Abstract
OBJECTIVE
We hypothesized that, despite increased activity, bone density would be low in athletes with amenorrhea, compared with athletes with eumenorrhea and control subjects, because of associated hypogonadism and would be associated with a decrease in bone formation and increases in bone-resorption markers.
METHODS
In a cross-sectional study, we examined bone-density measures (spine, hip, and whole body) and body composition by using dual-energy radiograph absorptiometry and assessed fasting levels of insulin-like growth factor I and bone-turnover markers (N-terminal propeptied of type 1 procollagen and N-telopeptide) in 21 athletes with amenorrhea, 18 athletes with eumenorrhea, and 18 control subjects. Subjects were 12 to 18 years of age and of comparable chronologic and bone age.
RESULTS
Athletes with amenorrhea had lower bone-density z scores at the spine and whole body, compared with athletes with eumenorrhea and control subjects, and lower hip z scores, compared with athletes with eumenorrhea. Lean mass did not differ between groups. However, athletes with amenorrhea had lower BMI z scores than did athletes with eumenorrhea and lower insulin-like growth factor I levels than did control subjects. Levels of both markers of bone turnover were lower in athletes with amenorrhea than in control subjects. BMI z scores, lean mass, insulin-like growth factor I levels, and diagnostic category were important independent predictors of bone mineral density z scores.
CONCLUSIONS
Although they showed no significant differences in lean mass, compared with athletes with eumenorrhea and control subjects, athletes with amenorrhea had lower bone density at the spine and whole body. Insulin-like growth factor I levels, body-composition parameters, and menstrual status were important predictors of bone density. Follow-up studies are necessary to determine whether amenorrhea in athletes adversely affects the rate of bone mass accrual and therefore peak bone mass.
Keywords: adolescents, athletes, bone density, bone-turnover markers, insulin-like growth factor I
Amenorrhea has been reported to occur in 23.5% of high school athletes,1 although the prevalence may vary depending on the type, intensity, and duration of exercise, as well as the athlete's nutritional status.1–4 In particular, activities such as gymnastics, running track, ballet, diving, swimming, and cheerleading are common among high school girls, require a thin physique, and are associated with the risk of amenorrhea. Running, cheerleading, and gymnastics also are associated with greater risk of stress fractures in adolescents.5 Low bone mineral density (BMD) has been reported for adult athletes with amenorrhea,6–14 particularly endurance athletes, but there are few data regarding bone metabolism in adolescent athletes with amenorrhea, particularly in comparison with nonathletic control subjects.
Maximal bone mass accrual occurs between 11 and 14 years of age in girls,15 with >90% of peak bone mass being achieved by the end of the second decade.15,16 The time to optimize BMD is thus early to middle adolescence, and insults occurring at this time may have severe consequences for peak bone mass. This is suggested by reports of greater bone mass deficits when amenorrhea in athletes develops in adolescence than when it occurs in adult life.17 Major contributors to the rapid increase in BMD in adolescents are the bone anabolic effects of increasing growth hormone and insulin-like growth factor I (IGF-I) levels in early to middle puberty and the antiresorptive effects of increasing estrogen levels. Adolescence is a high bone-turnover state, with increased levels of markers of both bone formation and bone resorption.18 Levels of these markers decrease to adult levels in late puberty. Bone-turnover marker levels have not been reported for adolescent athletes with amenorrhea.
Amenorrhea in athletes is associated with significant estrogen deficiency, and we hypothesize that this may affect bone mass severely when the disorder begins in early or middle adolescence. In adult athletes, strong associations between the severity of menstrual dysfunction and low BMD have been reported.11,19–21 Of importance, a permissive role of estrogen seems to be necessary for the bone anabolic effects of impact-loading exercise,22,23 which may be undone in a hypogonadal state. Therefore, BMD may not be preserved in athletes with amenorrhea. Predictors of low BMD have not been examined systematically in adolescent athletes with amenorrhea.
Disordered eating can occur in athletes, and Nichols et al1 reported disordered eating in 18.2% of high school athletes, compared with 13% of adolescents at large.24 Another study, however, reported a much higher prevalence (47%) of disordered eating in sports requiring a lean physique, with the prevalence decreasing to ~20% in sports not requiring leanness and among control subjects.25 Given this prevalence of disordered eating among athletes with amenorrhea,1,24–29 it is possible that alterations in body-composition and nutritional status also contribute to impaired bone metabolism. BMI and lean mass are body-composition measures that strongly predict BMD in adolescents,30 as do levels of IGF-I, a surrogate nutritional marker.31 Lean mass may or may not increase with endurance training32–34 and would affect bone accrual accordingly. IGF-I levels peak in middle puberty,35 with important bone anabolic effects, and low IGF-I levels at this time may have significant negative effects on bone metabolism. The contributions of changes in body composition and IGF-I levels to low BMD in adolescent athletes with amenorrhea have not been examined.
In this study, we determined BMD, levels of bone formation and bone-resorption markers, and predictors of BMD and bone-turnover markers in adolescent athletes with amenorrhea, athletes with eumenorrhea, and control subjects of comparable maturity. We hypothesized that, despite increased activity, BMD would be low in athletes with amenorrhea, compared with athletes with eumenorrhea and control subjects, because of associated hypogonadism and would be associated with a decrease in bone formation marker levels and an increase in bone-resorption marker levels. Given the important role of IGF-I and estrogen in optimizing bone density in adolescence, we hypothesized that low IGF-I levels and prolonged duration of amenorrhea would predict low BMD and low levels of bone-turnover markers.
METHODS
Subject Selection
Twenty-one adolescent athletes who met the criteria for diagnosis of amenorrhea, 18 adolescent athletes with eumenorrhea, and 18 control subjects were enrolled in the study. All subjects were 12 to 18 years of age, and athletes with amenorrhea and athletes with eumenorrhea were endurance athletes. Athletes with amenorrhea had a self-reported history of 1 of the following: (1) ≥4 hours of aerobic weight-bearing training of the legs weekly; (2) >30 miles of running weekly; or (3) >4 hours of specific endurance training weekly for a period of ≥6 months. These criteria were modified for adolescents from adult criteria.36 Athletes with amenorrhea had been amenorrheic for ≥3 consecutive cycles after initiation of menstruation36 and regular menstruation for ≥6 months or had not attained menarche by the age of 15.3 years (mean age at menarche + 2 SDs for girls in the United States).37 Athletes with eumenorrhea met the criteria for endurance athletes but did not have a history of amenorrhea or menarchal delay. Control subjects did not meet the criteria for endurance athletes and did not have a history of amenorrhea or menarchal delay. None of our subjects met Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, criteria for the diagnosis of anorexia nervosa or bulimia nervosa, on the basis of self-reports, reported history from their care providers, and interviews with our study psychiatrist. However, some form of disordered eating was noted for 15 athletes, of whom 13 were in the amenorrhea group and 2 were in the eumenorrhea group. Subjects receiving hormonal therapy or other medications known to affect bone metabolism, subjects with other conditions that could cause hypogonadism, and subjects with abnormal thyrotropin levels or elevated follicle-stimulating hormone levels (indicative of hypergonadotropic hypogonadism) were excluded from study participation. Subjects were recruited through advertisements in area newspapers and mailings to pediatricians, adolescent medicine physicians, nutritionists, and therapists in the New England area. The institutional review board of Partners Health Care approved the study. Informed assent and consent were obtained from all subjects and their parents, respectively.
Experimental Protocol
All subjects were evaluated during an outpatient visit to the General Clinical Research Center of Massachusetts General Hospital. We used a single Harpenden stadiometer to measure heights of subjects, using an average of 3 measurements. Weight was measured on a single electronic scale, with the subject in a hospital gown and in a fasting state. BMI was calculated by using the formula of [weight (in kilograms)]/[height (in meters)2]. Bone age was assessed by using the methods described by Greulich and Pyle38 and was determined by a single investigator (a pediatric endocrinologist), to minimize interobserver variation. In addition to obtaining a complete history of exercise from the study subjects, a modifiable activity questionnaire validated for use in adolescents39 was administered with the help of a General Clinical Research Center dietician, for the purpose of quantifying activity into a composite score for comparison across groups. However, this questionnaire does not take into account the intensity of activity or metabolic equivalents and therefore does not provide a measure of energy expenditure. To minimize underreporting and overreporting, subjects were assured that the contents of the questionnaire would not be made available to parents or care providers, unless specifically requested for medical reasons. General Clinical Research Center dieticians also administered a food frequency questionnaire to a subset of 14 athletes with amenorrhea, 14 athletes with eumenorrhea, and 15 control subjects, for assessment of daily calcium and vitamin D intake. Fasting levels were measured for IGF-I, N-telopeptide of type 1 collagen (a bone-resorption marker), and the N-terminal propeptide of type 1 procollagen (PINP) (a bone formation marker). We determined the duration of amenorrhea in postmenarchal athletes with amenorrhea as a surrogate marker of gonadal status. The mean duration of amenorrhea was 8.3 ± 6.7 months (range: 3–29 months).
Bone-Density and Body-Composition Measurements
Lumbar spine, hip, and whole-body (WB) BMD and bone mineral content (BMC) values and body-composition measures, including fat mass and lean mass, were measured by using dual-energy radiograph absorptiometry (4500A fan-beam densitometer, software version 11.2; Hologic, Waltham, MA); z scores were calculated by using reference databases available to Hologic.40 To correct for body size, bone mineral apparent density (BMAD) was calculated for the lumbar spine,41 and measures of WB BMC/height were examined. We used the approach described by Mølgaard et al,42 as modified by Ward et al43 for Hologic densitometers, to determine measures of BMC for bone area (BA) and BA for height. The coefficients of variation for lumbar spine and WB BMD were 1.1% and 0.8% and those for fat and lean mass were 2.1% and 1.0%, respectively.
Biochemical Measurements
An immunoradiometric assay (Diagnostic Systems Laboratories, Webster, TX) was used to measure IGF-I levels. The detection limit was 2.06 ng/mL, and the intraassay coefficient of variation was 3.9%. PINP levels were measured with a radioimmunoassay (Orion Diagnostica Oy, Espoo, Finland) with a detection limit of 2 μg/L and an intraassay coefficient of variation of 6.5% to 10.2%. Serum N-telopeptide levels were measured by using an enzyme immunoassay (Osteomark-Wampole Laboratories, Princeton, NJ) with a detection limit of 2.5 nmol of bone collagen equivalent and an intraassay coefficient of variation of 4.6%. Samples were stored at –80°C until analysis and were analyzed in duplicate.
Statistical Methods
Data are presented as mean ± SD and were analyzed by using JMP 4 (SAS Institute, Inc, Cary, NC). We used analysis of variance to determine differences between groups, followed by the Tukey-Kramer test to correct for multiple comparisons. Analysis of covariance was used to assess differences between diagnostic subtypes, controlling for BMI z scores. P values of <.05 were considered significant, and trends (P values between .05 and .10) also are reported. Univariate and mixed-model stepwise regression analyses (P = .15 to enter the model and P = .10 to leave the model) were used to determine predictors of bone-density and bone-turnover markers.
RESULTS
Baseline Characteristics
Athletes with amenorrhea did not differ from athletes with eumenorrhea and control subjects with respect to chronologic age or bone age (Table 1). Height and height z scores did not differ between groups. Athletes with amenorrhea had significantly lower BMI values, compared with athletes with eumenorrhea and control subjects, and lower BMI z scores, compared with athletes with eumenorrhea. Fat mass was lower in athletes with amenorrhea than in athletes with eumenorrhea and control subjects. Lean mass was higher in athletes with eumenorrhea than in athletes with amenorrhea and control subjects, but the differences were not statistically significant. Activity scores were greater for athletes with amenorrhea and athletes with eumenorrhea, compared with control subjects, as expected. IGF-I levels were lower in athletes with amenorrhea, compared with control subjects. Menarchal age trended higher in athletes with amenorrhea than in athletes with eumenorrhea and control subjects. Athletes with amenorrhea reported greater calcium and vitamin D intake than did athletes with eumenorrhea and control subjects, primarily through increased use of supplements. The proportion of girls with a history of fractures was greater for athletes with amenorrhea, but the difference did not reach statistical significance.
TABLE 1.
Baseline Characteristics of Athletes With Amenorrhea, Athletes With Eumenorrhea, and Control Subjects
| Athletes With Amenorrhea (N = 21) | Athletes With Eumenorrhea (N = 18) | Control Subjects (N = 18) | P | |
|---|---|---|---|---|
| Age, mean ± SD, y | 16.1 ± 1.5 | 15.6 ± 1.4 | 15.5 ± 1.4 | NS |
| Bone age, mean ± SD, y | 16.0 ± 1.0 | 16.0 ± 1.3 | 15.6 ± 1.6 | NS |
| BMI, mean ± SD, kg/m2 | 19.1 ± 1.3a,b | 21.9 ± 3.2 | 20.7 ± 2.5 | .003 |
| BMI z score, mean ± SD | –0.61 ± 0.29b | 0.16 ± 0.76 | –0.14 ± 0.57 | .0003 |
| Weight, mean ± SD, kg | 50.6 ± 4.3b | 58.1 ± 8.0 | 55.4 ± 7.0 | .003 |
| Height, mean ± SD, cm | 163.0 ± 5.8 | 163.0 ± 5.9 | 163.7 ± 5.6 | NS |
| Height z score, mean ± SD | 0.12 ± 0.89 | 0.14 ± 0.99 | 0.27 ± 0.90 | NS |
| Fat mass, mean ± SD, kg | 11.2 ± 2.7a,b | 16.2 ± 4.6 | 15.1 ± 5.3 | .001 |
| Lean mass, mean ± SD, kg | 39.2 ± 4.3 | 41.8 ± 5.3 | 39.7 ± 3.6 | NS |
| Activity score, mean ± SD | 23.8 ± 6.6a | 28.1 ± 10.3a | 9.9 ± 4.5 | <.0001 |
| IGF-I level, mean ± SD, ng/mL | 498.9 ± 210.1a | 612.2 ± 168.9 | 692.1 ± 208.9 | .02 |
| Age at menarche, mean ± SD, y | 12.9 ± 1.5 | 12.1 ± 0.8 | 12.2 ± 1.0 | .09 |
| Daily calcium intake, mean ± SD, mg | 1838 ± 995a,b | 839 ± 432 | 893 ± 326 | .0002 |
| Daily vitamin D intake, mean ± SD, μg | 7.94 ± 5.66a,b | 3.82 ± 2.69 | 4.40 ± 2.49 | .01 |
| Fractures, % | 23.8 | 5.3 | 16.7 | NS |
NS indicates not significant.
P < .05, compared with control subjects (Tukey-Kramer test to adjust for multiple comparisons).
P < .05, compared with athletes with eumenorrhea.
Bone-Density Measures and Bone-Turnover Markers
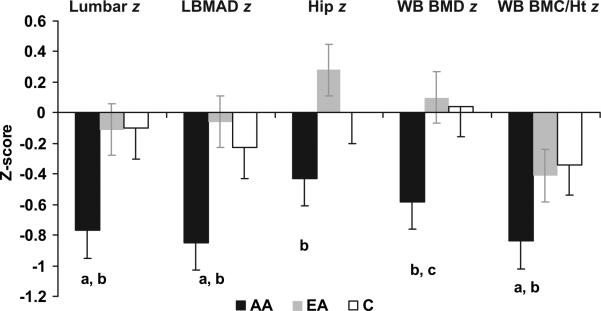
Athletes with amenorrhea had significantly lower lumbar BMD and BMAD z scores, compared with athletes with eumenorrhea and control subjects (Table 2 and Fig 1). Lumbar BMD z scores below –1 were observed for 38% of athletes with amenorrhea, compared with 11% of athletes with eumenorrhea and 11% of control subjects (P = .05). Two athletes with amenorrhea but none of the athletes with eumenorrhea or control subjects had lumbar BMD z scores below –2. Lumbar BMC for BA was lower in athletes with amenorrhea than in athletes with eumenorrhea. Hip BMD and hip BMD z score were significantly lower in athletes with amenorrhea than in athletes with eumenorrhea. Hip z scores below –1 were observed for 19% of athletes with amenorrhea, compared with 5.6% of athletes with eumenorrhea and 11% of control subjects (not significant). WB BMC/height was lower in athletes with amenorrhea than in athletes with eumenorrhea or control subjects, and the corresponding z score was lower in athletes with amenorrhea than in control subjects. WB BMC/height z scores below –1 were observed for 28.6% athletes with amenorrhea, compared with 16.7% of athletes with eumenorrhea and 11.1% of control subjects (not significant). WB BA for height was lower in athletes with amenorrhea than in athletes with eumenorrhea and trended lower in comparison with control subjects. None of our subjects had hip or WB BMC/height z scores below –2. In an analysis of covariance controlling for BMI z scores, hip BMD, WB BMD, and WB BMC/height and corresponding z scores remained lower in athletes with amenorrhea, compared with athletes with eumenorrhea (P < .05), and lumbar BMD z scores trended lower (P = .08). Overall, bone-density measures were higher in athletes with eumenorrhea than in control subjects, but these differences did not reach statistical significance. Levels of the bone formation marker PINP and the bone-resorption marker N-telopeptide were significantly lower in athletes with amenorrhea, compared with control subjects.
TABLE 2.
Bone-Density Measures for Athletes With Amenorrhea, Athletes With Eumenorrhea, and Control Subjects
| Athletes With Amenorrhea (N = 21) | Athletes With Eumenorrhea (N = 18) | Control Subjects (N = 18) | P | |
|---|---|---|---|---|
| Lumbar BMD, mean ± SD, g/cm2 | 0.90 ± 0.11a | 0.99 ± 0.09 | 0.96 ± 0.09 | .01 |
| Lumbar BMD z score, mean ± SD | –0.76 ± 0.85a,b | –0.11 ± 0.87 | –0.10 ± 0.65 | .02 |
| Lumbar BMAD, mean ± SD, g/cm3 | 0.143 ± 0.015c | 0.153 ± 0.014 | 0.154 ± 0.017 | .045 |
| Lumbar BMAD z score, mean ± SD | –0.85 ± 0.80a,b | –0.06 ± 0.84 | –0.23 ± 0.96 | .02 |
| Lumbar BA for height z score, mean ± SD | –0.08 ± 0.88 | 0.08 ± 1.10 | 0.16 ± 0.97 | NS |
| Lumbar BMC for BA z score, mean ± SD | 0.16 ± 0.67a | 0.86 ± 0.84 | 0.54 ± 0.91 | .03 |
| Hip BMD, mean ± SD, g/cm2 | 0.89 ± 0.10a | 1.00 ± 0.10 | 0.96 ± 0.09 | .004 |
| Hip BMD z score, mean ± SD | –0.43 ± 0.75a | 0.28 ± 1.0 | –0.00 ± 0.80 | .04 |
| WB BMD, mean ± SD, g/cm2 | 0.98 ± 0.07a,c | 1.05 ± 0.08 | 1.03 ± 0.07 | .01 |
| WB BMD z score, mean ± SD | –0.58 ± 0.72a,c | 0.10 ± 1.00 | 0.04 ± 0.74 | .02 |
| WB BMC/height, mean ± SD, g/cm | 10.97 ± 1.35a,b | 12.33 ± 1.17 | 12.01 ± 1.17 | .003 |
| WB BMC/height z score, mean ± SD | –0.79 ± 0.54b | –0.41 ± 0.71 | –0.34 ± 0.50 | .04 |
| WB BA for height z score, mean ± SD | –0.06 ± 0.97a,c | 0.74 ± 0.94a | 0.60 ± 0.86 | .02 |
| WB BMC for BA z score, mean ± SD | –0.44 ± 1.14 | 0.34 ± 1.25 | –0.15 ± 1.13 | NS |
| PINP level, mean ± SD, μg/L | 102.7 ± 69.7b | 135.6 ± 73.7 | 205.6 ± 143.9 | .01 |
| N-telopeptide level, mean ± SD, nmol of bone collagen equivalent | 139.4 ± 119.8b | 173.2 ± 128.1 | 295.7 ± 293.1 | .05 |
NS indicates not significant.
P < .05, compared with athletes with eumenorrhea (Tukey-Kramer test to adjust for multiple comparisons).
P < .05, compared with control subjects.
P < .1, compared with control subjects.
FIGURE 1.
Bone-density measures in athletes with amenorrhea (AA), athletes with eumenorrhea (EA), and control subjects (C) (mean ± SEM). Bone-density z scores for the lumbar spine and WB BMC/height (BMC/Ht) were lower in athletes with amenorrhea than in athletes with eumenorrhea and control subjects. Hip bone density was lower in athletes with amenorrhea than in athletes with eumenorrhea. a P < .05, compared with control subjects; b P < .05, compared with athletes with eumenorrhea; c P < .1, compared with control subjects.
In a subset analysis, we excluded the 5 athletes with amenorrhea with the lowest BMI values and the 5 athletes with eumenorrhea with the highest BMI values in their respective groups, to obtain amenorrhea (n = 16) and eumenorrhea (n = 13) groups that did not differ in BMI z scores (–0.50 ± 0.23 for athletes with amenorrhea and –0.28 ± 0.43 for athletes with eumenorrhea; not significant). The groups also did not differ with respect to activity scores, age, or IGF-I levels. The primary difference between the groups was therefore the presence or absence of amenorrhea or hypogonadism. Similar to findings for the larger group, athletes with amenorrhea in this subset had lower hip BMD, WB BMD, and WB BMC/height and corresponding z scores than did athletes with eumenorrhea (P < .05). Lumbar BMD trended lower in athletes with amenorrhea, compared with athletes with eumenorrhea (P = .08), in this subset analysis. We also compared athletes with amenorrhea with a history of disordered eating (n = 13) with athletes with amenorrhea without a history of disordered eating (n = 8), and we found no differences in bone-density measures between the subgroups.
Predictors of Bone-Density Measures and Bone-Turnover Markers
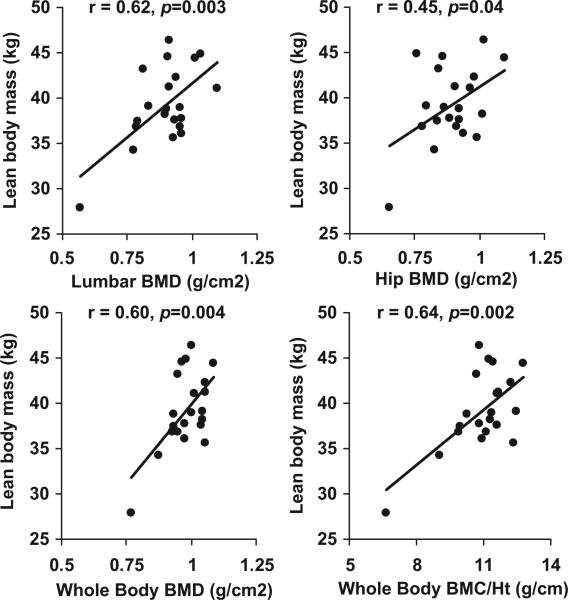
Simple correlation analyses indicating associations of bone-density measures and markers of bone turnover with various covariates are shown in Table 3, both for the group as a whole and for athletes with amenorrhea. Lean body mass was an important predictor of almost all bone-density measures, both for the group as a whole and for athletes with amenorrhea considered separately (Fig 2), whereas BMI z scores predicted bone-density measures for the group as a whole but not for athletes with amenorrhea. IGF-I levels predicted lumbar spine bone-density measures and PINP levels. Bone age was a predictor of lumbar and WB BMD and of levels of bone-turnover markers. Bone age was a stronger predictor than chronologic age for bone-density measures, and only correlations with the former are reported. Bone-turnover markers were also predicted by lean mass in athletes with amenorrhea. No correlations between bone-density measures and duration of amenorrhea, age at menarche, activity scores, or vitamin D or calcium intake were observed.
TABLE 3.
Correlation Coefficients for Associations of Bone-Density Measures With Nutritional Markers, Body-Composition Parameters, and Bone Age for All Subjects and for Athletes With Amenorrhea
| Correlation Coefficient |
||||
|---|---|---|---|---|
| Bone Age | Lean Mass | BMI z Score | IGF-I Level | |
| All subjects | ||||
| Lumbar BMD | 0.40a | 0.45b | 0.46b | 0.15 |
| Lumbar BMD z score | 0.05 | 0.38a | 0.37a | 0.23c |
| Lumbar BMAD | 0.37a | 0.17 | 0.42b | 0.25c |
| Lumbar BMAD z score | 0.25c | 0.13 | 0.46b | 0.28d |
| Hip BMD | 0.11 | 0.46b | 0.30d | 0.13 |
| Hip BMD z score | 0.11 | 0.45b | 0.26d | 0.08 |
| WB BMD | 0.40a | 0.49e | 0.27d | 0.07 |
| WB BMD z score | 0.16 | 0.45b | 0.17 | 0.11 |
| WB BMC/height | 0.39a | 0.60e | 0.39a | 0.08 |
| WB BMC/height z score | 0.07 | 0.50e | 0.26d | 0.02 |
| PINP level | –0.82e | –0.16 | –0.11 | 0.30d |
| N-telopeptide level | –0.69e | –0.02 | 0.03 | –0.02 |
| Athletes with amenorrhea | ||||
| Lumbar BMD | 0.55d | 0.62a | 0.06 | 0.39c |
| Lumbar BMD z score | 0.18 | 0.40c | 0.08 | 0.40c |
| Lumbar BMAD | 0.40 | 0.40c | 0.19 | 0.51d |
| Lumbar BMAD z score | 0.11 | 0.26 | 0.19 | 0.53d |
| Hip BMD | 0.31 | 0.45d | –0.37c | 0.35 |
| Hip BMD z score | 0.08 | 0.33 | –0.43d | 0.36 |
| WB BMD | 0.65d | 0.60a | –0.12 | 0.25 |
| WB BMD Z–score | 0.36 | 0.42c | –0.15 | 0.27 |
| WB BMC/height | 0.64d | 0.64a | 0.02 | 0.30 |
| WB BMC/height z score | 0.11 | 0.42c | –0.04 | 0.25 |
| PINP level | –0.84b | –0.46d | 0.09 | 0.17 |
| N-telopeptide level | –0.64d | –0.57a | –0.20 | –0.31 |
P < .01.
P < .001.
P < .1.
P < .05.
P < .0001.
FIGURE 2.
Associations between lean body mass and bone-density measures in athletes with amenorrhea. Lean body mass correlated positively with lumbar spine (r = 0.62; P = .003), hip (r = 0.45; P =. 04), and WB (r = 0.60; P = .004) BMD and with WB BMC/height (r = 0.64; P = .002).
Regression Modeling
Bone-density measures were entered into a regression model that included significant predictors of bone density, namely, diagnosis subtype, bone age, BMI z scores, lean mass, and IGF-I levels (Table 4). Lean mass and diagnostic subtype were the most significant predictors of lumbar, hip, and WB BMD measures, as well as the corresponding z scores. Bone age remained a significant predictor of lumbar and WB BMD values, lumbar BMAD, and WB BMC/height but not of the corresponding z scores. BMI z scores and IGF-I levels were independent predictors of lumbar BMAD measures. Bone age was the only significant predictor of PINP levels in regression modeling (r2 = 0.67; P < .0001). N-telopeptide levels were predicted by bone age (P < .0001), IGF-I levels (P = .03), and BMI z scores (P = .06); (cumulative r 2 = 0.56).
TABLE 4.
Regression Modeling With Covariates Including Diagnostic Category, Bone Age, BMI z Score, Lean Mass, Activity Score, and IGF-I Levels
| Parameter Estimate | P | Variability Explained, % | |
|---|---|---|---|
| Lumbar BMD | |||
| Lean body mass | 8.33 × 10–3 | .002 | 42.2 |
| Bone age | 0.024 | .004 | |
| Diagnostic category (athletes with amenorrhea vs athletes with eumenorrhea and control subjects) | –0.037 | .004 | |
| Lumbar BMD z score | |||
| Lean body mass | 6.84 × 10–2 | .004 | 26.2 |
| Diagnostic category (athletes with amenorrhea vs athletes with eumenorrhea and control subjects) | –0.245 | .02 | |
| Lumbar BMAD | |||
| BMI z score | 0.008 | .01 | 27.5 |
| Bone age | 0.003 | .03 | |
| IGF-I level | 2.00 × 10–5 | .04 | |
| Lumbar BMAD z score | |||
| BMI z score | 0.595 | .002 | 24.4 |
| IGF-I level | 9.26 × 10–4 | .09 | |
| Hip BMD | |||
| Lean body mass | 9.57 × 10–3 | .001 | 32.5 |
| Diagnostic category (athletes with amenorrhea and control subjects vs athletes with eumenorrhea) | –0.034 | .01 | |
| Hip BMD z score | |||
| Lean body mass | 7.67 × 10–2 | .001 | 30.0 |
| Diagnostic category (athletes with amenorrhea and control subjects vs athletes with eumenorrhea) | –0.237 | .03 | |
| WB BMD | |||
| Lean body mass | 6.72 × 10–3 | .001 | 42.8 |
| Diagnostic category (athletes with amenorrhea vs athletes with eumenorrhea and control subjects) | –0.027 | .005 | |
| Bone age | 0.165 | .007 | |
| WB BMD z score | |||
| Lean body mass | 8.12 × 10–2 | .0007 | 29.7 |
| Diagnostic category (athletes with amenorrhea vs athletes with eumenorrhea and control subjects) | –0.227 | .04 | |
| WB BMC/height | |||
| Lean body mass | 0.149 | .0001 | 54.5 |
| Diagnostic category (athletes with amenorrhea vs athletes with eumenorrhea and control subjects) | –0.503 | .0007 | |
| Bone age | 0.250 | .009 | |
| WB BMC/height z score | |||
| Lean body mass | 5.95 × 10–2 | .0001 | 35.7 |
| Diagnostic category (athletes with amenorrhea vs athletes with eumenorrhea and control subjects) | –0.153 | .03 |
There were no linear correlations of activity scores, duration of amenorrhea, or age at menarche with bone-density measures; however, when these parameters were added to the regression model described above for athletes with amenorrhea, duration of amenorrhea was observed to be a significant inverse predictor of lumbar BMD and BMAD and their corresponding z scores and of WB BMD and BMC/height. Age at menarche was a significant predictor of hip BMD and its z score.
DISCUSSION
We demonstrated lower bone-density measures at the spine and for the WB in adolescent athletes with amenorrhea, compared with athletes with eumenorrhea and control subjects, and lower bone density at the hip, compared with athletes with eumenorrhea. Our findings raise concerns regarding the deleterious effects of a hypogonadal state on bone metabolism, despite the known beneficial effects of exercise, particularly high-impact load exercises44 and weight-bearing exercises,45 on bone mass and geometric characteristics in adolescents. Lower bone density in athletes with amenorrhea was associated with lower levels of both bone formation and bone-resorption markers, indicating a state of reduced bone turnover. Important independent predictors of bone-density measures included body-composition parameters such as lean mass and BMI z scores, levels of IGF-I (a surrogate marker of nutritional status), and diagnostic category (athletes with amenorrhea, athletes with eumenorrhea, or control subjects). For athletes with amenorrhea, duration of amenorrhea was an independent inverse predictor of bone density, controlling for IGF-I levels, body composition, and other measures.
Although studies have examined bone metabolism in adult athletes,6–14 few studies have addressed this question in adolescent athletes, especially in comparison with a group of nonathletic control subjects. Because as many as 23.5% of high school athletes may have menstrual irregularities1 and because the adolescent years are a critical time for bone mass accrual,15 these data are important to obtain. Our data indicated that, whereas athletes with normal menstrual function have preservation of bone mass and may have slightly higher bone mass, compared with nonathletic control subjects, loss of menses in athletes is associated with lower bone-density measures. Athletes with amenorrhea had lower bone density than did athletes with eumenorrhea and control subjects, controlling for levels of IGF-I (a surrogate marker of nutritional status), body composition, and bone age in a regression model. Duration of amenorrhea did not correlate with BMD in athletes with amenorrhea in a simple correlational analysis. It is possible that our study was underpowered to detect this association or that this measure does not capture the extent of gonadal dysfunction in athletes with amenorrhea. Of importance, we measured the duration of amenorrhea since the last menstrual period and not the lifetime duration of oligomenorrhea/amenorrhea, which might have been more predictive of bone-density measures. When duration of amenorrhea was added to a regression model for athletes with amenorrhea that included BMI z scores, lean mass, bone age, IGF-I levels, activity scores, and menarchal age, it did significantly predict several measures of bone density in the larger regression model. These data suggest complex interactions between duration of amenorrhea and other covariates predicting bone density in athletes with amenorrhea.
We did not measure estradiol levels in this study, and the degree of hypoestrogenism may predict bone-density measures. However, because of the great variability in estradiol levels across the menstrual cycle, these levels may not be helpful, as evidenced by our studies with adolescent girls with anorexia nervosa.30 In those studies, we compared estradiol levels measured randomly in anorexia nervosa with estradiol levels measured in the early follicular phase in healthy control subjects, and we did not find an association between estradiol levels and bone-density measures.30 Nevertheless, the contribution of hypogonadism to low bone density in athletes with amenorrhea is indicated by (1) data from our regression models, as discussed above, and (2) greater deficits in bone-density measures in athletes with amenorrhea, compared with athletes with eumenorrhea, in our subset analysis of 16 athletes with amenorrhea and 13 athletes with eumenorrhea for whom BMI z scores, activity levels, and age did not differ and the only difference was the presence or absence of amenorrhea.
Athletes with amenorrhea have lower bone density not only in comparison with athletes with eumenorrhea but also in comparison with nonathletic control subjects with normal menstruation. Therefore, in addition to the beneficial effects of exercise on bone density being lost in girls who develop amenorrhea, amenorrhea may actually be deleterious to bone health. Nichols et al1 reported lower bone density at the femoral trochanter, although not at the spine, in athletes with amenorrhea, compared with athletes with eumenorrhea, but those authors did not compare the groups with nonathletic control subjects. Our data, in contrast, indicate lower bone density at the spine and in the WB in adolescent athletes with amenorrhea, compared with athletes with eumenorrhea and control subjects. Our study also raises concerns regarding the rate of bone mass accrual and subsequent peak bone mass in adolescent athletes with amenorrhea. Studies examining bone mass accrual over time in athletes are lacking and are important for determining whether peak bone mass is affected in these adolescents.
Important predictors of bone density other than amenorrhea included nutritional measures such as IGF-I levels and body-composition parameters such as lean mass and BMI z scores. Associations of BMI and lean mass with bone density are consistent with findings in other studies with athletes46 and other populations.30 Although subjects in our study were within a normal range for BMI, athletes with amenorrhea had lower BMI z scores than athletes with eumenorrhea and lower IGF-I levels than control subjects. Lean mass was an important predictor of bone density in athletes with amenorrhea and in the group as a whole. Although lean mass in athletes with eumenorrhea was somewhat greater than that in athletes with amenorrhea and control subjects, these differences were not statistically significant and, despite preservation of lean mass, athletes with amenorrhea had lower bone density, compared with athletes with eumenorrhea and control subjects. For athletes with amenorrhea, the relatively lower nutritional status, as evidenced by low IGF-I levels, and the state of hypogonadism likely contributed to lower bone density despite preservation of lean mass. Vitamin D intake and calcium intake were higher in athletes with amenorrhea, compared with athletes with eumenorrhea and control subjects, primarily through increased use of supplements; this likely reflects concerns of care providers regarding bone density and therefore increased prescription of these supplements for girls with amenorrhea. Of note, high cortisol levels have been reported in some studies of adult athletes with amenorrhea in comparison with athletes with eumenorrhea,47 although not in all studies,48 and cortisol has known deleterious effects on bone. Although we did not measure cortisol levels for our subjects, this would be important to examine in future studies.
The approach described by Mølgaard et al42 differentiates between reported low bone density resulting from short bones (based on height z scores) and that resulting from “thin” bones (based on measures of BA for height), and “light” bones (based on measures of BMC for BA). Whereas short bones are not necessarily at greater risk of fractures, thin bones (low BA for height) and light bones (low BMC for BA) have impaired strength. Athletes with amenorrhea had lower BMC for BA at the spine and lower WB BA for height than did athletes with eumenorrhea, although values did not differ from those in control subjects. Similar to other measures of bone density, beneficial effects of exercise on these measures seem to be lost in athletes who stop menstruating. Although athletes with amenorrhea did report more fractures than athletes with eumenorrhea, this difference was not statistically significant, and larger numbers of subjects are required to demonstrate whether fracture risk is increased in athletes with amenorrhea. Given that bone-density z scores were less than –2 in only 2 of our subjects with amenorrhea, it is unlikely that the bone-density measures we report in this study were low enough to cause increased fracture risk.
Markers of bone turnover were decreased in athletes with amenorrhea, compared with control subjects. This is in contrast to what may be expected, given the state of hypogonadism in athletes with amenorrhea. Estrogen inhibits bone resorption,49,50 and deficiency of estrogen, as in athletes with amenorrhea, would be expected to be associated with increased bone resorption. Overall, bone age was the strongest predictor of bone-turnover markers, and girls with greater bone age (and therefore less residual growth potential) had lower levels of bone-turnover markers. This is consistent with the fact that bone-turnover decreases in late adolescence, when little growth potential remains. Levels of IGF-I also predicted N-telopeptide levels, and the suboptimal nutritional state in athletes with amenorrhea seems to result in reduced bone turnover overall. In athletes with eumenorrhea, levels of bone-turnover markers were intermediate between those in control subjects and those in athletes with amenorrhea but did not differ significantly from either. This finding is unexpected, given that bone density was slightly greater in athletes with eumenorrhea than in the other 2 groups, leading to expectations of higher levels of bone formation markers and lower levels of bone-resorption markers in athletes with eumenorrhea, compared with both control subjects and athletes with amenorrhea. Somewhat lower levels of bone-turnover markers in athletes with eumenorrhea than in control subjects may be related to the somewhat lower IGF-I levels in athletes with eumenorrhea. However, these data need to be confirmed in future studies.
It is important to determine the factors that lead to hypogonadism in some but not all athletes. One possible contributor is the lower fat mass in athletes with amenorrhea, compared with athletes with eumenorrhea and control subjects, and we showed in other studies that fat mass is an important predictor of menstrual function.51 Importantly, a negative state of energy balance caused by nutrient intake that is inadequate to balance the expenditure associated with exercise (rather than the stress of exercise) is thought to be instrumental in the development of hypogonadotropic hypogonadism in athletes.52,53
A limitation of our study is the lack of details regarding nutritional intake and daily energy expenditure in our subjects. Although IGF-I levels and composite activity scores did not differ between athletes with amenorrhea and athletes with eumenorrhea, a complete assessment of energy intake and energy expenditure would have enabled us to determine whether athletes with amenorrhea were in a negative state of energy balance, accounting for their hypogonadotropic state. On the basis of the lower BMI and fat mass but equivalent activity scores in this group, compared with athletes with eumenorrhea, dietary intake in athletes with amenorrhea would be expected to be significantly lower than that in athletes with eumenorrhea, consistent with the greater frequency of disordered eating observed for athletes with amenorrhea. In addition, although screening questions ensured that athletes with amenorrhea and athletes with eumenorrhea met inclusion criteria for endurance athletes, the activity scores derived from the exercise questionnaire we used in this study did not take into account differences in types of exercise.54 Questionnaires that provide a composite measure of average daily energy expenditure, based on the nature of exercise and assigned metabolic equivalents, should be useful in this regard.55 Another limitation is that we concentrated on IGF-I levels and the hypogonadal state as possible predictors of bone metabolism in this study of adolescent athletes with amenorrhea, athletes with eumenorrhea, and control subjects, given the critical roles of IGF-I and estrogen in increasing bone density in adolescents. In future studies, it will be important to measure levels of other hormones known to affect bone density, such as 24-hour urinary free cortisol levels, thyroid hormone levels, and levels of some of the appetite-regulating peptides. It will also be important to obtain history regarding lifetime duration of oligomenorrhea, rather than just duration of amenorrhea, as an indicator of gonadal status. Finally, there is a possibility of referral bias in studies such as this, in which girls at some risk for low bone density may be more likely to be referred for assessment of bone density.
CONCLUSIONS
Our data indicate that amenorrhea in athletes negates the beneficial effects of weight-bearing exercise on bone mass and may in fact have deleterious effects on bone. This emphasizes the importance of optimizing nutritional and menstrual status in athletes. We demonstrate comprehensively the significant alterations in bone metabolism that occur in adolescent athletes with amenorrhea, compared with athletes with eumenorrhea and control subjects.
What's Known on This Subject.
Adult athletes with amenorrhea have low bone density, compared with athletes with eumenorrhea, and low bone density is predicted by the duration of amenorrhea and kind of athletic activity. Bone density is protected in athletes with eumenorrhea.
What This Study Adds.
This study demonstrates that adolescent endurance athletes with amenorrhea have lower bone density than athletes with eumenorrhea and nonathletic control subjects of similar age. Bone density is predicted by lean mass, BMI, IGF-I level, duration of amenorrhea, and bone age.
GEORGIA: CHILDREN PLOTTED TO HARM TEACHER, POLICE SAY.
“A group of third graders plotted to attack their teacher, bringing a broken steak knife, handcuffs, duct tape and other items for the job and assigning children tasks including covering the windows and cleaning up afterward, the police said. The plot by as many as nine boys and girls at Center Elementary School was a serious threat, said the Waycross police chief, Tony Tanner. ‘We did not hear anybody say they intended to kill her, but could they have accidentally killed her? Absolutely,’ Chief Tanner said. The children, ages 8 and 9, apparently were mad at the teacher after she scolded one of them for standing on a chair, he said. A prosecutor said they were too young to be charged with a crime. Nine children have been disciplined up to and including long-term suspension, said a spokeswoman for the Ware County school system.”
Associated Press. New York Times. April 2, 2008
Editor's note: Where do you think these children learned how to plot this crime? Could it have been on TV?
Noted by JFL, MD
ACKNOWLEDGMENTS
This work was supported in part by National Institutes of Health grants R01 DK062249, K23 RR018851, and M01 RR01066.
We thank the skilled nurses of the General Clinical Research Center of Massachusetts General Hospital and Ellen Anderson and the bionutrition team for help with carrying out this study. We also thank Rita Tsay and her team at the Clinical Research Center of Massachusetts Institute of Technology for performing the dual-energy radiograph absorptiometry scans and Jeff Breu at Massachusetts Institute of Technology for running our as-says. Finally, we thank our subjects, without whom this study would not have been possible.
Abbreviations
- WB
whole-body
- BA
bone area
- IGF-I
insulin-like growth factor I
- BMD
bone mineral density
- BMC
bone mineral content
- PINP
N-terminal propeptide of type 1 procollagen
- BMAD
bone mineral apparent density
Footnotes
The authors have indicated they have no financial relationships relevant to this article to disclose.
Drs Christo and Prabhakaran contributed equally to this work.
REFERENCES
- 1.Nichols JF, Rauh MJ, Lawson MJ, Ji M, Barkai HS. Prevalence of the female athlete triad syndrome among high school athletes. Arch Pediatr Adolesc Med. 2006;160(2):137–142. doi: 10.1001/archpedi.160.2.137. [DOI] [PubMed] [Google Scholar]
- 2.Loucks A, Horvath S. Athletic amenorrhea: a review. Med Sci Sports Exerc. 1985;17(1):56–72. [PubMed] [Google Scholar]
- 3.Otis C. Exercise-associated amenorrhea. Clin Sports Med. 1992;11(2):351–362. [PubMed] [Google Scholar]
- 4.Shangold M, Rebar R, Wentz A, Schiff I. Evaluation and management of menstrual dysfunction in athletes. JAMA. 1990;263(12):1665–1669. [PubMed] [Google Scholar]
- 5.Loud KJ, Gordon CM, Micheli LJ, Field AE. Correlates of stress fractures among preadolescent and adolescent girls. Pediatrics. 2005;(4):115. doi: 10.1542/peds.2004-1868. Available at: www.pediatrics.org/cgi/content/full/115/4/e399. [DOI] [PubMed]
- 6.Snow C, Rosen C, Robinson T. Serum IGF-I is higher in gymnasts than runners and predicts bone and lean mass. Med Sci Sports Exerc. 2000;32(11):1902–1907. doi: 10.1097/00005768-200011000-00013. [DOI] [PubMed] [Google Scholar]
- 7.Pettersson U, Stalnacke B, Ahlenius G, Henriksson-Larsen K, Lorentzon R. Low bone mass density at multiple skeletal sites, including the appendicular skeleton in amenorrheic runners. Calcif Tissue Int. 1999;64(2):117–125. doi: 10.1007/s002239900589. [DOI] [PubMed] [Google Scholar]
- 8.Cobb K, Bachrach L, Greendale G, et al. Disordered eating, menstrual irregularity, and bone mineral density in female runners. Med Sci Sports Exerc. 2003;35(5):711–719. doi: 10.1249/01.MSS.0000064935.68277.E7. [DOI] [PubMed] [Google Scholar]
- 9.Gremion G, Rizzoli R, Slosman D, Theintz G, Bonjour J. Oligo-amenorrheic long-distance runners may lose more bone in spine than in femur. Med Sci Sports Exerc. 2001;33(1):15–21. doi: 10.1097/00005768-200101000-00004. [DOI] [PubMed] [Google Scholar]
- 10.Grinspoon S, Miller K, Coyle C, et al. Severity of osteopenia in estrogen-deficient women with anorexia nervosa and hypothalamic amenorrhea. J Clin Endocrinol Metab. 1999;84(6):2049–2055. doi: 10.1210/jcem.84.6.5792. [DOI] [PubMed] [Google Scholar]
- 11.Rencken M, Chesnut CI, Drinkwater B. Bone density at multiple skeletal sites in amenorrheic athletes. JAMA. 1996;276(3):238–240. [PubMed] [Google Scholar]
- 12.Myburgh H, Hutchins J, Fataar A, Hough S, Noakes T. Low bone mineral density is an etiologic factor for stress fractures in athletes. Ann Intern Med. 1990;113(10):754–759. doi: 10.7326/0003-4819-113-10-754. [DOI] [PubMed] [Google Scholar]
- 13.Snead DB, Weltman A, Weltman JY, et al. Reproductive hormones and bone mineral density in women runners. J Appl Physiol. 1992;72(6):2149–2156. doi: 10.1152/jappl.1992.72.6.2149. [DOI] [PubMed] [Google Scholar]
- 14.Myburgh K, Bachrach L, Lewis B, Kent K, Marcus R. Low bone mineral density at axial and appendicular sites in amenorrheic athletes. Med Sci Sports Exerc. 1993;25(11):1197–1202. [PubMed] [Google Scholar]
- 15.Theintz G, Buchs B, Rizzoli R, et al. Longitudinal monitoring of bone mass accumulation in healthy adolescents: evidence for a marked reduction after 16 years of age at the levels of lumbar spine and femoral neck in female subjects. J Clin Endocrinol Metab. 1992;75(4):1060–1065. doi: 10.1210/jcem.75.4.1400871. [DOI] [PubMed] [Google Scholar]
- 16.Bachrach L. Acquisition of optimal bone mass in childhood and adolescence. Trends Endocrinol Metab. 2001;12(1):22–28. doi: 10.1016/s1043-2760(00)00336-2. [DOI] [PubMed] [Google Scholar]
- 17.Keen A, Drinkwater B. Irreversible bone loss in former amenorrheic athletes. Osteoporos Int. 1997;7(4):311–315. doi: 10.1007/BF01623770. [DOI] [PubMed] [Google Scholar]
- 18.Mora S, Pitukcheewanont P, Kaufman F, Nelson J, Gilsanz V. Biochemical markers of bone turnover and the volume and the density of bone in children at different stages of sexual development. J Bone Miner Res. 1999;14(10):1664–1671. doi: 10.1359/jbmr.1999.14.10.1664. [DOI] [PubMed] [Google Scholar]
- 19.Drinkwater B, Bruemner B, Chesnut CI. Menstrual history as a determinant of current bone density in young athletes. JAMA. 1990;263(4):545–548. [PubMed] [Google Scholar]
- 20.Wolman R, Clark P, McNally E, Harries M, Reeve J. Menstrual state and exercise as determinants of spinal trabecular bone density in female athletes. BMJ. 1990;301(6751):516–518. doi: 10.1136/bmj.301.6751.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jones K, Ravnikar V, Tulchinsky D, Schiff I. Comparison of bone density in amenorrheic women due to athletics, weight loss, and premature menopause. Obstet Gynecol. 1985;66(1):5–8. [PubMed] [Google Scholar]
- 22.Lee KC, Lanyon LE. Mechanical loading influences bone mass through estrogen receptor α. Exerc Sport Sci Rev. 2004;32(2):64–68. doi: 10.1097/00003677-200404000-00005. [DOI] [PubMed] [Google Scholar]
- 23.Lanyon L, Skerry T. Postmenopausal osteoporosis as a failure of bone's adaptation to functional loading: a hypothesis. J Bone Miner Res. 2001;16(11):1937–1947. doi: 10.1359/jbmr.2001.16.11.1937. [DOI] [PubMed] [Google Scholar]
- 24.Haines J, Neumark-Sztainer D, Eisenberg ME, Hannan PJ. Weight teasing and disordered eating behaviors in adolescents: longitudinal findings from Project EAT (Eating Among Teens). Pediatrics. 2006;(2):117. doi: 10.1542/peds.2005-1242. Available at: www.pediatrics.org/cgi/content/full/117/2/e209. [DOI] [PubMed]
- 25.Torstveit MK, Rosenvinge JH, Sundgot-Borgen J. Prevalence of eating disorders and the predictive power of risk models in female elite athletes: a controlled study. Scand J Med Sci Sports. 2008;18(1):108–118. doi: 10.1111/j.1600-0838.2007.00657.x. [DOI] [PubMed] [Google Scholar]
- 26.Drummer G, Rosen L, Heusner W. Pathogenic weight-control behaviors of young competitive swimmers. Phys Sportsmed. 1987;15(5):75–86. doi: 10.1080/00913847.1987.11709350. [DOI] [PubMed] [Google Scholar]
- 27.Rosen L, McKeag D, Hough D. Pathogenic weight-control behavior in female athletes. Phys Sportsmed. 1986;14(1):79–86. doi: 10.1080/00913847.1986.11708966. [DOI] [PubMed] [Google Scholar]
- 28.Rosen L, Hough D. Pathogenic weight-control behaviors of female college gymnasts. Phys Sportsmed. 1988;16(9):141–146. doi: 10.1080/00913847.1988.11709603. [DOI] [PubMed] [Google Scholar]
- 29.Sundgot-Borgen J. Eating disorders in female athletes. Sports Med. 1994;17(3):176–188. doi: 10.2165/00007256-199417030-00004. [DOI] [PubMed] [Google Scholar]
- 30.Misra M, Aggarwal A, Miller KK, et al. Effects of anorexia nervosa on clinical, hematologic, biochemical, and bone density parameters in community-dwelling adolescent girls. Pediatrics. 2004;114(6):1574–1583. doi: 10.1542/peds.2004-0540. [DOI] [PubMed] [Google Scholar]
- 31.Soyka L, Misra M, Frenchman A, et al. Abnormal bone mineral accrual in adolescent girls with anorexia nervosa. J Clin Endocrinol Metab. 2002;87(9):4177–4185. doi: 10.1210/jc.2001-011889. [DOI] [PubMed] [Google Scholar]
- 32.Elovainio R, Sundberg S. A five-year follow-up study on cardiorespiratory function in adolescent elite endurance runners. Acta Paediatr Scand. 1983;72(3):351–356. doi: 10.1111/j.1651-2227.1983.tb09727.x. [DOI] [PubMed] [Google Scholar]
- 33.Wilder N, Gilders R, Hagerman F, Deivert RG. The effects of a 10-week, periodized, off-season resistance-training program and creatine supplementation among collegiate football players. J Strength Cond Res. 2002;16(3):343–352. [PubMed] [Google Scholar]
- 34.Vicente-Rodriguez G, Dorado C, Ara I, et al. Artistic versus rhythmic gymnastics: effects on bone and muscle mass in young girls. Int J Sports Med. 2007;28(5):386–393. doi: 10.1055/s-2006-924397. [DOI] [PubMed] [Google Scholar]
- 35.Cara J, Rosenfield R, Furlanetto R. A longitudinal study of the relationship of plasma somatomedin-C concentration to the pubertal growth spurt. Am J Dis Child. 1987;141(5):562–564. doi: 10.1001/archpedi.1987.04460050104041. [DOI] [PubMed] [Google Scholar]
- 36.Rickenlund A, Carlstrom K, Ekblom B, Brismar TB, von Schoultz B, Hirschberg AL. Effects of oral contraceptives on body composition and physical performance in female athletes. J Clin Endocrinol Metab. 2004;89(9):4364–4370. doi: 10.1210/jc.2003-031334. [DOI] [PubMed] [Google Scholar]
- 37.Herman-Giddens M, Slora J, Wasserman R, et al. Secondary sexual characteristics and menses in young girls seen in office practice: a study from the Pediatric Research in Office Settings Network. Pediatrics. 1997;99(4):505–512. doi: 10.1542/peds.99.4.505. [DOI] [PubMed] [Google Scholar]
- 38.Greulich W, Pyle S. Radiographic Atlas of Skeletal Development of the Hand and Wrist. 2nd ed. Stanford University Press; Stanford, CA: 1959. [Google Scholar]
- 39.Aaron D, Kriska A, Dearwater S, Cauley J, Metz K, LaPorte R. Reproducibility and validity of an epidemiologic questionnaire to assess past year physical activity in adolescents. Am J Epidemiol. 1995;142(2):191–201. doi: 10.1093/oxfordjournals.aje.a117618. [DOI] [PubMed] [Google Scholar]
- 40.Kelly T, Specker B, Binkley T, et al. Pediatric BMD Reference Database for US White Children. Children's Bone Health Abstract; Sorrento, Italy: 2005. [Google Scholar]
- 41.Carter DR, Bouxsein ML, Marcus R. New approaches for interpreting projected bone densitometry data. J Bone Miner Res. 1992;7(2):137–145. doi: 10.1002/jbmr.5650070204. [DOI] [PubMed] [Google Scholar]
- 42.Mølgaard C, Thomsen BL, Prentice A, Cole TJ, Michaelsen KF. Whole body bone mineral content in healthy children and adolescents. Arch Dis Child. 1997;76(1):9–15. doi: 10.1136/adc.76.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ward KA, Ashby RL, Roberts SA, Adams JE, Zulf Mughal M. UK reference data for the Hologic QDR Discovery dual-energy x-ray absorptiometry scanner in healthy children and young adults aged 6–17 years. Arch Dis Child. 2007;92(1):53–59. doi: 10.1136/adc.2006.097642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lima F, De Falco V, Baima J, Carazzato JG, Pereira RM. Effect of impact load and active load on bone metabolism and body composition of adolescent athletes. Med Sci Sports Exerc. 2001;33(8):1318–1323. doi: 10.1097/00005768-200108000-00012. [DOI] [PubMed] [Google Scholar]
- 45.Duncan CS, Blimkie CJ, Cowell CT, Burke ST, Briody JN, Howman-Giles R. Bone mineral density in adolescent female athletes: relationship to exercise type and muscle strength. Med Sci Sports Exerc. 2002;34(2):286–294. doi: 10.1097/00005768-200202000-00017. [DOI] [PubMed] [Google Scholar]
- 46.Markou KB, Mylonas P, Theodoropoulou A, et al. The influence of intensive physical exercise on bone acquisition in adolescent elite female and male artistic gymnasts. J Clin Endocrinol Metab. 2004;89(9):4383–4387. doi: 10.1210/jc.2003-031865. [DOI] [PubMed] [Google Scholar]
- 47.Ding J, Sheckter C, Drinkwater B, Soules M, Bremner W. High serum cortisol levels in exercise-associated amenorrhea. Ann Intern Med. 1988;108(4):530–534. doi: 10.7326/0003-4819-108-4-530. [DOI] [PubMed] [Google Scholar]
- 48.Tomten S, Falch J, Birkeland K, Hemmersbach P, Hostmark A. Bone mineral density and menstrual irregularities: a comparative study on cortical and trabecular bone structures in runners with alleged normal eating behavior. Int J Sports Med. 1998;19(2):92–97. doi: 10.1055/s-2007-971888. [DOI] [PubMed] [Google Scholar]
- 49.Riggs B. The mechanisms of estrogen regulation of bone resorption. J Clin Invest. 2000;106(10):1203–1204. doi: 10.1172/JCI11468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Riggs BL, Khosla S, Melton LJ., III Sex steroids and the construction and conservation of the adult skeleton. Endocr Rev. 2002;23(3):279–302. doi: 10.1210/edrv.23.3.0465. [DOI] [PubMed] [Google Scholar]
- 51.Misra M, Prabhakaran R, Miller KK, et al. Role of cortisol in menstrual recovery in adolescent girls with anorexia nervosa. Pediatr Res. 2006;59(4):598–603. doi: 10.1203/01.pdr.0000203097.64918.63. [DOI] [PubMed] [Google Scholar]
- 52.Loucks AB. The response of luteinizing hormone pulsatility to 5 days of low energy availability disappears by 14 years of gynecological age. J Clin Endocrinol Metab. 2006;91(8):3158–3164. doi: 10.1210/jc.2006-0570. [DOI] [PubMed] [Google Scholar]
- 53.Loucks MK. Pros and cons of off-label promotion investigations and prosecutions. Food Drug Law J. 2006;61(3):577–583. [PubMed] [Google Scholar]
- 54.Kriska A, Caspersen CE. Introduction to a collection of physical activity questionnaires. Med Sci Sports Exercise. 1997;29(6):S5–S9. [PubMed] [Google Scholar]
- 55.Bouchard C, Tremblay A, Leblanc C, Lortie G, Savard R, Theriault G. A method to assess energy expenditure in children and adults. Am J Clin Nutr. 1983;37(3):461–467. doi: 10.1093/ajcn/37.3.461. [DOI] [PubMed] [Google Scholar]