Abstract
WHIM syndrome is a rare congenital immunodeficiency disorder characterized by warts, hypogammaglobulinemia, infections, and myelokathexis (neutropenia because of impaired egress from the BM); most patients also have severe panleukopenia. Because WHIM syndrome is caused by mutations in the chemokine receptor CXCR4 that result in increased agonist-dependent signaling, we hypothesized that the CXCR4 antagonist plerixafor (Mozobil [Genyzme Corporation], AMD3100), might be an effective treatment. To test this, we enrolled 3 unrelated adult patients with the most common WHIM mutation, CXCR4R334X, in a phase 1 dose-escalation study. Plerixafor increased absolute lymphocyte, monocyte, and neutrophil counts in blood to normal without significant side effects in all 3 patients. Peak responses occurred at 3-12 hours after injection and waned by 24 hours after injection which tracked the drug's pharmacokinetics. All 3 cell types increased in a dose-dependent manner with the rank order of responsiveness absolute lymphocyte > monocyte > neutrophil. These data provide the first pharmacologic evidence that panleukopenia in WHIM syndrome is caused by CXCL12-CXCR4 signaling-dependent leukocyte sequestration, and support continued study of plerixafor as mechanism-based therapy in this disease. This study is registered at http://www.clinicaltrials.gov as NCT00967785.
Introduction
WHIM syndrome is a rare primary immunodeficiency disorder characterized by warts, hypogammaglobulinemia, infections, and myelokathexis.1–3 Myelokathexis refers to the retention of mature neutrophils in BM, resulting in neutropenia. Most patients with WHIM syndrome are panleukopenic, which partially corrects if they undergo severe infection.4 The cause of almost all cases of WHIM syndrome is autosomal-dominant inheritance of carboxy-terminal truncation mutations that remove 10-19 amino acids from the chemokine receptor CXCR4.5 CXCR4R343X, shortened by 19 amino acids, is the most common and best-studied variant in WHIM syndrome. When expressed in cell lines lacking endogenous CXCR4, CXCR4R343X exhibits ∼ 2-fold enhanced signaling on exposure to its ligand CXCL12 (also known as stromal cell–derived factor-1).6 The mechanism involves loss of negative regulatory elements in the missing C-terminus and defective ligand-induced down-regulation of the receptor from the cell surface.7,8 Because CXCR4 normally regulates leukocyte trafficking and in particular is important for neutrophil adhesion in BM, increasing its function may cause myelokathexis and neutropenia. Consistent with this, gene transfer of CXCR4R343X phenocopies myelokathexis in both zebrafish and Nod-scid (ie, nonobese diabetes/severe combined immunodeficiency) mice.9,10
Current treatments for WHIM syndrome include G-CSF (filgrastim [Neupogen; Amgen Inc]) and intravenous immunoglobulin but are nonspecific, expensive, difficult to administer, and only partially effective.4 Recently, a small molecule CXCR4 antagonist named plerixafor (trade name Mozobil [Genyzme Corporation] and formerly known as AMD3100) was approved by the Food and Drug Administration (FDA) for mobilizing HSCs from BM to blood with G-CSF for the purpose of autologous transplantation after cytoreductive therapy in patients with non-Hodgkin lymphoma or multiple myeloma.11,12 In this role it is usually given at an FDA-approved dose of 0.24 mg/kg before harvesting HSC.
We have previously established that AMD3100 is equipotent and equieffective at blocking both endogenous and recombinant forms of wild-type CXCR4 and CXCR4R343X.6 Because mice lacking Cxcr4 are not viable and have defective myelopoiesis and B-cell lymphopoiesis as well as defects in cerebellar and vascular development,13–15 there are important safety concerns about chronically blocking CXCR4 with drugs in any disease. However, we hypothesized that partial blockade of CXCR4 with plerixafor would be sufficient to correct panleukopenia and possibly other phenotypes in patients with WHIM syndrome and would be safe. Here we test this hypothesis in a phase 1 clinical trial in adults.
Methods
Patients
All patients signed informed consent consistent with the Declaration of Helsinki under clinical protocols approved by the National Institute of Allergy and Infectious Diseases before taking part in research at the National Institutes of Health Clinical Center. Three unrelated white adults with WHIM syndrome were recruited: a 44-year-old woman (patient 1), a 30-year-old woman (patient 2), and a 51-year-old man (patient 3). Patient 1 was previously identified as P1 in Wetzler et al,3 whereas patients 2 and 3 are the only identified adult survivors from 2 previously unreported families. All 3 patients were found to have the 4 defining clinical features of WHIM syndrome and were heterozygous for the CXCR4R334X mutation.
Plerixafor pharmacokinetic analysis
Plasma plerixafor concentrations were determined by Tandem Labs by the use of a previously validated proprietary assay. Prism v5 (GraphPad Software) was used to plot the concentration versus time data, and WinNonlin v5 (Pharsight Corporation) was used to generate standard pharmacokinetic parameter values via the use of noncompartmental methods. See supplemental Methods for additional details (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Flow cytometry
Flow cytometry was performed on whole blood and purified cells after staining for 30 minutes at 4°C with directly conjugated antibodies (BD Biosciences/Pharmingen) with the use of standard techniques. See supplemental Methods for additional details.
Neutrophil functional assays
Reactive oxygen species (ROS) production was tested by flow cytometry by use of the ROS-sensitive fluorescent dye dihydrorhodamine (DHR). Leukocytes isolated by ammonium chloride lysis were loaded with DHR and then stimulated with phorbol myristate acetate (PMA; Sigma-Aldrich), as previously described.16,17 Neutrophils were purified from diluted whole blood by centrifugation over lymphocyte separation media followed by dextran sedimentation and hypotonic saline lysis, as previously described,17 and their ability to kill bacteria was assessed by incubation with Staphylococcus aureus for 90 minutes at 37°C.
Results
Study design
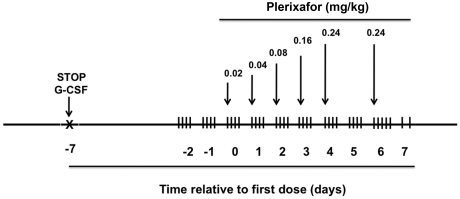
For safety and to find the lowest dose that might be used for chronic treatment, we designed a phase 1 dose-escalation study by using single daily injections at doses that had previously been tested in normal volunteers, up to a maximum of 0.24 mg/kg, the FDA-approved dose for HSC mobilization in patients with normal renal function (Figure 1). The primary end point of the protocol was safety, and the secondary end point was change in numbers of leukocytes in the blood with a doubling of average baseline value considered significant. Because there were no previous peer-reviewed published data on effects of plerixafor in children, we restricted the study to adults, defined as age 18 or older. G-CSF was 1 week before we initiated plerixafor. Patient 1 was receiving G-CSF on entry into the study, whereas patients 2 and 3 had not received G-CSF for several years prior to enrollment.
Figure 1.
Design of a phase 1 study of plerixafor treatment in adults with WHIM syndrome. Patients receiving G-CSF had the drug discontinued at the indicated time. Arrows indicate time of subcutaneous administration of plerixafor at the indicated dose. Vertical hash marks denote times of blood sampling for complete blood counts. Not shown: a sham saline injection of the same volume as the lowest drug dose was given on day −1 and the greatest drug dose on day +7.
To monitor leukocyte mobilization dynamics, complete blood count with differential was performed every 3 hours 4-5 times per day after the patients received plerixafor. Dose escalation was designed to stop if neutrophils reached 4000/μL. At 2 days after patients received the maximal dose, we determined the pharmacokinetics of plerixafor by readministering the maximal dose followed by 9 blood draws over the course of 24 hours. Erythrocyte sedimentation rate and serum chemistries were performed daily. Participants had to be free of infection, not pregnant (if female), willing to use 2 methods of contraception for 6 weeks before and after drug administration, and willing to temporarily discontinue G-CSF. Other exclusion criteria were the presence of hematologic malignancy, serious heart disease or conduction abnormalities, and poor renal function (creatinine clearance < 15 mL/min or requiring dialysis).
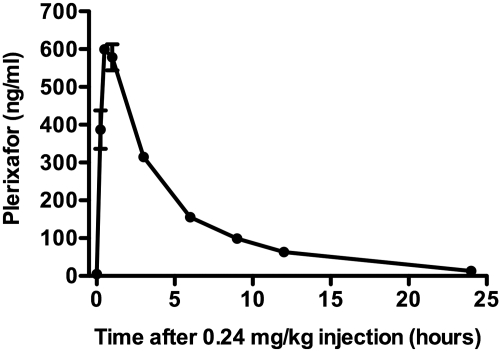
Plerixafor pharmacokinetics were normal in patients with WHIM syndrome
The pharmacokinetic data after a single subcutaneous dose of 0.24 mg/kg revealed a pattern of absorption, distribution, and elimination in WHIM patients 1 and 2 similar to that previously reported for normal volunteers (Figure 2).18–20 The average peak plasma concentration was 616 ng/mL at ∼ 30 minutes after injection. The mean half-life was 5.2 hours with an apparent clearance and volume of distribution of 5.2 L/h and 39.2 L, respectively. A 2-compartment model with first-order elimination was found to closely predict the measured values, consistent with previous studies in normal volunteers.18–20
Figure 2.
Plerixafor pharmacokinetics in WHIM patients. A single dose of 0.24 mg/kg of plerixafor was subcutaneously injected on day +6 as indicated in Figure 1. Plasma drug concentration was determined at the times indicated. Shown are the mean ± SEM of data from patient 1 and 2.
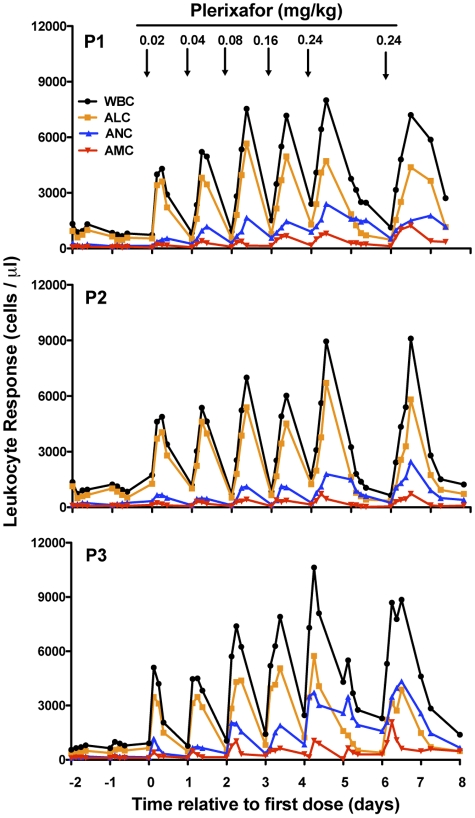
Plerixafor increased leukocyte counts in the blood of patients with WHIM syndrome
Off drug, all 3 subjects were severely panleukopenic at levels that were stable during the 2-day prelude (Figure 3). The 0.02 mg/kg dose of plerixafor induced a rapid increase in white blood cells (WBCs; peak 3-6 hours after the dose) in all subjects. Unexpectedly, lymphocytes accounted for most of the increase in the total WBC and by the 0.04 mg/kg dose exceeded the upper limit of normal, unlike in normal volunteers, in whom the majority of the increase in WBC is because of neutrophils.18–20 Monocytes were also highly responsive to plerixafor (peak 3-6 hours) in all 3 subjects and exceeded the upper limit of normal by the 0.04-0.08 mg/kg doses. The neutrophil increase was slower (peak 9-12 hours) and more sustained but never exceeded the upper limit of normal even at the highest dose.
Figure 3.
Plerixafor normalizes absolute blood leukocyte counts in patients with WHIM syndrome. All data collected from all 3 patients studied (P1, P2, and P3) are shown. Each point represents a single value obtained from that time point. Time zero is defined as the first day of plerixafor administration. Arrows indicate the time when the indicated dose of plerixafor in mg/kg was administered subcutaneously to the patient (Figure 1). Not shown: a sham saline injection of the same volume as the lowest dose was given on day −1 and as the greatest dose on day +7. WBC indicates white blood count; ALC, absolute lymphocyte count; ANC, absolute neutrophil count, and AMC, absolute monocyte count.
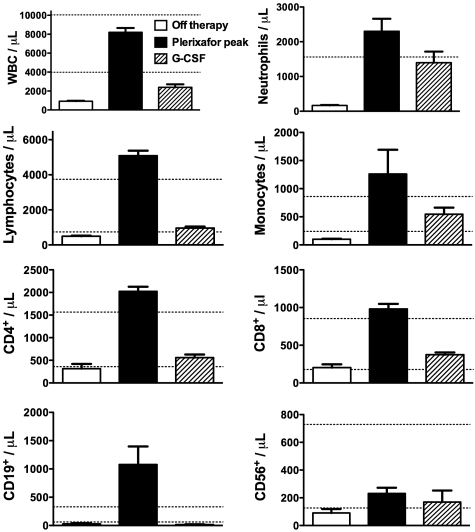
Nevertheless, even at the 0.02-mg/kg dose, the absolute neutrophil count (ANC) exceeded the safe level of > 500 neutrophils/μL of blood in all 3 subjects. Comparison of leukocyte dynamics after the 0.16-mg/kg and 0.24-mg/kg doses of plerixafor to leukocyte levels maintained by chronic daily G-CSF administration (Figure 4) revealed that peak ANC after plerixafor was similar to steady-state ANC on G-CSF; however, the peak absolute lymphocyte and monocyte counts (ALC and AMCs, respectively), and thus total peak WBC, were much greater after the administration of plerixafor than with G-CSF. All 3 major subsets of lymphocytes (CD19+ B cells and CD4+ and CD8+ T cells) were strongly mobilized by plerixafor but not by G-CSF (Figure 4, Table 1). T cells were primarily of the memory phenotype (CD45RO+), specifically the effector memory subtype (CD45RO+CD62L−CCR7−). CD4+CD25+ and CD4+CCR6+ T cells, which contain Treg cells and TH17 cells, respectively, were also strongly mobilized (data not shown). Mobilized B cells appeared to be primarily an immature phenotype (CD19+CD27−) and were largely composed of (CD27−IgD+IgM+) naive (transitional) type cells, although CD27−IgD−IgM+ immature B cells were also present. Mobilized monocytes were predominantly classic monocytes (CD14+CD16−), but inflammatory monocytes (CD14+CD16+) were also mobilized.
Figure 4.
Comparison of blood leukocyte counts in WHIM patients after treatment with plerixafor and G-CSF. Parameters on x-axis are defined as follows: (1) “Plerixafor peak,” the mean ± SEM of the peak absolute blood leukocyte subset counts of WHIM patients 1, 2, and 3 in response to administration of both 0.16 and 0.24 mg/kg doses of plerixafor in this study; (2) “Off therapy,” the mean ± SEM of absolute blood leukocyte subset counts for all values determined on days −1 and −2 of the phase 1 study (Figure 1); and (3) “G-CSF,” the mean ± SEM of all available absolute blood leukocyte subset counts of WHIM patients 1 and 2 during treatment with daily G-CSF at 1-3 μg/kg. Horizontal dotted lines indicate the normal range.
Table 1.
Leukocyte subset response to plerixafor
| Cell type | Cells/μL blood (mean ± SEM) |
|||
|---|---|---|---|---|
| Off therapy | Plerixafor peak | G-CSF* | Reference range | |
| CD4+ T cell | 313 ± 104 | 2025 ± 101 | 559 ± 70 | 359-1565 |
| CD4+CD45RA+ Naïve T cell | 46 ± 23 | 149 ± 53 | 91 ± 26 | 454-733 |
| CD4+CD45RO+ memory | 260 ± 73 | 1784 ± 115 | 459 ± 45 | 216-1048 |
| CD4+CD45RA−CD62L+CCR7+ central memory | 3 ± 1 | 9 ± 7 | 9 ± 7 | 16-49 |
| CD4+CD45RA−CD62L−CCR7− effector memory | 109 ± 25 | 591 ± 105 | 263 ± 15 | 57-130 |
| CD8+ T cell | 203 ± 43 | 981 ± 67 | 375 ± 30 | 178-853 |
| CD8+CD45RA+ Naïve T cell | 74 ± 29 | 295 ± 114 | 107 ± 22 | 231-371 |
| CD8+CD45RO+ memory | 95 ± 9 | 529 ± 51 | 155 ± 1.5 | 50-352 |
| CD8+CD45RA−CD62L+CCR7+ central memory | < 1 | < 1 | 1 | 0-12 |
| CD8+CD45RA−CD62L−CCR7− effector memory | 35 ± 8 | 170 ± 58 | 69 ± 19 | 22-75 |
| CD3−CD56+ NK cell | 91 ± 28 | 231 ± 41 | 169 ± 83 | 126-729 |
| CD19+ B cell | 28 ± 13 | 1077 ± 320 | 17 ± 5 | 61-329 |
| CD19+CD27+ memory | 1 ± 0 | 10 ± 1 | 2 ± 0 | 18-29 |
| CD19+CD27+CD38+IgD+ memory unswitched | < 1 | 3 ± 1 | < 1 | 0-2 |
| CD19+CD27+CD38+IgD− memory switched | < 1 | 2 ± 1 | < 1 | 4-21 |
| CD19+CD27− | 16 ± 5 | 306 ± 72 | 8 ± 0 | 90-176 |
| CD19+CD27−IgD+IgM+ transitional | 10 ± 4 | 217 ± 103 | 4 ± 0 | 42-85 |
| CD19+CD27−IgD−IgM+ immature | < 1 | 2 ± 1 | < 1 | 2-10 |
| CD14+ monocyte | 100 ± 9 | 1260 ± 433 | 545 ± 117 | 240-860 |
| CD14+CD16− classical monocyte | 70 ± 5 | 614 ± 249 | 184 ± 62 | 371-539 |
| CD14+CD16+ inflammatory monocyte | 4 ± 2 | 19 ± 9 | 11 ± 3 | 14-30 |
CD indicates cluster of differentiation; and NK, natural killer.
G-CSF is only based on patients 1 and 2, whereas off treatment and peak plerixafor numbers are based on all 3 patients and the reference range is based on 12-40 healthy volunteers. See Figure 4 legend for definition of Off therapy, plerixafor peak, and G-CSF.
Plerixafor did not impair neutrophil function in patients with WHIM syndrome
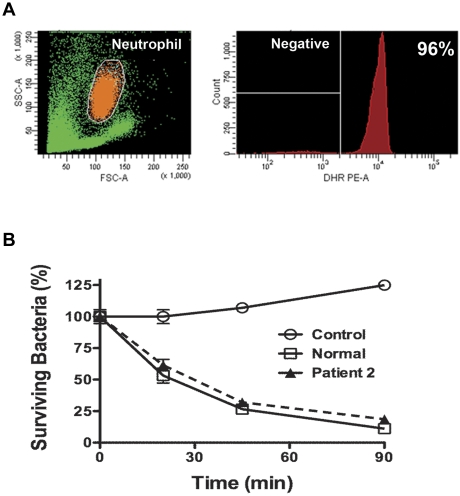
Virtually all blood neutrophils harvested at the peak of the plerixafor response were capable of producing normal amounts of ROS (Figure 5A). We also performed a classic neutrophil bacterial killing assay to measure staphylocidal activity at 2 target:effector ratios (2 and 8 bacteria/neutrophil). Neutrophils collected at the peak of the plerixafor response killed bacteria normally (Figure 5B).
Figure 5.
Neutrophils released by plerixafor function normally. (A) ROS production. DHR was loaded into isolated neutrophils, which were then stimulated with PMA. Data are from a single experiment in which purified blood neutrophils from patient 2 were used, gated as shown in orange on the left and displayed as a histogram on the right. Data are representative of 2 independent experiments, one each for patients 2 and 3, each tested on day +4 of the protocol (Figure 1). Neutrophils were obtained 3 hours after the administration of 0.24 mg/kg plerixafor. FSC indicates forward scatter; SSC, side scatter. (B) Staphylocidal assay. S aureus was incubated at either 2 or 8 bacteria/neutrophil for the time indicated on the x-axis in vitro at 37°C. Cells were then lysed and plated on agar to determine the viable bacterial count. Data are from a single experiment using neutrophils from patient 2 and a single healthy donor (normal) incubated at 2 bacteria/cell and are representative of 4 experiments performed, one for patients 2 and 3 at the 2 concentrations of bacteria. Patient neutrophils were obtained on day +4 of the protocol, 3 hours after the administration of 0.24 mg/kg plerixafor (Figure 1). Control, S aureus incubated without neutrophils.
Plerixafor was well-tolerated in patients with WHIM syndrome
In terms of safety parameters, there were no arrhythmias, palpitations, electrocardiogram changes, thrombocytopenia or changes in electrolytes, kidney function, or liver enzymes (supplemental Figure 1). Patients 1 and 3 had asymptomatic, postprandial elevations of glucose (range, 116-231 mg/dL) on the day after the greatest plerixafor dose; however, patient 1 had been previously diagnosed as prediabetic. Mild injection-site reactions occurred in all 3 patients, consisting of pruritic erythematous induration that resolved in 30-90 minutes without treatment (supplemental Figure 2). This was not dose-dependent but was more common in stomach than arm injection sites and was associated with shallower injection technique. Patients 1 and 3 exhibited insomnia and headache on days 5-7 that were alleviated with narcoleptics and analgesics. Patient 2 had lumbar and femoral pain with some associated nausea and emesis similar to previous treatment with G-CSF that was relieved by analgesics. There was no grade 3 or 4 toxicity.
Discussion
We have demonstrated, in a phase 1 dose-escalation drug treatment trial in WHIM syndrome, that plerixafor, which blocks the precise molecular target in the disease, appears to be safe and effective for 1 week in correcting panleukopenia, the major cellular mechanism of immunodeficiency in the disease.
With regard to scientific significance, the data provide the first in vivo pharmacologic evidence in support of the hypothesis that panleukopenia in WHIM syndrome is caused by increased CXCR4 signaling. This hypothesis draws its strength from multiple independent lines of evidence, including: (1) CXCL12-CXCR4 signaling is known to normally mediate neutrophil retention in the BM of mice9,21,22; (2) CXCR4 is the disease gene in WHIM syndrome5; (3) the CXCR4R334X variant found in our WHIM patients exhibits increased agonist-dependent signaling in multiple independent assays and mediates myelokathexis when expressed in mice and zebrafish9,10; (4) the administration of plerixafor mobilizes many leukocyte subsets, including HSCs, lymphocytes, monocytes, neutrophils, and eosinophils, to the blood in healthy subjects18–20; (5) plerixafor is a specific and equipotent antagonist of both wild-type CXCR4 and CXCR4R334X6; and (6) heterozygous Cxcr4+/− mice appear phenotypically normal.13–15
It is important to note that the rank order of responsiveness of leukocyte subsets to plerixafor (ALC > ANC > AMC) was consistent for all 3 of our WHIM patients but different from what has been reported for healthy subjects (ANC > ALC > AMC), which possibly reflects differential expression and activity of the mutant receptor in these lineages. The difference is not as great, however, when the rank order is determined not from peak levels after drug but as -fold increase from baseline cell counts in which the WHIM patients increased their baseline WBC by 8.8-fold, ANC by 13.9-fold, ALC by 10.3-fold, and AMC by 12.6-fold. In healthy volunteers and patients with HIV, the increase peaked at less than 4-fold for all of the aforementioned leukocyte subtypes even with prolonged administration.18–20,23 Remarkably, in our patients CD19+ B cells were almost completely absent from the blood before drug and increased 54-fold to above the upper limit of normal after peak drug effect. The sensitivity of B cells to plerixafor has not been previously reported, but our data reveal that CD19+ B cells express a high level of CXCR4 in both healthy donors and patients with WHIM (not shown), which may explain their responsiveness. The slower dynamics of the neutrophil compared with lymphocyte response may suggest that their movement is not as dependent on drug concentration and permit less frequent dosing to achieve the safe target level of ANC > 500/μL.
With regard to medical significance, our data support continued investigation of plerixafor as a safe and effective treatment for all phenotypes that define WHIM syndrome. Although our study was not designed to address clinical efficacy and was restricted to adults, plerixafor increased all major leukocyte lineages from below the lower limit of normal to above the upper limit of normal (ALC and AMC) or to the normal range (ANC). Importantly, the panleukopenia in all study subjects was rapidly corrected with very low doses of drug, 1/12th that of the FDA-approved dose for HSC mobilization. At the lowest dose, we estimate that at peak the drug achieves at most ∼ 30% receptor coverage, which is important for safety.
The goal in treatment in WHIM syndrome is to target the molecular mechanism, which should require only partial receptor blockade to reduce CXCR4 signaling toward normal. This may avert potential toxicities associated with complete loss of normal CXCR4 function observed in previous murine studies.13–15 The relatively high doses of plerixafor used in the dose-escalation phase of our study were accompanied by very few side effects compared with what has been reported in clinical trials in patients with cancer and HIV.11,12,23 Minor skin hyperemia occurring within minutes after injection at the site of injection was the only definitely associated side effect. This finding confirmed a previous report and resolved spontaneously within 90 minutes.20
Unlike in studies of healthy volunteers and HIV and cancer patients, there was no association of plerixafor with heart arrhythmia, thrombocytopenia, or gastrointestinal symptoms in our patients, which may indicate that these side effects may be because of the underlying illness or other drugs or that WHIM patients are more resistant to these side effects; additional WHIM patients will need to be treated with plerixafor to provide a definitive answer. Importantly, our patients did not develop any infections while they were taking the drug. Consistent with this finding, patient neutrophils mobilized to the blood by plerixafor were able to produce ROS ex vivo at normal levels in response to PMA and to kill S aureus ex vivo. The corollary of this is that in WHIM syndrome susceptibility to infections is probably because of defects in the number of neutrophils in the blood, not their function. Whether this is also the case for monocytes remains to be determined. For lymphocytes, there is evidence of dysfunction in WHIM syndrome because patients are usually hypogammaglobulinemic and may respond poorly to vaccines. Future studies will be needed to determine whether these problems can be resolved by simply mobilizing cells with plerixafor.
BM is probably the major source of neutrophil sequestration as suggested by direct BM examination of WHIM patients and, as mentioned previously, by direct data establishing a role for CXCR4 in neutrophil retention in BM in the mouse.9,21 The sequestration site for other leukocyte subsets mobilized by plerixafor in WHIM patients is not directly addressed by our study, nor has it been defined in healthy subjects receiving the drug. Monocytopenia in WHIM syndrome has only recently been recognized,24 and our results suggest that both classic and inflammatory monocyte numbers in the circulation are controlled by CXCR4. It is unknown whether monocytopenia causes any of the phenotypes in the syndrome, although monocytes are known to play a significant role in controlling infection, particularly by intracellular pathogens. The deficiency of naive (transitional B cells) in patient blood may explain the poor vaccine responses and hypogammaglobulinemia seen in WHIM syndrome, although the full mechanism of action may be more complicated because CXCR4 is also important in precisely locating B cells with T cells in the germinal centers where they are stimulated by antigen and undergo class switching.25,26 CD4+ and CD8+ effector memory T cells were both released in large numbers by plerixafor. Vaccination studies have identified these cells in mouse BM, which may serve as a reservoir.27,28 A recent study suggests that this may also occur in humans.29 Thus, BM is potentially a major source of these cells mobilized to the blood by plerixafor.
In conclusion, our study provides initial human in vivo evidence supporting CXCR4 signaling as the critical molecular mechanism in patients with WHIM syndrome and justifies continued study of plerixafor as a potentially safe and effective mechanism-based treatment for adult patients with this disease. A longer, therapeutic trial at the National Institutes of Health is currently underway. Future work will need to address optimal dosing, and long-term safety and efficacy in more patients, including children.
Supplementary Material
Acknowledgments
The authors thank the patients with WHIM syndrome and their families who have volunteered to make this study possible. They also thank Dr Kathryn Zoom for support.
This work was supported by the Division of Intramural Research of the National Institute of Allergy and Infectious Diseases and the Office of Rare Diseases Research, National Institutes of Health, and was funded in part with federal funds from the National Cancer Institute, National Institutes of Health under Contract number HHSN261200800001E. Plerixafor was provided at no cost for the study by Genzyme Corporation.
The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US government.
Footnotes
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: D.H.M., N.K., S.R.P., H.L.M., and P.M.M. designed the protocol; D.H.M., J.U., S.A., C.K., M.G., P.L., M.M.M., D.H., R.D., H.L.M., and P.M.M. provided patient recruitment and care; Q.L., J.O.F., S.R.P., and D.A.L.P. generated and analyzed experimental data; and D.H.M., T.A.F., D.B.K., H.L.M., and P.M.M. supervised the experiments and analyzed data. All authors participated, but D.H.M. and P.M.M. were principally responsible for the writing of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: David H. McDermott, MD, 9000 Rockville Pike, Bldg 10, Rm 11N107, Bethesda, MD 20892; e-mail: dmcdermott@nih.gov.
References
- 1.Zuelzer WW. “Myelokathexis”—a new form of chronic granulocytopenia. Report of a case. N Engl J Med. 1964;270:699–704. doi: 10.1056/NEJM196404022701402. [DOI] [PubMed] [Google Scholar]
- 2.Krill CE, Jr, Smith HD, Mauer AM. Chronic idiopathic granulocytopenia. N Engl J Med. 1964;270:973–979. doi: 10.1056/NEJM196405072701902. [DOI] [PubMed] [Google Scholar]
- 3.Wetzler M, Talpaz M, Kleinerman ES, et al. A new familial immunodeficiency disorder characterized by severe neutropenia, a defective marrow release mechanism, and hypogammaglobulinemia. Am J Med. 1990;89(5):663–672. doi: 10.1016/0002-9343(90)90187-i. [DOI] [PubMed] [Google Scholar]
- 4.Kawai T, Malech HL. WHIM syndrome: congenital immune deficiency disease. Curr Opin Hematol. 2009;16(1):20–26. doi: 10.1097/MOH.0b013e32831ac557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hernandez PA, Gorlin RJ, Lukens JN, et al. Mutations in the chemokine receptor gene CXCR4 are associated with WHIM syndrome, a combined immunodeficiency disease. Nat Genet. 2003;34(1):70–74. doi: 10.1038/ng1149. [DOI] [PubMed] [Google Scholar]
- 6.McDermott DH, Lopez J, Deng F, et al. AMD3100 is a potent antagonist at CXCR4(R334X), a hyperfunctional mutant chemokine receptor and cause of WHIM syndrome. J Cell Mol Med. 2011;15(10):2071–2081. doi: 10.1111/j.1582-4934.2010.01210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Balabanian K, Lagane B, Pablos JL, et al. WHIM syndromes with different genetic anomalies are accounted for by impaired CXCR4 desensitization to CXCL12. Blood. 2005;105(6):2449–2457. doi: 10.1182/blood-2004-06-2289. [DOI] [PubMed] [Google Scholar]
- 8.McCormick PJ, Segarra M, Gasperini P, Gulino AV, Tosato G. Impaired recruitment of Grk6 and beta-Arrestin 2 causes delayed internalization and desensitization of a WHIM syndrome-associated CXCR4 mutant receptor. PLoS One. 2009;4(12):e8102. doi: 10.1371/journal.pone.0008102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kawai T, Choi U, Cardwell L, et al. WHIM syndrome myelokathexis reproduced in the NOD/SCID mouse xenotransplant model engrafted with healthy human stem cells transduced with C-terminus-truncated CXCR4. Blood. 2007;109(1):78–84. doi: 10.1182/blood-2006-05-025296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Walters KB, Green JM, Surfus JC, Yoo SK, Huttenlocher A. Live imaging of neutrophil motility in a zebrafish model of WHIM syndrome. Blood. 2010;116(15):2803–2811. doi: 10.1182/blood-2010-03-276972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DiPersio JF, Micallef IN, Stiff PJ, et al. Phase III prospective randomized double-blind placebo-controlled trial of plerixafor plus granulocyte colony-stimulating factor compared with placebo plus granulocyte colony-stimulating factor for autologous stem-cell mobilization and transplantation for patients with non-Hodgkin's lymphoma. J Clin Oncol. 2009;27(28):4767–4773. doi: 10.1200/JCO.2008.20.7209. [DOI] [PubMed] [Google Scholar]
- 12.Dugan MJ, Maziarz RT, Bensinger WI, et al. Safety and preliminary efficacy of plerixafor (Mozobil) in combination with chemotherapy and G-CSF: an open-label, multicenter, exploratory trial in patients with multiple myeloma and non-Hodgkin's lymphoma undergoing stem cell mobilization. Bone Marrow Transplant. 2010;45(1):39–47. doi: 10.1038/bmt.2009.119. [DOI] [PubMed] [Google Scholar]
- 13.Ma Q, Jones D, Borghesani PR, et al. Impaired B-lymphopoiesis, myelopoiesis, and derailed cerebellar neuron migration in CXCR4- and SDF-1-deficient mice. Proc Natl Acad Sci U S A. 1998;95(16):9448–9453. doi: 10.1073/pnas.95.16.9448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tachibana K, Hirota S, Iizasa H, et al. The chemokine receptor CXCR4 is essential for vascularization of the gastrointestinal tract. Nature. 1998;393(6685):591–594. doi: 10.1038/31261. [DOI] [PubMed] [Google Scholar]
- 15.Zou YR, Kottmann AH, Kuroda M, Taniuchi I, Littman DR. Function of the chemokine receptor CXCR4 in haematopoiesis and in cerebellar development. Nature. 1998;393(6685):595–599. doi: 10.1038/31269. [DOI] [PubMed] [Google Scholar]
- 16.Jirapongsananuruk O, Malech HL, Kuhns DB, et al. Diagnostic paradigm for evaluation of male patients with chronic granulomatous disease, based on the dihydrorhodamine 123 assay. J Allergy Clin Immunol. 2003;111(2):374–379. doi: 10.1067/mai.2003.58. [DOI] [PubMed] [Google Scholar]
- 17.McDermott DH, De Ravin SS, Jun HS, et al. Severe congenital neutropenia resulting from G6PC3 deficiency with increased neutrophil CXCR4 expression and myelokathexis. Blood. 2010;116(15):2793–2802. doi: 10.1182/blood-2010-01-265942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hendrix CW, Flexner C, MacFarland RT, et al. Pharmacokinetics and safety of AMD-3100, a novel antagonist of the CXCR-4 chemokine receptor, in human volunteers. Antimicrob Agents Chemother. 2000;44(6):1667–1673. doi: 10.1128/aac.44.6.1667-1673.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hübel K, Liles WC, Broxmeyer HE, et al. Leukocytosis and mobilization of CD34+ hematopoietic progenitor cells by AMD3100, a CXCR4 antagonist. Support Cancer Ther. 2004;1(3):165–172. doi: 10.3816/SCT.2004.n.008. [DOI] [PubMed] [Google Scholar]
- 20.Liles WC, Broxmeyer HE, Rodger E, et al. Mobilization of hematopoietic progenitor cells in healthy volunteers by AMD3100, a CXCR4 antagonist. Blood. 2003;102(8):2728–2730. doi: 10.1182/blood-2003-02-0663. [DOI] [PubMed] [Google Scholar]
- 21.Martin C, Burdon PC, Bridger G, Gutierrez-Ramos JC, Williams TJ, Rankin SM. Chemokines acting via CXCR2 and CXCR4 control the release of neutrophils from the bone marrow and their return following senescence. Immunity. 2003;19(4):583–593. doi: 10.1016/s1074-7613(03)00263-2. [DOI] [PubMed] [Google Scholar]
- 22.Ma Q, Jones D, Springer TA. The chemokine receptor CXCR4 is required for the retention of B lineage and granulocytic precursors within the bone marrow microenvironment. Immunity. 1999;10(4):463–471. doi: 10.1016/s1074-7613(00)80046-1. [DOI] [PubMed] [Google Scholar]
- 23.Hendrix CW, Collier AC, Lederman MM, et al. Safety, pharmacokinetics, and antiviral activity of AMD3100, a selective CXCR4 receptor inhibitor, in HIV-1 infection. J Acquir Immune Defic Syndr. 2004;37(2):1253–1262. doi: 10.1097/01.qai.0000137371.80695.ef. [DOI] [PubMed] [Google Scholar]
- 24.Siedlar M, Rudzki Z, Strach M, et al. Familial occurrence of warts, hypogammaglobulinemia, infections, and myelokathexis (WHIM) syndrome. Arch Immunol Ther Exp (Warsz) 2008;56(6):419–425. doi: 10.1007/s00005-008-0046-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McGuire PJ, Cunningham-Rundles C, Ochs H, Diaz GA. Oligoclonality, impaired class switch and B-cell memory responses in WHIM syndrome. Clin Immunol. 2010;135(3):412–421. doi: 10.1016/j.clim.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Allen CD, Ansel KM, Low C, et al. Germinal center dark and light zone organization is mediated by CXCR4 and CXCR5. Nat Immunol. 2004;5(9):943–952. doi: 10.1038/ni1100. [DOI] [PubMed] [Google Scholar]
- 27.Tokoyoda K, Zehentmeier S, Hegazy AN, et al. Professional memory CD4+ T lymphocytes preferentially reside and rest in the bone marrow. Immunity. 2009;30(5):721–730. doi: 10.1016/j.immuni.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 28.Becker TC, Coley SM, Wherry EJ, Ahmed R. Bone marrow is a preferred site for homeostatic proliferation of memory CD8 T cells. J Immunol. 2005;174(3):1269–1273. doi: 10.4049/jimmunol.174.3.1269. [DOI] [PubMed] [Google Scholar]
- 29.Herndler-Brandstetter D, Landgraf K, Jenewein B, et al. Human bone marrow hosts polyfunctional memory CD4+ and CD8+ T cells with close contact to IL-15–producing cells. J Immunol. 2011;186(12):6965–6971. doi: 10.4049/jimmunol.1100243. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.