Abstract
Although current antiplatelet therapies provide potent antithrombotic effects, their efficacy is limited by a heightened risk of bleeding and failure to affect vascular remodeling after injury. New lines of research suggest that thrombosis and hemorrhage may be uncoupled at the interface of pathways controlling thrombosis and inflammation. Here, as one remarkable example, studies using a novel and highly selective pharmacologic inhibitor of the spleen tyrosine kinase Syk [PRT060318; 2-((1R,2S)-2-aminocyclohexylamino)-4-(m-tolylamino)pyrimidine-5-carboxamide] coupled with genetic experiments, demonstrate that Syk inhibition ameliorates both the acute and chronic responses to vascular injury without affecting hemostasis. Specifically, lack of Syk (murine radiation chimeras) attenuated shear-induced thrombus formation ex vivo, and PRT060318 strongly inhibited arterial thrombosis in vivo in multiple animal species while having minimal impact on bleeding. Furthermore, leukocyte-platelet–dependent responses to vascular injury, including inflammatory cell recruitment and neointima formation, were markedly inhibited by PRT060318. Thus, Syk controls acute and long-term responses to arterial vascular injury. The therapeutic potential of Syk may be exemplary of a new class of antiatherothrombotic agents that target the interface between thrombosis and inflammation.
Introduction
Experimental and clinical studies support close interrelationship between inflammation and thrombosis.1 Leukocyte–platelet interactions induce bidirectional signals that amplify proinflammatory and prothrombotic cellular responses2 and are critical, for example, in the initiation and progression of atherosclerosis3 as well as restenosis4 and in thrombotic events.5 Antithrombotic agents developed to date provide substantial benefits via inhibition of thrombosis, but they do not seem to impact the progression of underlying vascular disease.6,7
Several in vitro lines of evidence suggest that inflammatory and thrombotic signaling pathways converge on spleen tyrosine kinase (Syk), a 72-kDa signaling protein with kinase and scaffolding activities. In platelets, phosphorylation of Syk has been reported after activation by multiple receptors (eg, platelet collagen receptor glycoprotein VI [GPVI], which mediates platelet adhesion and activation to vascular collagen;8 platelet glycoprotein [GP]Ibα and GPIIb-IIIa9). Syk is also a mediator of signaling events induced by high shear stress,10 after engagement of FcγRIIA, FcRγ, FcαRI, and the C-type lectin receptor-2.8,11–14 In leukocytes, Syk promotes the recruitment of these cells to both inflamed and injured blood vessels. In the presence of activated endothelium, Syk regulates selectin-dependent leukocyte rolling.15 At sites of vascular injury with endothelial denudation and platelet deposition, leukocyte recruitment is mediated by the leukocyte β2-integrin Mac-1 and the platelet counter receptor GPIbα,4,16 both of which signal through Syk.17
Despite a well-described role for Syk in platelet and leukocyte biology, our understanding of the contribution of Syk to platelet-mediated thrombosis and vascular inflammation in vivo has remained limited. This discrepancy is probably explained by the severe phenotype associated with Syk deficiency (embryonic lethality, anemia, and petechial hemorrhages throughout the gut, impairment of B-cell development18,19) and the lack of a highly selective Syk inhibitor that led to contradictory results. For example, although the results obtained with pharmacologic inhibitor R406 support a role for Syk downstream of both GPVI and C-type lectin receptor-2 in human washed platelets,20 oral administration of R406 blocked Fc receptor signaling and reduced immune complex-mediated inflammation in whole blood, but it had no significant effect on collagen-induced platelet aggregation in platelet-rich plasma (PRP).21 Furthermore, the selectivity of R406 for Syk is open to question because it inhibits multiple tyrosine kinases.21,22 Similarly, the lack of a selective pharmacologic agent with favorable pharmacokinetic properties has limited the evaluation of Syk kinase activity in atherogenesis and restenosis.
To determine whether Syk has a functional role in thrombosis and vascular injury in vivo, multiple strategies were used. First, we studied ex vivo thrombosis in radiation chimeras mice obtained by injecting Syk−/− fetal liver cells into lethally irradiated recipient mice. Second, we identified a Syk inhibitor and established its potency and selectivity against the kinase activity of Syk. Third, using this small molecule, we found that the kinase activity of Syk is preferentially involved in the regulation of arterial thrombosis (ie, platelet adhesion, thrombus growth, and stability) in multiple animal species, minimally contributes to primary hemostasis, and that it promotes inflammation and cellular proliferation after vascular injury.
Methods
Materials
Collagen type I was from Chronolog; convulxin (CVXN) was from Centerchem, ELISA kits for RANTES and soluble CD40 ligand (sCD40L) were from Bender MedSystems, PE-labeled anti–human P-selectin (CD42) was from ID Labs, rabbit anti-SYK(525/526) antibody was from Cell Signaling Technology, rabbit anti-pLAT (191) was from BioSource, and mouse anti-SYK (4D10) was from Santa Cruz Biotechnology.
Impact of genetic modulation of Syk on arterial thrombosis
Radiation chimeras mice were obtained by injecting Syk−/− fetal liver cells into lethally irradiated recipient mice to overcome perinatal lethality of Syk−/− mice.23,24 Syk+/− and Syk+/+ mice were obtained by breeding (Syk+/− × Syk+/−). The murine ex vivo thrombosis perfusion chamber protocol (using nonanticoagulated samples of blood) has been described previously.25 In brief, naive blood was perfused through either a glass capillary (internal diameter, 282 μm) coated with type I collagen or tissue factor (Thromborel; Dade Behring) at arterial rates of shear (871 seconds−1) for 2.5 minutes. Measurement of the thrombotic deposits (mean thrombus volume expressed in cubic micrometers per square micrometer) was performed on Epon semithin cross sections. Thrombotic deposits formed 5 mm from the proximal part of the capillary were analyzed using computer-assisted morphometry as described previously.25 Mice were maintained in animal facilities at Portola Pharmaceuticals, Inc. Animal care and procedures were reviewed and approved by the Institutional Animal Care and Use Committees and performed in accordance with the guidelines of the American Association for Accreditation of Laboratory Animal Care and the National Institutes of Health.
PRT060318
The specificity of PRT060318 [2-((1R,2S)-2-aminocyclohexylamino)-4-(m-tolylamino)pyrimidine-5-carboxamide;26)] for Syk has been described previously.27 Activity (IC50) of PRT060318 on Syk, its most closely related kinases (ZAP-70, FAK, and Pyk2), and kinases of the Src family (cSRC, Fyn, Lyn, and Yes) were determined at ATP concentrations that corresponded to Km apparent for each individual kinase (15μM, Syk; 15μM, ZAP-70; 70μM, FAK; 90μM, Pyk2; 200μM, cSRC; 70μM, Fyn; 70μM, Lyn; and 45μM, Yes).
Analysis of PRT060318 activity on platelet function (human washed platelets)
Determination of PRT060318 activity on Syk signaling.
Platelet signaling assays were performed using a 96-well plate format because preliminary experiments showed that this approach routinely resulted in equal protein loading of drug-treated samples within an experiment. In brief, washed platelets were obtained as described previously28 and pretreated with eptifibatide (9μM final concentration to prevent platelet aggregation). One hundred microliters was added into each well containing different concentrations of PRT060318 (in 30% DMSO) for 20 minutes before activation with 150 ng/mL CVXN for 1 minute at room temperature (RT). Reactions were terminated by addition of 20μL of 5 × loading buffer. After boiling, samples were stored at −20°C until analyzed by Western blotting. Platelet lysates were subjected to SDS-PAGE (Invitrogen) under reducing conditions, followed by electroblot-transfer onto nitrocellulose membranes. Membranes were incubated for 1 hour at RT in blocking buffer (Zymed blocking buffer; Invitrogen) and then overnight at 4°C with 1:500 anti-pLAT pY191 primary antibody (BioSource; to measure inhibition of Syk kinase activity) or with 1:1000 pSyk pY525/526 (Cell Signaling Technology; to measure inhibition of the autophosphorylation of Syk) in blocking buffer. After washes with Tris-buffered saline NP-40, blots were incubated for 1 hour at RT in the presence of 1:10 000 mouse anti–rabbit secondary antibody in blocking buffer (Jackson Immuno Research Laboratories). After Tris-buffered saline NP-40 washes, blots were visualized by ECL using Western Lightening (PerkinElmer Life and Analytical Sciences). Quantification of protein bands was performed using densitometry (Epson Expression 1680 scanner; Epson) using Quantity One Version 4.5.0 software (Bio-Rad Laboratories).
Determination of PRT060318 activity on heat-aggregated IgG-induced platelet aggregation.
Aggregation was induced by 30 μg/mL human aggregated IgG (obtained by heating antibodies for 15 minutes at 63°C).
Analysis of PRT060318 activity on platelet function (human PRP)
PRP was obtained from trisodium citrated (0.32%) blood from human volunteers who gave written informed consent to the protocol (approved by the local Human Subjects Committee of Portola Pharmaceuticals). PRP was preincubated with PRT060318 (20 minutes) before all experiments. Aggregation was induced by 4 μg/mL collagen or 5μM ADP. Release of CD40L and RANTES was measured by ELISA after incubation of PRP with 4 μg/mL collagen for 45 minutes at 37°C.
Analysis of PRT060318 activity on platelet function (whole blood)
Human blood was collected in syringes containing 5μM final concentration of the factor Xa inhibitor C921-78 to preserve physiologic Ca2+ concentration and perfused through a collagen type I-coated capillary at 1500 second−1 for 5 minutes. End point evaluation of the thrombotic deposits was performed as described previously.29 For details, see supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Antithrombotic activity of PRT060318 in murine models
Photochemical carotid artery thrombosis.
Photochemical (Rose Bengal-green light laser) carotid artery injury was performed as described previously.30 PRT060318 (30 mg/kg in distilled water) or vehicle control was administered via oral gavage 2 hours before carotid injury. For details, see supplemental Methods.
Mouse FeCl3 thrombosis model.
Thrombosis on mesenteric arteries (1000-1300 seconds−1) was performed and recorded as described previously.29 In brief, arteries visualized using a Carl Zeiss Axiovert S100 inverted microscope (objective ×20) equipped with a 250-W HBO fluorescent lamp source (Opti Quip) with a narrow-band FITC filter set (Chroma Technology Corp) and a Hamamatsu chilled CCD C5985 intensified camera. Platelet vessel wall interactions and thrombus formation were analyzed in real time using Simple PCI software (Compix Inc Imaging System).
Thromboembolism model.
Anesthetized mice received a mixture of collagen (0.8 mg/kg) and epinephrine (60 μg/kg) injected into the jugular vein. Time from collagen plus epinephrine injection to death was recorded over a 30-minute period. PRT060318 (30 mg/kg) or its vehicle control was administered orally 2 hours before the intravenous injection of the platelet agonists.
Mouse bleeding times.
Tail bleeding times were measured by transecting the tails of anesthetized (50 mg/kg sodium pentobarbital) mice 3 or 5 mm from the tip, as described previously.31 In brief, mice were treated with PRT060318 (30 mg/kg in distilled water) or vehicle control, and the 3-mm transected tail tip was blotted with filter paper (Whatman) every 15 seconds. The time to cessation of bleeding recorded when no blood appeared on the filter paper for 2 consecutive 15-second periods. The 5-mm transected tail tip was placed into a beaker containing saline at 37°C, and the time to cessation of bleeding for persistent 3-minute period was determined.
Mouse femoral artery wire injury
Wire injury of the femoral artery was performed, as described previously.32 PRT060318 (30 mg/kg in distilled water) or vehicle control was administered via oral gavage 2 hours before injury and then twice daily for 10 days. For details, see supplemental Methods.
Atherosclerosis experiments: ApoE model of high-fat diet-induced atherosclerosis
To induce atherosclerosis, male apoE-deficient (ApoE−/−) mice consumed a high-fat diet (Clinton/Cybulsky Rodent Diet D12108 with 1.25% cholesterol; Research Diets) from 8 to 28 weeks of age. Mice were treated with PRT060318 (30 mg/kg in distilled water; n = 15) or vehicle control (n = 16) administered via oral gavage twice daily for 3 weeks followed by 1 week off from 8 weeks of age until 24 weeks of age, for a total of 16 weeks. As detailed in supplemental Methods, aortas were then harvested at 28 weeks of age for atherosclerotic lesion analysis using Sudan IV staining.
In vivo rabbit arterial thrombosis model
A pulsed Doppler flow probe was placed around the carotid artery. Rabbits were anesthetized and maintained at a surgical plan of anesthesia with ketamine (75 mg/kg), xylazine (8 mg/kg), and acepromazine (1.5 mg/kg). A custom-made rigid plastic cuff was placed on the artery ∼ 1 to 2 cm proximal to the probe to create a critical stenosis (50% reduction of the blood flow). The arterial injury was induced using a crush method (3 consecutive crushes 3 seconds each, 1 cm proximal to the Doppler flow probe). The cuff was then placed over the injured site, and the flow was monitored and recorded for the remainder of the experiment. Data are expressed as ratio of nonoccluded vessels (number of patents arteries/number of occluded arteries [blood flow = 0] over time). PRT060318 intravenous infusion regimen was initiated before vascular injury and established as follows: from 0 to 15 minutes, 20.67 mg/kg/h at 12.4 mL/kg/h and then from 15 minutes to the end, 7.33 mg/kg/h at 4.4 mL/kg/h.
In vivo swine arterial thrombosis model
After anesthesia and 111In-platelet labeling, carotid arterial crush injury of 4-month-old female pigs (Babcock 4-way cross stock) was performed, as described previously.33 In brief, injury was induced by 6 serial hemostat crushes of 5-second duration, interspersed with a 3-second rest period, with each subsequent injury visually abutting the prior injury site. The thrombi were then allowed to propagate for 30 minutes. At the end of the 30-minute period, injured arterial segments were harvested and assayed for 111In-content in a scintillation counter. The model used both carotid arteries where the first thrombus formed served as the vehicle control and second as the active drug-treated thrombus. The pigs were then infused with PRT060318 at a constant intravenous infusion rate of 8.90 mg/kg/h at 1 mL/kg/h for the duration of the study. The second carotid was injured next and thrombi allowed to propagate for 30 minutes. Platelet aggregation in PRP was performed before and on infusion of PRT060318 with ADP (20μM) or CVXN (250 ng/mL). Activity of PRT060318 on primary hemostasis was assessed in the ear bleeding time model before and on infusion of the drug.33
Statistics
Data are presented as the mean ± SD unless otherwise specified in the text. Comparisons between genotypes or treatment groups used a nonpaired t test. P values < .05 were considered significant.
Results
Lack of Syk attenuates shear-induced thrombus formation ex vivo
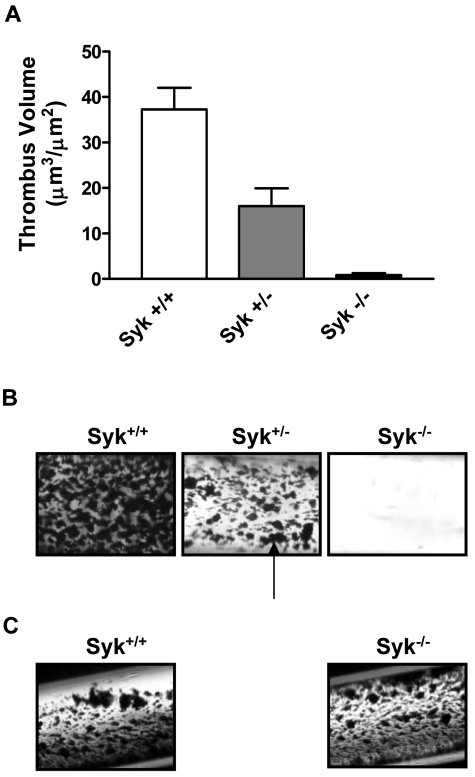
We investigated the role of Syk in thrombus formation under physiologic arterial shear rates (871 seconds−1) using a collagen-coated flow perfusion chamber assay.29 Perfusion of nonanticoagulated blood resulted in the rapid formation of platelet aggregates in wild-type (WT) mice (thrombus volume = 37.2 ± 4.7 μm3/μm2) that was essentially abolished in Syk−/− radiation chimeras (0.8 ± 0.4 μm3/μm2; P < .001; Figure 1 A-B). Interestingly, although no noticeable immunologic phenotype has been reported for Syk haploinsuffiency, the perfusion of blood from Syk+/− mice led to the formation of thrombi that were significantly smaller in size than their WT counterparts (16.0 ± 3.9 μm3/μm2; P < .01; Figure 1A-B). In contrast to collagen-coated capillaries, thrombi formed to a similar size in WT and Syk−/− radiation chimeras mice after perfusion on a tissue factor-coated surface (Figure 1C).
Figure 1.
Lack of Syk attenuates arterial thrombus formation ex vivo. (A) Histogram showing the critical role for Syk in mediating thrombosis on type I collagen. (B) Thrombotic deposits formed on collagen-coated capillaries exposed for 2.5 minutes to nonanticoagulated blood from Syk+/+ (n = 9), Syk+/− (n = 10), and radiation chimeras (Syk−/− radiation chimera; n = 7) mice at 871 seconds−1. The black arrow points to platelet-rich thrombi. Quantification was performed on semithin cross section of Epon-embedded thrombotic deposits via computer-assisted morphometry. (C) Photomicrograph of thrombotic deposits formed on tissue factor-coated capillaries at 871 seconds−1 in Syk+/+ and Syk−/− radiation chimera mice.
PRT060318, a selective, small-molecule inhibitor of Syk kinase
To further characterize the contributions of Syk to thrombosis, we used a highly selective small-molecule inhibitor of Syk kinase activity, PRT060318, that has an IC50 value that is > 200, 400, 300, and 80 times lower than that of cSRC (886nM), Fyn (1494nM), Lyn (611nM), and Yes (253nM), respectively.27
PRT060318 activity on platelet function
Washed platelets.
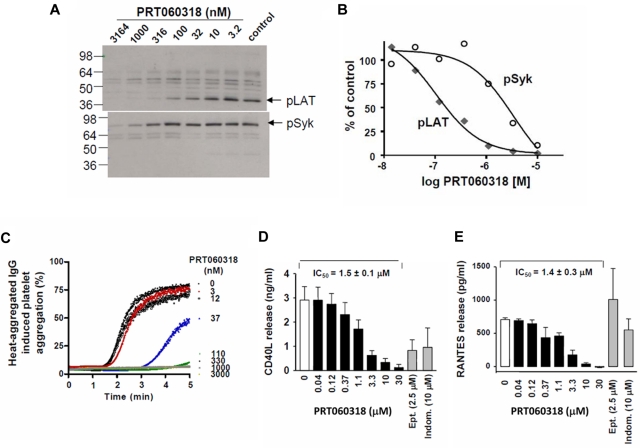
Engagement of the GPVI receptor by CVXN initiates an intracellular signaling cascade, including phosphorylation of LAT on tyrosine residue 191 and increases in intracellular calcium and P-selectin expression. PRT060318 inhibited CVXN-induced phosphorylation of LAT and Syk(525/6Y), with a mean IC50 value of 52 ± 18nM (n = 6) and 2637 ± 740nM (n = 3; Figure 2A-B), respectively. PRT060318 inhibited CVXN-induced P-selectin expression, with an IC50 value of 48nM (n = 4; data not shown) and activation of FcγRIIA by heat-aggregated IgG, with an IC50 value of 85nM (Figure 2C).
Figure 2.
Kinase specificity of PRT060318 and activity on human platelet function. (A) Integrilin-treated human washed platelets were incubated with 2 μL of various concentrations of PRT060318 (all in 30% DMSO) before activation with CVXN (150 ng/mL). Platelet lysates were subjected to SDS-PAGE gels, and Western blots were probed with anti-pLAT pY191 or pSyk pY525/526 antibodies. (B) Quantification of protein bands was performed using densitometry (Epson Expression 1680) using Quantity One Version 4.5.0 software (Bio-Rad Laboratories), and variations normalized to control bands (absence of PRT060318) were plotted against PRT060318 concentrations. (C) Representative dose response effect of PRT060318 on heat-aggregated IgG-induced platelet aggregation (n = 3; IC50 = 85nM in washed platelets). In PRP, PRT060318 inhibits collagen-induced platelet aggregation CD40L (D) and RANTES (E) release. Ept indicates eptifibatide; and Indom, indomethacin. For further data on PRT060318 specificity, please see Reilly et al.27
Platelet-rich plasma.
PRT060318 potently inhibited platelet aggregation induced by collagen (4 μg/mL), with an IC50 value of 0.57 ± 0.19μM, while not affecting ADP (5μM)–induced platelet aggregation, even when tested at 50μM. PRT060318 inhibited collagen-induced CD40L and RANTES release, with an IC50 value of 1.5 and 1.4μM (Figure 2D-E), respectively.
PRT060318 activity on the kinetics of thrombosis in vitro
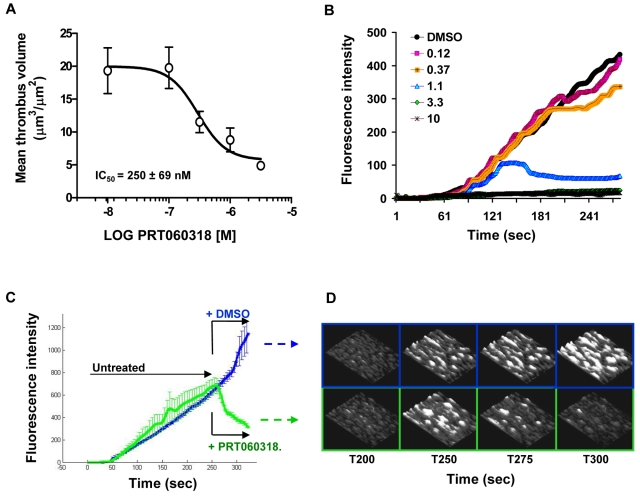
PRT060318 dose-dependently inhibited thrombus volume in collagen-coated capillaries (1500 seconds−1), with an IC50 value of 250nM (Figure 3A). Concentrations of PRT060318 that had no effect on the initial rate of thrombus formation-induced disaggregation of platelet thrombi, whereas higher concentrations (> 3.3μM) prevented thrombus growth on collagen (Figure 3B). Figure 3C-D indicates that perfusion of PRT060318-treated blood induced immediate dethrombosis of preformed platelet-rich thrombi.
Figure 3.
Syk inhibition affects both thrombus initiation and thrombus stability in human blood. (A) Effect of PRT060318 on thrombus volume after perfusion of human blood over collagen. Each point represents the mean ± SEM of 7 individuals. (B) Continuous, real-time thrombosis profiles of 1 representative experiment in which anticoagulated human blood was perfused through collagen-coated capillaries in presence of PRT060318 (0.12-10μM). PRT060318 affects thrombus stability (< 1μM) and thrombus growth (> 3μM). (C) Mean thrombosis profiles of experiments performed with blood from 4 individuals showing dethrombotic activity of PRT060318. After 250 seconds, blood treated with either DMSO (vehicle control) or 5μM PRT060318 was immediately perfused over the preformed, untreated thrombi. (D) Representative 3-dimensional photomicrographs corresponding to panel C. The base of the thrombi remained unaffected by the treatment indicating destabilization of the platelet–platelet interactions.
Inhibition of Syk kinase activity provides protection from arterial and venous thrombosis in murine models with minimal effects on bleeding time
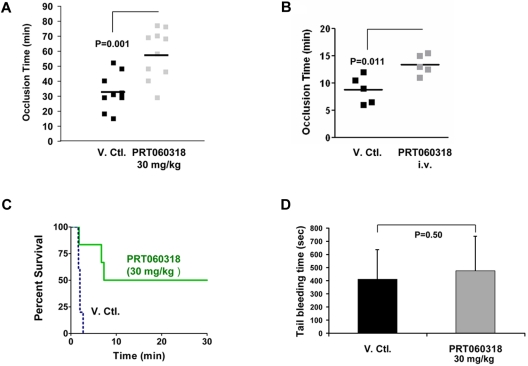
To examine the function of Syk in arterial thrombosis, we subjected the carotid arteries of mice administered vehicle or PRT060318 (30 mg/kg oral) to the Rose Bengal photochemical carotid artery injury model, which produces thrombosis because of local free radical release and oxidative endothelial cell injury.30 A formal pharmacokinetic study was performed to assess the plasma concentration of PRT060318 after oral dosing. Peak and trough concentrations were 4.7 to 7.2μM and 0.6 to 0.9μM, respectively (supplemental Table 1). As shown in Figure 4A, the time to occlusive thrombus formation was prolonged significantly in PRT060318-treated mice (58 ± 16 vs 33 ± 12 minutes; P = .001). In light of the weak thrombotic phenotype associated with Syk deficiency in an FeCl3 thrombosis model,34 we studied the contribution of the kinase activity of Syk in an FeCl3 thrombosis model applied to mesenteric arteries. In this model, PRT060318 was administered as a continuous intravenous infusion and targeted full inhibition (concentration achieved, 9.6 ± 0.9μM) of the kinase activity of Syk. PRT060318 delayed the time to occlusion (vehicle, 8.8 ± 1.1 vs PRT060318, 13.4 ± 0.8 minutes; P = .011; Figure 4B).
Figure 4.
PRT060318 inhibits arterial and venous thrombosis in the mouse. (A) PRT060318 (30 mg/kg oral) delays time to occlusion in carotid artery photochemical injury model (PRT060318, 58 ± 16 vs vehicle, 33 ± 12 minutes; P = .001). V.Ctl. indicates vehicle control. (B) PRT060318 (intravenous infusion) delays time to occlusion in FeCl3-injured mesenteric arteries (PRT060318, 13.4 ± 0.8 vs vehicle, 8.8 ± 1.1 minute; P = .011). (C) Inhibition of Syk by PRT060318 (30 mg/kg oral, n = 6) but not vehicle control (n = 5) prevents death after injection of a collagen + epinephrine mixture in WT mice. All vehicle control-treated animals died within 5 minutes of the intravenous. Injection, whereas 50% of the PRT060318-treated animals survived. (D) Tail bleeding times, performed by transecting the mouse tail 3 mm from the tip, are comparable in PRT060318 (30 mg/kg oral) and vehicle-treated mice (V. Ctl., n = 15; PRT060318, n = 14).
The function of Syk in venous thrombosis also was investigated in an internal jugular vein thromboembolism model. PRT060318 (30 mg/kg single oral dose) significantly protected WT mice from death induced by intravenous injection of a mixture of collagen and epinephrine (Figure 4C). The significant extent of protection in this model may be related to the simple and direct nature of the agonists used (collagen + epinephrine) and their direct stimulation of the Syk signaling pathway.
To assess the role of Syk in hemostasis, we examined tail bleeding times. There was no difference in tail bleeding times between PRT060318 (30 mg/kg single oral dose) and vehicle control-treated mice whether tails were transected 3 or 5 mm from the tip. For 3-mm transection, mean bleeding time for vehicle-treated mice was 411 ± 225 seconds compared with 474 ± 267 seconds (P = .50) for PRT060318-treated mice (Figure 4D). For 5-mm transection, mean bleeding time for vehicle-treated mice was 480 ± 378 seconds compared with 690 ± 186 seconds for PRT060318-treated mice (P = .17).
Femoral artery injury and neointima formation
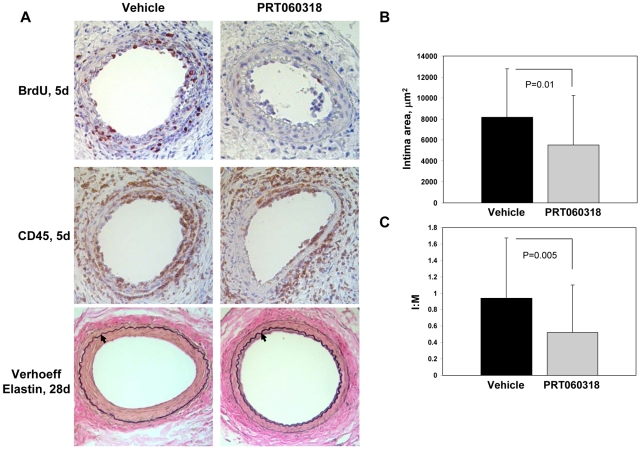
We investigated whether Syk activity modulates neointima formation by subjecting mice treated with PRT060318 (30 mg/kg via oral gavage twice daily for 10 days) to transluminal wire injury of the femoral artery. Wire injury is accompanied by endothelial denudation, platelet and fibrin deposition, prominent vascular inflammation, smooth muscle cell proliferation and migration, and neointima formation.32 We assessed platelet deposition 1 day after injury by staining for the platelet-specific marker GPIIb. There was no significant difference (P = .52) in GPIIb-positive area in arteries from vehicle-treated (2634 ± 809 μm2) and PRT060318-treated (2898 ± 933 μm2) mice. Wire injury of the femoral artery is accompanied by prominent vascular inflammation that peaks 3 to 5 days after injury.32 Our studies implicated Syk in this process because altered leukocyte accumulation within vessels was observed in injured arteries from PRT060318-treated compared with control vehicle-treated mice (Figure 5A; supplemental Figure 1A). At 5 days after injury, inflammatory cells (percentage of CD45-positive cells) accumulating in the intima (vehicle, 70.4 ± 15.8% vs PRT060318, 48.9 ± 12.1%; P = .004) and media (vehicle, 56.2 ± 24.3% vs PRT060318, 16.2 ± 20.4%; P < .001) were decreased significantly in PRT060318-treated mice (supplemental Figure 1A). We expanded the CD45 analysis by immunostaining with cell-specific markers. Accumulation of neutrophils (mAb-7/4–positive cells) in the developing neointima (vehicle, 50.7 ± 10.0% vs PRT060318, 12.2 ± 9.7%; P < .001) and media (vehicle, 33.3 ± 21.6% vs PRT060318, 7.4 ± 10.4%; P = .005) at 5 days was decreased by 76% and 78%, respectively, in PRT060318-treated compared with control vehicle-treated mice (supplemental Figure 2A-B). Accumulation of macrophages (Mac-3–positive area) in the developing neointima (vehicle, 41.9 ± 12.8% vs PRT060318, 3.8 ± 2.2%; P < .001) and media (vehicle, 19.3 ± 7.2% vs PRT060318, 13.8 ± 5.0%; P = .08) at 5 days was decreased by 91% and 28%, respectively, in PRT060318-treated mice (supplemental Figure 2C-D). We examined the inhibition of Syk kinase activity within the injured vessel wall by assaying the phosphorylation status of Vav, a downstream Syk target (supplemental Figure 3). Phospho-Vav–positive area was reduced significantly in the vessel wall of PRT060318-treated mice (vehicle, 30.5 ± 12.7 vs PRT060318, 14.7 ± 5.5%; P = .016).
Figure 5.
PRT060318 inhibits neointima formation after femoral artery wire injury. (A) Photomicrographs of injured femoral arteries from vehicle control and PRT060318-treated mice after wire injury. Verhoeff elastin staining 28 days after injury. Arrows indicate the internal elastic lamina. Images were captured using a microscope (model DM2000; Leica) and captured with an AxioCam MRc5 camera (Carl Zeiss) interfaced to a computer running Zeiss Axiovision Rel 4.5 software (original magnification, ×20). Immunostaining for CD45 and BrdU 5 days after injury. Quantitative morphometry, including intimal area (B) and intimal area:medial area (I:M) ratio (C), of mouse femoral arteries 28 days after injury.
Because there is evidence that Syk modulates vascular smooth muscle cell proliferation in vitro,35 we assessed cellular proliferation by quantifying incorporation of BrdU in vessels after injury (Figure 5A; supplemental Figure 1B). Five days after injury, substantial proliferation was evident in control vehicle-treated arteries (51.8 ± 5.6% of intimal cells and 29.8 ± 7.3% of medial cells). Inhibition of Syk with PRT060318 significantly attenuated intimal (43% decrease; P < .001) and medial (64% decrease; P < .001) proliferation at 5 days (supplemental Figure 1B). These data provide direct evidence that PRT060318 ameliorates the proliferative response to vascular injury.
In this model, neointimal thickening is evident 5 days after injury and progresses significantly between 5 and 28 days. Neointimal thickening was reduced significantly at 28 days (PRT060318, 5521 ± 4687 vs vehicle, 8187 ± 4604 μm2; P = .01; Figure 5) and the intima:media area ratio was decreased by 45% in PRT060318-treated compared with control vehicle-treated mice (PRT060318, 0.52 ± 0.58 vs vehicle, 0.94 ± 0.73; P = .005). Taken together, these data indicate that Syk activity modulates the recruitment and accumulation of neutrophils and macrophages, cellular proliferation, and neointima formation after vascular injury.
Inhibition of Syk inhibits atherosclerotic lesion formation
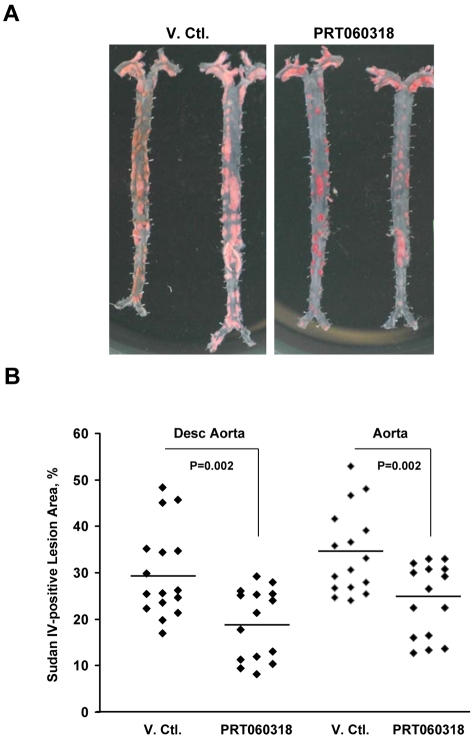
Because leukocyte–platelet interactions are critical in the initiation and progression of atherosclerosis3 as well as restenosis,16 we examined the development of atherosclerotic lesions in ApoE−/− male mice consuming a high-fat diet from 8 to 28 weeks of age. Mice were treated with PRT060318 (30 mg/kg in distilled water; n = 15) or vehicle control (n = 16) administered via oral gavage twice daily for 3 weeks followed by 1 week off from 8 weeks of age until 24 weeks of age, for a total of 16 weeks. Plasma lipid profiles did not differ significantly between vehicle control- and PRT060318-treated mice on high-fat diet (Table 1). Treatment with PRT060318 did not affect total white blood cell count (P = .94), hemoglobin (P = .25), or platelet count (P = .58). Importantly, mouse weight was monitored weekly and revealed no significant difference between vehicle or PRT060318 treatment (supplemental Figure 4). Aortas were harvested at 28 weeks of age for atherosclerotic lesion analysis. Inhibition of Syk activity with PRT060318 resulted in a 36% reduction in lesion area in en face analysis of Sudan IV-stained thoraco-abdominal aortas (vehicle, 29.9 ± 9.7% vs PRT060318, 19.1 ± 7.6%; P = .002) and a 30% reduction in lesion area when the entire aorta including the ascending and aortic arch was analyzed (vehicle, 34.3 ± 9.1% vs PRT060318, 24.2 ± 7.8%; P = .002; Figure 6).
Table 1.
Lipid and hematologic parameters in apoE−/− mice treated with PRT060318
| Parameter | Vehicle control (n = 4) | PRT060318 (n = 5) | P |
|---|---|---|---|
| Lipids | |||
| Total cholesterol, mg/dL | 334 ± 64 | 378 ± 96 | 0.44 |
| LDL cholesterol, mg/dL | 319 ± 61 | 334 ± 64 | 0.58 |
| Triglycerides, mg/dL | 44 ± 16 | 51 ± 11 | 0.48 |
| CBC | |||
| WBC, no./μL × 103 | 3.7 ± 1.1 | 3.8 ± 1.4 | 0.94 |
| Hemoglobin, g/L | 10.8 ± 2.1 | 12.4 ± 1.9 | 0.25 |
| Platelet count, no./μL × 103 | 577 ± 297 | 480 ± 136 | 0.58 |
PRT060318 (30 mg/kg in distilled water) or vehicle control was administered via oral gavage twice daily for 3 weeks followed by 1 week off, for a total of 16 weeks.
LDL indicates low-density lipoprotein; CBC, complete blood count; WBC, white blood cells; PMN, neutrophils; and HCT, hematocrit.
Figure 6.
Atherosclerotic lesion formation is attenuated by PRT060318. (A) Digital photograph (D70S camera [Nikon] with Tamron SP AF90mm F/2.8 Di Macro 1:1 lens) of Sudan IV staining of longitudinally opened and pinned thoraco-abdominal aortas harvested from ApoE−/− mice after 20 weeks of high-fat feeding. PRT060318 (30 mg/kg in distilled water; n = 15) or vehicle control (n = 16) were administered via oral gavage twice daily for 3 weeks followed by 1 week off, for a total of 16 weeks. (B) Quantification of percentage of lesion area in the descending thoracic and abdominal aorta as assessed by Sudan IV staining and computer-assisted imaging analysis.
Rabbit thrombosis studies
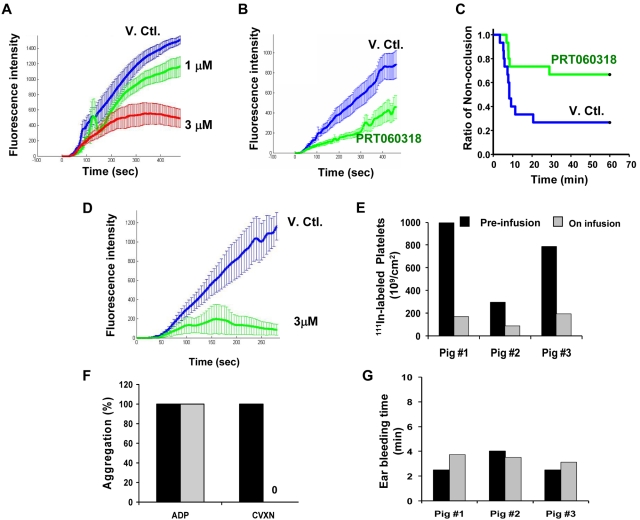
PRT060318 regimen targeted concentrations that showed in vitro maximal levels of inhibition of arterial thrombosis in the collagen-coated perfusion chamber assay (Figure 7A). Plasma concentrations achieved were 3.93 ± 0.54 (T0), 4.45 ± 0.59 (T20), 4.67 ± 0.64 (T40), and 4.98 ± 0.82μM at T60 minutes after injury, providing near maximal inhibition of collagen (10 μg/mL Horm collagen [Nycomed])–induced platelet aggregation (86%-92%) and smaller thrombi ex vivo in the perfusion chamber assay (Figure 7B). In vivo, this dose regimen led to a significant reduction in the occlusion event rate as 10 of 15 arteries in the PRT060318-treated group did not occlude versus 4 of 15 for vehicle-treated group (P = .021; Figure 7C).
Figure 7.
PRT060318 activity in rabbits and pigs. (A) In vitro (spiking experiments) mean thrombotic profiles in presence of vehicle control (V.Ctl.) or PRT060318 (1 and 3μM) in rabbits. (B) Ex vivo thrombotic profiles associated with V. Ctl. or PRT060318 infusion regimen (intravenous infusion regimen was initiated before vascular injury and established as follows: from 0 to 15 minutes, 20.67 mg/kg/h at 12.4 mL/kg/h and then from 15 minutes until the end, 7.33 mg/kg/h at 4.4 mL/kg/h). (C) Occlusion rate in vivo in the rabbit thrombosis model. P = .021 by Gehan–Breslow survival analysis with Bonferroni comparison. (D) In vitro mean thrombotic profiles of whole blood from 4 pigs treated with DMSO (blue curve) or 3μM PRT060318. Intravenous infusion of PRT060318 (8.90 mg/kg/h at 1 mL/kg/h) in pigs inhibited 111In-labeled platelet deposition in vivo in the pig thrombosis model (E), abolished ex vivo platelet aggregation induced by CVXN (250 ng/mL) but not ADP (20μM; F), and did not affect the ear bleeding time (G).
Pig thrombosis studies
The intravenous infusion rate of PRT060318 targeted plasma concentrations providing maximal inhibition of thrombosis in the in vitro perfusion chamber assay (Figure 7D). Animals tested halfway through the 30-minute infusion period showed concentrations of 1090, 1260, and 1680 ng/mL (mean = 3.9μM). These levels significantly inhibited the deposition of 111In-labeled platelets onto the injured vessel wall by > 75% in vivo (Figure 7E) and abolished ex vivo CVXN-induced platelet aggregation without affecting ADP-induced platelet aggregation (Figure 7F) and the activated clotting time (control, 132.5 ± 22 seconds; treatment, 130 ± 13 seconds) and without compromising ear bleeding time, a measure of primary hemostasis (Figure 7G).
Discussion
In this study, we have found that the modulation of Syk using Syk knockout radiation chimeras or on pharmacologic modulation of its tyrosine kinase activity inhibits thrombosis and vascular injury responses without affecting hemostasis. This conclusion is supported by the following data: (1) In vitro, Syk supports thrombus growth and stability on collagen under arterial shear rate conditions but does not play a role in a reaction driven by tissue factor. (2) In vivo, using different thrombosis models applied to multiple animal species, we showed that Syk mediates thrombosis on an injured blood vessel. Furthermore, Syk is involved in the long-term responses to vascular injury, including inflammatory cell recruitment, cellular proliferation, and neointima formation. (3) Finally, we established that modulation of these functions does not translate into detrimental effects on primary hemostasis.
Selectivity of PRT060318 for Syk and effect on thrombosis
PRT060318 is highly specific for Syk kinase, with an IC50 value of 3nM, which is ∼ 35 to 300 times lower than that of the closely related tyrosine kinases ZAP-70, FAK, and Pyk2 and 80 to 400 times lower than the Src kinases Fyn, Lyn, and Yes. PRT060318 selectively inhibited Syk kinase activity in purified enzyme assays, GPVI-dependent signaling pathways of platelet activation, and shear-induced thrombus growth on collagen and promoted dissolution of preformed thrombi. These observations are in agreement with the phenotype of the genetically engineered mice (Figure 1) and with known platelet GPVI biology (for review, see Watson et al8).
Because definitive conclusions regarding the importance of GPVI signaling in thrombosis are subject to the limitations of mouse models of thrombosis in general,36,37 we evaluated the effects of Syk inhibition on thrombosis in large animal species (rabbits and pigs) that exposed nondenatured proteins of the vessel wall to blood flow (ie, carotid artery crush-injury models). These 2 species were intentionally selected based on the homology of their whole blood thrombotic profile in the perfusion chamber compared with human blood. In both rabbit and pig models, intravenous infusion of PRT060318 targeted maximal inhibition of Syk kinase activity and significantly inhibited arterial thrombosis. The antithrombotic activity of PRT060318 in pigs was remarkable because it was achieved with complete inhibition of CVXN-induced platelet aggregation, with no effect on ADP-induced platelet aggregation, and no prolongation of ear bleeding time.
Although the plasma levels achieved in the 2 animal models may seem high (∼ 4μM), possibly confounding Syk specificity, 3 sets of data strongly suggest that PRT060318 selectivity for Syk remained. First, PRT060318 is 89% protein bound, leaving only ∼ 10% (ie, ∼ 400nM) free in plasma. Second, when PRT060318 was tested in a broad panel of kinases, at a concentration greater than 17-fold higher than its IC50 value for Syk, the next kinase inhibited was minimally affected (29% inhibition). Thus, under conditions that are fully inhibitory of Syk kinase activity, both related kinases (ZAP-70, FAK, and Pyk2) and nonrelated kinases in the Millipore panel are unlikely to be sufficiently inhibited to influence thrombotic processes. Accordingly, we reported previously27 that 10μM PRT060318 did not inhibit Lyn activity in human whole blood. Third, plasma levels achieved in the pig study had no effects on platelet aggregation induced by ADP and did not prolong bleeding time. Thus, it is expected that the majority of the antithrombotic effect of PRT060318 stems from on-target activity at the tyrosine kinase function of Syk and that Syk seems to be preferentially involved in mechanisms regulating arterial thrombosis, while having a lesser role in primary hemostasis.
The significant inhibition of arterial thrombosis in vivo by the Syk inhibitor may stem from a dual action. First, consistent with its role in GPVI signaling, Syk promotes platelet adhesion and activation by collagen. Second, Syk has a role in maintaining thrombus stability. In fact, PRT060318 affected thrombus stability at lower concentrations than was required to inhibit platelet adhesion (Figure 3B). Phosphorylation of Syk has been reported in both inside-out38 and outside-in39 signaling pathways involving GPIIb-IIIa, the key glycoprotein promoting thrombus stability.40 Because shear itself contributes to phosphorylation of Syk,10,41 it is possible that the primary contribution of Syk to thrombosis is to stabilize arterial thrombi in vivo.
Modulation of Syk affects vessel wall response to vascular injury
Leukocyte–platelet interactions are critical in the initiation and progression of atherosclerosis3,42 as well as restenosis.4 Recruitment of circulating leukocytes to vascular endothelium requires multistep adhesive and signaling events including selectin-mediated attachment and rolling, leukocyte activation, and integrin-mediated firm adhesion and diapedesis that result in the infiltration of inflammatory cells into the blood vessel wall.43 Firm attachment is mediated by members of the β2-integrin family, LFA-1 (αLβ2, CD11a/CD18), Mac-1 (αMβ2, CD11b/CD18), and p150,95 (αXβ2, CD11c/CD18).44 Importantly, leukocyte recruitment also occurs at sites of vascular injury where the lining endothelial cells have been denuded and platelets and fibrin have been deposited. A similar sequential adhesion model of leukocyte attachment to and transmigration across surface-adherent platelets has been proposed previously.45 The initial tethering and rolling of leukocytes on platelet P-selectin46 is followed by their firm adhesion and transplatelet migration, processes that are dependent on leukocyte Mac-145 and platelet GPIbα.16 We have reported previously that deficiency4 and antibody targeting of Mac-1–GPIbα16 inhibits neointima formation. Because Syk is downstream of both GPIbα and Mac-1 signaling,17 we investigated the effect of PRT060318 on the acute and mid-term (5-28 days) biologic responses to vascular injury. We found that treatment with PRT060318 for 10 days modulated the biologic response to vascular injury at 28 days. In this endothelial cell-denuding model that does not lead to vascular occlusion by platelet-rich thrombi, PRT060318 inhibited neointima formation by impairing inflammatory cell accumulation and cellular proliferation rather than by affecting platelet deposition (a process probably driven by thrombin generation). The finding that inhibition of Syk with PRT060318 attenuated atherosclerotic lesion formation indicates that Syk controls both acute and long-term responses to arterial vascular injury. Because leukocyte-platelet interactions are critical in the initiation and progression of atherosclerosis3 as well as restenosis16 and because Syk is involved in leukocyte rolling and adhesion to activated endothelium,15 the antiatherosclerotic effect of PRT060318 is not unexpected.
Unmet clinical need and Syk therapeutic window
Inhibition of Syk kinase activity with PRT060318 did not interfere with the hemostatic function of platelets, in agreement with reports made using genetically engineered mice,23 providing a novel mechanism to regulate thrombosis without impairing hemostasis (ie, reduced bleeding risk). These findings are probably explained by the fact that Syk does not play a role downstream of thrombin signaling, as shown in Figure 1C and illustrated by others using GPVI-deficient mice.47
The identification of a small-molecule inhibitor of thrombosis that does not affect hemostasis (ie, bleeding time) has important clinical implications. Thrombotic cardiovascular diseases, including myocardial infarction and stroke, are the leading cause of death in developed countries.48 Total U.S. healthcare expenditures in 2009 for coronary heart disease and stroke were a staggering US$165.4 billion and US$68.9 billion, respectively,48 with pharmacologic therapies estimated to exceed US$20 billion worldwide.48 Primary drug therapies include antiplatelet and anticoagulant agents6,7; however, current multidrug strategies are limited by significant bleeding risks that are associated with increased mortality.49 Although new antiplatelet (ie, prasugrel and ticagrelor) and anticoagulant (ie, dabigatran, rivaroxaban, and apixaban) agents are being developed on the basis of superior efficacy, these therapeutic advances are often, although not always, associated with an increase in the rate of bleeding or transfusion requirement.6,7 There is emerging experimental evidence distinguishing the molecular and cellular mechanisms of hemostasis and thrombosis.50 Nonetheless, programs exploiting alternative targets, including platelet receptors/ligands (eg, P2X1, CD150, Gas6, and CD40L) and coagulation factor XIIa that seek to prevent thrombotic occlusion without impairing hemostasis,50 have not provided new, safer drugs to date.
Thus, the kinase activity of Syk seems to have a preferential and critical function in both thrombotic and inflammatory responses to vascular injury in arteries. Dose–response studies are required to establish whether a strategy aimed at targeting the kinase activity of Syk in a chronic setting is doable and safe. The thrombotic phenotype associated with Syk heterozygous mice and that has not been associated with an impairment of the immune function indicates that a therapeutic window may exist that would allow for pharmacologic modulation of atherothrombotic events.
Supplementary Material
Acknowledgments
The authors thank Eduardo Escobar and John Malinowski, Department of Pharmacology, Portola Pharmaceuticals Inc, for support related to animal husbandry.
This work was supported in part by the National Institutes of Health (grants HL85816 and HL57506 Merit Award, D.I.S.).
Footnotes
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: P.A., T.M., S.H., D.P., U.S., C.L., Y.W., W.O., and D.I.S. designed research and analyzed data; P.A., T.M., D.S., K.A., N.N., S.D., G.S., Y.Y., H.G., Y.M., S.A.P, K.N., and C.S. performed research; A.P. contributed new reagents and analytic tools; and P.A. and D.I.S. wrote the manuscript.
Conflict-of-interest disclosure: P.A., D.S., K.A., N.N., S.D., G.S., Y.Y., S.H., A.P., D.P., and U.S. are all present or former employees of Portola Pharmaceuticals Inc. The remaining authors declare no competing financial interests.
The current affiliation for D.S. is Bayer Healthcare, Berkeley, CA. The current affiliation for N.N. is ChemGenex Pharmaceuticals, Menlo Park, CA. The current affiliation for Y.Y. is Department of Development Oncology Diagnostics, Genentech, South San Francisco, CA.
Correspondence: Daniel I. Simon, University Hospitals Harrington-McLaughlin Heart & Vascular Institute, Case Western Reserve University School of Medicine, 11100 Euclid Ave, Cleveland, OH 44106; e-mail: daniel.simon@uhhospitals.org.
References
- 1.Libby P, Simon DI. Inflammation and thrombosis: the clot thickens. Circulation. 2001;103(13):1718–1720. doi: 10.1161/01.cir.103.13.1718. [DOI] [PubMed] [Google Scholar]
- 2.McEver RP. Adhesive interactions of leukocytes, platelets, and the vessel wall during hemostasis and inflammation. Thromb Haemost. 2001;86(3):746–756. [PubMed] [Google Scholar]
- 3.Massberg S, Brand K, Gruner S, et al. A critical role of platelet adhesion in the initiation of atherosclerotic lesion formation. J Exp Med. 2002;196(7):887–896. doi: 10.1084/jem.20012044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Simon DI, Chen Z, Seifert P, Edelman ER, Ballantyne CM, Rogers C. Decreased neointimal formation in Mac-1(−/−) mice reveals a role for inflammation in vascular repair after angioplasty. J Clin Invest. 2000;105(3):293–300. doi: 10.1172/JCI7811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Palabrica T, Lobb R, Furie BC, et al. Leukocyte accumulation promoting fibrin deposition is mediated in vivo by P-selectin on adherent platelets. Nature. 1992;359(6398):848–851. doi: 10.1038/359848a0. [DOI] [PubMed] [Google Scholar]
- 6.Anderson JL, Adams CD, Antman EM, et al. ACC/AHA 2007 guidelines for the management of patients with unstable angina/non ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines for the Management of Patients With Unstable Angina/Non ST-Elevation Myocardial Infarction): developed in collaboration with the American College of Emergency Physicians, the Society for Cardiovascular Angiography and Interventions, and the Society of Thoracic Surgeons: endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation and the Society for Academic Emergency Medicine. Circulation. 2007;116(7):e148–304. doi: 10.1161/CIRCULATIONAHA.107.181940. [DOI] [PubMed] [Google Scholar]
- 7.Kushner FG, Hand M, Smith SC, Jr, et al. 2009 Focused Updates: ACC/AHA Guidelines for the Management of Patients With ST-Elevation Myocardial Infarction (updating the 2004 Guideline and 2007 Focused Update) and ACC/AHA/SCAI Guidelines on Percutaneous Coronary Intervention (updating the 2005 Guideline and 2007 Focused Update): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2009;120(22):2271–2306. doi: 10.1161/CIRCULATIONAHA.109.192663. [DOI] [PubMed] [Google Scholar]
- 8.Watson SP, Auger JM, McCarty OJ, Pearce AC. GPVI and integrin alphaIIb beta3 signaling in platelets. J Thromb Haemost. 2005;3(8):1752–1762. doi: 10.1111/j.1538-7836.2005.01429.x. [DOI] [PubMed] [Google Scholar]
- 9.Gibbins JM. Platelet adhesion signalling and the regulation of thrombus formation. J Cell Sci. 2004;117(Pt 16):3415–3425. doi: 10.1242/jcs.01325. [DOI] [PubMed] [Google Scholar]
- 10.Feng S, Lu X, Resendiz JC, Kroll MH. Pathological shear stress directly regulates platelet alphaIIbbeta3 signaling. Am J Physiol Cell Physiol. 2006;291(6):C1346–1354. doi: 10.1152/ajpcell.00559.2005. [DOI] [PubMed] [Google Scholar]
- 11.Yanaga F, Poole A, Asselin J, et al. Syk interacts with tyrosine-phosphorylated proteins in human platelets activated by collagen and cross-linking of the Fc gamma-IIA receptor. Biochem J. 1995;311(Pt 2):471–478. doi: 10.1042/bj3110471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gibbins J, Asselin J, Farndale R, Barnes M, Law CL, Watson SP. Tyrosine phosphorylation of the Fc receptor gamma-chain in collagen-stimulated platelets. J Biol Chem. 1996;271(30):18095–18099. doi: 10.1074/jbc.271.30.18095. [DOI] [PubMed] [Google Scholar]
- 13.Poole A, Gibbins JM, Turner M, et al. The Fc receptor gamma-chain and the tyrosine kinase Syk are essential for activation of mouse platelets by collagen. EMBO J. 1997;16(9):2333–2341. doi: 10.1093/emboj/16.9.2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Suzuki-Inoue K, Fuller GL, Garcia A, et al. A novel Syk-dependent mechanism of platelet activation by the C-type lectin receptor CLEC-2. Blood. 2006;107(2):542–549. doi: 10.1182/blood-2005-05-1994. [DOI] [PubMed] [Google Scholar]
- 15.Abbal C, Lambelet M, Bertaggia D, et al. Lipid raft adhesion receptors and Syk regulate selectin-dependent rolling under flow conditions. Blood. 2006;108(10):3352–3359. doi: 10.1182/blood-2006-04-013912. [DOI] [PubMed] [Google Scholar]
- 16.Wang Y, Sakuma M, Chen Z, et al. Leukocyte engagement of platelet glycoprotein Ibalpha via the integrin Mac-1 is critical for the biological response to vascular injury. Circulation. 2005;112(19):2993–3000. doi: 10.1161/CIRCULATIONAHA.105.571315. [DOI] [PubMed] [Google Scholar]
- 17.Mócsai A, Ruland J, Tybulewicz VL. The SYK tyrosine kinase: a crucial player in diverse biological functions. Nat Rev Immunol. 2010;10(6):387–402. doi: 10.1038/nri2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheng AM, Rowley B, Pao W, Hayday A, Bolen JB, Pawson T. Syk tyrosine kinase required for mouse viability and B-cell development. Nature. 1995;378(6554):303–306. doi: 10.1038/378303a0. [DOI] [PubMed] [Google Scholar]
- 19.Turner M, Mee PJ, Costello PS, et al. Perinatal lethality and blocked B-cell development in mice lacking the tyrosine kinase Syk. Nature. 1995;378(6554):298–302. doi: 10.1038/378298a0. [DOI] [PubMed] [Google Scholar]
- 20.Spalton JC, Mori J, Pollitt AY, Hughes CE, Eble JA, Watson SP. The novel Syk inhibitor R406 reveals mechanistic differences in the initiation of GPVI and CLEC-2 signaling in platelets. J Thromb Haemost. 2009;7(7):1192–1199. doi: 10.1111/j.1538-7836.2009.03451.x. [DOI] [PubMed] [Google Scholar]
- 21.Braselmann S, Taylor V, Zhao H, et al. R406, an orally available spleen tyrosine kinase inhibitor blocks fc receptor signaling and reduces immune complex-mediated inflammation. J Pharmacol Exp Ther. 2006;319(3):998–1008. doi: 10.1124/jpet.106.109058. [DOI] [PubMed] [Google Scholar]
- 22.Pamuk ON, Tsokos GC. Spleen tyrosine kinase inhibition in the treatment of autoimmune, allergic and autoinflammatory diseases. Arthritis Res Ther. 2010;12(6):222. doi: 10.1186/ar3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Law DA, Nannizzi-Alaimo L, Ministri K, et al. Genetic and pharmacological analyses of Syk function in alphaIIbbeta3 signaling in platelets. Blood. 1999;93(8):2645–2652. [PubMed] [Google Scholar]
- 24.Mócsai A, Zhou M, Meng F, Tybulewicz VL, Lowell CA. Syk is required for integrin signaling in neutrophils. Immunity. 2002;16(4):547–558. doi: 10.1016/s1074-7613(02)00303-5. [DOI] [PubMed] [Google Scholar]
- 25.Andre P, Delaney SM, LaRocca T, et al. P2Y12 regulates platelet adhesion/activation, thrombus growth, and thrombus stability in injured arteries. J Clin Invest. 2003;112(3):398–406. doi: 10.1172/JCI17864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hisamichi H, Kawazoe S, Naito R, et al. Synthetic studies on heteroaryl carboxamide derivatives as novel Syk inhibitors.. 226th ACS National Meeting; 2003; New York, NY. MEDI:068. [Google Scholar]
- 27.Reilly MP, Sinha U, Andre P, et al. PRT-060318, a novel Syk inhibitor, prevents heparin-induced thrombocytopenia and thrombosis in a transgenic mouse model. Blood. 2011;117(7):2241–2246. doi: 10.1182/blood-2010-03-274969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Prasad KS, Andre P, He M, Bao M, Manganello J, Phillips DR. Soluble CD40 ligand induces beta3 integrin tyrosine phosphorylation and triggers platelet activation by outside-in signaling. Proc Natl Acad Sci U S A. 2003;100(21):12367–12371. doi: 10.1073/pnas.2032886100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.André P, LaRocca T, Delaney SM, et al. Anticoagulants (thrombin inhibitors) and aspirin synergize with P2Y12 receptor antagonism in thrombosis. Circulation. 2003;108(21):2697–2703. doi: 10.1161/01.CIR.0000093279.36628.12. [DOI] [PubMed] [Google Scholar]
- 30.Falati S, Gross PL, Merrill-Skoloff G, et al. In vivo models of platelet function and thrombosis: study of real-time thrombus formation. Methods Mol Biol. 2004;272:187–197. doi: 10.1385/1-59259-782-3:187. [DOI] [PubMed] [Google Scholar]
- 31.Nieman MT, Warnock M, Hasan AA, et al. The preparation and characterization of novel peptide antagonists to thrombin and factor VIIa and activation of protease-activated receptor 1. J Pharmacol Exp Ther. 2004;311(2):492–501. doi: 10.1124/jpet.104.069229. [DOI] [PubMed] [Google Scholar]
- 32.Roque M, Fallon JT, Badimon JJ, Zhang WX, Taubman MB, Reis ED. Mouse model of femoral artery denudation injury associated with the rapid accumulation of adhesion molecules on the luminal surface and recruitment of neutrophils. Arterioscler Thromb Vasc Biol. 2000;20(2):335–342. doi: 10.1161/01.atv.20.2.335. [DOI] [PubMed] [Google Scholar]
- 33.Karnicki K, Leadley RJ, Jr, Baxi S, Peterson T, Wysokinski W, McBane RD., 2nd Comparison of PD0348292, a selective factor Xa inhibitor, to antiplatelet agents for the inhibition of arterial thrombosis. Thromb Haemost. 2008;99(4):759–766. doi: 10.1160/TH07-09-0576. [DOI] [PubMed] [Google Scholar]
- 34.Liu J, Joglekar M, Ware J, et al. Evaluation of the physiological significance of botrocetin/von Willebrand factor in vitro signaling. J Thromb Haemost. 2008;6(11):1915–1922. doi: 10.1111/j.1538-7836.2008.03135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee HM, Kim HJ, Park HJ, et al. Spleen tyrosine kinase participates in Src-mediated migration and proliferation by PDGF-BB in rat aortic smooth muscle cells. Arch Pharm Res. 2007;30(6):761–769. doi: 10.1007/BF02977640. [DOI] [PubMed] [Google Scholar]
- 36.Renné T, Pozgajova M, Gruner S, et al. Defective thrombus formation in mice lacking coagulation factor XII. J Exp Med. 2005;202(2):271–281. doi: 10.1084/jem.20050664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dubois C, Panicot-Dubois L, Merrill-Skoloff G, Furie B, Furie BC. Glycoprotein VI-dependent and -independent pathways of thrombus formation in vivo. Blood. 2006;107(10):3902–3906. doi: 10.1182/blood-2005-09-3687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Keely PJ, Parise LV. The alpha2beta1 integrin is a necessary co-receptor for collagen-induced activation of Syk and the subsequent phosphorylation of phospholipase Cgamma2 in platelets. J Biol Chem. 1996;271(43):26668–26676. [PubMed] [Google Scholar]
- 39.Clark EA, Shattil SJ, Ginsberg MH, Bolen J, Brugge JS. Regulation of the protein tyrosine kinase pp72syk by platelet agonists and the integrin alpha IIb beta 3. J Biol Chem. 1994;269(46):28859–28864. [PubMed] [Google Scholar]
- 40.Goto S, Tamura N, Ishida H, Ruggeri ZM. Dependence of platelet thrombus stability on sustained glycoprotein IIb/IIIa activation through adenosine 5′-diphosphate receptor stimulation and cyclic calcium signaling. J Am Coll Cardiol. 2006;47(1):155–162. doi: 10.1016/j.jacc.2005.08.055. [DOI] [PubMed] [Google Scholar]
- 41.Speich HE, Grgurevich S, Kueter TJ, Earhart AD, Slack SM, Jennings LK. Platelets undergo phosphorylation of Syk at Y525/526 and Y352 in response to pathophysiological shear stress. Am J Physiol Cell Physiol. 2008;295(4):C1045–1054. doi: 10.1152/ajpcell.90644.2007. [DOI] [PubMed] [Google Scholar]
- 42.Huo Y, Schober A, Forlow SB, et al. Circulating activated platelets exacerbate atherosclerosis in mice deficient in apolipoprotein E. Nat Med. 2003;9(1):61–67. doi: 10.1038/nm810. [DOI] [PubMed] [Google Scholar]
- 43.Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76(2):301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- 44.Plow EF, Zhang L. A MAC-1 attack: integrin functions directly challenged in knockout mice. J Clin Invest. 1997;99:1145–1146. doi: 10.1172/JCI119267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Diacovo TG, Roth SJ, Buccola JM, Bainton DF, Springer TA. Neutrophil rolling, arrest, and transmigration across activated, surface-adherent platelets via sequential action of P-selectin and the beta 2-integrin CD11b/CD18. Blood. 1996;88:146–157. [PubMed] [Google Scholar]
- 46.McEver RP, Cummings RD. Role of PSGL-1 binding to selectins in leukocyte recruitment. J Clin Invest. 1997;100:S97–S103. [PubMed] [Google Scholar]
- 47.Mangin P, Yap CL, Nonne C, et al. Thrombin overcomes the thrombosis defect associated with platelet GPVI/FcRgamma deficiency. Blood. 2006;107(11):4346–4353. doi: 10.1182/blood-2005-10-4244. [DOI] [PubMed] [Google Scholar]
- 48.Lloyd-Jones D, Adams R, Carnethon M, et al. Heart disease and stroke statistics–2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119(3):e21–181. doi: 10.1161/CIRCULATIONAHA.108.191261. [DOI] [PubMed] [Google Scholar]
- 49.Eikelboom JW, Mehta SR, Anand SS, Xie C, Fox KA, Yusuf S. Adverse impact of bleeding on prognosis in patients with acute coronary syndromes. Circulation. 2006;114(8):774–782. doi: 10.1161/CIRCULATIONAHA.106.612812. [DOI] [PubMed] [Google Scholar]
- 50.Sachs UJ, Nieswandt B. In vivo thrombus formation in murine models. Circ Res. 2007;100(7):979–991. doi: 10.1161/01.RES.0000261936.85776.5f. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.