlson Comorbidity Index (CCI).40 The algorithms used to identify these conditions reflect the Deyo41 adaptation of the CCI, and include several procedure codes from the Romano42 adaptation. A weight is assigned to each condition, and the weights are summed to obtain the index for each patient.
Socioeconomic information at the patient level is not available through SEER-Medicare. Instead, the SEER-Medicare dataset contains information from the 2000 Census reported at the tract level in which the patient lives, the percentage of the population living in poverty, and the percentage of those 25 years of age or older with some college. We used these as indicators of the socioeconomic status of individual patients in the cohort. The assigned metropolitan statistical area as recoded by SEER (large metropolitan, metropolitan, urban, and less urban/rural) was used as a geographic indicator.
First-line therapy
We used the Medicare NCH and Outpatient files to identify all claims containing a HCPCS “J” code for chemotherapy (J9000-J9999) or rituximab (J9310) within 30 days after the first such claim.27–29 Patients were then classified as having received chemotherapy with or without rituximab as first-line therapy on the basis of these claims. In addition, we identified specific chemotherapy agents used during the first 30 days of claims, consisting of cyclophosphamide (J8530, J9070, J9080, J9090-J9097), doxorubicin (J9000, J9001), vincristine (J9370, J9375, J9380), and mitoxantrone (J9293) according to treatment guidelines.43 These claims were used to classify chemotherapy regimens as CHOP or CHOP-like (cyclophosphamide, vincristine, prednisone [CVP] or cyclophosphamide, mitoxantrone, vincristine, prednisone [CNOP]) and “other” chemotherapy regimen. The use of prednisone was assumed when the other agents were present. All codes recommended by the NCI28 were used to identify radiation therapy. The end of first-line therapy was defined as the date of the last chemotherapy or radiation claim before the beginning of a period of at least 90 days in which there was no claim for either chemotherapy or radiation.
Follow-up and survival
Patients were followed from their date of cancer diagnosis until death, the end of their claims (December 31, 2007), or the end of their Medicare Part A and/or Part B coverage, whichever came first. The first day of the SEER month of diagnosis was assigned as the day of diagnosis. The observation period was divided into 2 discrete intervals: (1) from the date of diagnosis until 90 days after the end of first-line therapy and (2) from 91 days after the end of first-line therapy until the end of the observation period.
The date of death was assigned using the Medicare date because it is more current than the SEER date.44 In cases in which the Medicare date of death was missing, the SEER date was used. The cause of death was classified as cancer or noncancer using the “CODKM” variable in the SEER Patient Entitlement and Diagnosis Summary File through 2007. Cancer mortality included all deaths due to cancer, not just those due to lymphoma. Noncancer mortality included all other identified causes of death such as heart disease or diabetes; however, it excluded missing or unspecified cause of death. These patients were censored at the time of death in both the cancer and noncancer survival analyses because exploratory analysis showed that almost 90% of those with a known cause of death died of cancer. Consequently, including them in the group of noncancer deaths could have resulted in significant misclassification. All other patients were assumed to be alive at the end of the analysis period (December 31, 2007) if not censored earlier for other reasons such as switching to HMO coverage.
Time to second-line therapy
We defined second-line therapy as any new Medicare claim for chemotherapy or radiation > 90 days after the end of first-line therapy based on algorithms reported previously in the literature that used claims data to identify cancer relapse in leukemia45 and breast cancer.46 Therefore, to be considered at risk for receiving second-line therapy, patients had to survive at least 90 days after the end of first-line therapy without additional therapy.
Statistical analysis
Analysis (χ2) was used to test for the independence between patient characteristics specified as categorical variables and the 2 first-line treatment groups. ANOVA was used to test for independence of continuous variables. Unadjusted Kaplan-Meier survival plots were used to explore overall survival and time to next therapy stratified by the first-line treatment groups.
Cox proportional hazards models were used to examine adjusted associations between patient factors and both survival and time to next treatment. Rituximab patients tended to be diagnosed in later years and to have shorter observation periods; therefore, the primary survival analyses were limited to 2 years. Exploratory survival analysis showed that patients who received chemotherapy alone had significantly improved 2-year overall survival in later (2003-2005) compared with earlier (1999-2002) years of diagnosis. However, there was no change in 2-year overall survival between later and earlier years in the patients who received rituximab plus chemotherapy. Therefore, we included year of diagnosis, as well as an interaction term between year of diagnosis and type of first-line therapy, as independent variables in all of the models.
Additional survival analyses were conducted using the entire observation period, using cancer and noncancer mortality as the outcomes, and restricting the cohort to those who received CHOP/CHOP-like therapy. Finally, all of the survival analyses were repeated using propensity techniques,47 which entailed using multivariate logistic regression to obtain the propensity score, with rituximab plus chemotherapy as the outcome variable and all other patient variables except year of diagnosis and the interaction term between year of diagnosis and treatment as independent variables. In the propensity score survival analyses, patients were divided into propensity score quintiles, and propensity quintile was included in the multivariate survival models as an independent variable in lieu of all patient factors except treatment, year, and the interaction term.
This study was conducted as part of a protocol submitted to Quorum Review Institutional Review Board. On December 1, 2009, Quorum granted a determination of exemption for this protocol, based on the fact that the information in the data files is recorded in such a manner that subjects cannot be identified either directly or through identifiers linked to them.
Results
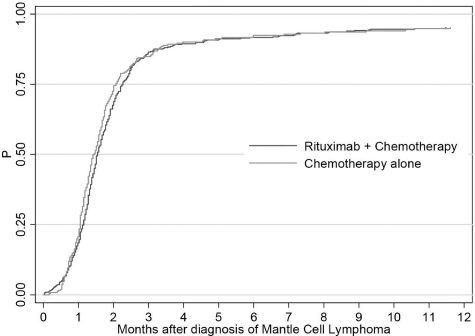
We identified 992 patients diagnosed with MCL between 1999 and 2005. Of these, 694 (70%) had at least one HCPCS claim for infused chemotherapy at any time during the observation period and 638 (92%) of these had their first claim within 180 days of diagnosis (Figure 1) and were included in the final cohort. (Table 1) Twenty-one patients were excluded because they had only ICD-9-CM diagnosis or procedure codes for chemotherapy, and a further 17 were excluded because they received only rituximab within 6 months after diagnosis.
Figure 1.
Time to first-line therapy.
Table 1.
Patient characteristics
| First-line treatment |
P | ||||||
|---|---|---|---|---|---|---|---|
| Overall (n = 638) |
Rituximab + chemotherapy (n = 407) |
Chemotherapy alone (n = 231) |
|||||
| n | % | n | % | n | % | ||
| Age at diagnosis, y | |||||||
| 66-70 | 150 | 23.5 | 84 | 20.6 | 66 | 28.6 | .11 |
| 71-75 | 183 | 28.7 | 122 | 30.0 | 61 | 26.4 | |
| 76-80 | 159 | 24.9 | 101 | 24.8 | 58 | 25.1 | |
| > 80 | 146 | 22.9 | 100 | 24.6 | 46 | 19.9 | |
| Mean & SD age | 74.8 | 6.1 | 75.2 | 6.1 | 74.2 | 6.0 | .04 |
| Sex | |||||||
| Male | 430 | 67.4 | 283 | 69.5 | 147 | 63.6 | .13 |
| Female | 208 | 32.6 | 124 | 30.5 | 84 | 36.4 | |
| Race/ethnicity | |||||||
| White | 580 | 90.9 | 370 | 90.9 | 210 | 90.9 | .47 |
| Black | 18 | 2.8 | Not reported, due to 1 or more cells containing < 11 patients | ||||
| Hispanic | 20 | 3.1 | |||||
| Other | 20 | 3.1 | |||||
| Year of diagnosis | |||||||
| 1999-2002 | 328 | 51.4 | 150 | 36.9 | 178 | 77.1 | < .0001 |
| 2003-2005 | 310 | 48.6 | 257 | 63.1 | 53 | 22.9 | |
| Extranodal involvement | |||||||
| Yes | 428 | 67.1 | 270 | 66.3 | 158 | 68.4 | .60 |
| No | 176 | 27.6 | 117 | 28.7 | 59 | 25.5 | |
| Unknown | 34 | 5.3 | 20 | 4.9 | 14 | 6.1 | |
| Stage at MCL diagnosis | |||||||
| I/II | 128 | 20.1 | 78 | 19.2 | 50 | 21.6 | .58 |
| III/IV | 476 | 74.6 | 309 | 75.9 | 167 | 72.3 | |
| Unknown | 34 | 5.3 | 20 | 4.9 | 14 | 6.1 | |
| Presence of “B” symptoms | |||||||
| No | 221 | 34.6 | 147 | 36.1 | 74 | 32.0 | .55 |
| Yes | 134 | 21.0 | 82 | 20.1 | 52 | 22.5 | |
| Unknown | 283 | 44.4 | 178 | 43.7 | 105 | 45.5 | |
| Anemia | |||||||
| No | 557 | 87.3 | 359 | 88.2 | 198 | 85.7 | .36 |
| Yes | 81 | 12.7 | 48 | 11.8 | 33 | 14.3 | |
| Leukocytosis | |||||||
| No | 560 | 87.8 | 360 | 88.5 | 200 | 86.6 | .49 |
| Yes | 78 | 12.2 | 47 | 11.5 | 31 | 13.4 | |
| NCI Comorbidity Index | |||||||
| 0 | 417 | 65.4 | 259 | 63.6 | 158 | 68.4 | .34 |
| 1 | 153 | 24.0 | 105 | 25.8 | 48 | 20.8 | |
| 2 | 32 | 5.0 | 18 | 4.4 | 14 | 6.1 | |
| ≥ 3 | 36 | 5.6 | 25 | 6.1 | 11 | 4.8 | |
| Performance status indicators | |||||||
| 0 | 541 | 84.8 | 351 | 86.2 | 190 | 82.3 | .18 |
| ≥ 1 | 97 | 15.2 | 56 | 13.8 | 41 | 17.7 | |
| Percent in census tract with some college | |||||||
| < 25% | 210 | 32.9 | 122 | 30.0 | 88 | 38.1 | .04 |
| > 25% | 428 | 67.1 | 285 | 70.0 | 143 | 61.9 | |
| Percent in census tract living in poverty | |||||||
| < 5 | 247 | 38.7 | 174 | 42.8 | 73 | 31.6 | .04 |
| 5-7 | 88 | 13.8 | 51 | 12.5 | 37 | 16.0 | |
| 8-12 | 135 | 21.2 | 78 | 19.2 | 57 | 24.7 | |
| > 12 | 166 | 26.0 | 103 | 25.3 | 63 | 27.3 | |
| Type of geographic area | |||||||
| Large metropolitan | 356 | 55.8 | 231 | 56.8 | 125 | 54.1 | .37 |
| Metropolitan | 178 | 27.9 | Not reported, due to 1 or more cells containing < 11 patients | ||||
| Urban | 37 | 5.8 | |||||
| Less urban/rural | 67 | 10.5 | 38 | 9.3 | 29 | 12.6 | |
Patients in the final cohort accounted for 1727 years of follow-up, with a median of 2.3 years. Overall, the median age at diagnosis was 74 years (mean, 75 years), 58% were diagnosed with stage IV disease, 67% had extranodal disease, and 64% received first-line rituximab in addition to their chemotherapy. Patients in the 2 first-line treatment groups were similar with respect to age, sex, race, extranodal involvement, presence of “B” symptoms, and NCI Comorbidity Index score. Those who received rituximab were diagnosed later in the study period. They were more likely to live in a census tract with a more highly educated and affluent population.
Treatment
The mean duration of first-line therapy was 21 weeks (95% CI, 19-22 weeks) overall, 20 weeks (95% CI, 18-22 weeks) for rituximab plus chemotherapy, and 21 weeks (95% CI, 19-23 weeks) for chemotherapy alone (P = .76 for the difference between the 2 treatment groups). In the rituximab plus chemotherapy group, the median number of rituximab administrations during first-line therapy was 6 (interquartile range [IQR] 4-7), and 51% (208 of 407) received CHOP. In the chemotherapy alone group, 34% (78/231) received CHOP alone (P < .001 for the difference between groups; Table 2). Only 3 patients received stem cell transplantation: 2 in the rituximab plus chemotherapy group and 1 in the chemotherapy alone group.
Table 2.
Chemotherapy regimens
| Chemotherapy regimen | First-line treatment |
|||||
|---|---|---|---|---|---|---|
| Rituximab + chemotherapy (n = 407) |
Chemotherapy alone (n = 231) |
Overall (n = 638) |
||||
| n | % | n | % | n | % | |
| CHOP | 208 | 51.1 | 78 | 33.8 | 286 | 44.8 |
| CNOP | 18 | 4.4 | 16 | 6.9 | 34 | 5.3 |
| CVP | 51 | 12.5 | 44 | 19.0 | 95 | 14.9 |
| Other* | 130 | 31.9 | 93 | 40.3 | 223 | 35.0 |
This group includes 70 patients (46 of 130 [35%] rituximab plus chemotherapy and 24 of 93 [26%] chemotherapy alone) who received cyclophosphamide alone or as part of a regimen other than CHOP, CNOP, or CVP. It also includes 35 patients (23 of 130 [18%] rituximab plus chemotherapy and 12 of 93 [13%] chemotherapy alone) who received either fludarabine or cytarabine without cyclophosphamide.
Survival
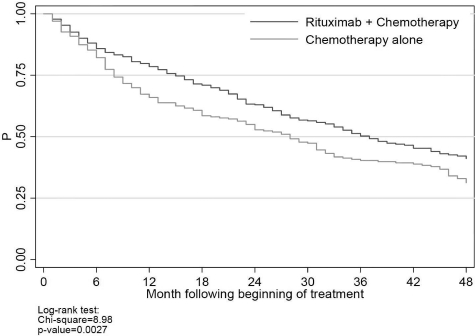
There were 411 deaths during the observation period: 307 (75%) were classified as cancer, 41 (10%) were classified as noncancer, and the remaining 63 (15%) were classified as missing or unknown. The latter were included in the overall survival analyses, but censored in the cancer and noncancer survival analyses. Median survival was 37 months (95% CI, 33-44 months) for rituximab plus chemotherapy and 27 months (95% CI, 20-31 months) for chemotherapy alone (Figure 2); the percentages of patients remaining alive 2 years after the beginning of first-line therapy were 63% and 52%, respectively (P < .001).
Figure 2.
Overall survival.
Multivariate survival analyses based on up to 2 years of follow-up from the beginning of first-line therapy showed that rituximab was associated with statistically significantly lower all-cause mortality (HR = 0.58; 95% CI 0.41-0.82; P < .01) and cancer mortality (HR = 0.56; 95% CI 0.37-0.84; P < .01), but not noncancer mortality (HR = 0.83; 95% CI 0.25-2.80; P = .77; Table 3). Advanced stage III/IV (compared with localized stage I/II) and older age were associated with higher cancer but not noncancer mortality. Female sex and later year of diagnosis were associated with lower cancer but not noncancer mortality. The presence of “B” symptoms was associated with lower all-cause mortality, but the HRs for cancer and noncancer mortality failed to meet the threshold for statistical significance (P ≤ .05). The interaction term between type of first-line therapy and year of diagnosis did not meet the threshold for statistical significance in any of the models. Results of multivariate analyses using the entire observation period and those using propensity techniques were comparable to those based on 2 years of follow-up using standard multivariate techniques (Figure 3).
Table 3.
Multivariate survival analysis: 2-year all-cause, cancer, and noncancer mortality
| All-cause mortality |
Cancer mortality |
Noncancer mortality |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | HR | 95% CI | P | ||||
| First-line treatment | ||||||||||||
| Chemotherapy alone | Reference | Reference | Reference | |||||||||
| Rituximab + chemotherapy | 0.58 | 0.41 | 0.82 | < .01 | 0.56 | 0.37 | 0.84 | < .01 | 0.83 | 0.25 | 2.80 | .77 |
| Year of diagnosis* | ||||||||||||
| 1999-2002 | Reference | Reference | Reference | |||||||||
| 2003-2005 | 0.64 | 0.39 | 1.05 | .08 | 0.55 | 0.30 | 1.00 | .05 | 0.72 | 0.12 | 4.24 | .72 |
| Age at diagnosis, y | ||||||||||||
| 66-70 | Reference | Reference | Reference | |||||||||
| 71-75 | 1.12 | 0.75 | 1.67 | .58 | 1.14 | 0.71 | 1.83 | .60 | 0.62 | 0.17 | 2.21 | .46 |
| 76-80 | 1.55 | 1.05 | 2.30 | .03 | 1.79 | 1.13 | 2.82 | .01 | 0.46 | 0.12 | 1.74 | .25 |
| > 80 | 2.04 | 1.37 | 3.05 | < .001 | 2.36 | 1.47 | 3.77 | < .001 | 0.60 | 0.16 | 2.17 | .43 |
| Sex | ||||||||||||
| Male | Reference | Reference | Reference | |||||||||
| Female | 0.64 | 0.48 | 0.85 | < .01 | 0.62 | 0.45 | 0.87 | < .01 | 1.07 | 0.41 | 2.78 | .89 |
| Race/ethnicity | ||||||||||||
| White | Reference | Reference | Reference | |||||||||
| Black | 0.85 | 0.39 | 1.88 | .69 | 0.67 | 0.24 | 1.89 | .45 | 2.30 | 0.25 | 20.95 | .46 |
| Hispanic | 0.64 | 0.30 | 1.39 | .26 | 0.73 | 0.32 | 1.69 | .46 | 2.53 | 0.24 | 26.88 | .44 |
| Other | 0.82 | 0.39 | 1.69 | .58 | 0.64 | 0.26 | 1.61 | .35 | 4.59 | 0.84 | 25.10 | .08 |
| Stage at diagnosis | ||||||||||||
| I/II | Reference | Reference | Reference | |||||||||
| III/IV | 1.44 | 0.98 | 2.12 | .06 | 1.61 | 1.00 | 2.58 | .05 | 1.07 | 0.37 | 3.10 | .91 |
| Unknown/not stated | 0.95 | 0.47 | 1.94 | .89 | 1.40 | 0.64 | 3.08 | .40 | 0.18 | 0.02 | 1.80 | .15 |
| Extranodal involvement | ||||||||||||
| No | Reference | Reference | Reference | |||||||||
| Yes | 1.11 | 0.80 | 1.53 | .55 | 1.31 | 0.88 | 1.95 | .18 | 0.18 | 0.06 | 0.53 | < .01 |
| Presence of “B” symptoms | ||||||||||||
| No | Reference | Reference | Reference | |||||||||
| Yes | 1.58 | 1.12 | 2.25 | .01 | 1.47 | 0.97 | 2.23 | .07 | 1.55 | 0.41 | 5.81 | .52 |
| Unknown | 1.01 | 0.74 | 1.37 | .96 | 1.07 | 0.76 | 1.52 | .69 | 1.75 | 0.60 | 5.11 | .31 |
| Anemia | ||||||||||||
| No | Reference | Reference | Reference | |||||||||
| Yes | 1.10 | 0.76 | 1.58 | .62 | 1.09 | 0.72 | 1.65 | .69 | 0.83 | 0.17 | 3.97 | .82 |
| Leukocytosis | ||||||||||||
| No | Reference | Reference | Reference | |||||||||
| Yes | 1.20 | 0.83 | 1.73 | .33 | 1.36 | 0.91 | 2.02 | .13 | 0.42 | 0.04 | 4.22 | .46 |
| NCI Comorbidity Index | ||||||||||||
| 0 | Reference | Reference | Reference | |||||||||
| 1 | 1.05 | 0.77 | 1.43 | .76 | 1.05 | 0.74 | 1.50 | .78 | 0.17 | 0.02 | 1.44 | .10 |
| 2 | 0.84 | 0.45 | 1.54 | .57 | 0.60 | 0.27 | 1.32 | .20 | 1.96 | 0.49 | 7.90 | .34 |
| ≥ 3 | 1.37 | 0.82 | 2.28 | .23 | 1.42 | 0.79 | 2.56 | .24 | 0.84 | 0.13 | 5.51 | .86 |
| Performance status indicators | ||||||||||||
| None | Reference | Reference | Reference | |||||||||
| ≥ 1 | 1.52 | 1.09 | 2.12 | .01 | 1.51 | 1.03 | 2.20 | .04 | 2.92 | 0.83 | 10.26 | .09 |
| Percent in census tract with some college | ||||||||||||
| < 25% | Reference | Reference | Reference | |||||||||
| > 25% | 0.95 | 0.72 | 1.25 | .71 | 0.87 | 0.63 | 1.19 | .38 | 1.78 | 0.59 | 5.36 | .30 |
| Percent in census tract living in poverty | ||||||||||||
| < 5% | Reference | Reference | Reference | |||||||||
| 5%-7% | 1.06 | 0.71 | 1.60 | .77 | 1.07 | 0.67 | 1.70 | .79 | 1.52 | 0.42 | 5.58 | .53 |
| 8%-12% | 1.20 | 0.83 | 1.72 | .33 | 1.31 | 0.87 | 1.97 | .20 | 1.01 | 0.27 | 3.83 | .99 |
| > 12% | 1.48 | 1.05 | 2.11 | .03 | 1.35 | 0.89 | 2.03 | .15 | 1.64 | 0.46 | 5.79 | .44 |
| Type of geographic area | ||||||||||||
| Large metropolitan | Reference | Reference | Reference | |||||||||
| Metropolitan | 0.82 | 0.60 | 1.11 | .19 | 0.79 | 0.56 | 1.13 | .20 | 0.25 | 0.07 | 0.90 | .03 |
| Urban | 1.04 | 0.61 | 1.76 | .89 | 0.69 | 0.34 | 1.41 | .31 | 0.55 | 0.06 | 4.79 | .58 |
| Less urban/rural | 0.69 | 0.43 | 1.10 | 0.12 | 0.78 | 0.46 | 1.30 | .34 | 0.27 | 0.03 | 2.37 | .23 |
All models also included an interaction term between type of first-line therapy and year of diagnosis. The interaction term did not meet the threshold for statistical significance (P ≤ .05) in any of the models.
Figure 3.
Multivariate survival analysis-sensitivity analysis on HRs for rituximab plus chemotherapy. This figure presents the results of 3 sets (all-cause mortality, cancer mortality, and noncancer mortality) of 4 multivariate survival analyses (2 for survival during the entire observation period and 2 for survival during the first 2 years of follow-up) designed to test the sensitivity of the findings reported in Table 3 to changes in the specification of the outcome variable and the approach to multivariate analysis. Standard multivariate survival analyses (*) were performed with all individual patient variables included in the model. Propensity multivariate survival analyses (**) were performed with propensity score quintile included in the model as a substitute for all patient variables except rituximab plus chemotherapy, year of diagnosis, and the interaction between year of diagnosis and rituximab plus chemotherapy. The third analysis in each of the 3 major groupings (all-cause mortality, cancer mortality, and noncancer mortality), labeled as Standard MV* 2 year follow-up, is the “baseline” analysis included in Table 3. The y-axis indicates the HR for rituximab plus chemotherapy compared with chemotherapy alone. Triangles represent the estimated HR for rituximab plus chemotherapy compared with chemotherapy alone from the corresponding model on the x-axis. Bars around each triangle represent the upper and lower bounds of the 95% CI for the HR. CIs that overlap the horizontal line at the HR of 1.0 indicate that the estimated HR for rituximab plus chemotherapy is not significant at P = .05.
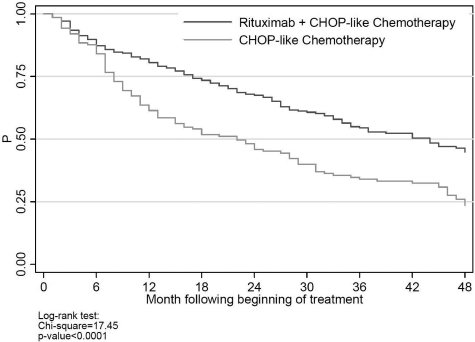
There were 415 patients (65%) who received CHOP/CHOP-like therapy. In unadjusted survival analysis (Figure 4), rituximab plus CHOP/CHOP-like chemotherapy was associated with significantly lower all-cause mortality than CHOP/CHOP-like chemotherapy alone. As shown in Figure 4, separation in the mortality curves coincided with the average time to the end of first-line therapy. In multivariate survival analysis, rituximab was associated with significantly lower all-cause and cancer mortality at 2 years (HR for cancer mortality = 0.39; 95% CI 0.23-0.67; P < 0. 001).
Figure 4.
Overall survival in patients receiving CHOP/CHOP-like chemotherapy.
Time to second-line treatment
Of the entire cohort, 502 (79%) patients survived at least 90 days after the end of first-line therapy with no chemotherapy or radiation during this period and were at risk of receiving second-line treatment. The median time to second-line treatment was 11 months and was similar in the 2 first-line treatment groups (P = .48). In multivariate analysis, there was no difference in the rate of second-line treatment between those who received first-line rituximab plus chemotherapy and those who received chemotherapy alone (HR = 0.89: 95% CI 0.67-1.20: P = .46).
Discussion
Using SEER-Medicare data, we identified a cohort of 638 patients who were diagnosed with MCL between 1999 and 2005 and who began chemotherapy with or without rituximab within 180 days of diagnosis. We performed a wide range of multivariate analyses to examine adjusted associations between rituximab use and survival, including varying the time horizon, looking separately at cancer and noncancer mortality, including only patients with CHOP/CHOP-like chemotherapy, and using propensity score quintile in lieu of most individual patient variables in the models. Our findings show that adding rituximab to first-line chemotherapy was associated with significantly improved overall and cancer survival at 2 years and over the entire observation period. The results of the analyses using propensity score techniques were consistent with those analyses that included individual patient factors. Rituximab had no impact on noncancer mortality or on the time to next treatment in the subset of patients who completed first-line therapy.
One important question is why did we not observe differences in time to second-line treatment in the presence of differences in overall survival? The Kaplan-Meier survival analyses (Figures 2 and 4) show that almost all of the separation in the survival curves of the 2 first-line treatment groups occurred within 6-9 months after the start of therapy. In our analysis of time to next treatment, we included only patients who survived at least 90 days without chemotherapy or radiation after the end of first-line therapy, and we began the observation period for this analysis at the end of that 90-day interval. We established these criteria to avoid misclassifying short interruptions in first-line therapy as second-line treatment. As a result, however, our criteria may have inadvertently introduced a “survivor bias” in that only the healthiest patients in each of the 2 treatment groups were included in the time to next treatment analysis.
Our study population and findings do differ considerably from those previously reported based on a randomized clinical trial of rituximab added to first-line therapy for MCL.14 For example, the median age was 74 years in this study, compared with 62 years in the previous study. In addition, 63% of patients in this study had stage IV disease, compared with 79% in the previous study, and the 2-year survival probability was 59% in this study compared with 77% in the previous study. In the GLSG trial, the Kaplan-Meier curve for overall survival shows virtually no separation between the 2 treatment groups during the limited follow-up (median = 18 months). In our study, the treatment groups began to separate within 6 months of beginning treatment. Older age is an independent prognostic factor for overall survival included in the MIPI,22 and in this study, we found that older age was associated with significantly higher overall and cancer mortality.
Several observational studies have examined patterns of rituximab use and outcomes in NHL using SEER-Medicare data.33,48,49 One common characteristic of studies conducted with SEER-Medicare data is that because almost all of those eligible for Medicare insurance qualify by virtue of older age, these studies usually include only patients ≥ 65 years of age or ≥ 66 years of age if the first year of Medicare claims is used to calculate an NCI Comorbidity Index score.26,27 Two SEER-Medicare studies in NHL, one in FL48 and one in CLL,33 used multivariate analysis to compare overall survival in patients receiving chemotherapy with or without rituximab. The FL study included > 1100 patients diagnosed with FL between 1999 and 2005 who received first-line chemotherapy with or without rituximab and who were followed for overall survival from the beginning of treatment until the end of the observation period (December 2007). The median age at diagnosis was 73 years, compared with age 52-57 years, depending on treatment group, in 3 phase 3 trials of front-line chemotherapy with or without rituximab in FL.9,11,12 The multivariate analysis of overall survival in the SEER-Medicare study showed a significant advantage for rituximab (HR = 0.62; P < .0001), which was similar to that reported by Marcus et al in their trial comparing R-CVP with CVP (HR = 0.60).11 In the SEER-Medicare CLL study, multivariate analysis of 1721 patients who received first-line chemotherapy with or without rituximab showed that immunochemotherapy was associated with a 25% lower risk of death compared with chemotherapy alone (HR = 0.75; 95% CI 0.62-0.91). When the analysis was restricted to 737 patients who received fludarabine-containing chemotherapy, rituximab plus chemotherapy was associated with a 42% reduction in mortality (HR = 0.58; 95% CI 0.40-0.84) versus chemotherapy alone. By comparison, Hallek et al13 reported an HR for overall survival of 0.67 (95% CI 0.48-0.92]) for rituximab plus fludarabine and chlorambucil (FC) compared with FC alone.
As with any study using observational data to examine the benefits of therapies or other interventions, the main threat to the validity of our findings is selection bias, in which unobserved factors influence both treatment selection and the outcome of interest.50 A limitation of SEER-Medicare is that presently it does not contain data on LDH, leukocyte count, and ECOG performance status, 3 of the 4 independent prognostic factors for MCL overall survival included in the MIPI.22 In lieu of these variables, we included claims-based indicators of elevated leukocyte count and poor performance status,35 and whereas we did find that having at least one indicator of poor performance was associated with a 52% increase in the risk of death at 2 years, the validity of these variables has not been confirmed using actual laboratory values or ECOG performance scores.
In this study, rituximab use increased rapidly over time, meaning that patients diagnosed in later years were more likely to receive it. This may have introduced several possible sources of bias, including shorter follow-up for patients who received rituximab plus chemotherapy and secular trends in the assignment of patients to different chemotherapy regimens and in the use of supportive care. Before deciding on the final specification of the multivariate models, we divided patients into those who received and those who did not receive rituximab with first-line chemotherapy. In the chemotherapy alone group, we found that a 2003-2005 year of diagnosis was associated with significantly lower mortality than a diagnosis in 1999-2002. In contrast, in the group that received rituximab plus chemotherapy, year of diagnosis was not associated with mortality. One possibility is that as rituximab became the standard of care, chemotherapy alone was more likely to be reserved for those with a better prognosis. This is supported by the fact that patients who received chemotherapy alone and were diagnosed in 2003-2005 tended to be younger and diagnosed with an earlier stage of disease that those who were diagnosed in 1999-2002. Although we adjusted for these factors in all the multivariate models, differences in age and stage at diagnosis suggest the possible existence of other unobserved differences such as in LDH, leukocyte count, and ECOG performance status. In this case failing to include year of diagnosis as an independent variable in the survival analyses could have resulted in underestimating the survival benefit of rituximab, because if selection bias does exist in our analyses, overall it appears to favor patients who received chemotherapy alone.
To adjust for differences in the length of follow-up between the treatment groups, we restricted the primary survival analyses to 2 years. We conducted analyses that only included patients with CHOP or CHOP-like therapy because the majority received these regimens, and we found results similar to those based on analyses that included all patients and the entire observation period. We did not look for dose-intensified regimens that have been shown to improve progression-free and overall survival, and only 3 patients in the cohort received stem cell transplantation. Based on the age distribution of our study population, it is likely that only a minority of patients would have qualified for dose-intensified regimens.
As discussed above, selection bias is the most critical issue in analyses of observational data. One potential indication of selection bias in survival analysis is that treatment differences in noncancer mortality are as large as, or larger than, the differences in cancer mortality.50 In our study, we found a statistically significant difference in cancer mortality favoring rituximab in each of the analyses we performed, but no difference in noncancer mortality in any of the analyses. Despite this finding, no approach can be sure to eliminate selection bias in observational research. Therefore, we cannot rule out the possibility that part of the difference in overall survival is because of unobserved differences between the treatment groups. It is not clear whether accounting for these differences would have increased or decreased the treatment effects we observed.
Our findings suggest that first-line chemotherapy including rituximab is associated with significantly improved survival in older patients diagnosed with MCL compared with chemotherapy alone. However, because of the limitations inherent in studies based on observational data, these findings should be confirmed in prospective clinical trials.
Acknowledgments
Editorial assistance on this manuscript was provided by Kim Merjan and Suzanne Griffiths.
This study used the linked SEER-Medicare database. The interpretation and reporting of these data are the sole responsibility of the authors. The authors acknowledge the efforts of the Applied Research Program, National Cancer Institute; the Office of Research, Development and Information, Centers for Medicare & Medicaid Services; Information Management Services Inc; and the SEER program tumor registries in the creation of the SEER-Medicare database.
Footnotes
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: R.G. and M.D. acquired the data and wrote the initial version of the manuscript; R.G., M.D., and M.G. developed the initial analysis plan; M.D. and J.M. provided comments on the analysis plan and additional clinical input during data analysis; M.G. did the programming and created all of the tables and figures; and all authors contributed to the revisions of the manuscript.
Conflict-of-interest disclosure: Acquisition of the data was funded by Genentech Inc through a contract with Outcomes Insights Inc. Development of the analysis plan, programming, and preparation of the manuscript were all performed without any additional funding, and Genentech Inc had no role whatsoever in any of the research activities pertaining to this study. R.G., M.D., and M.G. work for Outcomes Insights Inc in a research and consulting capacity. M.D. and J.M. did not receive any funding for this work.
Correspondence: Robert Griffiths, MS, ScD, Outcomes Insights Inc, 340 N Westlake Blvd, Suite 200, Westlake Village, CA 91362; e-mail: bob@outins.com.
References
- 1.European MCL Network. Dreyling M, Hiddemann W. Current treatment standards and emerging strategies in mantle cell lymphoma. Hematology Am Soc Hematol Educ Program. 2009:542–551. doi: 10.1182/asheducation-2009.1.542. [DOI] [PubMed] [Google Scholar]
- 2.Martin P, Chadburn A, Christos P, et al. Outcome of deferred initial therapy in mantle-cell lymphoma. J Clin Oncol. 2009;27(8):1209–1213. doi: 10.1200/JCO.2008.19.6121. [DOI] [PubMed] [Google Scholar]
- 3.Fernàndez V, Salamero O, Espinet B, et al. Genomic and gene expression profiling defines indolent forms of mantle cell lymphoma. Cancer Res. 2010;70(4):1408–1418. doi: 10.1158/0008-5472.CAN-09-3419. [DOI] [PubMed] [Google Scholar]
- 4.Hermine O, Hoster E, Walewski J, et al. Alternating courses of 3x CHOP and 3x DHAP plus rituximab followed by a high dose ARA-C containing myeloablative regimen and autologous stem cell transplantation (ASCT) is superior to 6 courses of CHOP plus rituximab followed by myeloablative radiochemotherapy and ASCT in mantle cell lymphoma: results of the MCL Younger Trial of the European Mantle Cell Lymphoma Network (MCL net).. Paper presented at: 52nd American Society of Hematology Annual Meeting and Exposition; December 4-7, 2010; Orlando, FL. [Google Scholar]
- 5.Coiffier B, Lepage E, Briere J, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med. 2002;346:235–242. doi: 10.1056/NEJMoa011795. [DOI] [PubMed] [Google Scholar]
- 6.Habermann T, Weller E, Morrison VA, et al. Rituximab-CHOP versus CHOP alone or with maintenance rituximab in older patients with diffuse large B-cell lymphoma. J Clin Oncol. 2006;24(19):3121–3127. doi: 10.1200/JCO.2005.05.1003. [DOI] [PubMed] [Google Scholar]
- 7.Pfreundschuh M, Trumper L, Osterborg A, et al. CHOP-like chemotherapy plus rituximab versus CHOP-like chemotherapy alone in young patients with good-prognosis diffuse large-B-cell lymphoma: A randomized controlled trial by the MabThera International Trial (MInT) Group. Lancet Oncol. 2006;7:379–391. doi: 10.1016/S1470-2045(06)70664-7. [DOI] [PubMed] [Google Scholar]
- 8.Coiffier B, Thieblemont C, Van Den Neste E, et al. Long-term outcome of patients in the LNH-98.5 trial, the first randomized study comparing rituximab-CHOP to standard CHOP chemotherapy in DLBCL patients: a study by the Groupe d'Etudes des Lymphomes de l'Adulte. Blood. 2010;116(12):2040–2045. doi: 10.1182/blood-2010-03-276246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hiddemann W, Kneba M, Dreyling M, et al. Frontline therapy with rituximab added to the combination of cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) significantly improves the outcome for patients with advanced-stage follicular lymphoma compared with therapy with CHOP alone: results of a prospective randomized study of the German Low-Grade Lymphoma Study Group. Blood. 2005;106:3725–3732. doi: 10.1182/blood-2005-01-0016. [DOI] [PubMed] [Google Scholar]
- 10.Herold M, Haas A, Srock S, et al. Rituximab added to first-line mitoxantrone, chlorambucil, and prednisolone chemotherapy followed by interferon maintenance prolongs survival in patients with advanced follicular lymphoma: an East German Study Group hematology and oncology study. J Clin Oncol. 2007;25(15):1986–1992. doi: 10.1200/JCO.2006.06.4618. [DOI] [PubMed] [Google Scholar]
- 11.Marcus R, Imrie K, Solal-Celigny P, et al. Phase III study of R-CVP compared with cyclophosphamide, vincristine, and prednisone alone in patients with previously untreated advanced follicular lymphoma. J Clin Oncol. 2008;26:4579–4586. doi: 10.1200/JCO.2007.13.5376. [DOI] [PubMed] [Google Scholar]
- 12.Salles G, Mounier N, de Guibert S, et al. Rituximab combined with chemotherapy and interferon in follicular lymphoma patients: results of the GELA-GOELAMS FL2000 study. Blood. 2008;112:4824–4831. doi: 10.1182/blood-2008-04-153189. [DOI] [PubMed] [Google Scholar]
- 13.Hallek M, Fischer K, Fingerle-Rowson G, et al. Addition of rituximab to fludarabine and cyclophosphamide in patients with chronic lymphocytic leukemia: a randomised, open-label, phase 3 trial. Lancet. 2010;376:1164–1174. doi: 10.1016/S0140-6736(10)61381-5. [DOI] [PubMed] [Google Scholar]
- 14.Lenz G, Dreyling M, Hoster E, et al. Immunochemotherapy with rituximab and cyclophosphamide, doxorubicin, vincristine, and prednisone significantly improves response and time to treatment failure, but not long-term outcome in patients with previously untreated mantle cell lymphoma: results of a prospective randomized trial of the German Low Grade Lymphoma Study Group (GLSG). J Clin Oncol. 2005;23(9):1984–1992. doi: 10.1200/JCO.2005.08.133. [DOI] [PubMed] [Google Scholar]
- 15.Schulz H, Bohlius JF, Trelle S, et al. Immunochemotherapy with rituximab and overall survival in patients with indolent or mantle cell lymphoma: a systematic review and meta-analysis. J Natl Cancer Inst. 2007;99(9):706–714. doi: 10.1093/jnci/djk152. [DOI] [PubMed] [Google Scholar]
- 16.Forstpointner R, Dreyling M, Repp R, et al. The addition of rituximab to a combination of fludarabine, cyclophosphamide, mitoxantrone (FCM) significantly increases the response rate and prolongs survival compared with FCM alone in patients with relapsed and refractory follicular and mantle cell lymphomas: results of a prospective randomized study of the German Low-Grade Lymphoma Study Group. Blood. 2004;104(10):3064–3071. doi: 10.1182/blood-2004-04-1323. [DOI] [PubMed] [Google Scholar]
- 17.Herold M, Pasold R, Srock S, et al. Results of a prospective randomised open label phase III study comparing rituximab plus mitoxantrone, chlorambucile, prednisolone chemotherapy (R-MCP) versus MCP alone in untreated advanced indolent non-Hodgkin's lymphoma (NHL) and mantle-cell lymphoma (MCL) [abstract]. Blood. 2004;104(11 suppl 1) Abstract 584. [Google Scholar]
- 18.Herrmann A, Hoster E, Zwingers T, et al. Improvement of overall survival in advanced mantle cell lymphoma. J Clin Oncol. 2009;27(4):511–518. doi: 10.1200/JCO.2008.16.8435. [DOI] [PubMed] [Google Scholar]
- 19.National Cancer Institute. Surveillance, epidemiology, and end results. Data set and software. [Accessed January 17, 2011]. Available from http://seer.cancer.gov/seerstat/
- 20.The International Non-Hodgkin's Lymphoma Prognostic Factors Project. A predictive model for aggressive non-Hodgkin's lymphoma. N Engl J Med. 1993;329:987–994. doi: 10.1056/NEJM199309303291402. [DOI] [PubMed] [Google Scholar]
- 21.Solal-Céligny P, Roy P, Colombat P, et al. Follicular lymphoma international prognostic index. Blood. 2004;104:1258–1265. doi: 10.1182/blood-2003-12-4434. [DOI] [PubMed] [Google Scholar]
- 22.Hoster E, Dreyling M, Klapper W, et al. A new prognostic index (MIPI) for patients with advanced-stage mantle cell lymphoma. Blood. 2008;111:558–565. doi: 10.1182/blood-2007-06-095331. [DOI] [PubMed] [Google Scholar]
- 23.Warren JL, Klabunde CN, Schrag D, Bach PB, Riley GF. Overview of the SEER-Medicare data: content, research applications, and generalizability to the United States elderly population. Med Care. 2002;40:IV, 3–18. doi: 10.1097/01.MLR.0000020942.47004.03. [DOI] [PubMed] [Google Scholar]
- 24.National Cancer Institute. About the SEER Program. [Accessed August 27, 2010]. Available from: http://seer.cancer.gov/about/
- 25.National Cancer Institute. SEER-Medicare: How the SEER & Medicare Data are Linked. [Accessed August 27, 2010]. Available from: http://healthservices.cancer.gov/seermedicare/overview/linked.html.
- 26.North American Association of Central Cancer Registries. Guidelines for ICD-O-3 Implementation. [Accessed February 24, 2011]. Available from: www.facs.org/cancer/coc/naaccr.pdf.
- 27.Warren JL, Harlan LC, Fahey A, et al. Utility of the SEER-Medicare data to identify chemotherapy use. Med Care. 2002;40(8 suppl):IV, 55–61. doi: 10.1097/01.MLR.0000020944.17670.D7. [DOI] [PubMed] [Google Scholar]
- 28.National Cancer Institute. Procedure Codes for SEER-Medicare Analysis. [Accessed December 7, 2010]. Available from: http://healthservices.cancer.gov/seermedicare/considerations/procedure_codes.html.
- 29.Practice Management Information Corporation. HCPCS. Los Angeles, CA: Practice Management Information Corporation; 2005. [Google Scholar]
- 30.Fritz A, Ries L, editors. SEER Program Code Manual. 3rd ed. [Accessed October 5, 2010]. Available from: http://seer.cancer.gov/manuals/codeman.pdf.
- 31.Young JL, Jr, Roffers SD, Ries LAG, Fritz AG. SEER Summary Staging Manual - 2000 Codes and Coding Instructions. (National Institutes of Health publication number 01-4969). [Accessed January 4, 2011]. Available from: http://seer.cancer.gov/tools/ssm/intro.pdf.
- 32.Practice Management Information Corporation. ICD-9-CM. 6th ed. Los Angeles, CA: Practice Management Information Corporation; 2005. [Google Scholar]
- 33.Danese MD, Griffiths RI, Gleeson M, et al. An observational study of outcomes after initial infused therapy in Medicare patients diagnosed with chronic lymphocytic leukemia. Blood. 2011;117(13):3505–3513. doi: 10.1182/blood-2010-08-301929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649–655. [PubMed] [Google Scholar]
- 35.Davidoff AJ, Tang M, Seal B, Edelman MJ. Chemotherapy and survival benefit in elderly patients with advanced non-small-cell lung cancer. J Clin Oncol. 2010;28(13):2191–2197. doi: 10.1200/JCO.2009.25.4052. [DOI] [PubMed] [Google Scholar]
- 36.Klabunde CN, Potosky AL, Legler JM, Warren JL. Development of a comorbidity index using physician claims data. J Clin Epidemiol. 2000;53:1258–1267. doi: 10.1016/s0895-4356(00)00256-0. [DOI] [PubMed] [Google Scholar]
- 37.Klabunde CN, Legler JM, Warren JL, Baldwin L-M, Schrag D. A refined comorbidity measurement algorithm for claims-based studies of breast, prostate, colorectal, and lung cancer patients. Ann Epidemiol. 2007;17:584–590. doi: 10.1016/j.annepidem.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 38.National Cancer Institute. SEER-medicare: calculation of comorbidity weights. [Accessed August 27, 2010]. Available from: http://healthservices.cancer.gov/seermedicare/program/comorbidity.html.
- 39.National Cancer Institute. Overview of the SEER Program. [Accessed August 27, 2010]. Available from: http://healthservices.cancer.gov/seermedicare/program/charlson.comorbidity.macro.txt.
- 40.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 41.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 42.Romano PS, Roos LL, Luft HS, Jollis JG, Doliszny K. A comparison of administrative versus clinical data: coronary artery bypass surgery as an example. Ischemic Heart Disease Patient Outcomes Research Team. J Clin Epidemiol. 1994;47:249–260. doi: 10.1016/0895-4356(94)90006-x. [DOI] [PubMed] [Google Scholar]
- 43.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology Non-Hodgkin's Lymphomas V. 1.2010. [Accessed January 4, 2011]. Available from: http://www.nccn.org/professionals/physician_gls/f_guidelines.asp. [DOI] [PubMed]
- 44.ResDAC. Research Data Assistance Center. ResDAC. Minneapolis, MN: 2008. May 15-16, CMS 301: Using SEER/Medicare Data for Research. [Google Scholar]
- 45.Earle CC, Nattinger AB, Potosky AL, et al. Identifying cancer relapse using SEER-Medicare data. Med Care. 2002;40(8 suppl):IV, 75–81. doi: 10.1097/00005650-200208001-00011. [DOI] [PubMed] [Google Scholar]
- 46.Stokes ME, Thompson D, Montoya EL, et al. Ten-year survival and cost following breast cancer recurrence: estimates from SEER-Medicare data. Value in Health. 2008;11:213–220. doi: 10.1111/j.1524-4733.2007.00226.x. [DOI] [PubMed] [Google Scholar]
- 47.Rubin DR. Estimating causal effects from large data sets using propensity scores. Ann Intern Med. 1997;127:757–763. doi: 10.7326/0003-4819-127-8_part_2-199710151-00064. [DOI] [PubMed] [Google Scholar]
- 48.Griffiths R, Gleeson M, Reyes C, Knopf K, Danese M. Survival in elderly follicular lymphoma patients who receive frontline chemo-immunotherapy. Am J Hematol. 2010;85(12):963–967. doi: 10.1002/ajh.21878. [DOI] [PubMed] [Google Scholar]
- 49.Griffiths R, Gleeson M, Knopf K, Danese M. Racial differences in treatment and survival in older patients with diffuse large B-cell lymphoma (DLBCL). BMC Cancer. 2010;10:625–639. doi: 10.1186/1471-2407-10-625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Giordano SH, Kuo Y-F, Duan Z, Hortobagyi GN, Freeman J, Goodwin JS. Limits of observational data in determining outcomes from cancer therapy. Cancer. 2008;112:2456–2466. doi: 10.1002/cncr.23452. [DOI] [PMC free article] [PubMed] [Google Scholar]