Abstract
Purpose
Most non–small cell lung cancer (NSCLC) tumors with an activating mutation of the epidermal growth factor receptor (EGFR) are initially responsive to EGFR tyrosine kinase inhibitors (TKIs) such as gefitinib but ultimately develop resistance to these drugs. Hepatocyte growth factor (HGF) induces EGFR-TKI resistance in NSCLC cells with such a mutation. We investigated strategies to overcome gefitinib resistance induced by HGF.
Experimental Design
Human NSCLC cells with an activating EGFR mutation (HCC827 cells) were engineered to stably express HGF (HCC827-HGF cells). The effects of TAK-701, a humanized monoclonal antibody to HGF, in combination with gefitinib were examined on signal transduction and cell growth in vitro or in vivo.
Results
HCC827-HGF cells secreted large amounts of HGF and exhibited resistance to gefitinib in vitro to an extent similar to that of HCC827 GR cells, in which the gene for the HGF receptor MET is amplified. A MET-TKI reversed gefitinib resistance in HCC827-HGF cells as well as in HCC827 GR cells, suggesting that MET activation induces gefitinib resistance in both cell lines. TAK-701 in combination with gefitinib inhibited the phosphorylation of MET, EGFR, ERK, and AKT in HCC827-HGF cells, resulting in suppression of cell growth and indicating that autocrine HGF-MET signaling contributes to gefitinib resistance in these cells. Combination therapy with TAK-701 and gefitinib also markedly inhibited the growth of HCC827-HGF tumors in vivo.
Conclusions
The addition of TAK-701 to gefitinib is a promising strategy to overcome EGFR-TKI resistance induced by HGF in NSCLC with an activating EGFR mutation.
Keywords: TAK-701, hepatocyte growth factor, gefitinib, resistance, non–small cell lung cancer
Introduction
Somatic mutations in the kinase domain of the epidermal growth factor receptor (EGFR) are associated with a high rate of response to EGFR tyrosine kinase inhibitors (TKIs) such as gefitinib and erlotinib in advanced non–small cell lung cancer (NSCLC) (1–3). Despite the therapeutic benefit of EGFR-TKIs in NSCLC, however, most patients ultimately develop resistance to these drugs. A secondary T790M mutation of EGFR and amplification of the MET gene are major causes of acquired resistance to EGFR-TKIs (4–7). In addition, hepatocyte growth factor (HGF), a ligand of the MET oncoprotein (8, 9), induces gefitinib resistance in EGFR mutation–positive NSCLC by activating MET and downstream signaling (10).
HGF was originally identified as a mitogenic protein for hepatocytes (11). Both HGF and its MET receptor are expressed, and often overexpressed, in a broad spectrum of human solid tumors including lung, mesothelioma, breast, and brain cancer (12–16). HGF thus acts as an autocrine or paracrine growth factor for these tumor cells (17, 18). TAK-701 is a potent humanized monoclonal antibody to HGF that blocks various HGF-induced biological activities as well as inhibits tumor growth in an autocrine HGF-MET–driven xenograft model.6 To identify strategies or agents capable of overcoming resistance to EGFR-TKIs induced by HGF, we have now established sublines of the EGFR mutation–positive human NSCLC cell line HCC827 that stably express transfected HGF cDNA. With the use of these cells, we investigated the effects of TAK-701 on HGF-MET signaling and gefitinib resistance induced by cell-derived HGF both in vitro and in vivo.
Materials and Methods
Cell culture and reagents
The human NSCLC cell lines HCC827 and HCC827 GR5 were obtained as described previously (6). The human glioblastoma cell line U87MG was obtained from American Type Culture Collection (Manassas, VA). HCC827 cells were cultured under a humidified atmosphere of 5% CO2 at 37°C in RPMI 1640 medium (Sigma, St. Louis, MO) supplemented with 10% fetal bovine serum. HCC827 GR5 cells were cultured in RPMI 1640 medium supplemented with 10% fetal bovine serum and 1 μM gefitinib. U87MG cells were cultured in Dulbecco’s modified Eagle’s medium (Gibco, Grand Island, NY) supplemented with 10% fetal bovine serum. TAK-701 was kindly provided by Takeda Pharmaceutical Co. Ltd. (Osaka, Japan), gefitinib was obtained from AstraZeneca (Macclesfield, UK), and PHA-665752 was from Tocris Bioscience (Bristol, UK).
Cell transfection
A full-length cDNA fragment encoding human HGF was obtained from U87MG cells by reverse transcription and the polymerase chain reaction with the primers HGF-F (5′-GCGGCCGCAGCACCATGTGGGTGACCAAA-3′) and HGF-R (5′-CGGGATCCCTATGACTGTGGTACCTTATAT-3′). The amplification product was verified by sequencing after its cloning into the pCR-Blunt II-TOPO vector (Invitrogen, Carlsbad, CA). The HGF cDNA was excised from pCR-Blunt II-TOPO and transferred to the pQCXIH retroviral vector (Clontech, Palo Alto, CA). Retroviruses encoding HGF were then produced and used to infect HCC827 cells as described (19). Cells stably expressing HGF were then isolated by selection with hygromycin at 500 μg/mL (InvivoGen, San Diego, CA).
ELISA for HGF
Cells (5 × 105) were seeded in six-well plates, cultured overnight in complete medium, and then incubated in serum-free medium for 24 h, after which the latter medium was collected and assayed for HGF with a Human HGF Quantikine ELISA Kit (R&D Systems, Minneapolis, MN). A standard curve for the enzyme-linked immunosorbent assay (ELISA) was generated with the supplied reagents, and HGF concentration was determined as the average value from triplicate samples.
Cell growth inhibition assay
Cells were transferred to 96-well flat-bottomed plates and cultured for 24 h before exposure for 72 h to various concentrations of gefitinib, TAK-701, or PHA-665752, as indicated. Tetra Color One (5 mM tetrazolium monosodium salt and 0.2 mM 1-methoxy-5-methyl phenazinium methylsulfate; Seikagaku Kogyo, Tokyo, Japan) was then added to each well, and the cells were incubated for 3 h at 37°C before measurement of absorbance at 490 nm with a Multiskan Spectrum instrument (Thermo Labsystems, Boston, MA). Absorbance values were expressed as a percentage of that for untreated cells.
Immunoblot analysis
Cells were washed twice with ice-cold PBS and then lysed with 1× Cell Lysis Buffer (Cell Signaling Technology, Danvers, MA) consisting of 20 mM Tris-HCl (pH 7.5), 150 mM NaCl, 1 mM EDTA (disodium salt), 1 mM EGTA, 1% Triton X-100, 2.5 mM sodium pyrophosphate, 1 mM β-glycerophosphate, 1 mM Na3VO4, leupeptin (1 μg/mL), and 1 mM phenylmethylsulfonyl fluoride. The protein concentration of cell lysates was determined with a BCA protein assay kit (Thermo Fisher Scientific, Rockford, IL), and equal amounts of protein were subjected to SDS-PAGE on 7.5% gels (Bio-Rad, Hercules, CA). The separated proteins were transferred to a nitrocellulose membrane, which was then incubated with Blocking One solution (Nacalai Tesque, Kyoto, Japan) for 20 min at room temperature before incubation overnight at 4°C with primary antibodies. Antibodies to phosphorylated EGFR (phosphotyrosine-1068), to phosphorylated MET (phosphotyrosine-1349), to phosphorylated or total forms of AKT, and to phosphorylated extracellular signal–regulated kinase (ERK) were obtained from Cell Signaling Technology; those to total ERK were from Santa Cruz Biotechnology (Santa Cruz, CA); those to total EGFR and to total MET were from Zymed/Invitrogen (Carlsbad, CA); and those to β-actin were from Sigma. The membrane was then washed with PBS containing 0.05% Tween 20 before incubation for 1 h at room temperature with horseradish peroxidase–conjugated secondary antibodies (GE Healthcare, Little Chalfont, UK). Immune complexes were finally detected with ECL Western Blotting Detection Reagents (GE Healthcare).
Growth inhibition assay in vivo
All animal studies were performed in accordance with the Recommendations for Handling of Laboratory Animals for Biomedical Research compiled by the Committee on Safety and Ethical Handling Regulations for Laboratory Animal Experiments, Kinki University. The ethical procedures followed met the requirements of the United Kingdom Co-ordinating Committee on Cancer Research guidelines (20). HCC827 cells were implanted subcutaneously into the right hind leg of 6-week-old female athymic nude mice (BALB/c nu/nu; CLEA Japan, Tokyo, Japan). Tumor volume was determined from caliper measurement of tumor length (L) and width (W) according to the formula LW2/2. Treatment was initiated when tumors in each group of animals achieved an average volume of 300 to 400 mm3. Treatment groups (each containing five mice) consisted of vehicle control, TAK-701 alone, gefitinib alone, and TAK-701 plus gefitinib. The mice were injected with TAK-701 (5 mg/kg) intraperitoneally twice a week for 7 weeks; control animals received PBS as vehicle. Gefitinib (50 mg/kg) was administered by oral gavage daily for 49 days; control animals received a 0.5% (w/v) aqueous solution of hydroxypropylmethylcellulose as vehicle. Both tumor size and body weight were measured twice per week.
Results
Cell-derived HGF induces gefitinib resistance in EGFR mutation–positive NSCLC cells
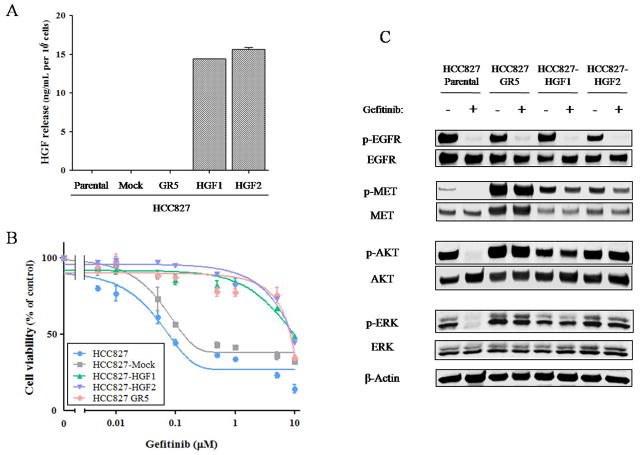
To investigate whether cell-derived HGF induces gefitinib resistance in NSCLC cells with an EGFR mutation, we established HCC827 cells (which are EGFR mutation positive) that stably express human HGF (HCC827-HGF1 and -HGF2 cells) or stably harbor the corresponding empty vector (HCC827-Mock cells). The secretion of HGF from these cell lines as well as from the parental (HCC827) cells and from an HCC827 subline with MET amplification (HCC827 GR5) was examined with the use of an ELISA. We found that HCC827-HGF1 and -HGF2 cells released large amounts of HGF into the culture medium, whereas the secretion of HGF from parental (HCC827), HCC827-Mock, or HCC827 GR5 cells was undetectable (Fig. 1A). To assess the effects of gefitinib on cell growth, we exposed these five cell lines to various concentrations of the drug and then measured cell viability. HCC827 GR5 as well as HCC827-HGF1 and -HGF2 cells showed a reduced sensitivity to gefitinib compared with HCC827 and HCC827-Mock cells, with median inhibitory concentrations of ~10 μM apparent for the former cell lines compared with ~0.1 μM for the latter (Fig. 1B). To investigate possible differences in signal transduction among these cell lines, we examined the effects of gefitinib on EGFR, MET, AKT, and ERK phosphorylation by immunoblot analysis (Fig. 1C). In the parental cells, gefitinib markedly inhibited the phosphorylation of EGFR, AKT, and ERK. In contrast, in the resistant cells (HCC827 GR5, HCC827-HGF1 and -HGF2), gefitinib alone had no effect on AKT and ERK phosphorylation, although it substantially reduced the level of EGFR phosphorylation. These data suggested that sustained AKT and ERK signaling in the presence of gefitinib contributes to gefitinib resistance in HCC827-HGF1 and -HGF2 cells as well as in HCC827 GR5 cells.
Figure 1.
Characterization of HCC827 isogenic cell lines. A, HCC827 isogenic cell lines (HCC827, HCC827-Mock, HCC827-HGF1 and -HGF2, and HCC827 GR5) were cultured overnight in medium containing 10% serum and then incubated for 24 h in serum-free medium, after which the culture supernatants were collected and assayed for HGF with an ELISA. Data are means ± SD from three independent experiments. B, HCC827 isogenic cell lines were cultured in medium containing 10% serum for 72 h in the presence of various concentrations of gefitinib, after which cell viability was assessed as described in Materials and Methods. The number of viable cells is expressed as a percentage of the value for untreated cells. Data are means ± SD from three independent experiments. C, HCC827 isogenic cell lines were incubated for 1 h with or without gefitinib (100 nM) in medium containing 10% serum, after which the cells were lysed and subjected to immunoblot analysis with antibodies to phosphorylated (p-) or total forms of EGFR, MET, AKT, or ERK or with those to β-actin (loading control).
TAK-701 abrogates gefitinib resistance induced by HGF
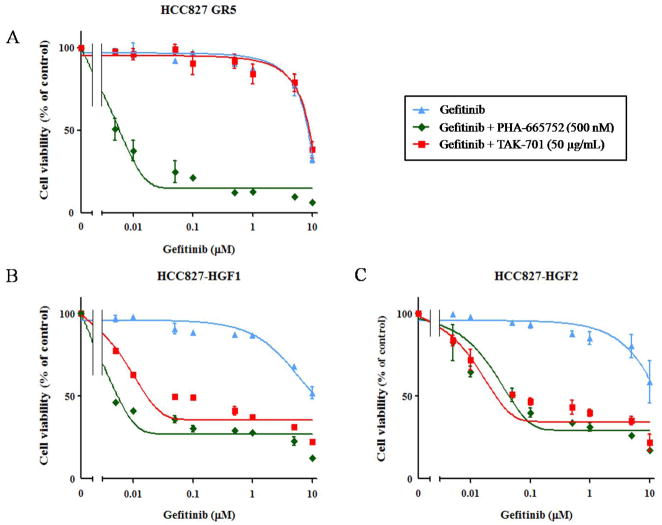
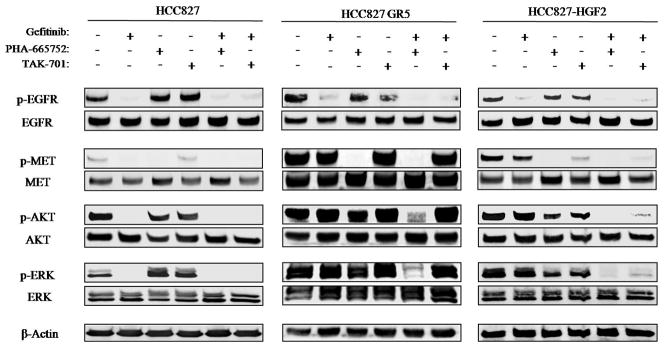
To investigate the roles of MET and HGF in gefitinib resistance in HCC827 GR5 as well as HCC827-HGF1 and -HGF2 cells, we exposed the cells to the MET-TKI PHA-665752 or to TAK-701, a humanized monoclonal antibody to HGF, in combination with gefitinib. Combined treatment with PHA-665752 and gefitinib was previously shown to result in substantial growth inhibition in HCC827 GR5 (MET amplification–positive) cells (6). We found that the combination of gefitinib and TAK-701 did not affect the growth of HCC827 GR5 cells (Fig. 2A). In HCC827-HGF1 and -HGF2 cells, however, TAK-701 and PHA-665752 each restored the sensitivity of cell growth to inhibition by gefitinib (Fig. 2B, C). To examine the effects of gefitinib, PHA-665752, and TAK-701 on cell signaling in the parental, HCC827 GR5, and HCC827-HGF2 cell lines, we again performed immunoblot analysis (Fig. 3). Consistent with previous observations (6), PHA-665752 in combination with gefitinib inhibited MET, AKT, and ERK phosphorylation in HCC827 GR5 cells. We further revealed that TAK-701 alone did not inhibit MET phosphorylation, and thus the combination of TAK-701 and gefitinib did not abrogate AKT and ERK phosphorylation, in HCC827 GR5 cells. In HCC827-HGF2 cells, however, TAK-701 as well as PHA-665752 inhibited MET phosphorylation, and the combined treatment with TAK-701 and gefitinib fully suppressed ERK and AKT phosphorylation. These results indicated that HGF-induced gefitinib resistance is mediated by HGF-MET signaling and is abrogated by treatment with TAK-701 in HCC827-HGF cells.
Figure 2.
Effects of the combination of gefitinib and either TAK-701 or PHA-665752 on the growth of gefitinib-resistant NSCLC cells. HCC827 GR5 cells (A), HCC827-HGF1 cells (B), or HCC827-HGF2 cells (C) were cultured for 72 h in medium containing 10% serum and various concentrations of gefitinib and either PHA-665752 (500 nM) or TAK-701 (50 μg/mL), after which cell viability was assessed. Data are means ± SD from three independent experiments.
Figure 3.
Effects of the combination of gefitinib and either TAK-701 or PHA-665752 on cell signaling in gefitinib-resistant NSCLC cells. HCC827 cells, HCC827 GR5 cells, or HCC827-HGF2 cells were incubated for 6 h in medium containing 10% serum in the absence or presence of gefitinib (1 μM), PHA-665752 (500 nM), or TAK-701 (50 μg/mL), as indicated. Cell lysates were then prepared and subjected to immunoblot analysis with antibodies to phosphorylated or total forms of EGFR, MET, AKT, or ERK or with those to β-actin.
Cell-derived HGF induces gefitinib resistance in NSCLC cells and TAK-701 restores the sensitivity of tumor growth to inhibition by gefitinib in vivo
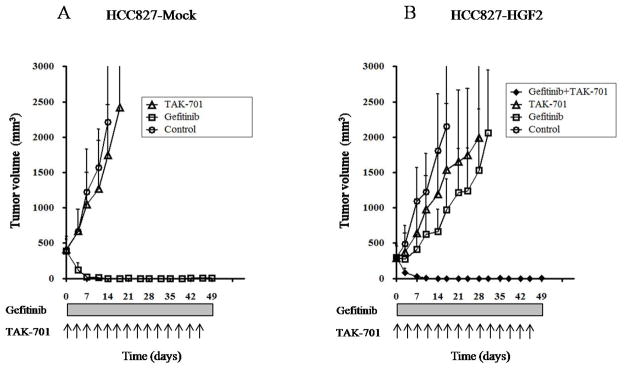
To examine the possible induction of gefitinib resistance by tumor cell–derived HGF and the efficacy of combined treatment with TAK-701 and gefitinib in vivo, we generated xenografts in nude mice by injection of HCC827-Mock or HCC827-HGF2 cells. We found that, whereas gefitinib markedly inhibited the growth of HCC827-Mock xenografts (Fig. 4A), HCC827-HGF2 xenografts were substantially resistant to gefitinib (Fig. 4B). TAK-701 alone had a minimal effect on tumor growth in both HCC827-Mock and HCC827-HGF2 xenograft models. However, the combination of gefitinib and TAK-701 induced marked regression of HCC827-HGF2 xenografts. These results thus suggested that HGF produced by NSCLC tumors harboring an EGFR mutation induces gefitinib resistance, and that TAK-701 abrogates such HGF-induced gefitinib resistance in vivo.
Figure 4.
Effects of the combination of TAK-701 and gefitinib on the growth of gefitinib-resistant NSCLC cells in vivo. Nude mice with tumor xenografts established by subcutaneous injection of HCC827-Mock (A) or HCC827-HGF2 (B) cells were treated for 7 weeks with vehicle (control), gefitinib (50 mg/kg), TAK-701 (5 mg/kg), or both drugs, as described in Materials and Methods. Tumor volume was determined at the indicated times after the onset of treatment. Data are means ± SEM from five mice per group.
Discussion
In the present study, we established HGF-overexpressing sublines of HCC827 cells and showed that these sublines are resistant to gefitinib both in vitro and in vivo. To investigate whether the resistance of HCC827-HGF cells to gefitinib is attributable to HGF-MET signaling, we examined the effects of the MET-TKI PHA-665752 and of TAK-701, a humanized monoclonal antibody to HGF, on signal transduction and cell growth. In both HCC827-HGF1 and -HGF2 cells as well as HCC827 GR5 cells, which are positive for MET amplification, gefitinib alone did not inhibit AKT or ERK phosphorylation, whereas gefitinib in combination with PHA-665752 markedly suppressed the phosphorylation of these signaling molecules. Consistent with these results, PHA-665752 restored the sensitivity of cell growth to inhibition by gefitinib in HCC827-HGF cells as well as in HCC827 GR5 cells. These results indicate that the gefitinib resistance of these cell lines is mediated by MET signaling. TAK-701 has been shown to potently inhibit HGF binding to MET in cancer cells and xenograft models dependent on autocrine HGF-MET signaling.6 TAK-701 did not inhibit the phosphorylation of MET in HCC827 GR5 cells, suggesting that the activation of MET in these cells is not dependent on HGF. Indeed, we were not able to detect the secretion of HGF from HCC827 GR5 cells. In contrast, TAK-701 suppressed MET phosphorylation, and thus the combination of TAK-701 and gefitinib markedly inhibited both AKT and ERK signaling, in HCC827-HGF cells, resulting in their growth inhibition. These results indicate that autocrine HGF-MET signaling contributes to gefitinib resistance in HCC827-HGF cells. Similar ligand-mediated gefitinib resistance has been described previously, with insulin-like growth factor (IGF) having been found to rescue NSCLC cells expressing wild-type EGFR from gefitinib-induced inhibition of cell growth (21). These observations suggest that ligand-dependent receptor tyrosine kinase (RTK) activation (by HGF or IGF), as well as ligand-independent RTK activation (by MET amplification), plays a pivotal role in the development of resistance to gefitinib. Further studies should reveal whether other ligand-RTK combinations contribute to gefitinib resistance.
We found that the baseline levels of both MET expression and MET phosphorylation in HCC827-HGF cells were lower than those in HCC827 GR5 cells (Fig. 1C), whereas HCC827-HGF cells were resistant to gefitinib to the same extent as were HCC827 GR5 cells in vitro (Fig. 1B). These results suggest that phosphorylated MET activates downstream signaling through different pathways in HCC827 GR5 and HCC827-HGF cells. MET was recently shown to signal through ERBB3 in MET amplification–positive NSCLC cells (6) or through Grb2-associated binder 1 (Gab1) in NSCLC cells with HGF-induced gefitinib resistance (22). Further studies are required to investigate whether the biological properties or the abilities of drugs to overcome gefitinib resistance are affected by differences in RTK downstream signaling.
In our HCC827-HGF xenograft model, we showed that HGF secreted from EGFR mutation–positive NSCLC cells drives tumor growth even in the presence of gefitinib, and that combination therapy with TAK-701 and gefitinib was able to greatly inhibit the growth of HCC827-HGF tumors. These results indicate that interruption of HGF-MET signaling with TAK-701 represents a powerful strategy to abrogate gefitinib resistance induced by HGF derived from tumor cells. HGF was previously shown to be expressed predominantly by adenocarcinoma cells in NSCLC specimens, although a low level of HGF staining was also apparent in stromal cells (23). Furthermore, marked expression of HGF has been detected in most lung cancers with intrinsic or acquired resistance to gefitinib (10, 24). These data suggest that our autocrine model systems based on stable overexpression of HGF are clinically relevant and should prove useful for the establishment of strategies to overcome gefitinib resistance. HGF is also produced by stromal cells of various tumor types (13, 25, 26). Indeed, HGF derived from fibroblasts injected into nude mice together with EGFR mutation–positive NSCLC cells induced gefitinib resistance in the NSCLC cells in vivo (27). Further studies are required to clarify the major source of HGF that contributes to gefitinib resistance in patients with EGFR mutation–positive lung cancer. Given that TAK-701 inhibits HGF binding to MET, TAK-701 may reverse gefitinib resistance induced by HGF derived not only from tumor cells but also from stromal cells.
In conclusion, we have shown that autocrine activation of MET by HGF confers resistance to gefitinib, and that TAK-701, a humanized monoclonal antibody to HGF, restored sensitivity to gefitinib in tumors with HGF-induced gefitinib resistance. TAK-701 is currently undergoing phase I trials as a single agent in patients with advanced solid tumors. Our results now indicate that the addition of TAK-701 to gefitinib is a potential strategy to overcome EGFR-TKI resistance induced by HGF and warrants clinical evaluation.
Translational Relevance.
Most non–small cell lung cancer (NSCLC) with an activating mutation of the epidermal growth factor receptor (EGFR) are initially responsive to EGFR tyrosine kinase inhibitors (TKIs) such as gefitinib but ultimately develop resistance to these drugs. Hepatocyte growth factor (HGF) induces EGFR-TKI resistance by activating its receptor, MET, and thereby eliciting downstream signaling. We show that EGFR mutation–positive NSCLC (HCC827) cells engineered to stably overexpress HGF exhibit a level of gefitinib resistance in vitro similar to that of HCC827 cells with MET amplification. TAK-701, a humanized monoclonal antibody to HGF, in combination with gefitinib inhibited the phosphorylation of MET, EGFR, ERK, and AKT in HCC827 cells overexpressing HGF, resulting in suppression of their growth both in vitro and in vivo. Our findings suggest that the addition of TAK-701 to gefitinib is a promising strategy to overcome EGFR-TKI resistance induced by HGF in NSCLC with an activating EGFR mutation.
Footnotes
Kitahara O, Nishizawa S, Ito Y, Toyoda Y, Misumi Y, Sato S, Inaoka T, Klakamp SL, Kokubo T, Hori A. TAK-701, a humanized monoclonal antibody to human hepatocyte growth factor, exhibits promising antitumor effects on multiple tumor types (in preparation).
References
- 1.Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–39. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 2.Paez JG, Janne PA, Lee JC, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304:1497–500. doi: 10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]
- 3.Pao W, Miller V, Zakowski M, et al. EGF receptor gene mutations are common in lung cancers from “never smokers” and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc Natl Acad Sci U S A. 2004;101:13306–11. doi: 10.1073/pnas.0405220101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kobayashi S, Boggon TJ, Dayaram T, et al. EGFR mutation and resistance of non-small-cell lung cancer to gefitinib. N Engl J Med. 2005;352:786–92. doi: 10.1056/NEJMoa044238. [DOI] [PubMed] [Google Scholar]
- 5.Pao W, Miller VA, Politi KA, et al. Acquired resistance of lung adenocarcinomas to gefitinib or erlotinib is associated with a second mutation in the EGFR kinase domain. PLoS Med. 2005;2:e73. doi: 10.1371/journal.pmed.0020073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Engelman JA, Zejnullahu K, Mitsudomi T, et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science. 2007;316:1039–43. doi: 10.1126/science.1141478. [DOI] [PubMed] [Google Scholar]
- 7.Bean J, Brennan C, Shih JY, et al. MET amplification occurs with or without T790M mutations in EGFR mutant lung tumors with acquired resistance to gefitinib or erlotinib. Proc Natl Acad Sci U S A. 2007;104:20932–7. doi: 10.1073/pnas.0710370104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bottaro DP, Rubin JS, Faletto DL, et al. Identification of the hepatocyte growth factor receptor as the c-met proto-oncogene product. Science. 1991;251:802–4. doi: 10.1126/science.1846706. [DOI] [PubMed] [Google Scholar]
- 9.Naldini L, Vigna E, Narsimhan RP, et al. Hepatocyte growth factor (HGF) stimulates the tyrosine kinase activity of the receptor encoded by the proto-oncogene c-MET. Oncogene. 1991;6:501–4. [PubMed] [Google Scholar]
- 10.Yano S, Wang W, Li Q, et al. Hepatocyte growth factor induces gefitinib resistance of lung adenocarcinoma with epidermal growth factor receptor-activating mutations. Cancer Res. 2008;68:9479–87. doi: 10.1158/0008-5472.CAN-08-1643. [DOI] [PubMed] [Google Scholar]
- 11.Nakamura T, Nishizawa T, Hagiya M, et al. Molecular cloning and expression of human hepatocyte growth factor. Nature. 1989;342:440–3. doi: 10.1038/342440a0. [DOI] [PubMed] [Google Scholar]
- 12.Maulik G, Shrikhande A, Kijima T, Ma PC, Morrison PT, Salgia R. Role of the hepatocyte growth factor receptor, c-Met, in oncogenesis and potential for therapeutic inhibition. Cytokine Growth Factor Rev. 2002;13:41–59. doi: 10.1016/s1359-6101(01)00029-6. [DOI] [PubMed] [Google Scholar]
- 13.Birchmeier C, Birchmeier W, Gherardi E, Vande Woude GF. Met, metastasis, motility and more. Nat Rev Mol Cell Biol. 2003;4:915–25. doi: 10.1038/nrm1261. [DOI] [PubMed] [Google Scholar]
- 14.Harvey P, Warn A, Newman P, Perry LJ, Ball RY, Warn RM. Immunoreactivity for hepatocyte growth factor/scatter factor and its receptor, met, in human lung carcinomas and malignant mesotheliomas. J Pathol. 1996;180:389–94. doi: 10.1002/(SICI)1096-9896(199612)180:4<389::AID-PATH685>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 15.Tuck AB, Park M, Sterns EE, Boag A, Elliott BE. Coexpression of hepatocyte growth factor and receptor (Met) in human breast carcinoma. Am J Pathol. 1996;148:225–32. [PMC free article] [PubMed] [Google Scholar]
- 16.Koochekpour S, Jeffers M, Rulong S, et al. Met and hepatocyte growth factor/scatter factor expression in human gliomas. Cancer Res. 1997;57:5391–8. [PubMed] [Google Scholar]
- 17.Danilkovitch-Miagkova A, Zbar B. Dysregulation of Met receptor tyrosine kinase activity in invasive tumors. J Clin Invest. 2002;109:863–7. doi: 10.1172/JCI15418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tsao MS, Zhu H, Giaid A, Viallet J, Nakamura T, Park M. Hepatocyte growth factor/scatter factor is an autocrine factor for human normal bronchial epithelial and lung carcinoma cells. Cell Growth Differ. 1993;4:571–9. [PubMed] [Google Scholar]
- 19.Tanaka K, Arao T, Maegawa M, et al. SRPX2 is overexpressed in gastric cancer and promotes cellular migration and adhesion. Int J Cancer. 2009;124:1072–80. doi: 10.1002/ijc.24065. [DOI] [PubMed] [Google Scholar]
- 20.United Kingdom Co-ordinating Committee on Cancer Research (UKCCCR) Br J Cancer. 2. Vol. 77. 1998. Guidelines for the Welfare of Animals in Experimental Neoplasia; pp. 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guix M, Faber AC, Wang SE, et al. Acquired resistance to EGFR tyrosine kinase inhibitors in cancer cells is mediated by loss of IGF-binding proteins. J Clin Invest. 2008;118:2609–19. doi: 10.1172/JCI34588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Turke AB, Zejnullahu K, Wu YL, et al. Preexistence and clonal selection of MET amplification in EGFR mutant NSCLC. Cancer Cell. 17:77–88. doi: 10.1016/j.ccr.2009.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsao MS, Yang Y, Marcus A, Liu N, Mou L. Hepatocyte growth factor is predominantly expressed by the carcinoma cells in non-small-cell lung cancer. Hum Pathol. 2001;32:57–65. doi: 10.1053/hupa.2001.21133. [DOI] [PubMed] [Google Scholar]
- 24.Onitsuka T, Uramoto H, Nose N, et al. Acquired resistance to gefitinib: The contribution of mechanisms other than the T790M, MET, and HGF status. Lung Cancer. 2009 doi: 10.1016/j.lungcan.2009.05.022. [DOI] [PubMed] [Google Scholar]
- 25.Bhowmick NA, Neilson EG, Moses HL. Stromal fibroblasts in cancer initiation and progression. Nature. 2004;432:332–7. doi: 10.1038/nature03096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matsumoto K, Nakamura T. Hepatocyte growth factor and the Met system as a mediator of tumor-stromal interactions. Int J Cancer. 2006;119:477–83. doi: 10.1002/ijc.21808. [DOI] [PubMed] [Google Scholar]
- 27.Wang W, Li Q, Yamada T, et al. Crosstalk to stromal fibroblasts induces resistance of lung cancer to epidermal growth factor receptor tyrosine kinase inhibitors. Clin Cancer Res. 2009;15:6630–8. doi: 10.1158/1078-0432.CCR-09-1001. [DOI] [PubMed] [Google Scholar]