Abstract
The circulating tumor cells (CTCs) appear to be a marker of metastasis development, especially, for highly aggressive and epidemically growing melanoma malignancy that is often metastatic at early stages. Recently, we introduced in vivo photoacoustic (PA) flow cytometry (PAFC) for label-free detection of mouse B16F10 CTCs in melanoma-bearing mice using melanin as an intrinsic marker. Here, we significantly improve the speed of PAFC by using a high pulse repetition rate laser operating at 820 and 1064 nm wavelengths. This platform was used in preclinical studies for label-free PA detection of low pigmented human CTCs. Demonstrated label-free PAFC detection, low level of background signals, and favorable safety standards for near infrared irradiation suggest that a fiber laser operating at 1064 nm at pulse repetition rates up to 0.5 MHz could be a promising source for portable clinical PAFC devices. The possible applications can include early diagnosis of melanoma at the parallel progression of primary tumor and CTCs, detection of cancer recurrence, residual disease, and real-time monitoring of therapy efficiency by counting CTCs before, during and after therapeutic intervention. Herewith, we also address sensitivity of label-free PAFC melanoma CTCs detection and introduce in vivo CTCs targeting by magnetic nanoparticles conjugated with specific antibody and magnetic cells enrichment.
Keywords: in vivo flow cytometry, photoacoustics, melanoma, metastasis, circulating tumor cells, high pulse repetition rate laser, magnetic nanoparticles, in vivo cell enrichment
Cutaneous melanoma is the third most common type of skin cancer after basal and squamous cell carcinoma it accounts for only 3% of all cases, but it leads to 65% of all deaths from skin cancer (1–7). The most alarming aspect of cutaneous melanoma is its potential to metastasize at a very early stage of disease. As a result, most of the melanoma deaths arise from metastases (1,2).
Appearance of circulating tumor cells (CTCs) has been suggested as an early marker of metastatic development, cancer recurrence, and therapeutic efficacy (2,8–10). The comprehensive review of 209 articles involving 53 studies that collected data on 5,433 patients have been well supported by this data (6). Among advanced technologies for detection of CTCs ex vivo (e.g., scanning cytometry, immunomagnetic enrichment, size filtration, negative cell sorting, and microfluidic chips (8–10) mostly reverse transcription–polymerase chain reaction (RT-PCR) combined with cell-enrichment techniques was broadly used for melanoma CTCs (1–7). Some difficulties in reproducing the results of the RT-PCR assay were associated with differences in sample processing and generation of false-positive signals due to contamination, amplification of pseudogenes, and illegitimate transcription (6). False-negative signals, in contrast, were related to the poor quality of source materials, and the genomic instability of malignant cells. Further, RT-PCR is an indirect method and cannot provide a direct evidence of the presence of intact CTCs in the blood.
In general, the ultimate sensitivity threshold of most ex vivo CTCs assays is limited by the small blood sample volume, typically a few milliliters, in which no less than one CTC can be detected. As a result, in the entire volume of blood (~5L in adult) existing tests with current threshold sensitivity of 1–10 cells/ml can miss up to 5,000–50,000 cells, which is sufficient for the rapid development of a barely treatable or already incurable metastasis (9–10). Invasive extraction of blood at discrete time points from an organism may lead to changes in CTC properties (e.g., morphology or marker expression) and prevents the long-term study of CTCs and metastasis development in the native biological environment. Little or no ability exists to collect blood samples of sufficient volume from clinically relevant anatomical sites such as the primary tumor area, lymph nodes, or bones.
Most of these problems can be solved by assessing large blood volume in vivo using the principles of flow cytometry with photothermal (PT), photoacoustic (PA) or fluorescent detection schematics proposed in 2004 by us and other groups (11–20). This approach provides the possibility of monitoring significantly larger blood volumes, and potentially the entire blood volume in 1–2 hours (the time it will take for 5 L of blood at 100 mL/min flow rate to pass through 2–3 mm peripheral blood vessels (15–17)). Unfortunately, translation to humans of fluorescence based CTCs labeling and detection techniques faces multiple challenges such as fluorescent probes toxicity, wide emission spectra of fluorophores in near-infrared (NIR) tissue transparency window, high intensity of continuous wave lasers used (~100 W/cm2 compared to safe level of 0.2–0.5 W/cm2 (21)), and assessment of only superficial (≤0.1–0.3 mm) microvessels having slow flow. For example, in a mouse model in 50-µm blood vessel having flow velocity of 5 mm/s, two days are required to continuously assess whole 2-mL blood volume.
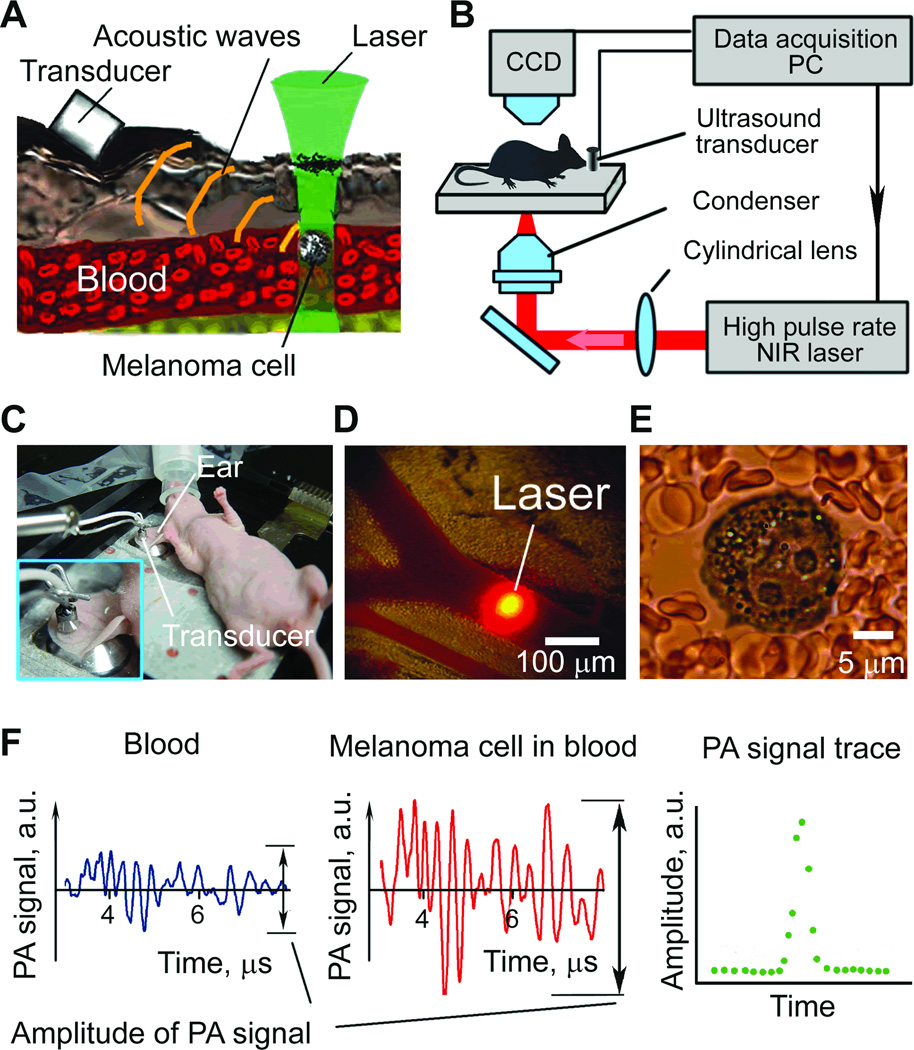
PT and, especially, PA methods are free of these limitations (13,16–18). These methods are based on nonradiative relaxation of absorbed laser energy into heat and accompanying acoustic effects (Fig. 1A). For nonfluorescent samples (e.g., melanin is weakly fluorescent), these methods offer the highest absorption sensitivity at the single-cell level absorption coefficient of 10–2 –10–3 cm–1). This level of absorption sensitivity makes it possible to noninvasively (i.e., a short-term temperature elevation of ≤ 0.5 °C (22–23)) detect individual nanoparticles, quantum dots, dyes and biomolecules at a threshold comparable with that of fluorescence methods (23–25). Several successful clinical trials on humans demonstrated a clinical significance and safety of PA technique including: 1) continuous monitoring of blood oxygenation in the internal jugular vein (10–20 mm in diameter, 5–10 mm deep in the presence of strong light scattering and attenuation in the 15–20 mm layer of overlaying tissue (26); 2) detection and imaging of breast tumor at a depth of 3 cm (27); and 3) monitoring of blood hemoglobin in the wrist area overlying the radial artery at a depth of 3 mm (28). PA technology is safe for human subjects as it requires a laser energy fluence of only 5–20 mJ/cm2 which is well within the laser safety standard (~30–100 mJ/cm2) in NIR range (e.g., 800–1100 nm) (21). Applied to a melanoma study, PA techniques have demonstrated promise for assessment of melanoma cells in vitro (8,16,29) and imaging of melanoma tumor in vivo in static conditions (30).
Fig. 1.
We have developed in vivo time-resolved PA flow cytometry (PAFC) (13–14) and demonstrated its potential for label-free counting of single CTCs (B16F10) in blood and lymph flow in melanoma-bearing mouse (16,31). In particular, the association between disseminated melanoma cells count in prenodal lymphatics and metatastatic status of sentinel lymph nodes supports the presence of melanoma cells in lymphatics as a novel prognostic marker of metastasis (18). We also developed advanced PAFC schematic with a high pulse-repetition rate diode laser at 904 nm and demonstrated an ultrasensitive, label-free, PA enumeration of melanoma CTCs in the mouse blood microvessels with diameter of 50–300 µm (16). However, relatively low pulse energy of diode lasers (few microjoules) makes generation of detectable signals from individual CTCs in larger vessels challengeable, for example, in 2–3 mm hand veins and arteries at depths of 1–3 mm (our primary clinical target) or, potentially, in ~ 1 cm jugular vein (26) where a probability to detect rare CTCs is higher due to higher flow velocity. To overcome these limitations, we recently developed the PAFC with high pulse repetition rate Yb: doped fiber laser having relatively high pulse energy (up to 100 µJ) at 1064 nm and demonstrated its application for detection of circulating nanoparticles (32). In this paper we extend applications of the high speed PAFC with high pulse repetition NIR lasers to in vivo real-time detection of human melanoma CTCs.
METHODS
PAFC setup
General principles of PAFC and its schematic were described in detail elsewhere (14,17). High-speed PAFC setup was built on the technical platform of an upright Olympus BX51 microscope (Olympus America, Inc., Center Valley, PA) equipped with two NIR lasers: 1) pulsed Yb-doped fiber laser MOPA-M-10 (MultiWave Photonics, SA, Portugal) with parameters: wavelength, 1064 nm; pulse rate, 1 Hz – 500 kHz; pulse width, 10 ns; pulse energy, 0.5 – 50 µJ; and 2) a diode-pumped solid state laser LUCE 820-10kHz (Bright Solutions, Cura Carpignano, Italy) with parameters: 820 nm1 Hz – 10 kHz, 10 ns, 76 J, respectively. Selection between laser sources was performed by a retractable 100% mirror installed in the laser path. Linear beams shaping was provided by a customized cylindrical lens. Laser beam sizes in the sample were 15 × 150 µm and 10 × 80 µm for 1064 and 820 nm, respectively. A CCD camera Cohu 2122 (Cohu Inc., Poway, CA, USA) was used to control beam focusing and shaping.
PA signal acquisition
PA signals were acquired by an ultrasound transducer (model 6528101, 3.5 MHz, 5.5 mm in diameter; Imasonic Inc., Besancon, France). The transducer was gently attached to the skin close to the laser beam using phosphate buffered saline (PBS) solution for acoustic matching (Fig. 1 C). For in vitro testing the transducer was placed directly on capillary or coverslip surface. Signals from transducer were amplified (amplifier model 5662B, 50kHz - 4 MHz; Panametrics) and recorded by PC (Dell Precision 690) with installed NI PCI-5124 12-bit, 200M Samples/s digitizer board (National Instruments, Inc., Austin, TX) controlled by custom software (LabView 8.5 complex, National Instruments, Inc., Austin, TX).
Melanoma cells and labeling procedure
The mouse melanoma B16F10 cells, and human melanoma HTB-65, SK-3, and SK-MEL-1 cells were obtained from a cell bank (American Type Culture Collection). The C8161 human melanoma cell line was a gift from the Harvard Medical School. Cells were cultured at 37°C in a humidified atmosphere containing 5% CO2 with Dulbecco’s modified Eagle medium (DMEM) (Gibco-Invitrogen) supplemented by 10% fetal bovine serum. Melanoma cells were treated with Trypsine for 5 min under 37°C and re-suspended in PBS. Cells were found to be 94–98% viable with Trypan Blue extrusion test. Cells for injection were concentrated by centrifugation of initial suspension.
Cells with increased melanin content were obtained by incubating B16F10 cells overnight in 5 mL of DMEM solution containing 3 mg of natural melanin (Sigma-Aldrich Co, USA) in the flask. Triple washing of the unincubated melanin cells solution with PBS cells were re-suspended as described earlier. Ninety percent of cells were found to be viable after incubation.
Melanoma cells were targeted in vitro and in vivo by 10-nm magnetic nanoparticles from Miltenyl Biotec Inc. (Auburn, CA) conjugated with monoclonal antihuman melanoma-associated chondroitin sulfate proteoglycan (MCSP) antibody. In vitro labeling efficiency was controlled by assessing PA contrast of individual cells (17). MCSP expression of SK-MEL-1 cells was assessed by fluorescent labeling of cells by phycoerythrin (PE) conjugated antihuman MCSP antibody (Miltenyi Biotec Inc) and fluorescent imaging.
In vitro models
In vitro PAFC studies were performed with cell suspension in PBS in thick (120 µm) slide. Cell solution was diluted to 106 cells/mL level to obtain loose distribution of cells. Microscope stage translation was used to move cells under the laser beam. Cell and beam positions were controlled by a CCD camera.
In vitro flow simulations were performed in 300-µm glass capillary (PYREX Disposable Micro-Sampling Pipets, 10 µl, Corning, USA). Linear flow velocity was set in the range 0.1–100mm/s by a syringe pump (KD Scientific model 780210, USA). To minimize refraction, a glass capillary was placed into a glycerol bath with a transducer immersed into glycerol close to the capillary surface.
Animal models
Nude mice (purchased from Harlan Sprague–Dawley; weighing 20–25 g) were used in accordance with protocols approved by the University of Arkansas for Medical Sciences Institutional Animal Care and Use Committee. For in vivo monitoring of circulating melanoma cells, mice were anesthetized with ketamine/xylazine, 50/10 mg/kg, i.p. or with Isoflurane inhalation (1.2%). An anesthetized mouse was placed on a temperature-controlled microscope stage (37°C). For ear vessels studies a mouse ear was spread over the stage glass window (Fig. 1C); for the abdominal wall vessels, two thin glass coverslips were used to stretch out a skin fold. The transducer was placed gently on the skin close to the laser beam. Subcutaneous inoculation of 106 mouse and human melanoma cells in a 50 µL suspension provided the mouse melanoma tumor in skin on the mouse back.
Navigation of the laser beam was performed either by a CCD camera in thin ear tissues (Fig. 1D) or with an NIR camera in abdominal wall tissues. Fine laser beam positioning and focusing was performed by searching maximum PA signals from blood vessels. Clearance rate for melanoma cells was detected by PAFC after injection of cells solution (105 of B16F10 or 5×105 of C8161 cells per injected 50 µl) into a tail vein. Recording of PA signal trace before injection for 30 minutes was used as a control. Monitoring of melanoma CTC in tumor –bearing mice was performed according to the same protocol, however, without control recording.
The permanent magnetic field in ear vessels was provided by a cylindrical Neodymium-Iron-Boron (NdFeB) magnet with Ni-Cu-Ni coating, 3.2 mm in diameter and 9.5 mm long and a surface field strength of 0.39 Tesla (MAGCRAFT).
Data treatment
Acoustic oscillations digitized by PC were processed in real time. Time domain recording and digital bandpass filtration were used to reduce background PA signals due to the scattered light and electromagnetic noise. Only oscillations in diapason from 750 kHz to 4 MHz were processed. Peak-to-peak amplitude of PA signal was determined as PA response on a laser pulse (Fig. 1F, left and middle). The resulting value was saved and the raw acoustic data was discarded to maximize the performance of the digitizer. The resulting PA signal trace (Fig. 1F, right) represented increase in PA amplitude for a melanoma cell passing under laser beam volume. PA signal trace was processed off-line by custom based software (MATLAB 7.7, Mathworks Inc.) to define peaks exceeding noise from the blood background using 3σ-criterion: signals with amplitude exceeding triple standard deviation of the background signal were considered to originate from circulating cells.
RESULTS
In Vitro Study of Melanoma Cells
The goals of in vitro study were to estimate: 1) PAFC’s sensitivity toward low and high pigmented melanoma cells in static conditions; 2) capability of PAFC to detect cells in fast flow; and 3) PAFC cell counting at various flow rates.
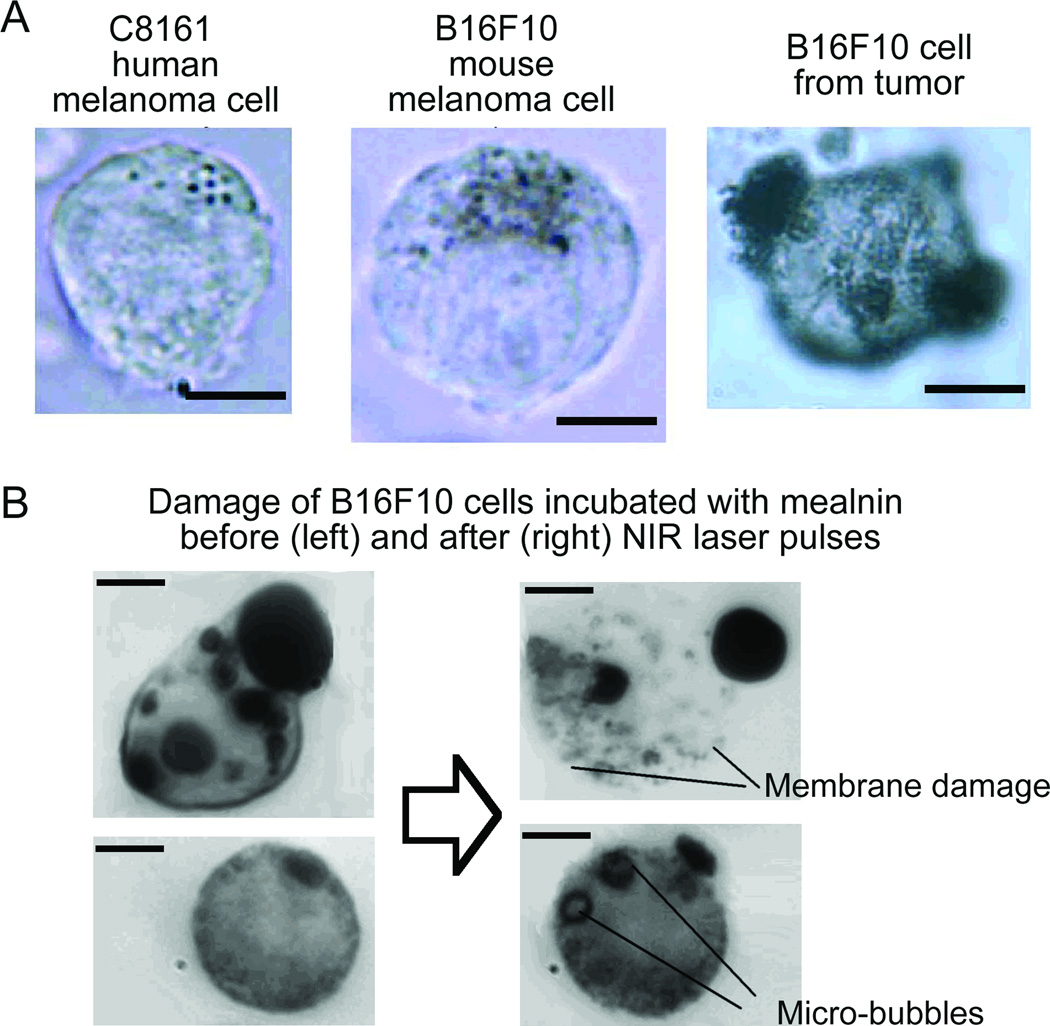
Individual melanoma cells in PBS suspensions in 120-µm thick glass slide or in 300-µm glass capillary were detected with PAFC in static and flow conditions, respectively. In PBS suspension at relatively low laser energy fluence of 0.02–0.05 J/cm2 only the strongly pigmented cells provided PA signals with signal-to-noise ratio (SNR ≥ 3) sufficient for their identification. At moderate laser energy, fluence of 0.1–0.5 J/cm2 98% cells were distinguished from pure solution. For higher levels of laser fluence (0.5-2.0 J/cm2) some cells were damaged by laser-induced nano-and microbubbles around the overheated melanin clusters accompanied by nonlinear PA signal amplification (16). Microscopic images revealed damaged membrane close to melanin particles or formation of stationary microbubbles in cytoplasm (Fig. 2B, right). The damage level depended on pigmentation and was further enhanced by absorption of multiple laser pulses compared to a single pulse mode. Nevertheless, at 1064nm, the damage thresholds was on average three to five times higher compared to that in 640–800 nm range (16), due to higher melanin absorbance at shorter wavelengths.
Fig. 2.
Acquisition of PA signals from individual cells (n = 300) in suspension (Fig. 2, top) with focused laser beam (beam diameter, ~10 µm) demonstrated high signal heterogeneity: there were 5–10% of cells with increased pigmentation producing PA at much higher (10-fold) amplitude compared to cells with less pigmentation. The average melanin content increased in the following sequence: cultured human melanoma cells C8161 - HTB-65 - B16F10 mouse cells and B16F10 cells derived from a primary tumor (Fig. 2 top, right). Approximately 98±1.9% of tumor–derived B16F10 cells were detectable with the PA technique, compared to 92±2.8% of the cultured B16F10 cells, 88±3.9% of the HTB-65, and 36 ± 15% C8161. These results are similar to the ones obtained with a single laser pulse previously: 92%, 87%, 76%, (no C8161 used), respectively (16). Noticeably, signal averaging with the high speed PAFC improved SNRs and increased the number of cells detected. The possibility of a further increase in absorbance and PA contrast was demonstrated by incubating cells with melanin (Fig. 2B). As it was expected, a higher absorbance increased PA signal amplitude 5–10-folds, while signal frequency spectrum shifted to 2 MHz range compared to 4 MHz for non-labeled B10F16 cells. This shift is likely related with features of generation of PA signals from larger melanin clusters compared to individual melanin nanoparticles.
High-Speed PA Detection of Moving Melanoma Cells In Vitro
First of all, we tested the capability of PAFC for label-free detection of melanoma cells in flow in vitro at flow velocities ranging from 0.1 to 100 mm/s using a 300-µm glass tube as a simple vessel phantom (33). Previous studies indicated the need in the use of a beam spot covering the whole vessel as in conventional flow cytometry to provide detection of all the cells without respect to the cell position in the vessel-cross section. The shaping beam spot as a narrow stripe with size of 15 × 150 µm was achieved by the use of cylindrical focusing optics.
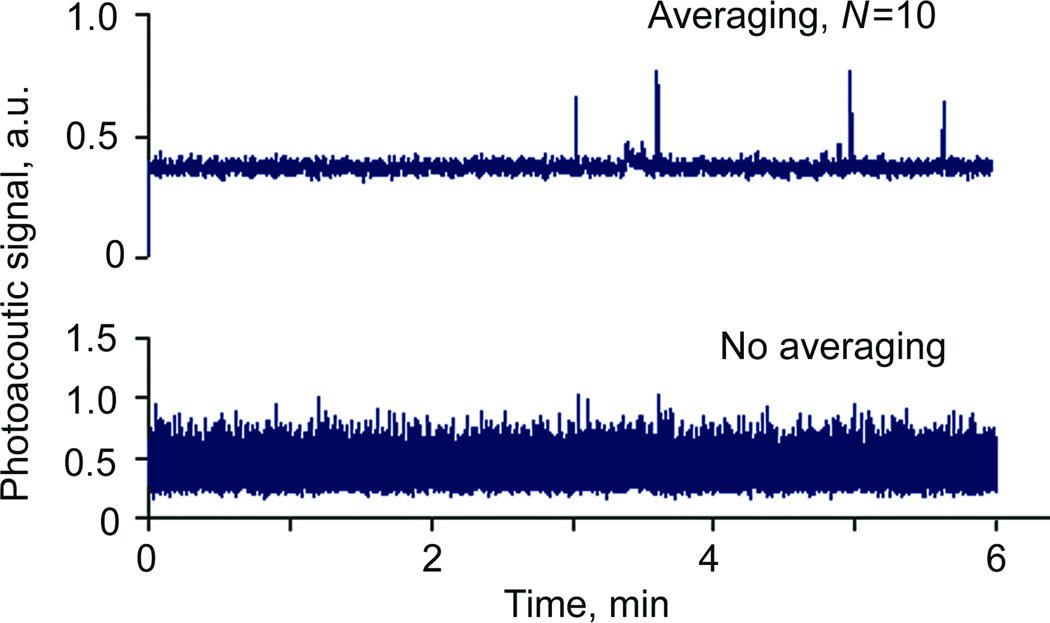
We experimentally verified that a high-pulse rate laser can improve performance of PAFC by addressing two critical issues. First, a short time interval between sequent laser pulses guaranteed that even at the very high flow speed topping few meters per second level there would be no cells passing the detection volume between laser pulses. For example, at linear flow rate of 5 mm/s (typical for human capillaries and animal microvessels), a cell could pass detection site without being exposed to the laser pulse of a laser operating at frequencies below 200 Hz. Second, high pulse rate increased SNR by acquiring several PA signals from the same circulating cell, with subsequent time- or frequency-domain averaging of the acquired PA signals. The maximal increase in SNRs is proportional to , where N is the number PA signals from the same cell. For example, with a 10 kHz laser rate ~140 PA signals can be acquired from a 20 µm cell passing a 50 µm wide laser beam at 5 mm/s linear velocity. Averaging of signals decreases electronic noise and provides increase in SNR ratio up to ~12-folds. Figure 3 illustrates the level of PA signals with and without averaging in PBS flow.
Fig. 3.
In vitro PA detection of melanoma cells in blood
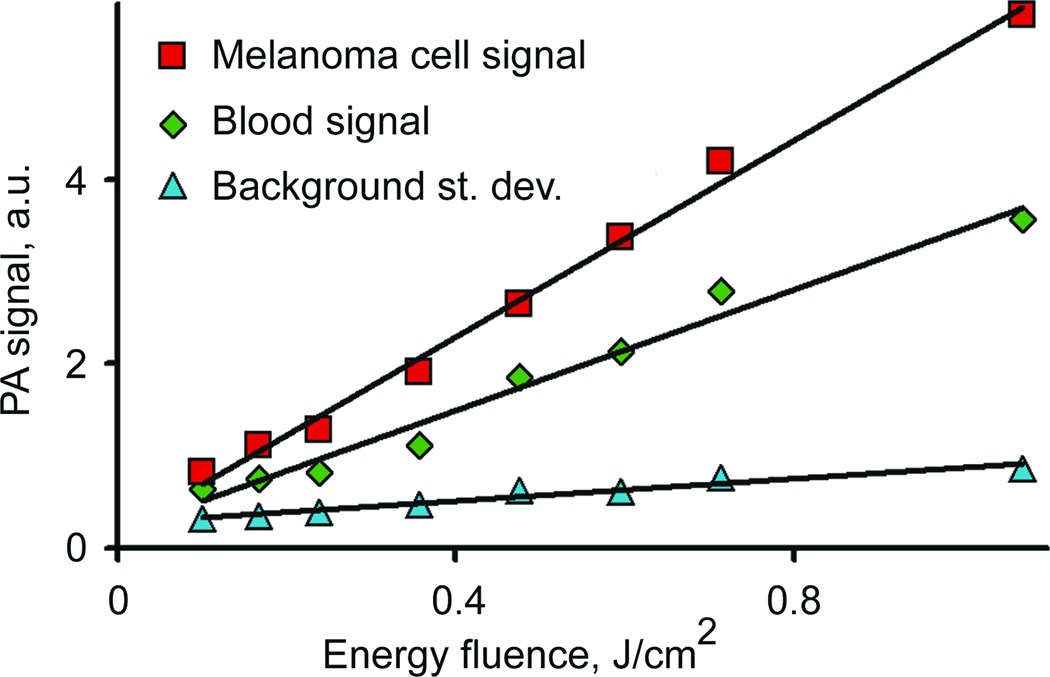
In read application, in vivo PA signals from melanoma cells have to be detected in the presence of background PA signals from blood decreasing sensitivity threshold of PAFC. Detection of rare pigmented melanoma cells in 120-µm thick blood layer revealed that the minimal laser fluence required for a single melanoma cell detection in blood at this condition is around 100 ± 20 mJ/cm2 level at 3σ-criterion (Fig. 4). The calibration curve was almost linear up to the fluence of 0.5–0.8 J/cm2. Above this level an increase in the laser fluence increased nonlinearly of PA signal generation (not shown in Fig. 4) due to formation of laser-induced nano- and microbubbles around intracellular melanin clusters (16). These estimations are valid only for the experimental conditions used (in vitro); however, the described methodology provided an insight for in vivo experiment.
Fig. 4.
In vivo PA detection of mimic CTCs, animal model
Here, we report our first results on monitoring melanoma cells in mouse circulation performed with a new high-speed PAFC. The main goal was to demonstrate the capability of this technique, to reveal its possible limitations, and determine ways for further improvement.
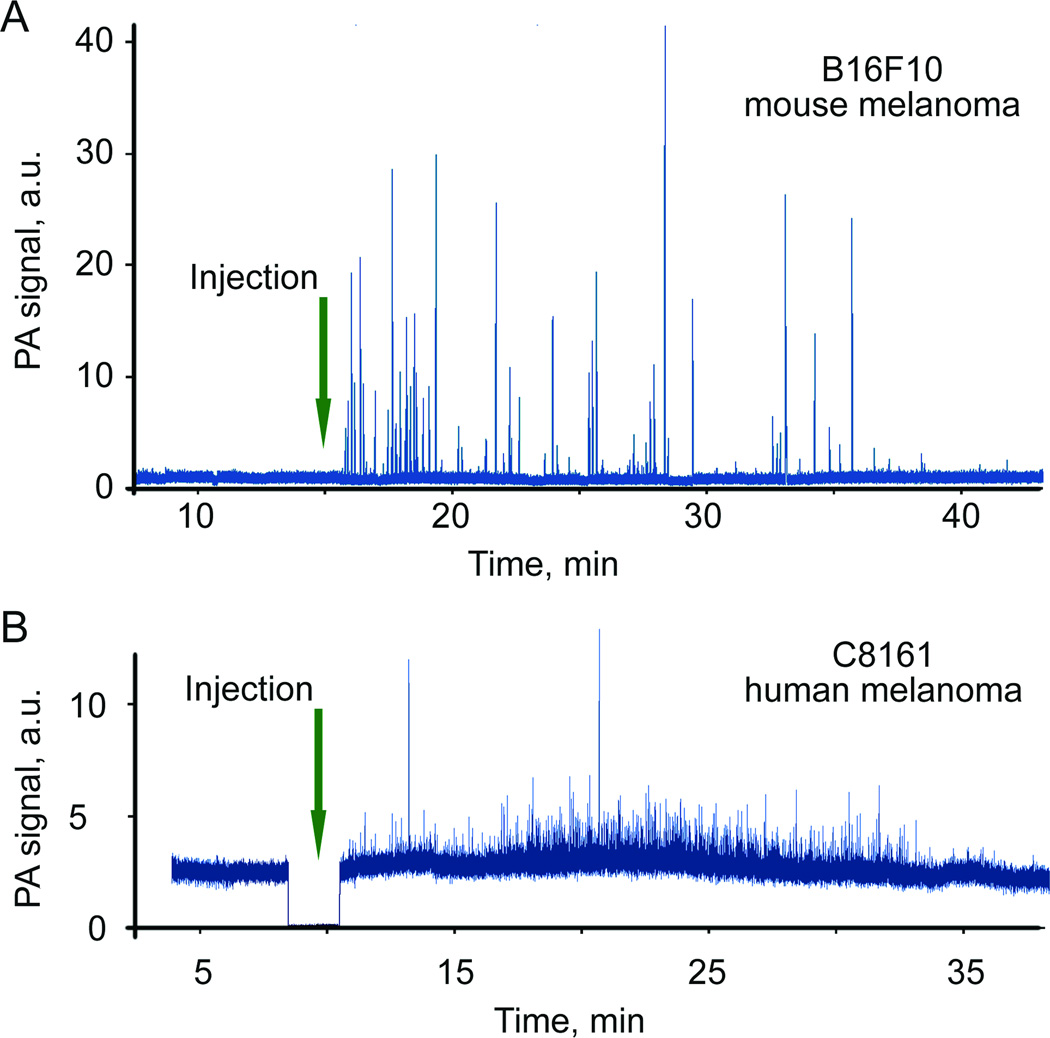
In vivo label-free detection of circulating both mouse (B16F10) and human (C8161) melanoma cells were provided by PA monitoring mouse ear blood microvessels after the injection of melanoma cells (mimic CTCs) into the tail vein. Our hypothesis was that emerging of PA signals above the background level (3σ-criterion) proves the presence of melanoma cells in blood. A laser at wavelength of 1064 nm was used for strongly pigmented melanoma cells, while laser at the 820 nm laser was used for low pigmented human melanoma cells. One group of mice (five animals) was used to monitor melanoma cells circulation in a mouse ear blood vessel, another group (three animals) was used for abdominal wall vessel monitoring. In most cases, PA signal traces from blood microvessels recorded for one hour prior injection did not reveal false positive signals. Pulse repetition rate of the 1064 laser varied in the range of 9–500 kHz, providing from 50 to 2500 PA signals for each cell passing detection zone. Typically, intensive and frequent PA signals were observed right after injection strongly pigmented B16F10 cells and relatively rare PA signals with smaller amplitude from low pigmented C8161 (Fig. 5). The rate of signals appearance had tendency to significantly decrease with strong fluctuations in rate (Fig. 6B).
Fig. 5.
Fig. 6.
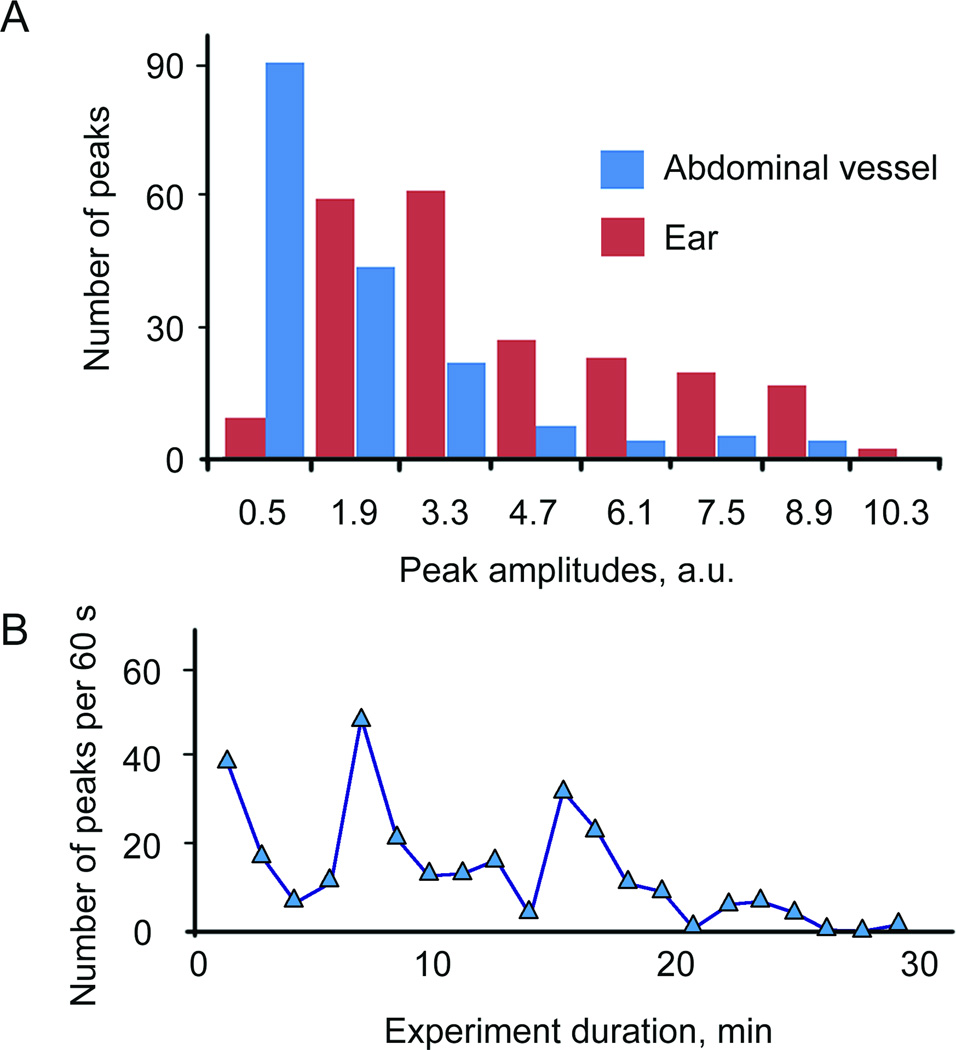
Because of the low melanin content in human melanoma C8161 cells, very rare PA signals were detected for these cells with a 1064 nm laser. Increase in detection sensitivity ~2.5 times was achieved with the use of a laser with wavelength of 820 nm where melanin absorption is higher compared to that at 1064 nm, while blood background absorption was lower approximately on 20–30% (32). Additionally, length of 820 nm laser spot in the sample was reduced compared to 1064 nm laser allowing further decreasing blood background due to smaller detection volume. The majority of the signals from B16F10 and C8161 had SNRs in the range of 5–20 and 2–5, respectively. Based on our previous (16) and current data including in vitro analysis of PA signal amplitudes distribution for many (100–300) individual cells, we estimated that PAFC is able to detect in average of 60–80% B16F10 cells and 20–35% of C8161cells depending on blood vessel sizes and its depth in skin. In particular, comparison of the data for cells circulation in abdominal wall and ear blood vessels demonstrated (Fig. 6A) that amplitude of PA peaks was higher for ear vessels. Obviously, stronger light scattering in thick abdominal vessel tissues decreased laser fluence and absolute PA amplitude. On the other hand, the number of PA signals was significantly higher in larger vessels (Fig. 6A) due to due to higher flow rate of blood.
In vivo PA detection of circulating metastatic cells in tumor-bearing mice
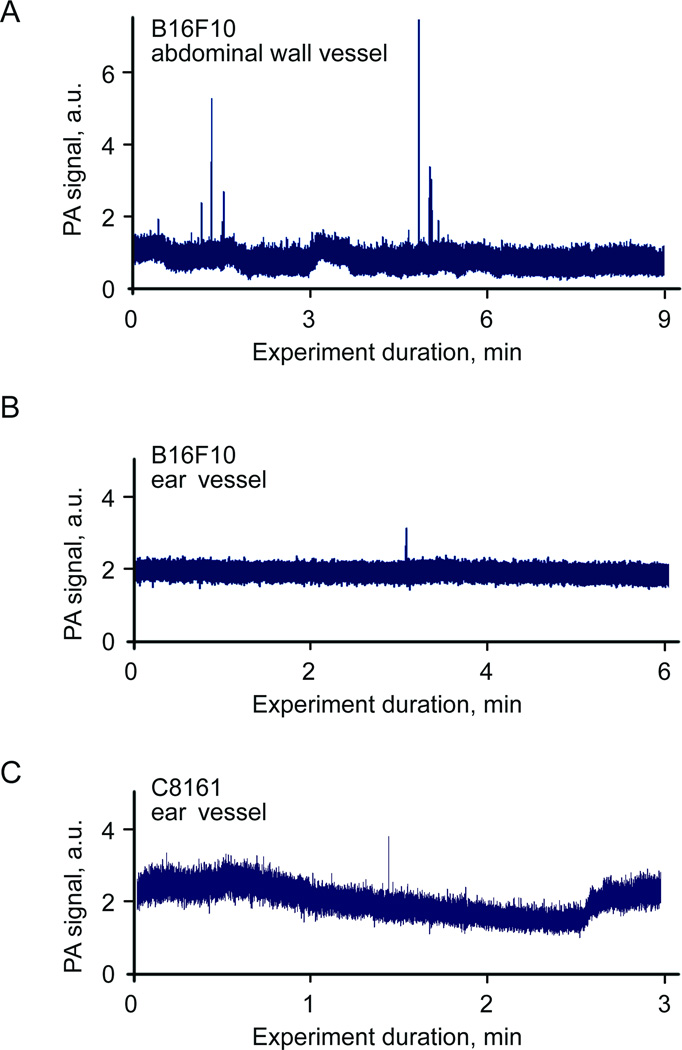
We applied advanced high-speed PAFC for label-free detection of the B16F10 melanoma CTCs using the same tumor-bearing mouse model described previously (16). As in the case of mimic CTCs (above) 3σ-criterion was used to distinguish PA signals from real CTCs and the blood background. With the criterion, there were no false-positive signals detected in control mice with no tumor. In mice with inoculated B16F10 melanoma cells we were able to detect CTCs in ear microcirculation on fourth day after inoculation. In particular, 7 CTCs were detected in abdominal wall blood vessel during 15 min long monitoring (30CTCs/hr), while in ear blood vessel there was only one CTC detected during 20 minutes (3 CTC/hr). These results (Figs. 7A,B) prove advantage of high-speed PAFC for monitoring fast moving CTCs in relatively large vessels. The demonstrated sensitivity of PAFC detection of metastatic B16F10 cells in abdominal vessels can be represented as following: seven cells were detected in 0.28 mL of blood (a 200-µm thick vessel with a maximal linear flow velocity of ~10 mm/s pumps ~0.28 mL of blood in 15 min, that is ~14% of total mouse blood assuming total blood volume of 2 mL). Thus, rough estimations give total of 70–100 cells in blood circulation at 4th day after tumor cells inoculation or one melanoma cell in 1.7×108 red blood cells (RBCs). However, it should be emphasized that sensitivity threshold as minimal number of CTCs detected in whole blood volume depends rather on monitoring time and flow rate in selected blood vessels. In particular, the capability of PAFC to monitor vessels during 3 h gives estimation threshold sensitivity as one CTC in whole mouse blood volume that is line with our previous experimental verification as 2 CTC/mL (16).
Fig. 7.
PAFC was also able to reveal presence of human melanoma C8161 CTCs in tumor-bearing model; however, as in the case of direct injection of C8161 cells there were just few PA signals reliably corresponding to C8161 cells. Thus, low intrinsic PA contrast of low pigmented melanoma cells impeded estimation of CTC rates.
In vivo enrichment and PA detection of melanoma cells targeted by functionalized magnetic nanoparticles
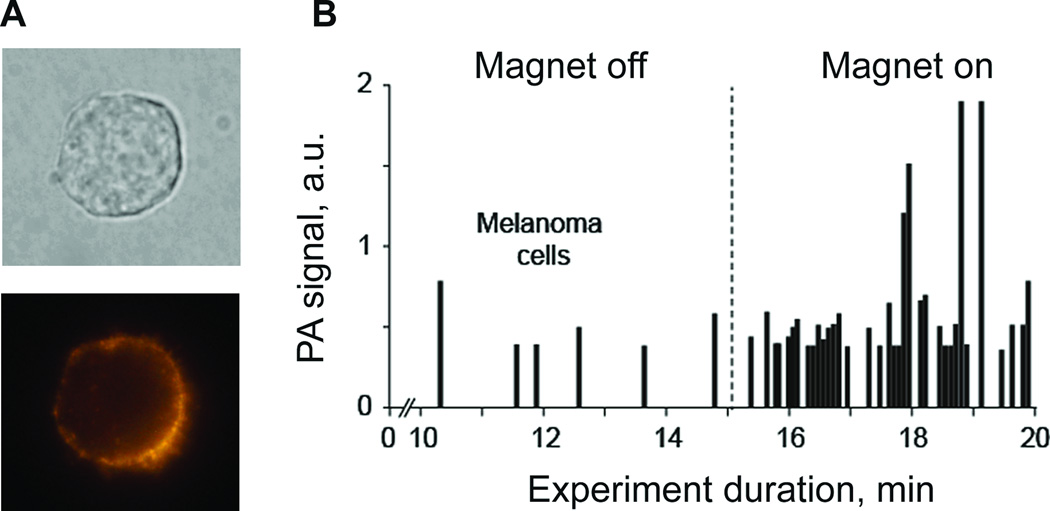
As PAFC is missing low pigmented melanoma cells, we further evaluated the capability of PAFC to detect CTCs targeted in vivo in circulation by functionalized nanoparticles. Previously, this approach was demonstrated for breast cancer cells only (17). For this study, we selected the human melanoma (SK-MEL-1) cells with profound expression of MCSP receptors. MSCP expression of the melanoma cells was assessed by conventional flowcytometry and fluorescent imaging of cells labeled by PE conjugated monoclonal antihuman MCSP antibody (Fig. 8A) and 90% of cells showed significant expression of MCSP. To verify cell targeting for PAFC, SK-MEL-1 melanoma cells were treated with unconjugated and conjugated magnetic nanoparticles in vitro at different times and temperatures. Next, all the cells were examined under PA microscope; PA signals from labeled cells were significantly higher than those from control unlabeled cells. A 85±8% targeting efficiency was achieved for the current cell line at 37°C after 30 min, compared to 3–5% of nonspecific binding; that is in line with flowcytometry data on MCSP expression.
Fig. 8.
The half-life of magnetic nanoparticles circulating in mouse blood after intravenous injection of 50 µL of nanoparticles PBS solution (concentration ~1012 particles per mL) was found to be 20 ± 5 min. No PA signals were observed from a 100-fold diluted solution of nanoparticles (1010 particles per mL) after intravenous injection and PAFC monitoring with 1064 nm laser (energy fluence of 500 mJ/cm2); suggesting that signals from unbound or nonspecifically bound nanoparticles were weaker than background signals from blood. Injection of 106 SK-mel-1 melanoma cancer cells in 50 µl of PBS gave rise to appearance of rare PA signals close to those observed for C8161 cells (Fig. 5B). At the lower level of energy fluence (50 mJ/cm2) no signals from melanoma cells were observed. Thus, PAFC parameters providing absence of signals from nanoparticles and cells circulating in mouse blood were found.
Next, we injected 105 unlabeled cancer cells in 50 µl of PBS, immediately followed by the injection of magnetic nanoparticles at a concentration of 1010/ml in 10 µl of PBS. None of the components produced PA signals during individual injection as was shown earlier. A gradual increase in the rate of high-PA signals appearance (SNR > 6) was observed within next 20–40 min after injection of the nanoparticles. These data suggest that targeting of CTC by nanoparticles increased their local concentration and provided PA contrast sufficient for detection. Gentle attaching a 0.7-T magnet to the mouse at the distance of ~0.3 mm from the laser spot led to an immediate increase in both the amplitude and rate of PA signals. It is likely that labeled cells moving in the vessel were completely trapped or slowed down in the strong magnetic field. Thus, a local increase in cells concentration provided further increase in PA contrast and appearance of PA signal with large amplitudes. As a result, under the action of the magnet, the rate of appearance of intensive PA signals associated with melanoma cells circulating in vivo increased from 1.1 cells/min to 12 cells/min (Fig. 8B), that is, ~10 times.
DISCUSSION
Here, we have presented the unique experimental data confirming the possibility of label-free detection of both human and mouse melanoma cells in fast blood circulation. The high speed PAFC reduces the rate of false-negative errors for fast moving CTCs in large blood vessels. Multiple PA signals received from the same cell allow increasing detection sensitivity through noise reduction. Eventually, high-speed PAFC with ability to assess large deep vessels should be able to drastically decrease time required for monitoring the whole blood volume, thus decreasing detection limits down to desired threshold (9): ultimately a few CTCs per whole blood volume (i..e 5 liter). We emphasize that with the use of melanin overexpression in melanoma cells as intrinsic cell marker in vivo label-free high-speed PAFC eliminates some potential problems of existing assays (e.g., low sensitivity due to assessment of small blood volume, toxicity concerns related with in vivo labeling procedure, etc.). This may provide precedent for a noninvasive in vivo blood cancer testing and, potentially purging (18) that focuses on melanoma, a highly aggressive, epidemic malignancy that is often widely metastatic at an early stage. Indeed, we demonstrated detection of B16F10 CTCs in blood circulation on the fourth day after subcutaneous inoculation of tumor cells.
Intrinsic PA contrast of melanin can be utilized in melanoma related research fields: 1) monitoring of melanoma cells dissemination in blood and in lymphatic as demonstrated previously (16,31); 2) study of cell pigmentation and melanin synthesis in vitro and in vivo; 3) correlating CTCs count to primary tumor and sentinel lymph node status (14,18). Clinical scenarios may include: 1) blood screening for early CTCs before development of metastases; 2) testing for cancer recurrence; 3) individualized assessment of therapy efficiency (e.g., chemo, radiation, or immunotherapy) through real-time CTC count; and 4) potential metastasis prevention by well-timed therapy including in vivo noninvasive laser PT killing of melanoma CTCs (16).
In vivo label-free PAFC of low pigmented C8161 human melanoma cells demonstrated increased false negativity rate of about 60–80% compared to 12% for strongly pigmented B16F10 mouse melanoma cells (16). As soon as both high and low pigmented cells can be simultaneously presented in melanoma patients, we assume that PAFC can miss up to 50–60% of cells in a label-free mode. Nevertheless, despite the high-false-negative rate of label-free PAFC of human melanoma, it may have tremendous clinical significance in blood testing for early melanoma diagnosis, for CTCs counting at parallel progression of primary tumor and metastasis and for diagnosis of melanoma recurrence. In most cases, the fact that CTCs are present in blood flow may have higher importance than accurate CTCs count. The missing of low pigmented melanoma CTCs should be more crucial for monitoring therapy efficiency. We believe that novel robust diagnostic platform using high pulse rate lasers and label-free noninvasive approach can be more quickly translated to humans. Indeed, noninvasive PA methods already demonstrated higher resolution, sensitivity, and the penetration depth (up to 3cm), compared to other optical modalities. According to our data a high-speed PAFC makes is possible to distinguish individual melanoma cells in blood circulation at low levels of laser fluence (~ 0.1 J/cm2) which satisfy laser safety standard (21). The clinical prototype could represent a portable fiber-based device that overlies the hand vessels. (e.g., see prototype in the supplementary information in ref. (16)).
Herewith we also address the problem of a high false negative rate of a label-free PAFC via molecular CTCs targeting by magnetic nanoparticles and their magnetic enrichment. Still, more studies are required to bring this approach to the bedside. Several other methods could be used to increase PA contrast of cells: 1) 5–10-fold increase in PA contrast by drug-induced activation of melanin production in melanoma cells (16); 2) melanogenesis activation in melanoma and nonmelanoma (e.g., breast cancer) cells via transfection with tyrosinase activating plasmids (16); 3) in vitro incubation of cells with melanin nanoparticles (Fig. 2); and 4) reduction of blood background PA signal by the change in blood oxygenation, the hematocrit, and osmolarity within physiological norms (16).
ACKNOWLEDGMENTS
This work was supported in part by the National Institute of Health grants, R01CA131164, R01EB009230, and R21CA139373, the National Science Foundation grant nos DBI-0852737, the Arkansas Biosciences Institute and Department of Defense grants: W88XWH-10-2-0130, W81XWH-10-BCRP-CA, and W81XWH-11-1-0129. We acknowledge the gift of C8161 human melanoma cells by Dr. Markus Frank, Harvard Medical School, MA.
REFERENCES
- 1.Cummins DL, Cummins JM, Pantle H, Silverman MA, Leonard AL, Chanmugam A. Cutaneous malignant melanoma. Mayo Clin Proc. 2006;81:500–507. doi: 10.4065/81.4.500. [DOI] [PubMed] [Google Scholar]
- 2.Menzies SW. Cutaneous melanoma: making a clinical diagnosis, present and future. Dermatol Ther. 2006;19:32–39. doi: 10.1111/j.1529-8019.2005.00054.x. [DOI] [PubMed] [Google Scholar]
- 3.Gray-Schopfer V, Wellbrock C, Marais R. Melanoma biology and new targeted therapy. Nature. 2007;445:851–857. doi: 10.1038/nature05661. [DOI] [PubMed] [Google Scholar]
- 4.Smith B, Selby P, Southgate J, Pittman K, Bradley C, Blair GE. Detection of melanoma-cells in peripheral-blood by means of reverse-transcriptase and polymerase chain-reaction. Lancet. 1991;338:1227–1229. doi: 10.1016/0140-6736(91)92100-g. [DOI] [PubMed] [Google Scholar]
- 5.Visus C, Andres R, Mayordomo JI, Martinez-Lorenzo MJ, Murillo L, Saez-Gutierrez B, Diestre C, Marcos I, Astier P, Godino J, et al. Prognostic role of circulating melanoma cells detected by reverse transcriptase-polymerase chain reaction for tyrosinase mRNA in patients with melanoma. Melanoma Res. 2007;17:83–89. doi: 10.1097/CMR.0b013e3280a60878. [DOI] [PubMed] [Google Scholar]
- 6.Mocellin S, Hoon D, Ambrosi A, Nitti D, Rossi CR. The prognostic value of circulating tumor cells in patients with melanoma: a systematic review and meta-analysis. Clin Cancer Res. 2006;12:4605–4613. doi: 10.1158/1078-0432.CCR-06-0823. [DOI] [PubMed] [Google Scholar]
- 7.Ma J, Lin JY, Alloo A, Wilson BJ, Schatton T, Zhan Q, Murphy GF, Waaga-Gasser A-M, Gasser M, Stephen Hodi F, et al. Isolation of tumorigenic circulating melanoma cells. Biochem Biophys Res Comm. 2010;402:711–717. doi: 10.1016/j.bbrc.2010.10.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weight RM, Viator JA, Dale PS, Caldwell CW, Lisle AE. Photoacoustic detection of metastatic melanoma cells in the human circulatory system. Opt Lett. 2006;31:2998–3000. doi: 10.1364/ol.31.002998. [DOI] [PubMed] [Google Scholar]
- 9.Alunni-Fabbroni M, Sandri MT. Circulating tumour cells in clinical practice: Methods of detection and possible characterization. Methods. 2010;50:289–297. doi: 10.1016/j.ymeth.2010.01.027. [DOI] [PubMed] [Google Scholar]
- 10.Stott SL, Hsu C-H, Tsukrov DI, Yu M, Miyamoto DT, Waltman BA, Rothenberg SM, Shah AM, Smas ME, Korir GK, et al. Isolation of circulating tumor cells using a microvortex-generating herringbone-chip. Proc Natl Acad Sci U S A. 2010;107:18392–18397. doi: 10.1073/pnas.1012539107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zharov VP, Galanzha EI, Tuchin VV. Photothermal imaging of moving cells in lymph and blood flow in vivo. Proc SPIE. 2004;5320:256–263. [Google Scholar]
- 12.Georgakoudi I, Solban N, Novak J, Rice WL, Wei X, Hasan T, Lin CP. In vivo flow cytometry: a new method for enumerating circulating cancer cells. Cancer Res. 2004;64:5044–5047. doi: 10.1158/0008-5472.CAN-04-1058. [DOI] [PubMed] [Google Scholar]
- 13.Zharov VP, Galanzha EI, Tuchin VV. In vivo photothermal flow cytometry: Imaging and detection of individual cells in blood and lymph flow. J Cell Biochem. 2006;97:916–932. doi: 10.1002/jcb.20766. [DOI] [PubMed] [Google Scholar]
- 14.Zharov VP, Galanzha EI, Shashkov EV, Kim JW, Khlebtsov NG, Tuchin VV. Photoacoustic flow cytometry: principle and application for real-time detection of circulating single nanoparticles, pathogens, and contrast dyes in vivo. J Biomed Opt. 2007;12:051503. doi: 10.1117/1.2793746. [DOI] [PubMed] [Google Scholar]
- 15.He W, Wang HF, Hartmann LC, Cheng JX, Low PS. In vivo quantitation of rare circulating tumor cells by multiphoton intravital flow cytometry. Proc Natl Acad Sci U S A. 2007;104:11760–11765. doi: 10.1073/pnas.0703875104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Galanzha EI, Shashkov EV, Spring PM, Suen JY, Zharov VP. In vivo, noninvasive, label-free detection and eradication of circulating metastatic melanoma cells using two-color photoacoustic flow cytometry with a diode laser. Cancer Res. 2009;69:7926–7934. doi: 10.1158/0008-5472.CAN-08-4900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Galanzha EI, Shashkov EV, Kelly T, Kim J-W, Yang L, Zharov VP. In vivo magnetic enrichment and multiplex photoacoustic detection of circulating tumour cells. Nat Nano. 2009;4:855–860. doi: 10.1038/nnano.2009.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Galanzha EI, Kim JW, Zharov VP. Nanotechnology-based molecular photoacoustic and photothermal flow cytometry platform for in vivo detection and killing circulating cancer stem cells. J Biophotonics. 2009;1:725–735. doi: 10.1002/jbio.200910078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhong CF, Tkaczyk ER, Thomas T, Ye JY, Myc A, Bielinska AU, Cao Z, Majoros I, Keszler B, Baker JR, et al. Quantitative two-photon flow cytometry - in vitro and in vivo. J Biomed Opt. 2008:13. doi: 10.1117/1.2931077. [DOI] [PubMed] [Google Scholar]
- 20.Chang YC, Ye JY, Thomas TP, Cao ZY, Kotlyar A, Tkaczyk ER, Baker JR, Norris TB. Fiber-optic multiphoton flow cytometry in whole blood and in vivo. J Biomed Opt. 2010:15. doi: 10.1117/1.3463481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.ANSI_Z136.1. ANSI Z136.1-2007. Washington, DC: American National Standards Institute; 2007. American national standard for the safe use of lasers. [Google Scholar]
- 22.Zharov VP, Letokhov VS. Laser optoacoustic spectroscopy. New York: Springer-Verlag; 1986. p. 327. [Google Scholar]
- 23.Wang LV, editor. Photoacoustic Imaging and Spectroscopy CRC. 2009. [Google Scholar]
- 24.Shashkov EV, Everts M, Galanzha EI, Zharov VP. Quantum dots as multimodal photoacoustic and photothermal contrast agents. Nano Lett. 2008;8:3953–3958. doi: 10.1021/nl802442x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Razansky D, Distel M, Vinegoni C, Ma R, Perrimon N, Koster RW, Ntziachristos V. Multispectral optoacoustic tomography of deep-seated fluorescent proteins in vivo. Nat Photonics. 2009;3:412–417. [Google Scholar]
- 26.Petrov YY, Petrova IY, Patrikeev IA, Esenaliev RO, Prough DS. Multiwavelength optoacoustic system for noninvasive monitoring of cerebral venous oxygenation: a pilot clinical test in the internal jugular vein. Opt Lett. 2006;31:1827–1829. doi: 10.1364/ol.31.001827. [DOI] [PubMed] [Google Scholar]
- 27.Ermilov SA, Khamapirad T, Conjusteau A, Leonard MH, Lacewell R, Mehta K, Miller T, Oraevsky AA. Laser optoacoustic imaging system for detection of breast cancer. J Biomed Opt. 2009;14:024007. doi: 10.1117/1.3086616. [DOI] [PubMed] [Google Scholar]
- 28.Petrova IY, Esenaliev RO, Petrov YY, Brecht HPE, Svensen CH, Olsson J, Deyo DJ, Prough DS. Optoacoustic monitoring of blood hemoglobin concentration: a pilot clinical study. Opt Lett. 2005;30:1677–1679. doi: 10.1364/ol.30.001677. [DOI] [PubMed] [Google Scholar]
- 29.Ara G, Anderson R, Mandel K, Ottesen M, Oseroff A. Irradiation of pigmented melanoma cells with high intensity pulsed radiation generates acoustic waves and kills cells. Lasers Surg Med. 1990;10:52–59. doi: 10.1002/lsm.1900100112. [DOI] [PubMed] [Google Scholar]
- 30.Wang LV. Multiscale photoacoustic microscopy and computed tomography. Nat Photonics. 2009;3:503–509. doi: 10.1038/nphoton.2009.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Galanzha EI, Shashkov EV, Tuchin VV, Zharov VP. In vivo multispectral, multiparameter, photoacoustic lymph flow cytometry with natural cell focusing, label-free detection and multicolor nanoparticle probes. Cytometry Part A. 2008;73A:884–894. doi: 10.1002/cyto.a.20587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nedosekin DA, Sarimollaoglu M, Shashkov EV, Galanzha EI, Zharov VP. Ultra-fast photoacoustic flow cytometry with a 0.5 MHz pulse repetition rate nanosecond laser. Opt Express. 2010;18:8605–8620. doi: 10.1364/OE.18.008605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zharov VP, Galanzha EI, Tuchin VV. Photothermal flow cytometry in vitro for detection and imaging of individual moving cells. Cytometry Part A. 2007;71A:191–206. doi: 10.1002/cyto.a.20384. [DOI] [PubMed] [Google Scholar]