Abstract
Interhemispheric communication consists of a complex balance of facilitation and inhibition that is modulated in a task-dependent manner. However, it remains unclear how individual differences in interhemispheric interactions relate to motor performance. To assess interhemispheric inhibition, we utilized the ipsilateral silent period technique (iSP; evoked by suprathreshold transcranial magnetic stimulation), which elicits inhibition of volitional motor activity. Participants performed three force production tasks: 1) unimanual (right hand) constant force, 2) bimanual constant force, (bimanual simultaneous) and 3) bimanual with right hand constant force and left hand sine wave tracking (bimanual independent). We found that individuals with greater IHI capacity demonstrated reduced mirror EMG activity in the left hand during unimanual right hand contraction. However, these same individuals demonstrated the poorest performance during the bimanual independent force production task. We suggest that a high capacity for IHI from one motor cortex to another can effectively prevent “motor overflow” during unimanual tasks, but it can also limit interhemispheric cooperation during independently controlled bimanual tasks.
Keywords: ipsilateral silent period, force production, motor overflow
INTRODUCTION
Imagine a concert pianist with exceptional bimanual coordination yet exquisite individuated finger control. Such skillful bimanual acts require that hand movements are coordinated in time and space, with minimal intermanual crosstalk or interference [1]. The ability for the two cortical hemispheres to coordinate such movements relies heavily upon callosally mediated communication [2]. This interhemispheric communication consists of a complex balance of facilitatory and inhibitory interactions. Action potentials transmitted transcallosally are primarily excitatory; however the fiber tracts between the primary motor cortices (M1s) project primarily to inhibitory interneurons and thus the effect of transcallosal potentials between M1s are predominately inhibitory [3]. While some degree of interhemispheric inhibition (IHI) is necessary to prevent interference of control processes between the two cortices [4], emerging work has begun to indicate that low levels of IHI may be beneficial for the performance of independently controlled bimanual tasks [5, 6].
Interhemispheric inhibition has been shown to increase during tasks when the upper limbs have different movement goals. For example, during unilateral movement IHI increases to suppress unwanted motor (or mirror) activity in the opposite limb [7, 8]. Furthermore, several studies report increased IHI during multi-limb movements where each effector has different movement goals as compared to movements where the limbs move in synchrony [9, 10]. Thus it stands to reason that by increasing IHI during such movements, individuals are able to maintain partitioned cortical activity resulting in less interference between the right and left motor cortices. While these studies demonstrate that IHI is modulated across task conditions, no motor performance metrics were presented. Thus it remains unclear how the amount of IHI is related to manual motor performance.
Emerging work has begun to indicate that the relationship between IHI and motor performance is complex. Poorer motor performance is reported in individuals with compromised inhibitory capacity such as children with premyelinated interhemispheric fibers [11] or patients with multiple sclerosis [12]. On the other hand, IHI is also reduced in musicians (experts in manual motor control) compared to non-musicians [5, 13], and studies demonstrate that IHI is decreased with a concomitant improvement in bimanual motor control following brief training interventions [6,14]. The latter studies demonstrate that interhemispheric inhibitory projections can show plastic changes that favor the execution of a practiced task by decreasing the amount of IHI.
Along with others, we have shown that IHI is highly correlated with the microstructure of callosal fibers connecting the homologous M1’s [15, 16]. Recent work utilizing diffusion tensor imaging has lent insight into the relationship between callosal microstructure and motor performance across a range of unimanual and bimanual tasks. Johansen-Berg and colleagues [17] reported that participants with better microstructural integrity of callosal fibers connecting primary and secondary motor cortices were better able to synchronize the two hands during a simultaneous bimanual tapping task (a task requiring relatively low IHI). Conversely, we [18] have recently shown that individuals with increased microstructural quality of callosal regions connecting sensorimotor cortical areas perform more poorly on unimanual and asynchronous bimanual finger tapping tasks (which require relatively high IHI). These studies, taken together with the work of Nordstrom and colleagues [5, 13], suggest that IHI is likely related to performance of unimanual and bimanual movements in a task-dependent fashion.
The use of an individual differences approach has recently been touted as a fruitful avenue for understanding how brain structural and neurophysiological characteristics affect motor performance [19]. Here we utilized such an approach to investigate the relationship(s) between IHI and performance on three different force production tasks: 1) unimanual (right hand) constant force production, 2) bimanual constant force production (bimanual simultaneous), and 3) bimanual with the right hand performing constant force and the left hand tracking a sine wave target (bimanual independent). Interhemispheric inhibition was assessed by eliciting iSPs in the dominant hand during all force production conditions. The iSP reflects transcallosal inhibition of volitional motor activity, and is particularly well-suited to investigate interhemispheric control of voluntary cortical motor output [3, 10]. We hypothesized that individuals with greater IHI capacity would be able to inhibit motor overflow during unimanual contraction to a greater extent. However, we predicted that these same individuals would demonstrate poorer performance on the bimanual independent task.
METHODS
Participants
Sixteen young adults (range: 19–28 yr of age; mean: 22.1 ± 3.1 yr; 8 males) participated in the current study. All individuals were right-hand dominant as assessed by the Edinburgh Handedness Inventory (mean: 77.4 ± 0.09) [20]. Any individuals with a history of neurological insult (e.g. head injury, history of migraines, epilepsy) or those taking medications with contraindications for transcranial magnetic stimulation were excluded, as were any individuals with musical training greater than 3 years. This experiment was approved by the Medical Institutional Review Board (IRBMED) of the University of Michigan. Participants gave their informed written consent prior to the experiment and were compensated for their time.
Experimental Task
Participants were seated in a chair with both their dominant and non-dominant forearms resting on a table. The shoulders were abducted at approximately 45°, the elbows were flexed at approximately 90°, and the forearms were pronated with the palms of the hand lying flat on the custom apparatus. The wrist, third, fourth, and fifth fingers were constrained from moving, isolating force production to the index finger. A pre-amplified force transducer (OMEGA LC509-015 Beam Load Cell) was positioned at the lateral aspect of the proximal interphalangeal joint of the isolated index finger to record compressive isometric force (output: 0.5–9.5 Vdc; excitation: 24 Vdc ± 4 Vdc).
Surface electromyography and motor evoked potentials (MEPs) elicited with transcranial magnetic stimulation (TMS) were recorded from the first dorsal interosseous (FDI) muscle of both hands using 4 mm Ag/AgCl- electrodes placed on the muscle in a belly-tendon arrangement. Task-related surface EMG and MEP data were recorded using Biopac hardware and AcqKnowledge software (BIOPAC Systems Inc., Goleta, CA). The raw EMG signal was collected and digitized at 2000 Hz, amplified and band pass filtered (10–1000 Hz). Data were collected at 2 kHz as it has previously been shown to produce the most accurate estimate of mean consecutive difference (MCD) of pre-stimulus EMG activity, which is used to calculate the iSP [21]. To assess maximal voluntary contractile (MVC) force of the FDI participants were instructed to press as hard as possible on the load cell using index finger abduction of the dominant hand for three consecutive 6-second trials. The force applied to the load cell was displayed on the video monitor, providing online visual feedback during the MVC trials. The highest force sample in each trial was averaged across three MVC trials providing an estimate of the participants’ MVC. A minimum 90-second rest period was provided in between each MVC trial. The same procedure was subsequently performed on the non-dominant hand.
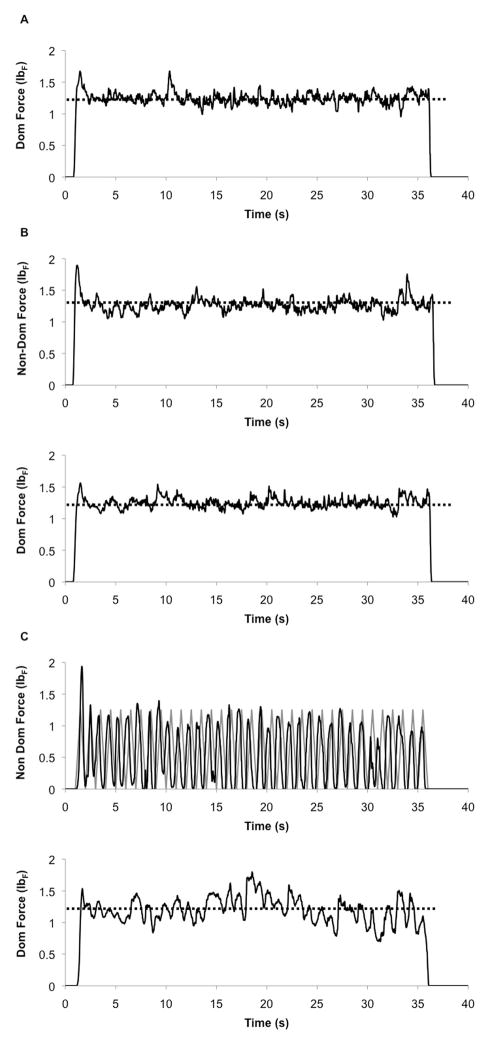
Participants performed three different force production tasks: 1) unimanual isometric force of the dominant hand at a constant force target level (Figure 1A), 2) bimanual simultaneous isometric force with both hands producing the same constant force target level (bimanual simultaneous task; Figure 1B), and 3) dominant hand isometric force at a constant force target level while the non-dominant hand matched a 1 HZ sine wave (bimanual independent task; Figure 1C). Thus, in all conditions the dominant hand always performed the same constant force production task while the non-dominant hand’s involvement varied across the three conditions. The order of conditions was counter-balanced across participants. For all conditions the force target levels used in the experiment were scaled to 20% of each individual’s MVC (during the bimanual independent task the peak force of the non-dominant sine wave-matching hand was also 20% MVC). Thus the dominant hand always produced a constant isometric force at 20% MVC across the three conditions while the varying role of the non-dominant hand (i.e. the right M1) was expected to modulate IHI demands. Feedback windows were presented on one video monitor and were aligned parallel on top of each other with the upper window providing feedback of the non-dominant hand force trace while the lower window provided feedback for the dominant hand. During the unimanual and bimanual simultaneous force production tasks participants viewed a yellow, horizontal target line that spanned the width of a video monitor (placed at 20% of MVC for each respective hand). During the bimanual independent movement condition the target for the dominant hand was identical to the other two conditions while the target for the non-dominant hand was a yellow 1-Hz sine wave target line with the peak force of each sine wave placed at 20% of MVC. Force output was sampled at 200 Hz; for each instant in time that force was sampled (.005s), a green pixel appeared in the center of the respective feedback window corresponding to the amount of force produced. This provided participants with on-line, real-time visual feedback of their performance with minimal horizontal saccades required. For each condition participants were instructed to match their green force trace to the respective yellow target line for three 35-second trials per condition. Participants were provided rest breaks of five minutes in between each trial (a total of 9 trials) to minimize fatigue.
Figure 1.
Representative force traces for the: A) dominant hand unimanual force condition, B) non-dominant (upper panel) and dominant hand (lower panel) during a bimanual simultaneous force production trial and, C) non-dominant (upper panel) and dominant hand (lower panel) during the bimanual independent force production task. For all isometric force conditions the horizontal dotted line indicates the force target, during the bimanual independent force task the gray line indicates the 1 Hz sine wave target for the non-dominant hand. Dom = dominant hand; Non-Dom = non-dominant hand; lbF = pound-force.
Interhemispheric Inhibition
Interhemispheric inhibition was measured by evoking an ipsilateral silent period (iSP) in the dominant hand FDI. The iSP is elicited by focal TMS of the M1 ipsilateral to the hand making a voluntary contraction, leading to a brief suppression of voluntary activity in the electromyogram signal of this muscle [3].
Prior to performing the experimental paradigm, we used a Magstim Rapid magnetic stimulator (The Magstim Company Ltd, Spring Gardens, Whitland, Carmarthenshire, UK) and a focal figure of eight coil (diameter of each wing 70 mm) to identify the right M1 hotspot for the left FDI. The coil was placed tangential to the scalp with the handle pointing backwards and 45° away from the midline [22]. The optimum site in the right M1 (hotspot) for eliciting motor responses in the left FDI was identified at supra-threshold intensity. This location was marked on the scalp and subsequently utilized to elicit iSPs from the right FDI as it has previously been shown that the topography of the contralateral MEP and the iSP correspond closely [23]. Resting motor threshold (RMT) was determined to the nearest 1% of the maximum stimulator output. Using standard protocol, the RMT was defined as the minimum stimulus intensity which elicited MEPs > 50 μV in at least 5 out of 10 consecutive trials [24].
During the unimanual and bimanual simultaneous force conditions, iSPs were elicited from the dominant hand once participants reached the target force level by stimulation given at 120% of resting motor threshold to the right motor cortex [15]. During the bimanual independent condition stimulations were always applied during the upward phase of the non-dominant hand sine wave force production task. During each 35-second trial, a minimum of five iSPs were evoked with an interstimulus interval jittered between 5–8 seconds [21, 25]. During the unimanual condition, EMG data were also collected and monitored from the non-dominant hand to ensure full relaxation and that the TMS coil was always in the appropriate location over the right M1. During one trial in two different participants, MEPs ceased to be elicited from the left FDI. On both occasions data collection was halted and the right M1 hotspot for the FDI was relocated.
Data Analysis
Force data
The initial 5 seconds of each force time series was removed to eliminate the transitory performance in achieving the force target. Force data were digitally filtered using a 4th order dual pass Butterworth filter with a low-pass cutoff frequency of 20 Hz. All data processing and subsequent time and frequency analyses were performed using software written in MATLAB (The MathWorks Inc., Natick, MA). Similar to previous work, motor performance was assessed by calculating the within-trial mean (normalized to the force target goal and expressed as a percentage throughout the remainder of this manuscript; i.e. accuracy) and the root mean square error (RMSE; i.e. variability) from the target [26]. We also performed a power spectral density analysis to assess peak frequency of the non-dominant hand during the bimanual independent condition to ensure that individuals were adhering to the 1 Hz frequency.
Similar to previous work, “motor overflow” was assessed for both groups during the unimanual dominant hand force production condition by taking the rectified integral of the non-dominant (resting) hand EMG normalized to baseline measures of EMG activity for the same hand [27]. Thus motor overflow is expressed as a percentage of the baseline EMG (i.e. 100% would indicate no change relative to baseline) to decrease inter-subject variability due to differences in skin-electrode impedance, background noise, or arousal.
Ipsilateral Silent Period (iSP)
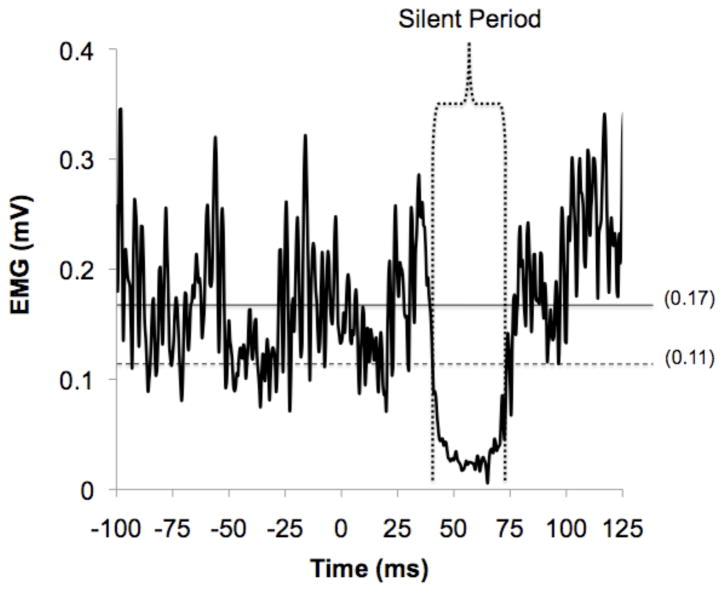
The iSP was calculated by using an objective, graphical method described in detail by Garvey and colleagues [21]. Single EMG trials (15 total per participant) were rectified and averaged across trials. Using a custom MATLAB program (The MathWorks Inc., Natick, MA), upper and lower variation limits of the EMG signal were calculated by determining the mean consecutive difference (MCD) of EMG data points 100ms prior to stimulation: mean pre-stimulus EMG ± (|MCD| × 1.77). These limits encompassed 95% of possible pre-stimulus EMG data points (equivalent to two standard deviations). Onset and offset of iSP were identified using the following criteria (see Figure 2): (1) time of onset was the first of 5 consecutive data points to fall below the lower variation limit; (2) all subsequent data points were considered part of the iSP until there was a return of sustained EMG activity; (3) time of offset was defined as the first data point to fall above the lower variation limit if 50% or more of the data points in the following 5 ms window were also above the variation limit [21]. In addition to measuring the onset, offset and duration of the iSP, the depth of the iSP was defined in two ways as previously described by Jung and Ziemann [25]: (1) the minimum EMG level during the iSP (diSP-max), and (2) the average EMG level during the iSP (diSP). The diSP and diSP-max were expressed as percentages of the mean prestimulus EMG. Therefore, the larger the diSP and diSP-max, the greater the suppression of ipsilateral EMG activity during the iSP. This is interpreted as increased interhemispheric inhibition [21].
Figure 2.
EMG data from one representative subject illustrating the TMS-induced iSP. Data displayed are the rectified average from 15 stimulation trials (applied at time 0) during 20% MVC isometric contraction. The horizontal solid line represents mean pre-stimulus EMG activity (0.17 mV). The horizontal dotted line represents the lower variability limit (0.11 mV). Vertical dotted lines encompass the ipsilateral silent period.
Statistical Analysis
Separate repeated measures analyses of variance were used to analyze measures of IHI and dominant hand force production with iSP onset, iSP duration, diSP, diSP-max, RMSE, and normalized mean force production treated as within-subject variables across the three conditions. Significance was set at an alpha of 0.05 (SPSS 18.0) and the Huyn-Feldt epsilon was computed to test for sphericity; we interpreted corrected P values in cases of violation. Significant main effects were subjected to post-hoc paired t-tests and Bonferroni-corrected for multiple comparisons. We performed linear regression to investigate the relationship between diSP and dominant hand variability (RMSE) on the three different force production tasks. We also correlated non-dominant hand EMG activity during the unimanual force condition (i.e. motor overflow) with diSP. All correlation analyses using diSP as a predictor were corrected for multiple comparisons (α = 0.05/4). Additionally, we used linear regression to test for associations between each hand’s force output during the bimanual simultaneous and bimanual independent force production tasks. Thus, we correlated force output between the dominant and non-dominant hand within each participant for the two bimanual conditions. All data are presented as mean ± standard deviation unless otherwise noted.
RESULTS
Force Data
The maximal voluntary contraction values from the dominant and non-dominant FDI were 4.3 ± 0.6 lbF and 3.9 ± 0.5 lbF, respectively. While a paired t-test revealed a trend towards higher MVC in the dominant hand, no statistical difference was observed (t1,15 = 2.1; P < 0.07).
Participants’ force production parameters including accuracy and variability can be viewed in Table 1. Additionally, during the bimanual independent condition participants’ non-dominant hand average frequency was 0.93 ± 0.01 Hz, indicating that they were able to adhere to the relatively difficult bimanual force production task (Figure 1C).
Table 1.
Constant isometric force production variables describing dominant hand performance across the three conditions. The dominant hand was most accurate (normalized mean force) and demonstrated the least variability (RMSE) during the unimanual condition. The force output timecourse was significantly correlated between the two hands during the bimanual simultaneous condition, but not during the bimanual independent condition.
| Force Condition | Normalized Mean Force (%) | Variability (RMSE) | B/w Hand Correlations (r) |
|---|---|---|---|
| Unimanual | 93.7 ± 1.5a | 0.031 ± 0.004a | - |
| Bim. Simultaneous | 90.9 ± 2.0a | 0.048 ± 0.005b | 0.69* |
| Bim. Independent | 74.2 ± 3.4b | 0.088 ± 0.008c | 0.34 |
Values are mean ± standard deviation. Bim. = bimanual; B/w = between. The same letter within each column indicates metrics of force performance that were not significantly different from each other.
P < 0.01.
A repeated measures ANOVA revealed a significant main effect of condition on dominant hand force variability (F2,30 = 45.8; P < 0.001) and accuracy (F2,30 = 31.8; P < 0.001). Post hoc tests for dominant hand force variability revealed a significant difference between all conditions (t1,15 > 5.0; P < 0.001; Table 1), such that variability was greatest during the bimanual independent task and lowest during the unimanual task. Post hoc paired t-tests for normalized dominant hand accuracy revealed that force production was significantly lower during the bimanual independent condition than either the unimanual (t1,15 = 6.7; P < 0.001) or the bimanual simultaneous conditions (t1,15 = 5.3; P < 0.001). No difference in accuracy was observed between the unimanual and bimanual simultaneous conditions (t1,15 = 1.7; P > 0.15; Table 1).
iSP Data
Resting motor threshold (RMT) was 63.6% ± 4.9% of maximal stimulator output, thus average stimulation intensity to elicit iSPs was 76.3% ± 5.7% (i.e. 120% of RMT). Ipsilateral silent periods were reliably produced in all 16 participants. An average of 15.6 stimulations (and a maximum of 19) were required to elicit the 15 iSPs used for data analysis within each condition. Participants’ IHI characteristics including iSP onset, iSP duration, diSP and diSP-max can be viewed in Table 2. A repeated measures ANOVA revealed no significant main effect of condition on iSP onset (F2,30 = .37; P > 0.68), iSP duration (F2,30 = 0.02; P > 0.96), diSP (F2,30 = 1.3; P > 0.27), nor diSP-max (F2,30 = .45; P > 0.62).
Table 2.
Characteristics of dominant hand iSPs across the three force production conditions. No differences were noted among any of the dependent measures.
| Force Condition | iSP Onset (ms) | iSP Duration (ms) | diSP (%) | diSP-max (%) |
|---|---|---|---|---|
| Unimanual | 38.4 (3.2) | 27.4 (8.7) | 73.6 (6.3) | 91.9 (4.4) |
| Bim. Simultaneous | 37.9 (2.6) | 27.1 (10.1) | 71.4 (9.6) | 92.4 (4.2) |
| Bim. Independent | 38.2 (2.7) | 26.9 (7.5) | 70.0 (9.2) | 91.2 (5.4) |
Values are mean ± standard deviation. Bim. = bimanual.
Relationships between EMG activity, force variability and IHI
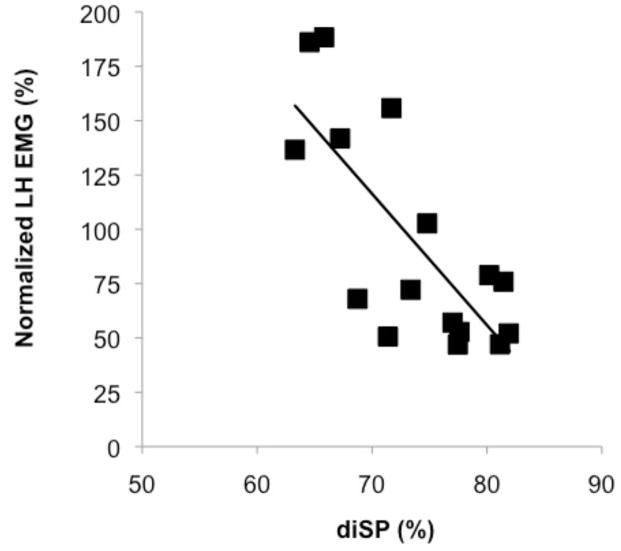
We first performed linear regression relating IHI, as assessed by diSP during the unimanual condition, to motor overflow observed during the dominant hand unimanual force production task. During unimanual contractions muscle activity was slightly decreased in the non-dominant hand relative to baseline (94.6 ± 24.6% of baseline activity). Furthermore, motor overflow and IHI were significantly inversely correlated (r = −0.76; P < 0.001; Figure 3) demonstrating that increased IHI resulted in decreased motor overflow.
Figure 3.
Scatterplot of interhemispheric inhibition, as assessed by diSP, and non-dominant hand EMG (normalized to baseline) during unimanual dominant hand contraction. A significant, negative association was found (r = −0.76; P < 0.001) indicating that individuals with greater IHI were able to suppress activity in the resting non-dominant hand (motor overflow) during unimanual dominant hand force production. IEMG = integral of the rectified EMG.
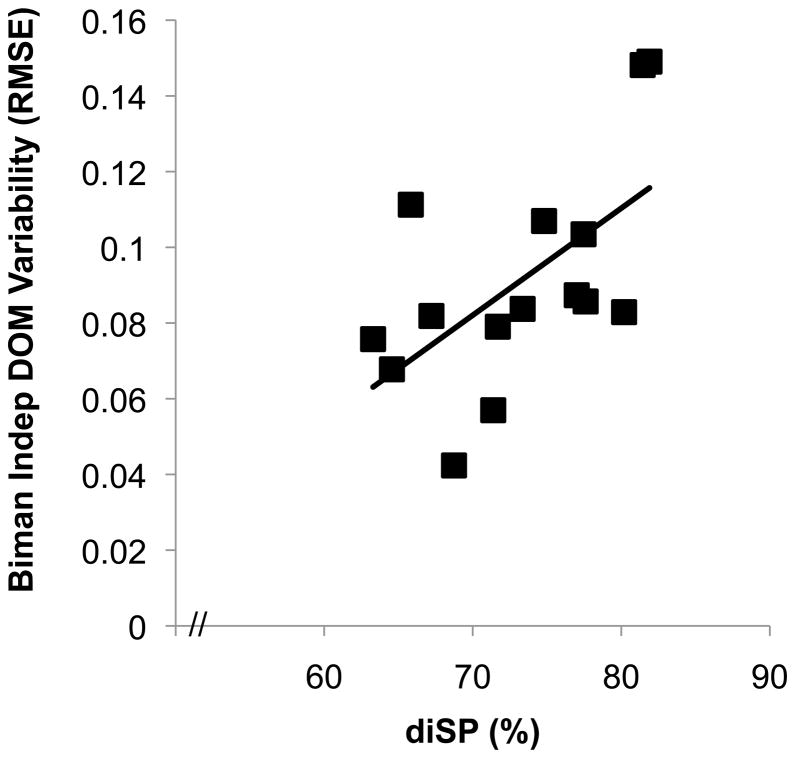
In addition, we regressed diSP against dominant hand force variability during each respective force condition. We found no relationship between diSP and dominant hand variability during the unimanual (r = −0.13; P > 0.4) or the bimanual simultaneous condition (r = −0.19; P > 0.25). In contrast, a significant relationship was observed between diSP and dominant hand variability during the bimanual independent condition (r = 0.59; P < 0.01; Figure 4) indicating that individuals with increased IHI were poorer at maintaining steady force during this particular task.
Figure 4.
Scatterplot of interhemispheric inhibition, as assessed by diSP, and dominant hand variability during the bimanual independent task. A significant, positive relationship was observed (r = 0.59; P < 0.01) indicating that individuals with greater IHI had greater dominant hand force variability during the bimanual independent task. Bim; = bimanual; Indep = independent; DOM = dominant hand.
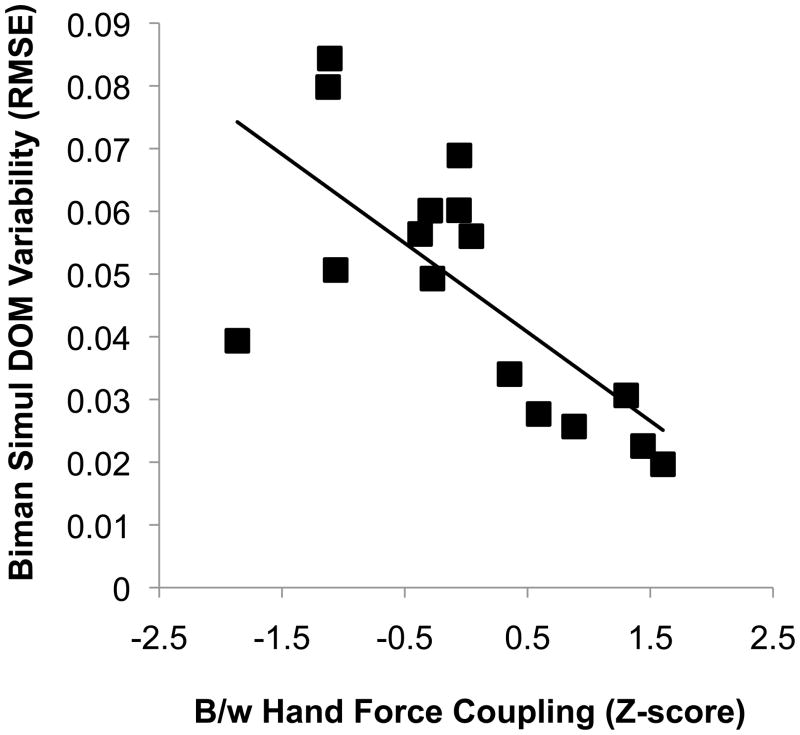
For both bimanual tasks we also performed linear regression to describe associations between dominant and non-dominant hand force output. The time course of force produced by each hand was significantly correlated during the bimanual simultaneous condition (r = 0.69; P < 0.01), but not during the bimanual independent condition (r = 0.34; P > 0.1). For each of these correlations we performed an r-to-Z transformation and regressed dominant hand force variability (RMSE) against between-hand force relationships (as assessed by their Z-score) within each bimanual condition. The extent to which the time course of force production was correlated between the two hands during the bimanual simultaneous condition was significantly inversely related to dominant hand variability (r = −0.7; P < 0.001; Figure 5). That is, participants with greater linked bimanual force output performed the task with less dominant hand variability during the simultaneous condition. A similar trend was observed for the bimanual independent condition, however the relationship was not significant (r = −0.33; P > 0.1).
Figure 5.
Scatterplot of dominant hand force variability (RMSE) during the bimanual simultaneous force production task and between-hand force coupling (assessed with a Fisher r-to-Z transformation). A significant, inverse association (r = −0.70; P < 0.01) was observed demonstrating that individuals performed the task better when they coupled the output of the two hands to a greater extent. Biman = bimanual; Simul = simultaneous; DOM = dominant hand.
DISCUSSION
We report a task-specific relationship between IHI and independent bimanual motor control. Consistent with our hypotheses, during unimanual force production individuals with greater IHI were able to more effectively reduce motor overflow. Conversely, those individuals with greater IHI were poorer at the bimanual independent task. We suggest that, while some degree of IHI is required for independent bimanual control, too high a level of IHI is detrimental during such tasks. Additionally, we found a continuum of variability in dominant hand force production across the three conditions investigated. Specifically, dominant hand variability increased (with a concomitant decrease in mean force production) when the non-dominant hand was required to produce either an identical or an independent force trajectory as compared to the unimanual task.
Interhemispheric interactions vary with task complexity [28]. Inhibition between the primary motor cortices is a necessity for the skilled performance of unimanual and bimanual motor control, particularly for tasks that require independent spatio-temporal paths for each hand [29]. While some level of IHI is necessary, we show that greater IHI is negatively associated with the ability to maintain steady force production when the contralateral hand is performing an independent action. This is in agreement with previous work demonstrating that interference related to bimanual force production arises from callosal interactions [30]. The current results suggest that individuals with greater IHI likely have a reduced capacity for interhemispheric cooperation, resulting in poorer performance on motor tasks requiring high levels of coordination. That is to say, these individuals may be “over-inhibiting” the contralateral cortex, thereby preventing each hemisphere from accurately activating its respective neuronal circuitry. Thus, while some level of IHI is required to coordinate movement, it appears that the ability to suppress IHI and increase interhemispheric cooperation favors the execution of bimanual tasks.
Studies conducted with highly trained (and developing) musicians support the hypothesis that reduced IHI is beneficial for bimanual tasks where the two hands have independent movement goals [5,13]. Long-term training within critical developmental periods has the potential to induce regionally specific neural adaptations, both at the anatomical and functional level. For example, the anterior half of the corpus callosum is larger in adult musicians who began training prior to the age of 7 when compared to control participants [31]. Whereas musical training appears to increase the size of anatomically specific regions in the brain, IHI is reduced in musicians compared to non-musicians [5]. Thus, it stands to reason that reduced IHI lends itself to better individuated bimanual control as we show in the current study. An exciting, though meager, body of literature indicates that interhemispheric physiology is also malleable via training interventions over a relatively short time-course. For example, following just two days of practice on a novel bimanual force production task, participants demonstrated task-selective reductions in IHI [6]. Furthermore, Hortobagyi et al. [14] reported a significant decrease in IHI and a concomitant increase in ipsilateral primary motor cortex excitability following 20 sessions of unimanual submaximal force production. A recent study revealed a reduction in asymmetry of cortical activity, assessed by functional MRI, following just five 1-hour training sessions in older adults (mean age = 66.1 years old), suggesting that the capability for plasticity of interhemispheric interactions remains even with advanced age [32]. This plasticity at the neuronal level is likely reflective of changes at the neurotransmitter level.
Interhemispheric inhibition is mediated by transcallosal glutamatergic pathways that synapse onto pyramidal tract neurons through gamma-aminobutyric acid (GABA) inhibitory interneurons [33]. Stagg and colleagues [34] reported that participants with higher baseline levels of GABA in M1 had slower reaction times. Furthermore, a positive correlation was observed between transcranial direct current stimulation (tDCS)-induced GABA decrease in M1 and degree of motor learning, such that subjects who demonstrated a greater decrease in M1 GABA following stimulation showed faster short-term learning [34]. It is possible that training-related decreases in IHI [6, 14] and GABA levels [34, 35] represent a change in the balance of interhemispheric facilitatory and inhibitory communication. In the current study, individuals who coupled the force output of the two hands to a greater extent, potentially via greater interhemispheric facilitation, performed the bimanual simultaneous task with less force variability. Future work would benefit from exploring an apparent increase in cooperative action between bilateral motor cortices as a result of manual training and/or non-invasive stimulation.
Callosally-mediated interhemispheric inhibition is a complex process that has traditionally been measured in humans using either the single-pulse iSP or with paired-pulse TMS to each primary motor cortex at an interstimulus interval of 8–12 ms [cf. 36; short interval interhemispheric inhibition, or SIHI]. While both the iSP and SIHI are reflective of transcallosal inhibition, they do not appear to represent the same phenomenon, nor are the two values correlated with each other within individuals [22]. The iSP represents interruption of voluntary cortical activity and likely relies upon GABAB receptors [33]. Conversely SIHI is mediated by GABAA receptors [37] and reflects inhibition of synchronized activation of the corticospinal system induced by the conditioning stimulus (the first stimulus applied). Due to the fact that the iSP reflects inhibition of volitional motor activity, it appears to be particularly well-suited to investigate interhemispheric control of voluntary cortical motor output [10] and was the method of choice in the current study.
It is also worth noting that multiple secondary motor regions appear to be involved in the interhemispheric inhibitory network [38], in addition to the primary motor cortices. Although full characterization of the transcallosal inhibitory sensorimotor network is still lacking, neuroimaging data suggest that it includes the supplementary and pre-supplementary motor areas [38, 39], the dorsal premotor cortices [40, 41] and the somatosensory cortices [42]. Thus, the findings here may be specific to just one of several interhemispheric motor control pathways.
It was somewhat surprising that we did not observe modulation of IHI across the different force production tasks in the current study as has previously been shown [10]. The current study and that of Giovannelli utilized identical stimulation parameters (120% of resting motor threshold) and both elicited iSPs from the dominant hand FDI. However, there is a clear difference in the force production tasks between the two studies. Participants in the Giovannelli study were required to produce maximal force with the dominant hand FDI across all conditions, whereas participants in the current study were only producing force at 20% of their maximal output. This was necessary to ensure that fatigue did not contaminate the multiple condition effects in the current study and better represents force production of everyday tasks. It may be that extremely high levels of interhemispheric and descending motor output (associated with maximal force production) are required for task-dependent modulation of IHI as reported by Giovannelli et al. [10].
An additional caveat to note is the attentional demand required to perform two tasks simultaneously, particularly during the bimanual independent force production task used in the current study. It is well established that performing two tasks simultaneously often degrades performance of one or both tasks, potentially due to competition for shared attentional resources [43]. In the current study performance of the dominant hand became more variable as the involvement of the non-dominant hand increased. Therefore, while the increase in dominant hand variability may be due to interhemispheric M1 interactions, it is also quite possible that the dual-task nature of the bimanual condition played a significant role. This concern is somewhat tempered by the fact that we did not observe any association between IHI and dominant hand force variability during the bimanual simultaneous condition (also a dual task).
Conclusions
Skillful bimanual control requires that hand movements are coordinated in time and space, with minimization of intermanual interference through interhemispheric inhibition. We report that isometric dominant hand force variability was increased when the non-dominant hand was required to produce either an identical or an independent force trajectory as compared to the dominant hand. Our results shed light on the task-specific nature of interhemispheric inhibition for tasks with different movement requirements. As shown in the current study, IHI is necessary for the performance of unimanual movements to reduce motor overflow. Furthermore, although some level of IHI is necessary for the performance of bimanual tasks where each hand is performing an independent movement, it is clear that reduced IHI is beneficial to performance in this context. Finally, as the acallosal literature has shown [29], interhemispheric communication and IHI are not a necessity for the performance of bimanual movements where the two hands have identical movement goals. This final statement may be slightly tempered by the fact that during the bimanual simultaneous task individuals who coupled the force output of the two hands to a greater extent, potentially by relying on more interhemispheric cooperation, performed the task with less force variability.
Research Highlights.
Interhemispheric inhibition (IHI) is necessary for individuated movements
The relationship between IHI and motor performance is poorly understood
We correlate IHI with performance on both unimanual and bimanual force tasks
Increased IHI reduces motor overflow during unimanual force production
Conversely, increased IHI limits performance on independent bimanual force production
Acknowledgments
The authors thank Scott Carr and Yanin Vongkancom for their assistance with equipment fabrication and data collection.
GRANTS
This work was supported by the National Institutes of Health (T32-AG00114).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bloom JS, Hynd GW. The role of the corpus callosum in interhemispheric transfer of information: excitation or inhibition? Neuropsychol Rev. 2005;15:59–71. doi: 10.1007/s11065-005-6252-y. [DOI] [PubMed] [Google Scholar]
- 2.Carson RG. Neural pathways mediating bilateral interactions between the upper limbs. Brain Research Reviews. 2005;49:641–62. doi: 10.1016/j.brainresrev.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 3.Meyer BU, Roricht S, Grafin von Einsiedel H, Kruggel F, Weindl A. Inhibitory and excitatory interhemispheric transfers between motor cortical areas in normal humans and patients with abnormalities of the corpus callosum. Brain. 1995;118:429–440. doi: 10.1093/brain/118.2.429. [DOI] [PubMed] [Google Scholar]
- 4.Fling BW, Peltier SJ, Bo J, Welsh RC, Seidler RD. Age differences in interhemispheric interactions: callosal structure, physiological function, and behavior. Front in Neurosci. 2011;5:38. doi: 10.3389/fnins.2011.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ridding MC, Brouwer B, Nordstrom MA. Reduced interhemispheric inhibition in musicians. Exp Brain Res. 2000;133:249–253. doi: 10.1007/s002210000428. [DOI] [PubMed] [Google Scholar]
- 6.Shim JK, Kim SW, Oh SJ, Kang N, Zatsiorsky VM, Latash ML. Plastic changes in interhemispheric inhibition with practice of a two-hand force production task: a transcranial magnetic stimulation study. Neurosci Lett. 2005;374:104–108. doi: 10.1016/j.neulet.2004.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cincotta M, Ziemann U. Neurophysiology of unimanual motor control and mirror movements. Clin Neurophys. 2008;119:744–762. doi: 10.1016/j.clinph.2007.11.047. [DOI] [PubMed] [Google Scholar]
- 8.Vercauteren K, Pleysier T, Van Belle L, Swinnen SP, Wenderoth N. Unimanual muscle activation increases interhemispheric inhibition from the active to the resting hemisphere. Neurosci Lett. 2008;445:209–213. doi: 10.1016/j.neulet.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 9.Stinear JW, Byblow WD. Disinhibition in the human motor cortex is enhanced by synchronous upper limb movements. J Physiol. 2002;543:307–316. doi: 10.1113/jphysiol.2002.023986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giovannelli F, Borgheresi A, Balestrieri F, Zaccara G, Viggiano MP, Cincotta M, Ziemann U. Modulation of interhemispheric inhibition by volitional motor activity: an ipsilateral silent period study. J Physiol. 2009;587:5393–5410. doi: 10.1113/jphysiol.2009.175885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koerte I, Heinen F, Fuchs T, Laubender RP, Pomschar A, Stahl R, Berweck S, Winkler P, Hufschmidt A, Reiser MF, Ertl-Wagner B. Anisotropy of callosal motor fibers in combination with transcranial magnetic stimulation in the course of motor development. Invest Radiol. 2009;44(5):279–84. doi: 10.1097/RLI.0b013e31819e9362. [DOI] [PubMed] [Google Scholar]
- 12.Boroojerdi B, Hungs M, Mull M, Topper R, Noth J. Interhemispheric inhibition in patients with multiple sclerosis. Electroencephalogr Clin Neurophysiol. 1998;109(3):230–237. doi: 10.1016/s0924-980x(98)00013-7. [DOI] [PubMed] [Google Scholar]
- 13.Nordstrom MA, Butler SL. Reduced intracortical inhibition and facilitation of corticospinal neurons in musicians. Exp Brain Res. 2002;144:336–342. doi: 10.1007/s00221-002-1051-7. [DOI] [PubMed] [Google Scholar]
- 14.Hortobagyi T, Richardson SP, Lomarev M, Shamim E, Meunier S, Russman H, Dang N, Hallett M. Interhemispheric Plasticity in Humans. Med Sci Sports Exerc. 2011;43(7):1188–99. doi: 10.1249/MSS.0b013e31820a94b8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fling BW, Benson BL, Seidler RD. Transcallosal sensorimotor fiber tract structure-function relationships. Hum Br Map. doi: 10.1002/hbm.21437. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wahl M, Lauterbach-Soon B, Hattingen E, Jung P, Singer O, Volz S, Klein JC, Steinmetz H, Ziemann U. Human motor corpus callosum: topography, somatotopy, and link between microstructure and function. J Neurosci. 2007;27:12132–12138. doi: 10.1523/JNEUROSCI.2320-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johansen-Berg H, Della-Maggiore V, Behrens TE, Smith SM, Paus T. Integrity of white matter in the corpus callosum correlates with bimanual co-ordination skills. Neuroimage. 2007;36 (Suppl 2):T16–21. doi: 10.1016/j.neuroimage.2007.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fling BW, Walsh CM, Bangert AS, Reuter-Lorenz PA, Welsh RC, Seidler RD. Differential Callosal Contributions to Bimanual Control in Young and Older Adults. J Cogn Neurosci. 2011;23(9):2171–85. doi: 10.1162/jocn.2010.21600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kanai R, Rees G. The structural basis of inter-individual differences in human behavior and cognition. Nat Rev Neurosci. 2011;2(4):231–242. doi: 10.1038/nrn3000. [DOI] [PubMed] [Google Scholar]
- 20.Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 21.Garvey MA, Ziemann U, Becker DA, Barker CA, Bartko JJ. New graphical method to measure silent periods evoked by transcranial magnetic stimulation. Clin Neurophysiol. 2001;112:1451–1460. doi: 10.1016/s1388-2457(01)00581-8. [DOI] [PubMed] [Google Scholar]
- 22.Chen R, Yung D, Li JY. Organization of ipsilateral excitatory and inhibitory pathways in the human motor cortex. J Neurophysiol. 2003;89:1256–1264. doi: 10.1152/jn.00950.2002. [DOI] [PubMed] [Google Scholar]
- 23.Wassermann EM, Fuhr P, Cohen LG, Hallett M. Effects of transcranial magnetic stimulation on ipsilateral muscles. Neurology. 1991;41(11):1795–1799. doi: 10.1212/wnl.41.11.1795. [DOI] [PubMed] [Google Scholar]
- 24.Triggs WJ, Calvanio R, Macdonell RA, Cros D, Chiappa KH. Physiological motor asymmetry in human handedness: evidence from transcranial magnetic stimulation. Brain Res. 1994;636:270–276. doi: 10.1016/0006-8993(94)91026-x. [DOI] [PubMed] [Google Scholar]
- 25.Jung P, Ziemann U. Differences of the ipsilateral silent period in small hand muscles. Muscle Nerve. 2006;34:431–436. doi: 10.1002/mus.20604. [DOI] [PubMed] [Google Scholar]
- 26.Vaillancourt DE, Newell KM. Aging and the time and frequency structure of force output variability. J Appl Physiol. 2003;94:903–912. doi: 10.1152/japplphysiol.00166.2002. [DOI] [PubMed] [Google Scholar]
- 27.Carey JR, Allison JD, Mundale MO. Electromyographic study of muscular overflow during precision handgrip. Phys Ther. 1983;63:505–511. doi: 10.1093/ptj/63.4.505. [DOI] [PubMed] [Google Scholar]
- 28.Verstynen T, Ivry RB. Network dynamics mediating ipsilateral motor cortex activity during unimanual actions. J Cogn Neurosci. 2011;23(9):2468–80. doi: 10.1162/jocn.2011.21612. [DOI] [PubMed] [Google Scholar]
- 29.Kennerley SW, Diedrichsen J, Hazeltine E, Semjen A, Ivry RB. Callosotomy patients exhibit temporal uncoupling during continuous bimanual movements. Nat Neurosci. 2002;5:376–381. doi: 10.1038/nn822. [DOI] [PubMed] [Google Scholar]
- 30.Diedrichsen J, Hazeltine E, Nurss WK, Ivry RB. The role of the corpus callosum in the coupling of bimanual isometric force pulses. J Neurophysiol. 2003;90:2409–2418. doi: 10.1152/jn.00250.2003. [DOI] [PubMed] [Google Scholar]
- 31.Schlaug G, Jancke L, Huang Y, Staiger JF, Steinmetz H. Increased corpus callosum size in musicians. Neuropsychologia. 1995;33:1047–1055. doi: 10.1016/0028-3932(95)00045-5. [DOI] [PubMed] [Google Scholar]
- 32.Erickson KI, Colcombe SJ, Wadhwa R, Bherer L, Peterson MS, Scalf PE, Kim JS, Alvarado M, Kramer AF. Training-induced plasticity in older adults: effects of training on hemispheric asymmetry. Neurobiol Aging. 2007;28:272–283. doi: 10.1016/j.neurobiolaging.2005.12.012. [DOI] [PubMed] [Google Scholar]
- 33.Werhahn KJ, Kunesch E, Noachtar S, Benecke R, Classen J. Differential effects on motorcortical inhibition induced by blockade of GABA uptake in humans. J Physiol. 1999;517 (Pt 2):591–597. doi: 10.1111/j.1469-7793.1999.0591t.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stagg CJ, Bachtiar V, Johansen-Berg H. The Role of GABA in Human Motor Learning. Curr Biol. 2011;21:480–4. doi: 10.1016/j.cub.2011.01.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Floyer-Lea A, Wylezinska M, Kincses T, Matthews PM. Rapid-modulation of GABA concentration in human sensorimotor cortex during motor learning. J Neurophysiol. 2006;95(3):1639–44. doi: 10.1152/jn.00346.2005. [DOI] [PubMed] [Google Scholar]
- 36.Chen R. Interactions between inhibitory and excitatory circuits in the human motor cortex. Exp Brain Res. 2004;154(1):1–10. doi: 10.1007/s00221-003-1684-1. [DOI] [PubMed] [Google Scholar]
- 37.Hanajima R, Ugawa Y, Terao Y, Sakai K, Furubayashi T, Machii K, Kanazawa I. Paired-pulse magnetic stimulation of the human motor cortex: differences among I waves. J Physiol. 1998;509(Pt 2):607–18. doi: 10.1111/j.1469-7793.1998.607bn.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grefkes C, Eickhoff SB, Nowak DA, Dafotakis M, Fink GR. Dynamic intra-and interhemispheric interactions during unilateral and bilateral hand movements assessed with fMRI and DCM. Neuroimage. 2008;41(4):1382–94. doi: 10.1016/j.neuroimage.2008.03.048. [DOI] [PubMed] [Google Scholar]
- 39.Kikuchi T, Matsumoto R, Mikuni N, Yokoyama Y, Matsumoto A, Ikeda A, Fukuyama H, Miyamoto S, Hashimoto N. Asymmetric bilateral effect of the supplementary motor area proper in the human motor system. Clin Neurophysiol. 2011 doi: 10.1016/j.clinph.2011.06.011. Epub ahead of print Jul 26. [DOI] [PubMed] [Google Scholar]
- 40.Giovannelli F, Borgheresi A, Balestrieri F, Ragazzoni A, Zaccara G, Cincotta M, Ziemann U. Role of the right dorsal premotor cortex in “physiological” mirror EMG activity. Exp Brain Res. 2006;175(4):633–40. doi: 10.1007/s00221-006-0581-9. [DOI] [PubMed] [Google Scholar]
- 41.Koch G, Franca M, Del Olmo MF, Cheeran B, Milton R, Alvarez Sauco M, Rothwell JC. Time course of functional connectivity between dorsal premotor and contralateral motor cortex during movement selection. J Neurosci. 2006;12;26(28):7452–9. doi: 10.1523/JNEUROSCI.1158-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ni Z, Gunraj C, Nelson AJ, Yeh IJ, Castillo G, Hoque T, Chen R. Two phases of interhemispheric inhibition between motor related cortical areas and the primary motor cortex in human. Cereb Cortex. 2009;19(7):1654–65. doi: 10.1093/cercor/bhn201. [DOI] [PubMed] [Google Scholar]
- 43.Hiraga CY, Garry MI, Carson RG, Summers JJ. Dual-task interference: attentional and neurophysiological influences. Behav Br Res. 2009;205:10–18. doi: 10.1016/j.bbr.2009.07.019. [DOI] [PubMed] [Google Scholar]