Abstract
The properties of oxaloacetate (OA) transport into mitochondria from potato (Solanum tuberosum) tuber and pea (Pisum sativum) leaves were studied by measuring the uptake of 14C-labeled OA into liposomes with incorporated mitochondrial membrane proteins preloaded with various dicarboxylates or citrate. OA was found to be transported in an obligatory counterexchange with malate, 2-oxoglutarate, succinate, citrate, or aspartate. Phtalonate inhibited all of these countertransports. OA-malate countertransport was inhibited by 4,4′-dithiocyanostilbene-2,2′-disulfonate and pyridoxal phosphate, and also by p-chloromercuribenzene sulfonate and mersalyl, indicating that a lysine and a cysteine residue of the translocator protein are involved in the transport. From these and other inhibition studies, we concluded that plant mitochondria contain an OA translocator that differs from all other known mitochondrial translocators. Major functions of this translocator are the export of reducing equivalents from the mitochondria via the malate-OA shuttle and the export of citrate via the citrate-OA shuttle. In the cytosol, citrate can then be converted either into 2-oxoglutarate for use as a carbon skeleton for nitrate assimilation or into acetyl-coenzyme A for use as a precursor for fatty acid elongation or isoprenoid biosynthesis.
Mitochondria from various plant tissues can reduce added OA at the expense of NADH generated in the mitochondrial matrix (Douce and Bonner, 1972; Woo and Osmond, 1976; Day and Wiskich, 1981a; Journet et al., 1981; Ebbighausen et al., 1985). In this way mitochondria contained in a plant cell are able to export reducing equivalents via a malate-OA shuttle, e.g. to supply the necessary reducing equivalents for the reduction of hydroxypyruvate in the peroxisomes, which is part of the photorespiratory cycle (Raghavendra et al., 1998). It has been estimated that during photosynthesis of a leaf under ambient conditions, about one-half of the reducing equivalents required in the peroxisomes are delivered via a mitochondrial malate-OA shuttle and the remainder by a chloroplastic malate-OA shuttle (Krömer and Heldt, 1991; Hanning and Heldt, 1993).
The mechanism of the transport of malate and OA across the inner mitochondrial membrane is still largely unknown. Silicon-layer-filtering centrifugation, a method successfully applied for the characterization of dicarboxylate transport in animal mitochondria (Palmieri and Klingenberg, 1979), cannot be used in plant mitochondria because the transport of OA and malate in these organelles is so rapid that the kinetics cannot be resolved. Therefore, our present knowledge about mitochondrial OA transport derives mainly from indirect studies, e.g. from the inhibition of mitochondrial respiration by the addition of OA (Ebbighausen et al., 1985), from the concentration dependence of OA conversion into malate by intact mitochondria (Oliver and Walker, 1984), and from the effect of phtalonate, a powerful inhibitor of OA uptake, on these parameters (Day and Wiskich, 1981b; Oliver and Walker, 1984; Proudlove and Moore, 1984; Ebbighausen et al., 1985).
Because there were no other methods available, the permeability of isolated plant mitochondria for various dicarboxylates had been previously studied by measuring mitochondrial swelling in isoosmolar solutions of dicarboxylates. These studies found that malate or OA was taken up rapidly into the mitochondria without the requirement for an anion to be transported in the opposite direction. It was sufficient when valinomycin and K+ were added to compensate for the anion uptake (Zoglowek et al., 1988). These results indicated that OA and malate are each transported by electrogenic uniport and are probably linked to each other for the sake of charge compensations.
Because it seemed possible that these results represented an artifact, we reinvestigated the properties of mitochondrial OA-malate transport with proteoliposomes that had incorporated mitochondrial transport proteins. This method had been used previously for the study of plant mitochondrial translocators, such as for transport of dicarboxylates (Vivekananda et al., 1988), aspartate-glutamate (Vivekananda and Oliver, 1989), monocarboxylates (Vivekananda and Oliver, 1990), 2-oxoglutarate (Genchi et al., 1991), tricarboxylates (McIntosh and Oliver, 1992), and phosphate (McIntosh and Oliver, 1994). The proteoliposome method has the advantage that by a defined preloading of the liposomes a counterexchange of anions can be measured and the kinetics of transport can be resolved even at room temperature. We show that the mitochondrial OA translocator catalyzes a strict counterexchange not only with malate but also with other dicarboxylates and citrate. We describe herein the properties of this transport.
The present experiments were mostly on mitochondria from potato (Solanum tuberosum) tubers, but for the sake of comparison we also performed them with mitochondria from pea (Pisum sativum) leaves and show that OA transport in mitochondria of different origins is essentially the same.
MATERIALS AND METHODS
Isolation of Mitochondria
Mitochondria were prepared from potato (Solanum tuberosum) tubers or from the leaves of 3-week-old pea (Pisum sativum) seedlings as described previously (Ebbighausen et al., 1985). The mitochondria were purified by density-gradient centrifugation in a medium containing 0.3 m Suc, 10 mm KH2PO4, pH 7.2, 1.0 mm EDTA, and 28% to 32% Percoll. These mitochondria were essentially free of plastidial contamination and were kept in a medium containing 0.3 m Suc, 10 mm EDTA, and 0.1% (w/v) defatted BSA.
Preparation of Substrate-Loaded Reconstituted Proteoliposomes
Acetone-washed soybean phospholipids (0.1 g mL−1) in a medium containing 100 mm Tricine-KOH, pH 7.2, 240 to 290 mm potassium gluconate-KOH, pH 7.2, and di- or tricarboxylates at the concentrations indicated in the single experiments (total osmolarity of the medium was 600 milliosmoles) were sonicated (model B15 sonicator using a microtip, an output control of 3, and 50% power; Branson Ultrasonics, Danbury, CT) at 1-s intervals in an ice bath. The sonication time was 1 min for 1-mL samples up to 10 min for larger samples. For each experiment control samples of liposomes were prepared in a sonication medium containing 290 mm potassium gluconate without the addition of di- or tricarboxylates.
For the preparation of reconstituted proteoliposomes a mitochondrial stock suspension equivalent to 0.6 to 1.0 mg of protein was solubilized in 7 μL of 20% (w/v) Triton X-100 added to 1 mL of liposomal suspension (lipid:detergent, 75:1) and was immediately frozen in liquid nitrogen (Kasahara and Hinkle, 1977). The sample was then thawed in an ice bath. After a second sonication (using the sonicator at 20% power for 20 s) the external anions (di- and tricarboxylates) were removed by passing the proteoliposomes over a 1.45-mL Dowex AG 1×8 (100–200 mesh; acetate form) anion-exchange column in a Pasteur pipette that had been preequilibrated with a solution containing 150 mm sodium gluconate, 70 mm potassium gluconate, and 10 mm Tricine-KOH, pH 7.2 (equilibration buffer). The substrate-loaded reconstituted proteoliposomes were eluted from the ion-exchange column with the equilibration buffer.
Transport Measurement
The experiments were performed at room temperature (20°C). Transport (60 s if not stated otherwise) was started by the addition of 14C-labeled OA (50 μCi [0.7–1.1 kBq]/μmol) prepared according to the method of Hatch et al. (1984) at the concentrations indicated in the tables and figures to the proteoliposomal suspension. To terminate metabolite transport, 200 μL of the liposomal suspension was placed at a defined time (1 min if not stated otherwise) on a 0.2-mL Dowex anion-exchange column contained in a Pasteur pipette and within about 30 s passed through. The liposomes were eluted from the ion-exchange column with 1.0 mL of the above-mentioned equilibration buffer.
The eluates were counted for 14C radioactivity in a liquid-scintillation counter, and the uptake of metabolites into the proteoliposomes was evaluated from the specific activity of the 14C-labeled metabolite applied. Phtalonic acid was synthesized and generously supplied by Prof. L.F. Tietze, Göttingen, Germany.
RESULTS AND DISCUSSION
OA Transport Proceeds by Obligate Counterexchange
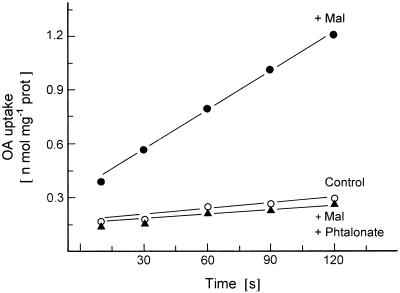
The time course of [14C]OA uptake into reconstituted proteoliposomes was investigated (Fig. 1). Liposomes preloaded with malate took up OA rapidly and with an almost linear rate for 120 s, whereas very little uptake was observed with liposomes lacking internal malate. This result demonstrated that the uptake of OA required a counterexchange with a dicarboxylate from the inside.
Figure 1.
[14C]OA transport into liposomes with incorporated membrane proteins from potato tuber mitochondria. The time dependence of OA transport in liposomes preloaded with 30 mm malate (Mal) was compared with the uptake of malate-preloaded liposomes incubated with 1 mm phtalonate for 10 min at room temperature before transport measurement. In the control experiment the liposomes were not preloaded. For details, see text. prot, Protein.
In earlier experiments phtalonate was shown to act as a powerful inhibitor of OA transport into plant mitochondria (Day and Wiskich, 1981b; Oliver and Walker, 1984; Proudlove and Moore, 1984). The transport of OA into chloroplasts was not affected by this inhibitor (Hatch et al., 1984). As shown in Figure 1, the transport of OA into malate-preloaded liposomes was strongly inhibited by phtalonate. Essentially the same result was also obtained with mitochondria from pea leaves (data not shown).
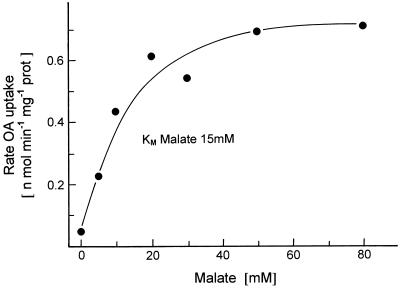
Figure 2 shows that OA uptake into liposomes is dependent on the internal malate concentration. Liposomes preloaded with various concentrations of malate showed a half-maximal rate of [14C]OA uptake at an internal malate concentration of about 15 mm. To measure high rates of OA uptake, we preloaded the liposomes with 50 mm malate (or other dicarboxylates) in subsequent experiments unless otherwise stated.
Figure 2.
Dependence of [14C]OA transport into liposomes with incorporated membrane proteins from potato tuber mitochondria on the preloading with malate of indicated concentrations. For details, see text. prot, Protein.
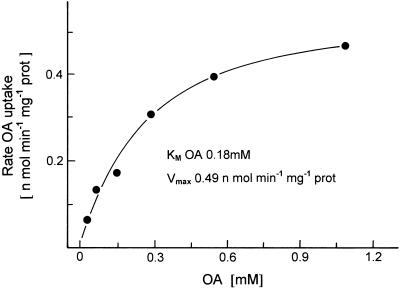
Figure 3 shows the dependence of OA uptake on its external concentration. A half-maximal rate of OA uptake was observed at a concentration of 0.18 mm. In a number of similar results, the Kms for the uptake of OA into mitochondria from potato tubers and pea leaves were found to be 0.18 ± 0.01 mm (n = 7) and 0.20 ± 0.06 mm (n = 10), respectively. With intact mitochondria from potato tubers and pea leaves the Kms for OA uptake were determined earlier as 0.04 and 0.006 mm, respectively (Ebbighausen et al., 1985). The Vmax of the transport into the liposomes was 3 orders of magnitude lower than in intact mitochondria. The reconstituted translocator in the liposomes reflects an artificial system, and it is therefore not surprising that the kinetic properties (Km and Vmax) of the translocator are altered.
Figure 3.
Dependence of [14C]OA transport into liposomes with incorporated membrane proteins from potato tubers on the external OA concentration. The liposomes were preloaded with 30 mm malate. prot, Protein.
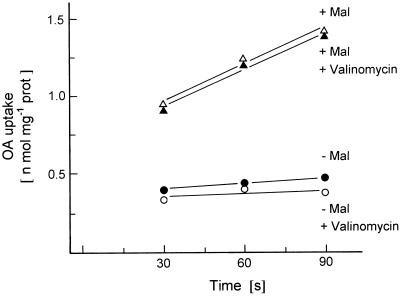
As shown in Figure 4, the presence of valinomycin plus K+ ions did not increase the uptake of [14C]OA into liposomes preloaded or not with malate, indicating that the OA translocator incorporated in the liposomal membrane catalyzed an obligatory antiport with a dicarboxylate. This contradicted results from earlier studies in which measurements of mitochondrial swelling in isoosmolar solutions of dicarboxylates suggested that OA could be transported into the mitochondria without counterexchange with dicarboxylates if K+ ions plus valinomycin were added for charge compensation (Zoglowek et al., 1988). It seems now that these results were artificial, because metabolite concentrations of 100 mm had to be used in these experiments. The chloroplast triose-P translocator catalyzing a strict counterexchange of metabolites may have been converted into an uniport when metabolites were present at very high concentrations (Schwarz et al., 1994).
Figure 4.
Effect of valinomycin (1 mm) on the transport of [14C]OA into liposomes, which were either preloaded with malate (+ Mal) or not (− Mal). The liposomes prepared with membrane proteins from potato tubers were preincubated for 10 min with 1 mm valinomycin (+ Val) or not. The medium contained about 200 mm K+ ions. prot, Protein.
Specificity of OA-Malate Transport
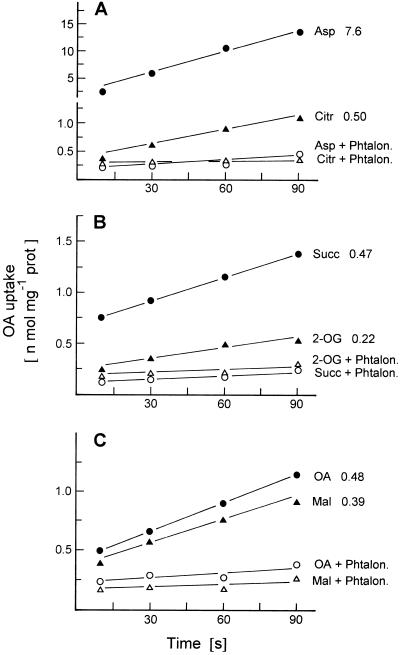
In the experiment shown in Figure 5, we investigated which substances participate in the countertransport with OA by measuring the rate of OA uptake into liposomes that had been preloaded with various di- and tricarboxylates. The results show that OA is taken up in counterexchange not only with malate or OA but also with aspartate, citrate, succinate, and, to a lesser extent, 2-oxoglutarate. In each case the countertransport of OA was inhibited by phtalonate, suggesting that these various countertransports were catalyzed by the same translocator. The countertransport of OA with aspartate was more rapid than with other dicarboxylates and with citrate. We obtained essentially the same results with pea leaf mitochondria.
Figure 5.
Effect of preloading with carboxylates and of phtalonate on the transport of [14C]OA into liposomes with membrane proteins from potato tuber mitochondria. The liposomes were preloaded with the carboxylate indicated (50 mm) for 10 min at room temperature before transport measurement. Citr, Citrate; Phtalon., phtalonate; Succ, succinate; prot, protein; Mal, malate.
Inhibition of OA-Malate Transport
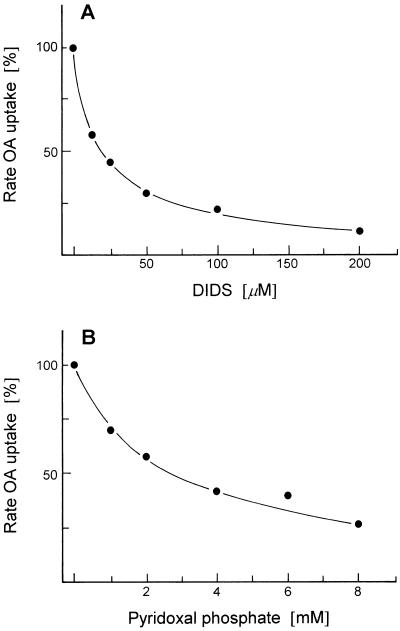
To further characterize OA-malate transport and compare it with other known mitochondrial translocators, we studied the effect of various inhibitors on the transport of OA into malate-preloaded liposomes. In these experiments the malate-preloaded liposomes were incubated with the inhibitors for 10 min at room temperature before OA transport was started by adding [14C]OA. DIDS and pyridoxal phosphate react with Lys residues. As shown in Figure 6A, DIDS was a powerful inhibitor: 25 μm was enough for approximately a 50% inhibition of transport. Pyridoxal phosphate also inhibited OA-malate transport but only at higher concentrations: 3 mm pyridoxal phosphate was required for 50% inhibition (Fig. 6B). In earlier experiments similar concentrations of pyridoxal phosphate inhibited the 2-oxoglutarate translocator (Genchi et al., 1991) and the tricarboxylate translocator (McIntosh and Oliver, 1992) in plant mitochondria. The inhibition of transport by DIDS and by pyridoxal phosphate suggests that a Lys residue has a function in transport.
Figure 6.
Effect of the Lys-specific inhibitors DIDS and pyridoxal phosphate on the transport of [14C]OA into liposomes with membrane proteins from potato tuber mitochondria. The malate-preloaded liposomes were incubated with the indicated concentration of inhibitor for 10 min at room temperature before transport measurement.
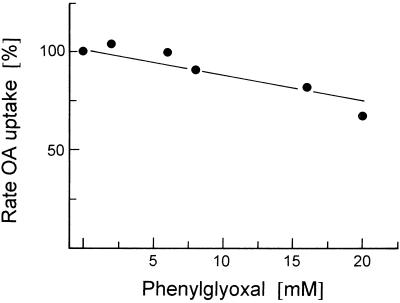
Phenylglyoxal, a substance reacting with Arg groups, was found to inhibit various mitochondrial translocators, such as the phosphate translocator from animals (Kaplan et al., 1986) and plants (McIntosh and Oliver, 1994), the tricarboxylate translocator (Kaplan et al., 1990; Azzi et al., 1993), and the aspartate-glutamate translocator (Bisaccia et al., 1992) (both from animals). As shown in Figure 7, phenylglyoxal had only a minor inhibitory effect, indicating that Arg residues did not have a major function in OA-malate transport. In this respect, the OA-malate translocator appeared to be different from the above-listed mitochondrial translocators. Diethylpyrocarbonate, reacting with His residues, also had only a minor inhibitory effect on OA-malate transport (data not shown).
Figure 7.
Effect of the Arg-specific inhibitor phenylglyoxal on the transport of [14C]OA into liposomes with membrane proteins from potato tuber mitochondria. The malate-preloaded liposomes were incubated with the indicated concentration of inhibitor for 10 min at room temperature before transport measurement.
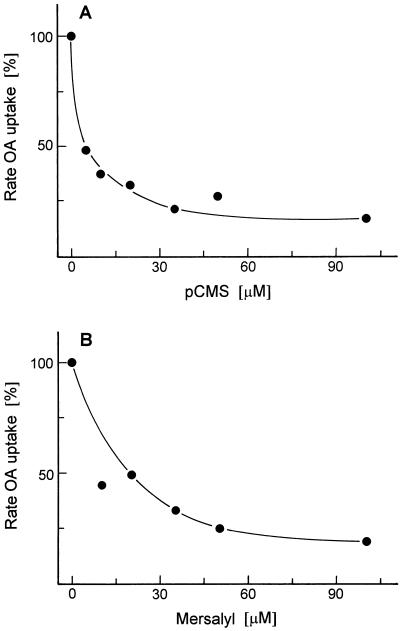
The binding of dicarboxylate anions to the binding site of the OA-malate translocator requires cationic groups. Because Arg and His residues are apparently not involved in binding site function, one may conclude that Lys residues provide the positive charges for substrate binding. p-Chloromercuribenzene sulfonate and mersalyl, reacting with the SH groups of Cys residues, strongly inhibited OA-malate transport (Fig. 8), indicating that a Cys residue participates in the transport process. In earlier experiments the tricarboxylate translocator of plant mitochondria was found to be insensitive to mersalyl (McIntosh and Oliver, 1992), whereas several animal mitochondrial translocators, such as those for transport of dicarboxylates (Kaplan and Pedersen, 1985), phosphate (Stappen and Krämer, 1993), aspartate-glutamate (Bisaccia et al., 1992), and pyruvate (Bolli et al., 1989), were strongly inhibited by low concentrations of mersalyl.
Figure 8.
Effect of the Cys-specific inhibitors p-chloromercuribenzene sulfonate (pCMS) (A) and mersalyl (B) on the transport of [14C]OA into liposomes with membrane proteins from potato tuber mitochondria. The malate-preloaded liposomes were incubated with the indicated concentration of inhibitor for 10 min at room temperature before transport measurement.
Inhibitors that act at very low concentrations may be useful tools for the identification of the OA-malate translocator protein. In a preliminary experiment (results not shown here) we attempted to label the OA-malate translocator contained in reconstituted liposomes by incubation with 15 μm [3H]DIDS. After isolation of the protein from the liposomes and SDS-PAGE, most of the radioactivity label was found in protein bands of 28 and 55 to 58 kD. This suggests that one of the two protein bands may represent the OA-malate translocator, the larger molecule perhaps as a dimer; but further studies are required for verification.
Properties and Functions of the OA-Malate Translocator
As mentioned in the introduction, it has not been possible for technical reasons to study the properties of the OA-malate translocator in intact mitochondria. Although reconstituted liposomes represent an artificial system with many drawbacks, this is at present the best choice for a more detailed study of this translocator. The inhibition studies revealed that the OA-malate translocation is different from all other known mitochondrial metabolite translocators. In contrast to earlier findings, our results provide convincing evidence that the OA-malate translocator catalyzes an obligatory countertransport of OA with malate. In addition to malate, 2-oxoglutarate, succinate, citrate, and, at particularly high rates, aspartate are also transported. However, it could not be determined whether these transports are catalyzed by a single translocator or by several with overlapping specificity.
An OA-malate countertransport enables the export of redox equivalents from the mitochondria to the cytosol via a malate-OA shuttle. In photosynthesizing cells this shuttle plays a role in photorespiratory metabolism by facilitating the transfer of reducing equivalents from the mitochondria to the peroxisomes as required for the reduction of hydroxypyruvate (Raghavendra et al., 1998). In nonphotosynthesizing cells such a redox transfer may provide the reducing equivalents for nitrate reductase present in the cytosol (Woo et al., 1980; Weger and Turpin, 1989). Because of a redox gradient between the NADH/NAD systems in the mitochondria and the cytosol of an intact plant cell, the malate-OA shuttle seems to be unsuited for import of redox equivalents into the mitochondria (Hanning and Heldt, 1993).
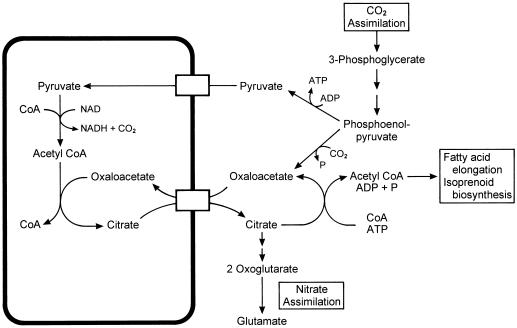
A countertransport of OA with citrate seems to be important for generating 2-oxoglutarate as carbon skeletons for nitrate assimilation (Fig. 9). From studies of isolated plant mitochondria we have shown previously that these mitochondria convert pyruvate plus OA into citrate, which was released from the mitochondria at a high rate (Hanning and Heldt, 1993). The cytosolic isoenzymes aconitase and isocitrate dehydrogenase (Chen and Gadal, 1990) may convert citrate first into 2-oxoglutarate and finally to glutamate, the key product of nitrate assimilation. Alternatively, the citrate exported from the mitochondria can be converted in the cytosol to acetyl-CoA via citrate lyase (Kaethner and ap Rees, 1985), a precursor for fatty acid elongation proceeding at the membranes of the ER (Ohlrogge and Brause, 1995) and also for isoprenoid biosynthesis (McGarvey and Croteau, 1995). The current study has shown that OA transport has an important function in plant metabolism.
Figure 9.
Metabolic scheme of the function of an OA-citrate countertransport.
Abbreviations:
- DIDS
4,4′-diisothiocyanostilbene-2,2′-disulfonate
- OA
oxaloacetate
Footnotes
H.W.H. was supported by the Deutsche Forschungsgemeinschaft.
LITERATURE CITED
- Azzi A, Glerum M, Koller R, Mertens W, Spycher S. The mitochondrial tricarboxylate carrier. J Bioenerg Biomembr. 1993;25:515–524. doi: 10.1007/BF01108408. [DOI] [PubMed] [Google Scholar]
- Bisaccia F, De Palma A, Palmieri F. Identification and purification of the aspartate/glutamate carrier from bovine heart mitochondria. Biochim Biophys Acta. 1992;1106:291–296. doi: 10.1016/0005-2736(92)90008-a. [DOI] [PubMed] [Google Scholar]
- Bolli R, Nalecz KA, Azzi A. Monocarboxylate and α-ketoglutarate carriers from bovine heart mitochondria. J Biol Chem. 1989;264:18024–18030. [PubMed] [Google Scholar]
- Chen RD, Gadal P. Do mitochondria provide the 2-oxoglutarate needed for glutamate synthesis in higher plant chloroplasts? Plant Physiol Bio Chem. 1990;28:141–145. [Google Scholar]
- Day DA, Wiskich JT. Glycine metabolism and oxaloacetate transport by pea leaf mitochondria. Plant Physiol. 1981a;68:425–429. doi: 10.1104/pp.68.2.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day DA, Wiskich JT. Effect of phthalonic acid on the respiration and metabolite transport in higher plant mitochondria. Arch Biochem Biophys. 1981b;211:100–107. doi: 10.1016/0003-9861(81)90434-3. [DOI] [PubMed] [Google Scholar]
- Douce R, Bonner WD. Oxaloacetate control of Krebs cycle in purified plant mitochondria. Biochem Biophys Res Commun. 1972;47:619–624. doi: 10.1016/0006-291x(72)90923-0. [DOI] [PubMed] [Google Scholar]
- Ebbighausen H, Chen J, Heldt HW. Oxaloacetate translocator in plant mitochondria. Biochim Biophys Acta. 1985;810:184–199. [Google Scholar]
- Genchi G, De Santis A, Ponzone C, Palmieri F. Partial purification and reconstitution of the α-ketoglutarate carrier from corn (Zea mays L.) mitochondria. Plant Physiol. 1991;96:1003–1007. doi: 10.1104/pp.96.4.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanning I, Heldt HW. On the function of mitochondrial metabolism during photosynthesis in spinach (Spinacia oleracea L.) leaves. Plant Physiol. 1993;103:1147–1154. doi: 10.1104/pp.103.4.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch MD, Dröscher L, Flügge UI, Heldt HW. A specific translocator for oxaloacetate in chloroplasts. FEBS Lett. 1984;178:15–19. [Google Scholar]
- Journet EP, Neuburger M, Douce R. Role of glutamate-oxaloacetate transaminase and malate dehydrogenase in the regeneration of NAD+ for glycine oxidation by spinach leaf mitochondria. Plant Physiol. 1981;67:467–469. doi: 10.1104/pp.67.3.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaethner TM, ap Rees T. Intracellular location of citrate lyase in leaves of Pisum sativum L. Planta. 1985;163:290–294. doi: 10.1007/BF00393520. [DOI] [PubMed] [Google Scholar]
- Kaplan RS, Mayor JA, Johnston N, Oliveira DL. Purification and characterization of the reconstitutively active tricarboxylate transporter from rat liver mitochondria. J Biol Chem. 1990;265:13379–13385. [PubMed] [Google Scholar]
- Kaplan RS, Pedersen PL. Isolation and reconstitution of the n-butylmalonate-sensitive dicarboxylate transporter from rat liver mitochondria. J Biol Chem. 1985;260:10293–10298. [PubMed] [Google Scholar]
- Kaplan RS, Pratt RD, Pedersen PL. Purification and characterization of the reconstitutively active phosphate transporter from rat liver mitochondria. J Biol Chem. 1986;261:12767–12773. [PubMed] [Google Scholar]
- Kasahara M, Hinkle PC. Reconstitution and purification of the d-glucose transporter from human erythrocytes. J Biol Chem. 1977;257:7384–7390. [PubMed] [Google Scholar]
- Krömer S, Heldt HW. Respiration of pea leaf mitochondria and redox transfer between the mitochondrial and extramitochondrial compartment. Biochim Biophys Acta. 1991;1057:42–50. [Google Scholar]
- McGarvey DJ, Croteau R. Terpenoid metabolism. Plant Cell. 1995;7:1015–1026. doi: 10.1105/tpc.7.7.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh CA, Oliver DJ. Isolation and characterization of the tricarboxylate transporter from pea mitochondria. Plant Physiol. 1992;100:2030–2034. doi: 10.1104/pp.100.4.2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh CA, Oliver DJ. The phosphate transporter from pea mitochondria. Plant Physiol. 1994;105:47–52. doi: 10.1104/pp.105.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlrogge J, Browse J. Lipid biosynthesis. Plant Cell. 1995;7:957–970. doi: 10.1105/tpc.7.7.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver DJ, Walker GH. Characterization of the transport of oxaloacetate by pea leaf mitochondria. Plant Physiol. 1984;76:409–413. doi: 10.1104/pp.76.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmieri F, Klingenberg M. Direct methods for measuring metabolite transport and distribution in mitochondria. Methods Enzymol. 1979;56:279–301. doi: 10.1016/0076-6879(79)56029-7. [DOI] [PubMed] [Google Scholar]
- Proudlove MO, Moore AL. Metabolite fluxes across the inner membrane of plant mitochondria, inhibition by phthalonic acid. Planta. 1984;160:407–414. doi: 10.1007/BF00429756. [DOI] [PubMed] [Google Scholar]
- Raghavendra AS, Reumann S, Heldt HW. Participation of mitochondrial metabolism in photorespiration. A reconstituted system of peroxisomes and mitochondria from spinach leaves. Plant Physiol. 1998;116:1333–1337. doi: 10.1104/pp.116.4.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz M, Gross A, Steinkamp T, Flügge I, Wagner R. Ion channel properties of the reconstituted triose phosphate/phosphate translocator. J Biol Chem. 1994;269:29481–29489. [PubMed] [Google Scholar]
- Stappen R, Krämer R. Functional properties of the reconstituted phosphate carrier from bovine heart mitochondria: evidence for asymmetric orientation and characterization of three different transport modes. Biochim Biophys Acta. 1993;1149:40–48. doi: 10.1016/0005-2736(93)90022-r. [DOI] [PubMed] [Google Scholar]
- Vivekananda J, Beck CF, Oliver DJ. Monoclonal antibodies as tools in membrane biochemistry: identification and partial characterization of the dicarboxylate transporter from pea leaf mitochondria. J Biol Chem. 1988;263:4782–4788. [PubMed] [Google Scholar]
- Vivekananda J, Oliver DJ. Isolation and partial characterization of the glutamate/aspartate transporter from pea leaf mitochondria using a specific monoclonal antibody. Plant Physiol. 1989;91:272–277. doi: 10.1104/pp.91.1.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivekananda J, Oliver DJ. Detection of the monocarboxylate transporter from pea mitochondria by means of a specific monoclonal antibody. FEBS Lett. 1990;260:217–219. [Google Scholar]
- Weger HG, Turpin DH. Mitochondrial respiration can support NO3− and NO2− reduction during photosynthesis. Plant Physiol. 1989;89:409–415. doi: 10.1104/pp.89.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo KC, Jokinen M, Canvin DT. Reduction of nitrate via a dicarboxylate shuttle in a reconstituted system of supernatant and mitochondria from spinach leaves. Plant Physiol. 1980;65:433–436. doi: 10.1104/pp.65.3.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo KC, Osmond CB. Glycine decarboxylation in mitochondria from spinach leaves. Aust J Plant Physiol. 1976;3:771–785. [Google Scholar]
- Zoglowek C, Krömer S, Heldt HW. Oxaloacetate and malate transport by plant mitochondria. Plant Physiol. 1988;87:109–115. doi: 10.1104/pp.87.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]