SUMMARY
Listeria monocytogenes infection generates T helper-1 (Th1) effector memory cells and CC chemokine receptor 7 (CCR7)+ cells resembling central memory cells. We tracked endogenous L. monocytogenes-specific CD4+ T cells to determine how these memory cells are formed. Two effector cell populations were already present several days after infection. One highly expressed the T-bet transcription factor and produced Th1 memory cells in an interleukin-2 (IL-2) receptor-dependent fashion. The other resided in the T cell areas, expressed CCR7 and CXC chemokine receptor 5 (CXCR5), and like follicular helper cells depended on the Bcl6 transcription factor and inducible costimulator ligand on B cells. The CCR7+ CXCR5+ effector cells produced similar memory cells that generated diverse effector cell populations in a secondary response. Thus, Th1 effector memory and follicular helper-like central memory cells are produced from early effector cell populations that diverge in response to signals from the IL-2 receptor, Bcl6, and B cells.
INTRODUCTION
Bacterial infection generally results in immunity (Ahmed and Gray, 1996), which is mediated by bacterial antigen-specific lymphocytes that proliferate and differentiate into long-lived memory cells. In the case of CD4+ T cells, this process involves initial proliferation of naïve cells with T cell antigen receptors (TCR) specific for microbe-derived peptides bound to host major histocompatibility complex II molecules (pMHCII) (Jenkins et al., 2001). The progeny of the naïve cells then differentiate into specialized effector cells such as interferon-γ (IFN-γ)-producing T helper-1 (Th1) cells under the influence of cytokines such as interleukin (IL)-12 produced by the innate immune cells. Some effector cells then survive after the infection is cleared to become long-lived quiescent memory cells (Ahmed and Gray, 1996).
Memory T cells exist in at least two subsets referred to as central (TCM cells) and effector memory cells (TEM cells) (Sallusto et al., 2004). TEM cells express receptors needed for migration into non-lymphoid organs, and when stimulated with the relevant pMHCII ligand, immediately produce microbicidal lymphokines (Reinhardt et al., 2001). TCM cells express CC chemokine receptor 7 (CCR7) and L-selectin, which direct recirculation through lymph nodes. When stimulated with the relevant pMHCII ligand, TCM cells do not produce microbicidal lymphokines immediately but proliferate to produce new effector cells, which then acquire this function (Sallusto, 2004).
Th1 effector memory cells (Th1EM) arise from earlier Th1 effector cells (Harrington et al., 2008; Lohning et al., 2008; Surh and Sprent, 2008). TEM cell formation is favored by strong TCR signaling (Catron et al., 2006; Sarkar et al., 2007), perhaps due to a shift to glycolytic metabolism (Araki et al., 2009; Pearce et al., 2009). IL-2 receptor signaling also plays a role in Th1 effector cell development (Khoruts et al., 1998), perhaps as a consequence of signal transducer and activator of transcription-5 (STAT5)-mediated upregulation of the IL-12 receptor β2-chain and the Th1 cell-associated T-bet (Liao et al., 2011) and Blimp-1 transcription factors (Gong and Malek, 2007; Pipkin et al., 2010), which repress the Bcl6 transcription factor (Shaffer et al., 2002).
Much less is known about the formation of TCM cells. These cells may be the progeny of effector cells that receive weaker TCR signals from antigen-presenting cells that display low numbers of pMHCII ligands (Catron et al., 2006; van Faassen et al., 2005). Recent work indicates that TCM cells defined by expression of CCR7 also express the B cell follicle homing receptor CXC chemokine receptor 5 (CXCR5) (Chevalier et al., 2011) and are potent helper cells for B cells (Chevalier et al., 2011; MacLeod et al., 2011). Although expression of CXCR5 suggests that TCM cells are related to follicular helper cells (TFH) (Morita et al., 2011), which depend on Bcl6 and provide helper signals to germinal center B cells (Crotty, 2011), this relationship has not been demonstrated in vivo. Here, we addressed this issue by tracing the derivation of pMHCII-specific TCM and TEM cells induced during acute systemic infection with Listeria monocytogenes (Lm).
RESULTS
Detection of LLOp:I-Ab-specific CD4+ memory T cells
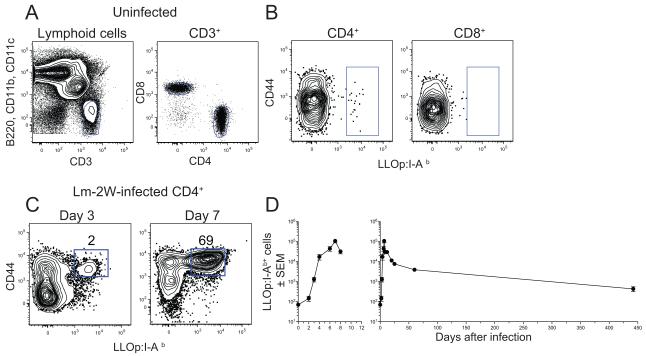
We used a pMHCII tetramer-based approach to identify CD4+ T cells specific for 2 pMHCII ligands produced in C57BL/6 (B6) mice during infection with a vaccine strain of Lm bacteria. This strain, called Lm-2W, contains a mutation in the actA gene (Portnoy et al., 2002), and was engineered to secrete a fusion protein containing the immunogenic 2W peptide (Rees et al., 1999) under the control of the hly promoter (Ertelt et al., 2009). Following infection, these bacteria are taken into phagosomes and then completely eliminated by innate and adaptive immune mechanisms (Portnoy et al., 2002). During this process, the 2W peptide and peptide 190-201 from listeriolysin O (LLOp) are produced by antigen processing and bind to I-Ab MHCII molecules on antigen-presenting cells. We detected CD4+ T cells expressing TCRs specific for these ligands by staining spleen and lymph node cells from individual mice with fluorochrome-labeled LLOp:I-Ab or 2W:I-Ab tetramers and anti-fluorochrome magnetic beads, and enriching the tetramer-bound cells on a magnetized column (Moon et al., 2007). The cells that bound to the column were stained with antibodies specific for CD3 and a mixture of non-T cell lineage-specific markers to aid in identification of genuine CD3+ T cells (Figure 1A).
Figure 1. Detection of LLOp:I-Ab-specific CD4+ T cells.
(A) Gate used to identify CD3+ non-T cell lineage− T cells (left) and CD4+ and CD8+ T cells within that population (right) from the bound fraction after enrichment with LLOp:I-Ab+ tetramer. (B) CD4+ T cells (left) or CD8+ T cells (right) identified as in (A) from an uninfected B6 mouse with gates on LLOp:I-Ab+ cells. (C) CD4+ T cells from B6 mice at the indicated times after intravenous infection with Lm-2W bacteria with gates on LLOp:I-Ab+ cells. The percentages of cells in the indicated gates are shown. (D) Mean number (± SEM, n ≥ 3 for each data point) of CD4+ LLO:I-Ab+ T cells in the spleen and lymph nodes over the first 8 (left) or 440 days (right) after intravenous infection with Lm-2W bacteria.
We first established the kinetics of the expansion, contraction, and memory phases of the LLOp:I-Ab-specific CD4+ T cell population. B6 mice that were not infected contained a small population of LLOp:I-Ab tetramer-binding CD3+ CD4+ cells in the spleen and lymph nodes, most which were CD44low as expected for naive cells (Figure 1B). LLOp:I-Ab tetramer-binding cells were not detected among the MHCI-restricted CD8+ T cells in the bound fraction (Figure 1B), indicating that the CD4+ cells that bound the tetramer did so via the TCR. Naïve mice contained about 80 LLOp:I-Ab-specific CD4+ T cells, which upregulated CD44 following intravenous injection of 107 Lm-2W bacteria, and increased in number in the spleen and lymph nodes beginning on day 3 to a peak of ~100,000 cells by day 7 post infection (Figure 1C and D). The population then contracted by day 20 to about 10,000 CD44high memory cells, which then slowly declined over the next year (Figure 1D). Thus, LLOp:I-Ab-specific CD4+ T cells undergo the expansion, contraction, and slow memory decline phases exhibited by other pMHCII-specific CD4+ T cell populations (Homann et al., 2001; Pepper et al., 2010).
CD4+ memory T cell heterogeneity
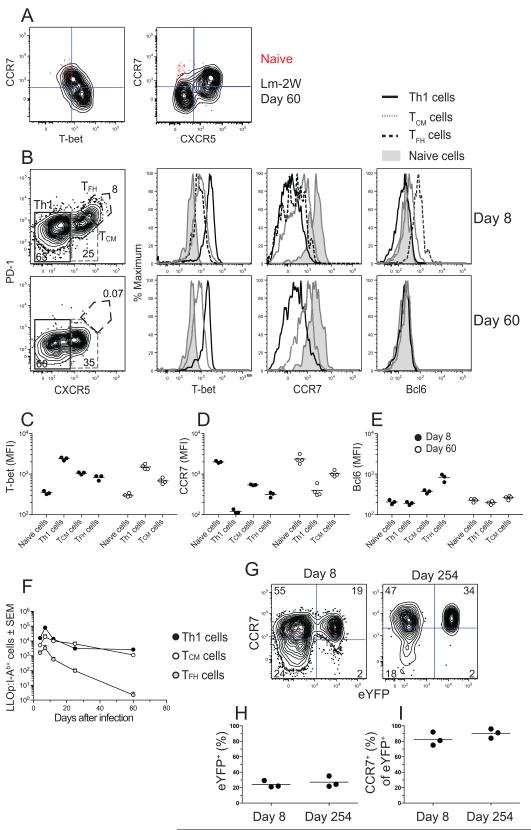
We next determined whether the LLOp:I-Ab-specific memory cell population was heterogeneous. Naïve LLOp:I-Ab-specific cells expressed CCR7 but not T-bet or CXCR5 (Figure 2A). In contrast, the LLOp:I-Ab-specific memory cell population in mice infected 60 days earlier with Lm-2W bacteria consisted of T-bethigh CCR7− and T-betlow CCR7+ subsets observed in a previous study of the 2W:I-Ab-specific memory cell population (Pepper et al., 2010). In addition, we found that the CCR7+ cells, but not the CCR7− cells expressed CXCR5 (Figure 2A).
Figure 2. Identification of TEM and TCM cells.
(A) Plots of CCR7 versus T-bet (left) or CXCR5 (right) on LLOp:I-Ab-specific CD44low naïve cells from uninfected B6 mice (red dots) or LLOp:I-Ab-specific CD44high memory cells from mice infected with Lm-2W bacteria 60 days before analysis (black contours). (B) Plots of CXCR5 versus PD-1 used to identify CXCR5− PD-1− Th1 cells, CXCR5intermediate PD-1− TCM cells, and CXCR5high PD-1+ TFH cells on days 8 or 60 after intravenous infection with Lm-2W bacteria, with histograms of T-bet, CCR7, and Bcl6 on Th1 cells (black line), TCM cells (gray line), TFH cells (dashed line), or CD44low naïve cells (shaded histogram). (C-E) Mean fluorescence intensities (MFI) of T-bet (C), CCR7 (D), and Bcl6 (E) on the indicated cell types from 3 individual mice on days 8 (filled circles) or 60 (open circles) after intravenous infection with Lm-2W bacteria. (F) Mean number (± SEM, n ≥ 3) of LLO:I-Ab-specific Th1 (filled circles), TCM (open circles), or TFH (gray circles) cells identified as shown in (B). (G) CCR7 and eYFP expression by 2W:I-Ab-specific CD4+ T cells in 2W:I-Ab tetramer-enriched samples from r7UP mice infected with Lm-2W bacteria, treated with tamoxifen on days 4-8 post-infection, and analyzed on days 8 or 254 post-infection. (H, I) Percentage of eYFP+ cells among 2W:I-Ab-specific cells (H) or CCR7+ cells among eYFP+ 2W:I-Ab-specific cells (I) from individual mice.
We next analyzed the peak of the primary response to determine if effector cells with the characteristics of the later memory cells were present. PD-1 was also tested to detect TFH cells, which express this marker and the largest amounts of CXCR5 (Crotty, 2011). As shown in Figure 2B, the LLOp:I-Ab-specific effector cells present on day 8 after infection were split about equally into CXCR5− and CXCR5+ populations. The CXCR5− cells were Th1 effector cells based on expression of large amounts of T-bet and lack of CCR7 (Figure 2B-D). About 10% of the CXCR5+ cells were TFH cells based on expression of PD-1, the largest amounts of CXCR5, and the TFH lineage-defining transcription factor Bcl6 (Figure 2B and E). The TFH cells expressed more T-bet than naïve cells but less than the Th1 cells (Figure 2B and C). The CXCR5+ cells that lacked PD-1 also expressed this intermediate amount of T-bet as well as low amounts of Bcl6 (Figure 2B, C, and E). These cells expressed the most CCR7 of any of the effector cell populations (Figure 2B and D). Thus, the LLOp:I-Ab-specific effector cell population present on day 8 after infection consisted of T-bethigh CCR7− and T-betlow CCR7+ subsets with the characteristics of the 2 later memory cell populations, along with an additional TFH subset. The T-bethigh CCR7− cells present at the peak of the primary response will be referred to as Th1 effector cells and the T-betlow CCR7+ CXCR5+ PD-1− cells as TCM precursor cells based on their expression of CCR7.
This analysis was repeated 60 days after infection to determine if any of the effector populations entered the memory pool. At this time, about half of the memory cells resembled the Th1 effector cell population (Figure 2B-E) and will therefore be referred to as Th1EM cells. The other memory cells closely resembled the CXCR5+ T- betlow CCR7+ TCM precursor cells present on day 8, with the exception of reduced expression of Bcl6 (Figure 2B-E). The CXCR5+ PD-1+ TFH population that was present at the peak of the response was not detected 60 days after infection (Figure 2B). A more detailed time course experiment showed that T-bethigh CXCR5− Th1 and CXCR5+ PD-1− CCR7+ TCM precursors cells peaked at day 7 and contracted to lower numbers by days 12-25 that were maintained until day 60 (Figure 2F). In contrast, the CXCR5+ PD-1+ Bcl6high TFH population peaked at day 7 but declined progressively until disappearing by day 60 (Figure 2F). These results suggested that some Th1 effector cells survived the contraction phase to become Th1EM cells, while some TCM precursor cells became TCM cells. TFH cells did not appear to enter the memory cell pool.
We produced a transgenic mouse (called the r7UP mouse) to formally test the possibility that some TCM precursor cells become TCM cells. These mice contain a bacterial artificial chromosome with Cre-recombinase-estrogen receptor 2-fusion protein sequence (Cre-ERT2) inserted between exon 3 and the 3′ untranslated region of the Ccr7 gene (Supplemental Figure 1A). These mice also contain an enhanced yellow fluorescent protein (eYFP) (Tsien, 1998) transgene with a floxed stop cassette controlled by the constitutive ROSA 26 promoter (Srinivas et al., 2001) (Supplemental Figure 1A). Administration of tamoxifen allows the Cre-ERT2 protein, which is normally sequestered in the cytosol to enter the nucleus, leading to excision of the stop cassette (Srinivas et al., 2001) and permanent expression of eYFP in cells that expressed CCR7 at the time of tamoxifen treatment.
As shown in Supplemental Figure 1B, about half of the CCR7+ CD4+ T cells in uninfected r7UP mice treated with tamoxifen for 5 days expressed eYFP, while none of the CCR7− CD4+ T cells in these mice or the CD4+ T cells in tamoxifen-treated B6 mice expressed eYFP. In addition, all the eYFP+ CD44high LLOp:I-Ab-specific cells in r7UP mice 20 days after infection with Lm-2W on day 0 and treatment with tamoxifen on days 16-20 expressed CXCR5 (Supplemental Figure 1C). The CCR7+ eYFP− CD4+ T cells in tamoxifen-treated r7UP mice were likely cells that had not yet deleted the stop cassette upstream of the eYFP gene after 5 days of tamoxifen treatment as observed in T cells in other Cre-ERT2 transgenic mice (Rubtsov et al., 2010). To test the capacity of CCR7+ effector cells to become memory cells, r7UP mice were infected with Lm-2W bacteria and labeled with tamoxifen during the period of maximal effector cell generation from days 4-8 when CXCR5+ PD-1− cells expressed the most CCR7 of any CD44high pMHCII-specific subset (Figure 2D). We focused on the 2W:I-Ab-specific population because its larger size provided a practical advantage. About 25% of the 2W:I-Ab-specific effector cells present on day 8 were eYFP+ (Figure 2G and H) and virtually all of these cells expressed CCR7 (Figure 2G and I). Two hundred and fifty four days after infection and 246 days after the cessation of tamoxifen treatment, about 30% of the 2W:I-Ab-specific memory cells were again eYFP+ (Figure 2G and H) and the vast majority retained CCR7 (Figure 2G and I). Therefore, some early CCR7+ effector cells produce stable CCR7+ TCM cells.
CXCR5+ memory cells are TCM cells
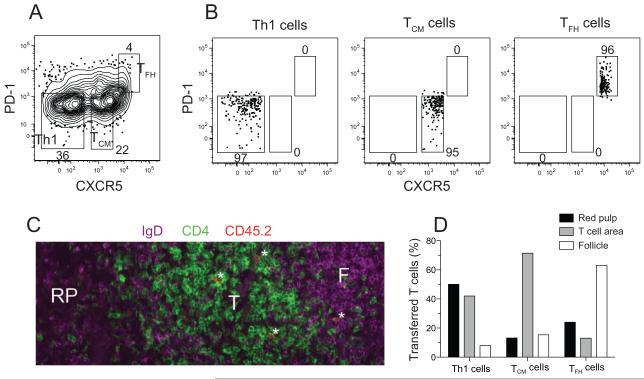
Expression of CXCR5 suggested that TCM precursor cells might be a type of TFH cell. If so, then TCM precursor cells would be located in B cell follicles. To test this possibility, CD44high CD4+ CXCR5− PD-1− Th1, CXCR5intermediate PD-1− TCM, and CXCR5high PD-1+ TFH cells (Figure 3A) were purified by flow cytometric sorting (Figure 3B) from CD45.2+ B6 mice 10 days after infection with Lm-2W organisms and injected into naive CD45.1+ recipients. These populations contained effector cells specific for Lm-2W-derived pMHCII ligands and other memory cells. One day after transfer, Th1 cells were located predominantly in the red pulp and T cell areas (identified as areas with sparse CD4+ T cells and IgD+ B cells or dense CD4+ T cells, respectively, Figure 3C) (Figure 3D), while TFH cells were located primarily in follicles (identified as areas with dense IgD+ B cells, Figure 3C) (Figure 3D). In contrast, CXCR5+ PD-1− TCM cells were located predominantly in the T cell areas (Figure 3D), formally demonstrating that TCM cells are not TFH cells.
Figure 3. TCM precursor cells are located in the T cell areas.
(A) Gates used to sort purify the indicated subsets from the spleen and lymph nodes of B6 mice 10 days after Lm-2W infection. CCR7 staining was also used as a sorting parameter (not shown) with Th1 cells sorted as CCR7− cells, TCM cells as CCR7+ cells, and TFH cells as CCR7low cells. (B) Post-sort analysis of the indicated populations. Four x 106 Th1, 3 x 106 TCM, or 106 TFH cells were transferred into CD45.1+ recipients. (C) CD4 (green), IgD (purple), and CD45.2 (red) expression in a spleen section from a naïve B6 mouse that received TCM cells one day before analysis. CD45.2+ cells that were also CD4+ appeared orange. A T cell area (T), follicle (F), and red pulp area (RP) are indicated. Three CD4+ CD45.2+ transferred T cells in the T cell area and one in the follicle are labeled with asterisks. (D) Percentage of Th1 (151 cells), TCM precursor (91 cells), and TFH (38 cells) located in the indicated areas one day after transfer.
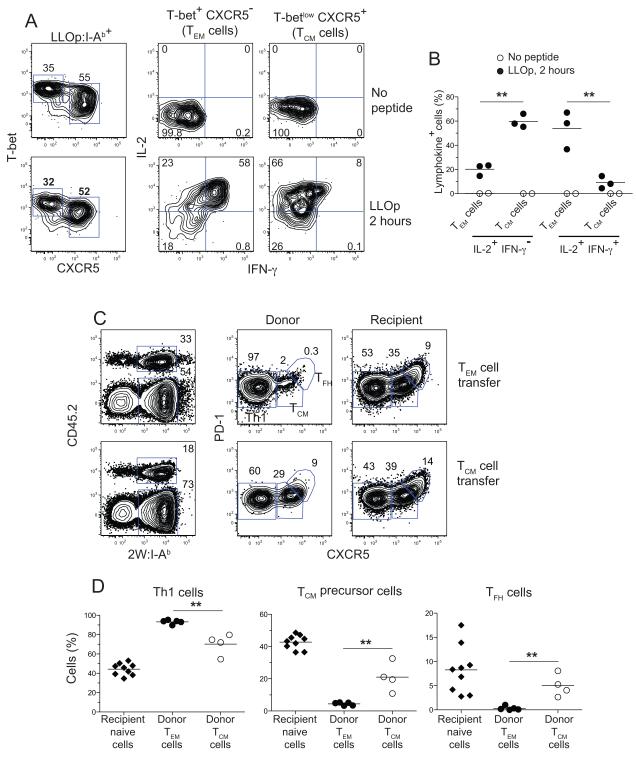
We also determined whether CXCR5+ memory cells behaved like TCM cells with respect to lymphokine production (Sallusto et al., 2004). Mice infected with Lm-2W bacteria 92 days earlier were injected intravenously with LLOp to restimulate LLOp:I-Ab-specific memory cells. As shown in Figure 4A and B, neither the T-bethigh CXCR5− Th1 nor the T-betlow CXCR5+ LLOp:I-Ab-specific memory cells were producing IL-2 or IFN-γ before peptide injection. Both memory cell types retained their pre-injection patterns of T-bet and CXCR5 expression 2 hours after LLOp challenge (Figure 4A), allowing analysis of their lymphokine production. About 60% of the T-bet+ CXCR5− Th1 memory cells produced IFN-γ and IL-2 and ~20% made IL-2 but not IFN-γ 2 hours after injection of LLOp (Figure 4A and B). In contrast, about 10% of the T-betlow CXCR5+ memory cells produced IFN-γ and IL-2 while ~60% made IL-2 but not IFN-γ. Therefore, the LLOp:I-Ab-specific CXCR5+ memory cells are less potent IFN-γ producers than Th1EM cells and rapidly produce IL-2 as expected for TCM cells.
Figure 4. Secondary responses by memory cells.
(A) Contour plots showing T-bet and CXCR5 expression by LLOp:I-Ab-specific T cells (left panels), or intracellular IFN-γ and IL-2 staining (lower panels) in LLOp:I-Ab-specific TEM (middle panels) or TCM cells (right panels) from day 92 Lm-2W-infected mice before (upper panels) or 2 hours after intravenous injection of LLOp (lower panels). (B) Percentage of LLOp:I-Ab-specific TCM or TEM cells producing the indicated lymphokines before (filled circles) or 2 hours after intravenous injection of LLOp (open circles). **, p < 0.01. (C) 4.8 x 105 CD44high CD4+ CCR7+ CXCR5+ PD-1− T or 4.3 x 105 CD44high CD4+ CCR7− CXCR5− CM PD-1− TEM cells were sorted from CD45.2+ B6 mice infected 30 days earlier with Lm-2W bacteria, and transferred into CD45.1+ mice, which were then challenged with Lm-2W bacteria. Representative plots used to identify donor (upper gates, left panels) or recipient (lower gates, left panels) 2W:I-Ab-specific cells 6 days after challenge are shown, along with plots of PD-1 versus CXCR5 used to identify Th1 effector cells, TCM precursors, and TFH cells of donor (middle) or recipient (right) origin. (D) Percentage of Th1 effector, TCM precursor, or TFH cell progeny from donor TEM, donor TCM, or recipient naïve cells. **, p < 0.01.
Finally, we used an adoptive transfer approach to test whether CXCR5+ memory cells produced diverse effector cell progeny during the secondary response. CD44high CD4+ CXCR5+ CCR7+ (containing 2W:I-Ab-specfiic T cells) and CD44high CD4+ CXCR5− CCR7− (containing 2W:I-Ab-specfiic Th1EM cells) cells were sorted from B6 mice 40 days after infection with Lm-2W organisms and injected into naive CD45.1+ recipients. The recipient mice were then challenged with Lm-2W organisms, and the phenotype of the donor and recipient 2W:I-Ab-specific T cells (Figure 4C) was assessed 6 days later. As shown in Figure 4C and D, 96% of the effector cell progeny of CCR7− CXCR5− Th1EM cells were Th1 cells. In contrast, the progeny of CXCR5+ memory cells consisted of 70% Th1 cells, 21% TCM precursor cells, and 5% TFH cells, whereas the naïve cells of the recipients produced 44% Th1, 43% TCM precursor, and 8% TFH cells. Therefore, although CXCR5+ memory cells have a tendency to produce Th1 effector cells, they are more efficient than TEM cells at generating TCM precursors and TFH cells.
Development of Th1EM and TCM cells
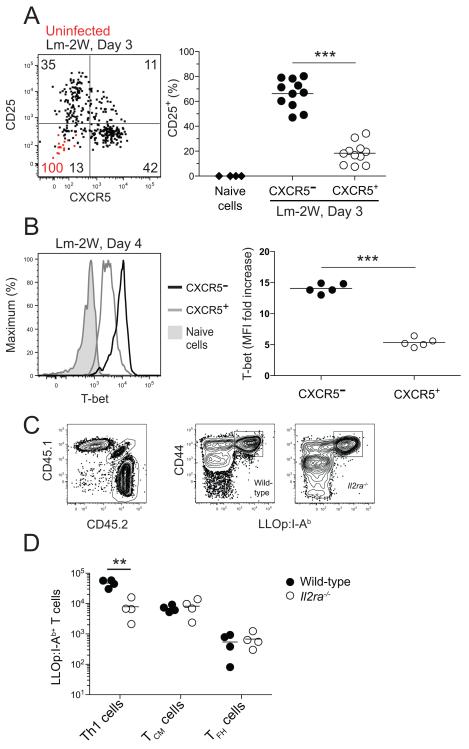
The results were consistent with the possibility that Th1 effector and TCM precursor cells generated early in the primary response gave rise to Th1EM and TCM cells. We examined very early times after Lm-2W infection to get insight into the origins of these populations. The IL-2 receptor alpha chain (CD25) was included in the analysis because it has been implicated in Th1 development in other systems (Khoruts et al., 1998; Liao et al., 2011). Naïve LLOp:I-Ab-specific cells in uninfected mice did not express CXCR5 or CD25 (Figure 5A). Remarkably, the LLOp:I-Ab-specific population had already split equally into CXCR5− and CXCR5+ subsets only 3 days after infection (Figure 5A) when expansion of LLOp:I-Ab-specific T cells was first detected (Figure 1D). Notably, most of the CXCR5− cells also expressed CD25 and most of the CXCR5+ cells did not (Figure 5A). On day 4 after infection, the CXCR5− cells expressed more T-bet than CXCR5+ cells (Figure 5B).
Figure 5. Development of Th1 effector cells depends on CD25.
(A) Dot plot of CD25 versus CXCR5 expression by LLOp:I-Ab-specific cells from naïve mice (red dots) or from mice 3 days after intravenous infection with Lm-2W bacteria (black dots). The values on the plots represent the percentage of cells in the indicated quadrants from naïve (red) or Lm-2W-infected (black) mice. The scatter plot shows the percentage of CD25+ total naïve CD4+ T cells from uninfected B6 mice (diamonds) or CXCR5− (filled circles) or CXCR5+ (open circles) LLOp:I-Ab-specific cells from mice 3 days after intravenous infection with Lm-2W bacteria. ***, p < 0.001. (B) T-bet expression in total naïve CD4+ T cells from uninfected B6 mice (shaded), or CXCR5− (black) or CXCR5+ (gray) LLOp:I-Ab-specific cells from mice 4 days after intravenous infection with Lm-2W bacteria, with a scatter plot showing the fold increase of T-bet mean fluorescence intensity (MFI) of the indicated LLOp:I-Ab-specific populations over the T-bet MFI of naïve CD4+ T cells. ***, p < 0.001. (C) Plots of CD45.1 versus CD45.2 expression and CD44 expression versus LLOp:I-Ab tetramer staining of CD4+ T cells in a tetramer-enriched sample from a radiation chimera produced with CD45.2+ wild-type and CD45.1+ CD45.2+ Il2ra−/− bone marrow, 5 days after intravenous infection with Lm-2W bacteria. (D) Number of LLOp:I-Ab-specific Th1, TCM, and TFH cells identified as in Figure 2B of wild-type (filled circles) or Il2ra−/− (open circles) origin from individual mice, 5 days after intravenous infection with Lm-2W bacteria. **, p < 0.01.
These findings raised the possibility that CD25 was required for the development of the CXCR5− Th1 effector cells. This premise was tested in chimeras containing a mixture of wild-type and CD25-deficient T cells so that both populations could be monitored under conditions where normal regulatory T cells were present to prevent the autoimmunity that occurs in intact CD25-deficient mice (Willerford et al., 1995). As shown in Figure 5C and D, CD25-deficient LLOp:I-Ab-specific naïve cells were about one tenth as good as wild-type cells at generating T-bethigh CXCR5− Th1 cells in these chimeras 5 days after Lm-2W infection, but were as good as wild-type cells at generating CXCR5+ PD-1− TCM precursor cells and CXCR5+ PD-1+ TFH cells. CD25 is therefore required for the optimal development of T-bethigh CXCR5− Th1 cells but not the other effector cell populations.
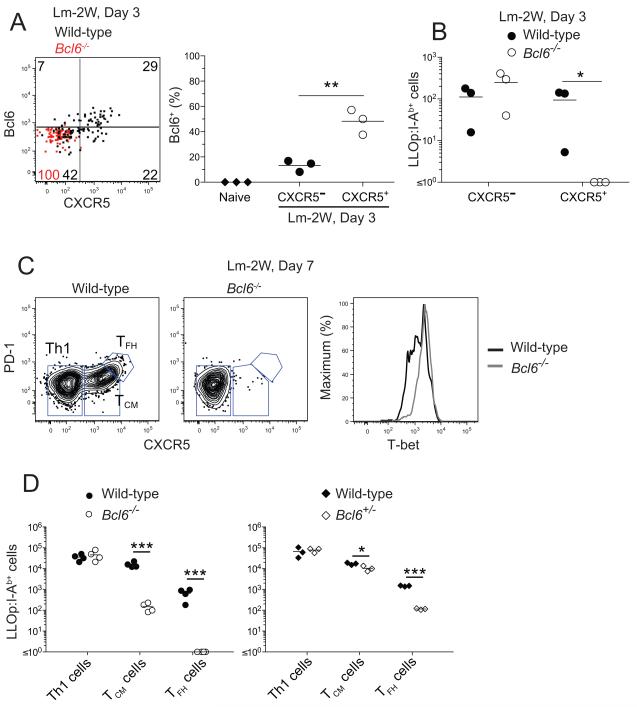
We also examined the role of Bcl6 in the development of TCM cells. Bcl6 was expressed in higher amounts in the CXCR5+ subset of LLOp:I-Ab-specific cells 3 days after Lm-2W infection than in the CXCR5− subset (Figure 6A). Radiation bone marrow chimeras containing a mixture of wild-type and Bcl6−/− (Dent et al., 1997) cells were used to test whether Bcl6 was required for the formation of CXCR5+ T cells. Indeed, Bcl6-deficient CD4+ T cells failed to generate early LLOp:I-Ab-specific CXCR5+ cells on day 3 (Figure 6A and B). Similarly, CXCR5+ PD-1− T + CM precursors and CXCR5 PD-1+ TFH cells were generated at only 1% of the amount generated from wild-type CD4+ T cells 7 days after Lm-2W infection (Figure 6C and D). The Bcl6-deficient LLOp:I-Ab-specific T cells present on day 7 were all T-bethigh indicating that T-betlow TCM cell precursors were truly absent and had not simply lost CXCR5 (Figure 6C). We also produced radiation bone marrow chimeras containing a mixture of wild-type and Bcl6+/- T cells to test the dependence of the two types of CXCR5+ effector cells on the amount of Bcl6 expressed. As shown in Figure 6D, LLOp:I-Ab-specific Bcl6+/- TCM precursor cells formed only slightly less well than wild-type cells 7 days after Lm-2W infection, while Bcl6+/- TFH cells were generated at 10% of the amount generated from wild-type T cells. These results demonstrate that TCM precursors and TFH cells both depend on Bcl6 although TCM precursors are less dependent.
Figure 6. Development of TCM cells depends on Bcl6.
(A) Plot of Bcl6 versus CXCR5 expression by wild-type (black dots) or Bcl6−/− (red dots) LLOp:I-Ab-specific cells from a wild-type plus Bcl6−/− mixed radiation bone marrow chimera 3 days after intravenous infection with Lm-2W bacteria with a scatter plot showing the percentage of Bcl6+ naïve CD4+ T cells from uninfected B6 mice (diamonds) or CXCR5− (filled circles) or CXCR5+ (open circles) LLOp:I-Ab-specific cells from wild-type mice 3 days after infection. (B) Number of wild-type (filled circles) or Bcl6−/− (open circles) CXCR5− or CXCR5+ LLOp:I-Ab-specific cells from 3 wild-type plus Bcl6−/− mixed radiation bone marrow chimeras, 3 days after intravenous infection with Lm-2W bacteria. *, p < 0.05. (C) Plots of CXCR5 versus PD-1 staining used to identify wild-type or Bcl6−/− LLOp:I-Ab-specific Th1 effector cells, T −/− CM precursor cells, and TFH cells from wild-type plus Bcl6 mixed radiation bone marrow chimeras 7 days after Lm-2W infection. T-bet expression by wild-type or Bcl6−/− LLOp:I-Ab-specific cells in these chimeras is also shown. (D) Number of LLOp:I-Ab-specific Th1, TCM, and TFH cells identified as in (C) from individual wild-type plus Bcl6−/− (left) or wild-type plus Bcl6+/- (right) mixed radiation bone marrow chimeras, 7 days after intravenous infection with Lm-2W bacteria. *, p < 0.05, ***, p < 0.001.
ICOS signals from B cells sustain TCM cell differentiation
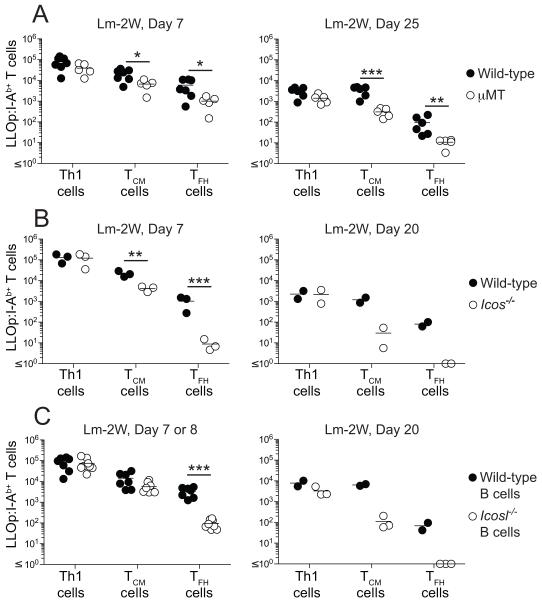
The Bcl6 requirement of TCM precursors and TFH cells prompted us to determine whether TCM precursor cells also required signals from B cells and the ICOS costimulatory receptor (Akiba et al., 2005; Vinuesa et al., 2005) as described for TFH cells (Nurieva et al., 2008). Seven and 25 days after Lm-2W infection, LLOp:I-Ab-specific TCM precursor cells and TFH cells were produced in μMT mice lacking B cells (Kitamura et al., 1991) at about 10% of the number produced in wild-type mice (Figure 7A). Therefore, B cells are necessary for optimal generation of TCM and TFH cells.
Figure 7. Development of TCM cells depends on ICOS signals from B cells.
(A-C) Number of LLOp:I-Ab-specific Th1, TCM, and TFH cells identified as in Figure 2B from individual (A) wild-type or B cell-deficient μMT mice, (B) wild-type plus Icos−/− mixed radiation bone marrow chimeras, or (C) wild-type plus μMT or Icosl−/− plus μMT mixed radiation bone marrow chimeras, at the indicated times after intravenous infection with Lm-2W bacteria. *, p < 0.05, **, p < 0.01, ***, p < 0.001
The role of ICOS was tested in bone marrow chimeras reconstituted with an equal mixture of ICOS-deficient (Tafuri et al., 2001) and wild-type bone marrow cells so that ICOS-deficient T cells could be analyzed under conditions where wild-type T cells were present to promote a normal germinal center response. ICOS-deficient and wild-type Th1 effector cells were generated equally well 7 days after Lm-2W infection, and maintained as Th1EM cells on day 20 (Figure 7B). In contrast, ICOS-deficient TCM cell precursors were generated at about 30% of the amount on day 7 and survived as TCM cells on day 20 at about 3% of the amount generated from the comparable wild-type populations. TFH cells were even more dependent on ICOS, forming from ICOS-deficient cells at about 1% of the amount formed from wild-type cells on days 7 and 20 after infection.
We next determined whether B cells were the critical source of ICOSL. Chimeras were made by reconstituting lethally irradiated B6 mice with an equal mixture of ICOSL-deficient (Mak et al., 2003) and wild-type or μMT bone marrow cells incapable of forming B cells (Kitamura et al., 1991). All of the B cells in the chimeras made with wild-type bone marrow expressed ICOSL, and all of the B cells in the chimeras made with ICOSL-deficient bone marrow cells lacked ICOSL, while half of the dendritic cells in those chimeras expressed ICOSL. Seven or 8 days after Lm-2W infection, the number of LLOp:I-Ab-specific TCM precursor cells was slightly but not significantly lower in chimeras containing ICOSL-deficient B cells than in chimeras containing wild-type B cells (Figure 7C). However, by day 20, the number of LLOp:I-Ab-specific TCM cells formed in chimeras containing ICOSL-deficient B cells was only 1% of the number formed in chimeras containing wild-type B cells (Figure 7C). The number of TFH cells generated in chimeras containing ICOSL-deficient B cells was also only 1% of the number formed in chimeras containing wild-type B cells. The formation of TCM cells and TFH cells in chimeras lacking ICOSL on B cells therefore mirrored the production of these populations from ICOS-deficient T cells. Together, these experiments demonstrate that the interaction of ICOS on the T cells with ICOSL on B cells is necessary for optimal generation of TFH cells and the maintenance of TCM cells.
Discussion
Our results demonstrate that pMHCII-specific T-bethigh CXCR5− and T-betlow CXCR5+ CD4+ T cells were already present at the peak of the primary response to a vaccine strain of Lm. Several observations indicate that some of these effector cells survived the contraction phase to become Th1EM and TCM cells. First, the 2 memory cell populations that survived the contraction phase had phenotypes that were very similar to the 2 effector cell populations with the exception that T-betlow CXCR5+ memory cells lacked Bcl6. Second, several other groups have demonstrated that memory cells can be derived from IFN-γ-producing Th1 effector cells (Harrington et al., 2008; Lohning et al., 2008). Third, our studies of the r7UP mouse revealed that CCR7+ TCM cells were derived from CCR7+ effector cells present at the peak of clonal expansion. Our results indicate that TFH cells fail to enter the memory cell pool although we cannot rule out that possibility that small numbers of these cells lose expression of PD-1 and make a minor contribution to the CXCR5+ memory cell population.
The bifurcation of Lm pMHCII-specific effector cells into CXCR5− and CXCR5+ subsets was already apparent at the time of the earliest proliferation at day 3 as recently described in another CD4+ T cell response (Choi et al., 2011). Although it is not clear what caused this early bifurcation, an asymmetric division of a CD25+ mother cell into CD25+ and CD25− daughter cells is a possibility (Chang et al., 2007). Once this split occurred, then work by others suggests a scenario in which IL-2 receptor signaling in the CD25+ cells activates STAT5 (Hou et al., 1995; Lin et al., 1995), which induces the IL-12 receptor and T-bet (Liao et al., 2011), and suppresses Bcl6 and CXCR5 by inducing Blimp-1 (Choi et al., 2011; Gong and Malek, 2007; Shaffer et al., 2000). This sequence of events would be predicted to cause the early CD25+ CXCR5− cells to retain T-bet and differentiate into Th1 effector cells, a fraction of which then survive as Th1EM cells.
Conversely, the lack of IL-2 receptor signaling in the early CD25− cells may have been conducive to Bcl6 expression, which we found was essential for formation of early CXCR5+ effector cells and subsequent PD-1− TCM precursor and PD-1+ TFH cell progeny. Although it is not clear what drives the split between PD-1− TCM precursors and PD-1+ TFH cells, our finding that TFH cells require more Bcl6 and earlier ICOS signals than TCM precursor cells may be a clue. TFH cells tend to express TCRs with higher affinity for pMHCII ligands than other cells in the population (Fazilleau et al., 2009). The formation of these cells is also promoted by additional pMHCII presentation by B cells (Deenick et al., 2010; Johnston et al., 2009). Therefore, early CXCR5+ effector cells that receive very strong TCR and ICOS signals may express and maintain the large amounts of Bcl6 needed to commit to the T fate. Other early CXCR5+ FH effector cells that receive less strong TCR and ICOS signals may express enough Bcl6 needed to become TCM cells but not enough to maintain Bcl6 and become TFH cells.
Although TCM and TFH cells express CXCR5 and depend on Bcl6, our results show that these are different cells. For example, TCM cells were located in the T cell areas, not in the follicles like TFH cells. In addition, we found that TCM cells did not express Bcl6 and were relatively inefficient at producing TFH cells during the secondary response. Indeed, Th1 effector cells were the dominant progeny produced by TCM cells when exposed to antigen. These properties may be related to the fact that TCM cells expressed low amounts of T-bet at the time of challenge, and thus were partially committed to the Th1 lineage. Notably, however, T cells produced some CXCR5+ CM effector cells indicating that they were more flexible than Th1EM cells, which only produced Th1 effector cells. The reports that CXCR5+ Bcl6− TCM-like memory cells present in humans (Chevalier et al., 2011), and similar cells in mice immunized with soluble antigen (MacLeod et al., 2011), are potent helpers of B cell proliferation and antibody production is further evidence of the poly-functionality of TCM cells.
An implication of this work is that B cells enhance the production of TCM cells, adding to other studies showing a role for B cells in memory T cell formation (Linton et al., 2000; Whitmire et al., 2009). In our study, it was very likely that many B cells in the spleen had access to Lm bacteria soon after intravenous injection. It will be of interest to determine if TCM cells fail to form during intracellular infections where B cells have less access to bacteria.
EXPERIMENTAL PROCEDURES
Mice
Six- to eight-week-old C57BL/6 (B6), 129x1/SvJ, B6.129S2-Ighmtm1Cgn/J (μMT) (Kitamura et al., 1991), B6.129P2-Icostm1Mak/J (ICOS-deficient) (Tafuri et al., 2001), B6.129P2-Icosltm1Mak/J (ICOSL-deficient) (Mak et al., 2003), and B6.SJL-Ptprca Pep3b/BoyJ (CD45.1) mice were from the Jackson Laboratory or the National Cancer Institute. B6.129S6-Bcl6 mice (Bcl6-deficient) (Dent et al., 1997) were obtained from Matthew Mescher (University of Minnesota). Foxp3-GFP Cd25−/− CD45.1+/CD45.2+ mice were obtained from Daniel Campbell (University of Washington). All mice were housed in specific pathogen-free conditions in accordance with guidelines of the University of Minnesota and National Institutes of Health. The Institutional Animal Care and Use Committee of the University of Minnesota approved all animal experiments.
Generation of r7UP transgenic mice
Pierre Chambon (IGBMC) provided the Cre-ERT2 plasmid. Recombination of the Cre-ERT2 coding sequence into the 3′-untranslated region (UTR) of CCR7 in bacterial artificial chromosome clone RP23-80024 (Invitrogen, Carlsbad, CA) was performed as described (Kaplan et al., 2005). Primers used for generation of the recombination cassette were: 5′ A box, 5′- TTAAGGCGCGCCGCTCCTATGCATCAGCATTGA -3′; 3′ A box, 5′- GGCGGATCCCGGATGTGTGCACCACATTAAGGCTC -3′; 5′ B box, 5′- GCCACAGCTTGAGCACAGACTCTCCATCCACCGAA -3′; 3′ B box, 5′- TATTAAGGCCGGCCCTGCAGGTGTATGTGCAAGAC -3′; 5′ I box, 5′- GGTGCACACATCCGGGATCCGCCCCTCTCC -3′; 3′ I box, 5′- AGAGTCTGTGCTCAAGCTGTGGCAGGGAAACCCTC -3′. The recombined construct was injected into the pro-nuclei of B6 mice in the University of Minnesota Mouse Genetics Laboratory. Transgenic mice were then crossed to the B6;129Gt(ROSA)26Sortm2Sho/J strain. eYFP expression was induced by daily intraperitoneal injection of tamoxifen (0.05 mg/g) for 5 days.
Bone marrow irradiation chimeras
Bone marrow cells were harvested from femurs, tibias, and humeri. T cells were depleted from bone marrow cell suspensions with anti-Thy1.2 (30-H12, Bio X Cell) and low toxicity rabbit complement (Cedarlane Laboratories). In some cases, CD45.1+ CD45.2+ wild-type bone marrow cells were mixed with an equal number of CD45.2+ Icos−/−, Bcl6−/−, or Bcl6+/-bone marrow cells. Five-10 x106 total bone marrow cells were injected into lethally-irradiated (1,000 rads) CD45.1+ mice. In other cases, CD45.2+ wild-type bone marrow cells were mixed with an equal number of CD45.1+ CD45.2+ Il2ra−/− bone marrow cells and injected into lethally-irradiated (1,000 rads) CD45.1+ mice. In other cases, wild-type or Icosl−/−bone marrow cells were mixed with an equal number of μMT bone marrow cells and 5-10×106 total bone marrow cells were injected into lethally-irradiated (1,000 rads) μMT mice. Variations in the absolute numbers of tetramer-binding T cells due to slight differences in chimerism were corrected with the formula, c = (r / p) X 50%, where p is the percent of cells among donor-derived cells obtained experimentally, r is the absolute number of tetramer-binding T cells obtained experimentally, and c is the absolute number after the correction.
Infections
Mice were injected intravenously with 107 actA-deficient Lm bacteria engineered to secrete a fusion protein containing an immunogenic peptide called 2W (Lm-2W) (Ertelt et al., 2009).
Tetramer production
Biotin-labeled soluble I-Ab molecules containing 2W peptide (EAWGALANWAVDSA) covalently attached to the I-Ab beta chain were produced in Drosophila melanogaster S2 cells, then purified and made into tetramers with streptavidin-phycoerythrin or streptavidin-allophycocyanin (Prozyme) as described (Moon et al., 2007). Biotin-labeled soluble I-Ab molecules containing LLO190-201 (NEKYAQAYPNVS, LLOp) were made in a similar fashion except that the I-Ab alpha chain contained a cysteine substitution at position 72, which allowed a disulfide bond to form between this cysteine and the cysteine 2 residues after the LLOp, in effect locking the peptide into the correct binding register (Stadinski et al., 2010).
Cell enrichment and flow cytometry
All antibodies were from eBioscience unless noted. Spleen and lymph node cells were prepared and then were stained for 1 hour at room temperature with LLOp:I-Ab- or 2W:I-Ab-streptavidin-allophycocyanin tetramers and 2 μg each of peridinin chlorophyll protein-cyanine 5.5-conjugated antibody to CCR7 (anti-CCR7; 4B12) and phycoerythrin-conjugated antibody to CXCR5 (2G8; Becton-Dickinson). Samples were then enriched for bead-bound cells on magnetized columns and a portion was removed for counting as described (Moon et al., 2007). For identification of surface phenotype, the rest of the sample underwent surface staining on ice with a mixture of antibodies specific for B220 (RA3-6B2), CD11b (MI-70), CD11c (N418); CD8α (5H10; Caltag); PD-1 (J43); CD4 (RM4-5); CD3ε (145-2C11); CD25 (PC61.5); CD44 (IM7); CD45.1 (A20), and/or CD45.2 (104), each conjugated with a different fluorochrome. In experiments designed to detect transcription factors, the cells were surface stained with some of the aforementioned antibodies, then treated with Foxp3 Fixation/Permeabilization Concentrate and Diluent (eBioscience), and stained for 1 hour on ice with antibodies against T-bet (4B10; Biolegend) and/or Bcl6 (K112-91; Becton-Dickinson). In experiments designed to detect cytokines, the cells were surface stained, then treated with BD Cytofix/Cytoperm (Becton-Dickinson) overnight, and stained for 1 hour on ice with antibodies against IL-2 (JES6-5H4) and IFN-γ (XMG1.2) in Perm Wash solution (Becton-Dickinson). In all cases, cells were then analyzed on an LSR II or Fortessa (Becton Dickinson) flow cytometer. Data were analyzed with FlowJo software (TreeStar).
Lymphokine production
Mice were infected intravenously with Lm-2W bacteria. Ninety-two days later, the mice were injected intravenously with 100 μg of LLOp. Spleen and lymph nodes were analyzed for the presence of intracellular IL-2 and IFN-γ in LLOp:I-Ab-specific T cells as described previously (Pepper et al., 2010).
Cell transfer
Flow cytometric sorting was used to purify T cell populations before adoptive transfer. For analysis of memory cell function in the secondary response, spleens and lymph nodes were collected from B6 mice infected intravenously at least 20 d earlier with Lm-2W bacteria and stained with fluorochrome-labeled antibodies to CD4, CD44, CCR7, PD-1, and CXCR5. CD44high CXCR5− CCR7− CD4+ Th1EM cells and CD44high CXCR5+ CCR7+ TCM cells were then sorted with a FACSAria (Becton Dickinson) flow cytometer. Sorted cells were then injected intravenously into CD45.1+ recipients, which were then infected intravenously with 107 Lm-2W bacteria. Six days later, the phenotype of 2W:I-Ab-specific T cells was determined after tetramer-based cell enrichment as described above.
For analysis of TCM cell location, B6 mice were infected intravenously with Lm-2W bacteria. Eight days later, spleen and lymph node cells from these mice were stained with fluorochrome-labeled antibodies against CD4, CD44, CXCR5, CCR7, and PD-1. CD4+ CD44low CXCR5− CCR7− PD-1− Th1 cells, CXCR5intermediate CCR7high PD-1− TCM cells, and CXCR5high CCR7low PD-1+ TFH cells were then sorted and transferred into CD45.1+ recipients. Seven micron spleen sections were prepared as previously described (Pape et al., 2007) and stained with fluorescein isothiocyanate-anti-IgD (11-26; eBioscience), biotin-anti-CD4 (GK1.5; eBioscience), and allophycocyanin-anti-CD45.2 (104; eBioscience, followed by Cy3-streptavidin (Invitrogen). Stained sections were imaged as previously described (Pape et al., 2007) with a Leica DM5500B automated upright microscope and a Leica DFC340FX Digital Camera.
Statistical analysis
Differences between 2 data sets were analyzed by a paired or unpaired two-tailed Student’s t-test using Prism (Graphpad) software.
Supplementary Material
Acknowledgements
The authors thank Jennifer Walter and Rebecca Speier for technical help, Terri Martin and the CFI core flow cytometry facility for assistance with sorting and flow cytometry experiments, and Kathryn Pape for assistance with immunohistology. This work was supported by grants to MKJ (NIH R01-AI39614, R37-AI27998, R01-AI66018), AP (NIH T32-AI07313 and a Kunze Fellowship), and BI (NIH R01-AR056632 and Dermatology Foundation Fellowship).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Ahmed R, Gray D. Immunological memory and protective immunity: understanding their relation. Science. 1996;272:54–60. doi: 10.1126/science.272.5258.54. [DOI] [PubMed] [Google Scholar]
- Akiba H, Takeda K, Kojima Y, Usui Y, Harada N, Yamazaki T, Ma J, Tezuka K, Yagita H, Okumura K. The role of ICOS in the CXCR5+ follicular B helper T cell maintenance in vivo. J. Immunol. 2005;175:2340–2348. doi: 10.4049/jimmunol.175.4.2340. [DOI] [PubMed] [Google Scholar]
- Araki K, Turner AP, Shaffer VO, Gangappa S, Keller SA, Bachmann MF, Larsen CP, Ahmed R. mTOR regulates memory CD8 T-cell differentiation. Nature. 2009;460:108–112. doi: 10.1038/nature08155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catron DM, Rusch LK, Hataye J, Itano AA, Jenkins MK. CD4+ T cells that enter the draining lymph nodes after antigen injection participate in the primary response and become central-memory cells. J. Exp. Med. 2006;203:1045–1054. doi: 10.1084/jem.20051954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang JT, Palanivel VR, Kinjyo I, Schambach F, Intlekofer AM, Banerjee A, Longworth SA, Vinup KE, Mrass P, Oliaro J, et al. Asymmetric T lymphocyte division in the initiation of adaptive immune responses. Science. 2007;315:1687–1691. doi: 10.1126/science.1139393. [DOI] [PubMed] [Google Scholar]
- Chevalier N, Jarrossay D, Ho E, Avery DT, Ma CS, Yu D, Sallusto F, Tangye SG, Mackay CR. CXCR5 expressing human central memory CD4 T cells and their relevance for humoral immune responses. J. Immunol. 2011;186:5556–5568. doi: 10.4049/jimmunol.1002828. [DOI] [PubMed] [Google Scholar]
- Choi YS, Kageyama R, Eto D, Escobar TC, Johnston RJ, Monticelli L, Lao C, Crotty S. ICOS receptor instructs T follicular helper cell versus effector cell differentiation via induction of the transcriptional repressor Bcl6. Immunity. 2011;34:932–946. doi: 10.1016/j.immuni.2011.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crotty S. Follicular helper CD4 T cells (TFH) Annu. Rev. Immunol. 2011;29:621–663. doi: 10.1146/annurev-immunol-031210-101400. [DOI] [PubMed] [Google Scholar]
- Deenick EK, Chan A, Ma CS, Gatto D, Schwartzberg PL, Brink R, Tangye SG. Follicular helper T cell differentiation requires continuous antigen presentation that is independent of unique B cell signaling. Immunity. 2010;33:241–253. doi: 10.1016/j.immuni.2010.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dent AL, Shaffer AL, Yu X, Allman D, Staudt LM. Control of inflammation, cytokine expression, and germinal center formation by BCL-6. Science. 1997;276:589–592. doi: 10.1126/science.276.5312.589. [DOI] [PubMed] [Google Scholar]
- Ertelt JM, Rowe JH, Johanns TM, Lai JC, McLachlan JB, Way SS. Selective priming and expansion of antigen-specific Foxp3- CD4+ T cells during Listeria monocytogenes infection. J. Immunol. 2009;182:3032–3038. doi: 10.4049/jimmunol.0803402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazilleau N, McHeyzer-Williams LJ, Rosen H, McHeyzer-Williams MG. The function of follicular helper T cells is regulated by the strength of T cell antigen receptor binding. Nat. Immunol. 2009;10:375–384. doi: 10.1038/ni.1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong D, Malek TR. Cytokine-dependent Blimp-1 expression in activated T cells inhibits IL-2 production. J. Immunol. 2007;178:242–252. doi: 10.4049/jimmunol.178.1.242. [DOI] [PubMed] [Google Scholar]
- Harrington LE, Janowski KM, Oliver JR, Zajac AJ, Weaver CT. Memory CD4 T cells emerge from effector T-cell progenitors. Nature. 2008;452:356–360. doi: 10.1038/nature06672. [DOI] [PubMed] [Google Scholar]
- Homann D, Teyton L, Oldstone MB. Differential regulation of antiviral T-cell immunity results in stable CD8+ but declining CD4+ T-cell memory. Nat. Med. 2001;7:913–919. doi: 10.1038/90950. [DOI] [PubMed] [Google Scholar]
- Hou J, Schindler U, Henzel WJ, Wong SC, McKnight SL. Identification and purification of human Stat proteins activated in response to interleukin-2. Immunity. 1995;2:321–329. doi: 10.1016/1074-7613(95)90140-x. [DOI] [PubMed] [Google Scholar]
- Jenkins MK, Khoruts A, Ingulli E, Mueller DL, McSorley SJ, Reinhardt RL, Itano A, Pape KA. In vivo activation of antigen-specific CD4 T cells. Annu. Rev. Immunol. 2001;19:23–45. doi: 10.1146/annurev.immunol.19.1.23. [DOI] [PubMed] [Google Scholar]
- Johnston RJ, Poholek AC, DiToro D, Yusuf I, Eto D, Barnett B, Dent AL, Craft J, Crotty S. Bcl6 and Blimp-1 are reciprocal and antagonistic regulators of T follicular helper cell differentiation. Science. 2009;325:1006–1010. doi: 10.1126/science.1175870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan DH, Jenison MC, Saeland S, Shlomchik WD, Shlomchik MJ. Epidermal langerhans cell-deficient mice develop enhanced contact hypersensitivity. Immunity. 2005;23:611–620. doi: 10.1016/j.immuni.2005.10.008. [DOI] [PubMed] [Google Scholar]
- Khoruts A, Mondino A, Pape KA, Reiner SL, Jenkins MK. A natural immunological adjuvant enhances T cell clonal expansion through a CD28-dependent, interleukin (IL)-2-independent mechanism. J. Exp. Med. 1998;187:225–236. doi: 10.1084/jem.187.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura D, Roes J, Kuhn R, Rajewsky K. A B cell-deficient mouse by targeted disruption of the membrane exon of the immunoglobulin mu chain gene. Nature. 1991;350:423–426. doi: 10.1038/350423a0. [DOI] [PubMed] [Google Scholar]
- Liao W, Lin JX, Wang L, Li P, Leonard WJ. Modulation of cytokine receptors by IL-2 broadly regulates differentiation into helper T cell lineages. Nat. Immunol. 2011;12:551–559. doi: 10.1038/ni.2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JX, Migone TS, Tsang M, Friedmann M, Weatherbee JA, Zhou L, Yamauchi A, Bloom ET, Mietz J, John S, et al. The role of shared receptor motifs and common Stat proteins in the generation of cytokine pleiotropy and redundancy by IL-2, IL-4, IL-7, IL-13, and IL-15. Immunity. 1995;2:331–339. doi: 10.1016/1074-7613(95)90141-8. [DOI] [PubMed] [Google Scholar]
- Linton PJ, Harbertson J, Bradley LM. A critical role for B cells in the development of memory CD4 cells. J. Immunol. 2000;165:5558–5565. doi: 10.4049/jimmunol.165.10.5558. [DOI] [PubMed] [Google Scholar]
- Lohning M, Hegazy AN, Pinschewer DD, Busse D, Lang KS, Hofer T, Radbruch A, Zinkernagel RM, Hengartner H. Long-lived virus-reactive memory T cells generated from purified cytokine-secreting T helper type 1 and type 2 effectors. J. Exp. Med. 2008;205:53–61. doi: 10.1084/jem.20071855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLeod MK, David A, McKee AS, Crawford F, Kappler JW, Marrack P. Memory CD4 T cells that express CXCR5 provide accelerated help to B cells. J. Immunol. 2011;186:2889–2896. doi: 10.4049/jimmunol.1002955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak TW, Shahinian A, Yoshinaga SK, Wakeham A, Boucher LM, Pintilie M, Duncan G, Gajewska BU, Gronski M, Eriksson U, et al. Costimulation through the inducible costimulator ligand is essential for both T helper and B cell functions in T cell-dependent B cell responses. Nat. Immunol. 2003;4:765–772. doi: 10.1038/ni947. [DOI] [PubMed] [Google Scholar]
- Moon JJ, Chu HH, Pepper M, McSorley SJ, Jameson SC, Kedl RM, Jenkins MK. Naive CD4(+) T cell frequency varies for different epitopes and predicts repertoire diversity and response magnitude. Immunity. 2007;27:203–213. doi: 10.1016/j.immuni.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita R, Schmitt N, Bentebibel SE, Ranganathan R, Bourdery L, Zurawski G, Foucat E, Dullaers M, Oh S, Sabzghabaei N, et al. Human blood CXCR5(+)CD4(+) T cells are counterparts of T follicular cells and contain specific subsets that differentially support antibody secretion. Immunity. 2011;34:108–121. doi: 10.1016/j.immuni.2010.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurieva RI, Chung Y, Hwang D, Yang XO, Kang HS, Ma L, Wang YH, Watowich SS, Jetten AM, Tian Q, et al. Generation of T follicular helper cells is mediated by interleukin-21 but independent of T helper 1, 2, or 17 cell lineages. Immunity. 2008;29:138–149. doi: 10.1016/j.immuni.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pape KA, Catron DM, Itano AA, Jenkins MK. The humoral immune response is initiated in lymph nodes by B cells that acquire soluble antigen directly in the follicles. Immunity. 2007;26:491–502. doi: 10.1016/j.immuni.2007.02.011. [DOI] [PubMed] [Google Scholar]
- Pearce EL, Walsh MC, Cejas PJ, Harms GM, Shen H, Wang LS, Jones RG, Choi Y. Enhancing CD8 T-cell memory by modulating fatty acid metabolism. Nature. 2009;460:103–107. doi: 10.1038/nature08097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepper M, Linehan JL, Pagan AJ, Zell T, Dileepan T, Cleary PP, Jenkins MK. Different routes of bacterial infection induce long-lived TH1 memory cells and short-lived TH17 cells. Nat. Immunol. 2010;11:83–89. doi: 10.1038/ni.1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pipkin ME, Sacks JA, Cruz-Guilloty F, Lichtenheld MG, Bevan MJ, Rao A. Interleukin-2 and inflammation induce distinct transcriptional programs that promote the differentiation of effector cytolytic T cells. Immunity. 2010;32:79–90. doi: 10.1016/j.immuni.2009.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portnoy DA, Auerbuch V, Glomski IJ. The cell biology of Listeria monocytogenes infection: the intersection of bacterial pathogenesis and cell-mediated immunity. J. Cell. Biol. 2002;158:409–414. doi: 10.1083/jcb.200205009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees W, Bender J, Teague TK, Kedl RM, Crawford F, Marrack P, Kappler J. An inverse relationship between T cell receptor affinity and antigen dose during CD4(+) T cell responses in vivo and in vitro. Proc. Natl. Acad. Sci. U S A. 1999;96:9781–9786. doi: 10.1073/pnas.96.17.9781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhardt RL, Khoruts A, Merica R, Zell T, Jenkins MK. Visualizing the generation of memory CD4 T cells in the whole body. Nature. 2001;410:101–105. doi: 10.1038/35065111. [DOI] [PubMed] [Google Scholar]
- Rubtsov YP, Niec RE, Josefowicz S, Li L, Darce J, Mathis D, Benoist C, Rudensky AY. Stability of the regulatory T cell lineage in vivo. Science. 2010;329:1667–1671. doi: 10.1126/science.1191996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu. Rev. Immunol. 2004;22:745–763. doi: 10.1146/annurev.immunol.22.012703.104702. [DOI] [PubMed] [Google Scholar]
- Sarkar S, Teichgraber V, Kalia V, Polley A, Masopust D, Harrington LE, Ahmed R, Wherry EJ. Strength of stimulus and clonal competition impact the rate of memory CD8 T cell differentiation. J. Immunol. 2007;179:6704–6714. doi: 10.4049/jimmunol.179.10.6704. [DOI] [PubMed] [Google Scholar]
- Shaffer AL, Lin KI, Kuo TC, Yu X, Hurt EM, Rosenwald A, Giltnane JM, Yang L, Zhao H, Calame K, et al. Blimp-1 orchestrates plasma cell differentiation by extinguishing the mature B cell gene expression program. Immunity. 2002;17:51–62. doi: 10.1016/s1074-7613(02)00335-7. [DOI] [PubMed] [Google Scholar]
- Shaffer AL, Yu X, He Y, Boldrick J, Chan EP, Staudt LM. BCL-6 represses genes that function in lymphocyte differentiation, inflammation, and cell cycle control. Immunity. 2000;13:199–212. doi: 10.1016/s1074-7613(00)00020-0. [DOI] [PubMed] [Google Scholar]
- Srinivas S, Watanabe T, Lin CS, William CM, Tanabe Y, Jessell TM, Costantini F. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev. Biol. 2001;1:4. doi: 10.1186/1471-213X-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadinski BD, Zhang L, Crawford F, Marrack P, Eisenbarth GS, Kappler JW. Diabetogenic T cells recognize insulin bound to IAg7 in an unexpected, weakly binding register. Proc. Natl. Acad. Sci. U S A. 2010;107:10978–10983. doi: 10.1073/pnas.1006545107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surh CD, Sprent J. Homeostasis of naive and memory T cells. Immunity. 2008;29:848–862. doi: 10.1016/j.immuni.2008.11.002. [DOI] [PubMed] [Google Scholar]
- Tafuri A, Shahinian A, Bladt F, Yoshinaga SK, Jordana M, Wakeham A, Boucher LM, Bouchard D, Chan VS, Duncan G, et al. ICOS is essential for effective T-helper-cell responses. Nature. 2001;409:105–109. doi: 10.1038/35051113. [DOI] [PubMed] [Google Scholar]
- Tsien RY. The green fluorescent protein. Annu. Rev. Biochem. 1998;67:509–544. doi: 10.1146/annurev.biochem.67.1.509. [DOI] [PubMed] [Google Scholar]
- van Faassen H, Saldanha M, Gilbertson D, Dudani R, Krishnan L, Sad S. Reducing the stimulation of CD8+ T cells during infection with intracellular bacteria promotes differentiation primarily into a central (CD62LhighCD44high) subset. J. Immunol. 2005;174:5341–5350. doi: 10.4049/jimmunol.174.9.5341. [DOI] [PubMed] [Google Scholar]
- Vinuesa CG, Cook MC, Angelucci C, Athanasopoulos V, Rui L, Hill KM, Yu D, Domaschenz H, Whittle B, Lambe T, et al. A RING-type ubiquitin ligase family member required to repress follicular helper T cells and autoimmunity. Nature. 2005;435:452–458. doi: 10.1038/nature03555. [DOI] [PubMed] [Google Scholar]
- Whitmire JK, Asano MS, Kaech SM, Sarkar S, Hannum LG, Shlomchik MJ, Ahmed R. Requirement of B cells for generating CD4+ T cell memory. J. Immunol. 2009;182:1868–1876. doi: 10.4049/jimmunol.0802501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willerford DM, Chen J, Ferry JA, Davidson L, Ma A, Alt FW. Interleukin-2 receptor alpha chain regulates the size and content of the peripheral lymphoid compartment. Immunity. 1995;3:521–530. doi: 10.1016/1074-7613(95)90180-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.