Abstract
Our goal was to examine the effect of deficiency of the lipoprotein receptor, scavenger receptor class B type I (SR-BI), on progesterone secretion in human granulosa cells (HGL5). Scrambled or SR-BI small interfering RNA [knockdown (KD)] cells were exposed to dimethylsulfoxide [DMSO, vehicle for forskolin (Fo)], Fo, serum, high-density lipoprotein, low-density lipoprotein (LDL), or Fo plus lipoproteins or serum for 24 h. Progesterone secretion was lower in all of the SR-BI KD cells regardless of treatment. We examined progesterone secretion in SR-BI KD, LDL receptor KD, and double KD cells incubated with DMSO, Fo, LDL, or Fo + LDL for 6–24 h. As compared with scrambled cells, progesterone secretion was lower in SR-BI and double KD cells regardless of treatment; whereas progesterone secretion was only lower in LDL receptor KD cells incubated with LDL and Fo + LDL. We measured phosphorylation of hormone-sensitive lipase (pHSL) expression, intracellular total cholesterol (TC) mass, and progesterone secretion in scrambled and SR-BI KD cells incubated with DMSO or Fo for 2–24 h. The expression of pHSL was similar between the cells and conditions. The mean change in TC mass and progesterone secretion was lower in SR-BI KD cells exposed to DMSO and Fo. Incubating SR-BI KD cells with 22-hydroxy cholesterol did not overcome the reduction in progesterone secretion. At different time points, RNA expression of steroidogenic acute regulatory protein, side-chain cleavage, and 3β-hydroxysteroid dehydrogenase was significantly lower in SR-BI KD cells incubated with Fo. In conclusion, SR-BI protein deficiency, in part, might explain progesterone deficiency in some infertile women.
Deficiency of the lipoprotein receptor, SR-BI, significantly decreases progesterone secretion in cultured human granulosa cells, in part, by down-regulating expression of key steroidogenic genes.
Deficiency of the lipoprotein receptor, scavenger receptor class B type I (SR-BI), might possibly explain, at least in part, some aspects of infertility in humans. SR-BI is a physiologically relevant lipoprotein receptor that mediates the uptake of cholesteryl esters (CE) from the core of lipoproteins (1). SR-BI has also been shown to colocalize in the peri-nuclear region of cells, with as yet an undefined function (2,3). It has been shown to be highly expressed in liver and steroidogenic tissues, with particularly high levels found in ovarian tissues (4). SR-BI deficiency is significantly associated with infertility in female mice (5). These female knockout mice have been shown to ovulate dysfunctional oocytes, and embryogenesis is abnormal (6). Interestingly, fertility can be restored with either the addition of probucol, a cholesterol lowering antioxidant drug (3), in the chow diet or by genetically restoring liver SR-BI protein expression (7).
Little is known regarding the role of SR-BI in human fertility. We were the first to show that infertile women with low expression of SR-BI RNA in granulosa cells isolated during oocyte retrievals had significantly lower plasma estradiol levels and lower number of retrieved and fertilized oocytes (8). Other evidence for the role of SR-BI on aspects of ovarian steroidogenesis has been based on the results of ex vivo studies in nonhuman primates and rat granulosa cells. For instance, Cherian-Shaw reported that SR-BI RNA levels increased steadily by ∼30-fold in macaque granulosa cells 24 h after stimulation by human chorionic gonadotropin (hCG), whereas low-density lipoprotein receptor (LDLR) expression initially increased but then decreased to low basal levels during this early time period (9). Earlier work by Azhar et al. (10) showed that induction of SR-BI expression in rat granulosa cells was significantly associated with increased CE uptake from high-density lipoprotein (HDL) and with increased total progestin secretion. These investigators subsequently showed that LDL receptor deficiency had minimal effect on murine ovarian progesterone secretion (11).
The goal of our study was to define the effect of SR-BI protein deficiency on progesterone secretion in cultured human granulosa HGL5 cells. In agreement with other investigators, we have found that LDL is the preferential lipoprotein supporting steroidogenesis (12,13,14,15,16). SR-BI, in contrast to the LDLR, appears to have a major effect on progesterone secretion. Deficiency of SR-BI significantly reduced RNA expression of p450 side-chain cleavage (SCC), 3β-hydroxysteroid dehydrogenase (3βHSD), and steroidogenic acute regulatory protein (StAR) especially after forskolin (Fo) stimulation.
Materials and Methods
Materials. Human lipoproteins (total HDL and total LDL) were purchased from Intracel, Inc. (Frederick, MD). Fo was purchased from Sigma (St. Louis, MO). All other chemical reagents were purchased from Sigma.
Cell culture
HGL5 cells. HGL5 cells were generously provided by Dr. Bruce Carr, University of Texas Southwestern. Cells were cultured in DMEM/F12 medium (Invitrogen, Carlsbad, CA) supplemented with 10% Ultra-low IgG fetal bovine serum (Invitrogen), 1% ITS+premix (BD Biosciences, Bedford, MA), 100 U/ml penicillin, 100 μg/ml streptomycin, and 1 μg/ml gentamicin (all from Invitrogen). Cells were plated in 12-well plates at density 1.5 × 105 cells per well for 2 h before transfection of oligonucleotides. The HGL5 cell line has been well-characterized and shown to secrete progesterone (17). The use of the HGL5 cell line overcomes a major problem of cell variability in primary human granulosa cells.
Knockdown of SR-BI and LDL receptor (LDLR)
The small interfering RNA (siRNA) and scrambled control duplexes were purchased from Qiagen (Valencia, CA). HGL5 cells were seeded in 12-well plates at density 1.5 × 105 and transfected with 10 nm SR-BI siRNA or 10 nm LDLR siRNA (final concentration in 550 μl) using HiPerfect transfection reagent (Qiagen) following the manufacturer’s protocol. A negative control siRNA with no homology to any known mammalian gene (AllStars Negative Control siRNA) was used at the 10 nm concentration. For double knockdown (KD) experiments, we transfected 10 nm of each siRNA for a total 20 nm siRNA. The cells were incubated with the transfection reagents in DMEM/F12 medium supplemented with 10% Ultra-low IgG fetal bovine serum, 1% ITS+premix, 100 U/ml penicillin, 100 μg/ml streptomycin, and 1 μg/ml gentamicin for 72 h before use in experiments. Cell viability was measured by trypan blue exclusion assay.
Cell experiments
Control cells were considered those incubated with scrambled oligonucleotides. Knockdown of the cells was carried out for 72 h in complete medium as indicated above. Control medium was DMEM/F12 medium (without serum and supplements) containing dimethylsulfoxide (DMSO), the vehicle used to dissolve Fo. The experimental design was to stimulate scrambled or KD cells with control medium or the same medium containing either Fo (10 μm), HDL (50 μg/ml), LDL (50 μg/ml), serum (10%), HDL + Fo, LDL + Fo, or serum + Fo, 22-hydroxycholesterol (22-OH cholesterol, 20 μm) or Fo + 22-OH cholesterol for varying periods of time. The experiments were terminated by collecting the medium and subjecting it to centrifugation to pellet nonadherent cells. An aliquot of the medium was used for progesterone measurements using commercially available RIA kits, and values were normalized to cell protein. Cell lysates were harvested for Western blotting, and total RNA was extracted for real-time PCR measurements.
Cholesteryl ester hydrolase assays
Cellular lipids were extracted with hexane-isopropanol (3:2) (18), and the distribution of intracellular esterified (EC) and unesterified (UC) cholesterol mass was measured by gas chromatography using stigmasterol as an internal control (19). Cell proteins were measured using the BCA method, and cholesterol mass values were normalized to mg cell protein.
Western blotting
Total cell lysates were prepared using 5% SDS, 50 mm Tris-Cl (pH 7.6) buffer in the presence of protease inhibitor cocktail (1:100), phenylmethylsulfonylfluoride (1 mg/ml) and sodium orthovanadate (100 μm) (20). Aliquots of the lysates (5 μg protein per lane) were subjected to SDS-PAGE in 10% gels and then transferred onto polyvinylidene fluoride membranes. Blots were blocked with 5% milk for 1 h, incubated with rabbit polyclonal anti-SR-BI (Novus Biological, Littleton, CO; 1:1000) or rabbit monoclonal anti-LDLR (Novus; 1:1000) at 4 C overnight, rinsed three times with TBS-0.1% Tween, reacted with antirabbit HR-peroxidase labeled IgG (Cell Signaling Technology, Danvers, MA) at room temperature for an additional hour, and then rinsed three more times with TBS-0.1% Tween. Bands were visualized using an Amersham ECL chemiluminescence kit (GE Healthcare, Piscataway, NJ), quantitated by densitometric scanning, and normalized to β-actin expression.
Fractionation experiment
To assess whether SR-BI siRNA transfection affected the different pools of SR-BI, we fractionated HGL5 cells after transfection with scrambled and SR-BI siRNA. The fractionation protocol was performed using a commercially available kit per the manufacturer (Plasma Membrane Protein Extraction Kit, Abcam, Cambridge, MA). In brief, plasma membrane proteins were purified from post-nuclear supernatants of SR-BI KD or scrambled siRNA transfected HGL5 cells. Cytosolic and plasma membrane fractions were separated by SDS-PAGE and transferred to polyvinylidene fluoride membranes for Western blot analysis of SR-BI and β-actin.
Real-time PCR
Aliquots of total RNA (50 ng) extracted from granulosa cells at different experimental conditions were reverse-transcribed in a reaction volume of 12 μl using 2.5 μm random hexamer, 500 μm dNTPs, 5.5 mm MgCl2, 10 U ribonuclease inhibitor, and 25 U MMLV reverse transcriptase. The reactions were carried out in thermal controller (50 C for 60 min and 92 C for 10 min). The resulting cDNAs were diluted with water. All probes and primers, including the internal control ribosomal protein L19 (RPL19) were synthesized by Applied Biosystems (Foster City, CA). The target gene and the RPL19 were detected in the same reaction. The PCR protocol consisted of 40 cycles of denaturing at 95 C for 15 s and annealing/extending at 60 C for 1 min per cycle. Detection of the gene expression was performed during the 2nd step in a two step RT-PCR protocol. To quantify mRNA levels, a standard curve was constructed using pooled HGL5 cDNA generated from nontransfected cells. Data were analyzed as the inverse log ([Ct-Y intercept]/slope of the standard curve) and expressed as a ratio of the target gene to endogenous control (21). Primer and probe sequences are as follows: RPL19 forward: CCCCAATGAGACCAATGAAATC; RPL19 reverse: CAGCCCATCTTTGATGAGCTT; RPL19 probe: ATGCCAACTCCCGTCAGCAGATC; 3βHSD forward: CCAGAACGGCCACGAAGA; 3βHSD reverse: AGCTTTTTGCTGTACGGGTATG; 3βHSD probe: AGCCTCTGGAAAACACATGGCCCAStAR forward: CCACCCCTAGCACGTGGAT; StAR reverse: TCCTGGTCACTGTAGAGAGTCTCTTC; StAR probe: CGGAGCTCTCTACTCGGTTCTC; SCC forward: CTTCTTCGACCCGGAAAATTT; SCC reverse: ATCCGCCGTCCCAGACA; SCC probe: ACCCAACCCGATGGCTGAGCAA.
Progesterone assays
Progesterone levels in culture media were measured using commercially available RIA kits (Siemens Healthcare Diagnostics Inc., Deerfield, IL). The intra- and interassay variability was 4.1% and 5.2%, respectively.
Statistics
Each experiment was performed using replicate wells, and each experiment was performed at least three times at different times. The data shown in the figures are expressed as the mean ± se at each time point. Generalized linear mixed models were performed to account for the correlation among measurements within the experiment under exactly the same conditions, or over time, from the same condition. Statistical comparisons for treatment effects (such as scrambled vs. SR-BI KD) and the treatment conditions (such as DMSO vs. Fo) were evaluated with linear combinations of the estimates based on the mixed models. P values ≤0.05 were considered statistically significant. All analyses were performed using Stata 10.1 statistical software (StataCorp, College Station, TX, 2009).
Results
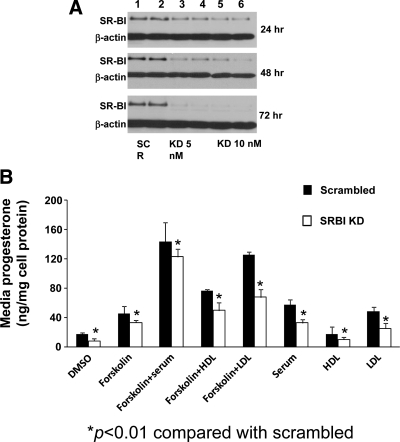
Our first approach was to knockdown SR-BI protein expression in HGL5 cells by transfecting cells with SR-BI specific siRNA (0–10 nM) or scrambled siRNA oligonucleotides (0–10 nM) for varying periods of time (24–72 h). As shown in Fig. 1A, SR-BI protein expression was markedly lower, in a dose- and time-dependent manner, as compared with cells transfected with scrambled siRNA. SR-BI protein expression was maximally reduced (97%) in cells transfected for 72 h with 10 nM SR-BI siRNA. The Western blot is representative of at least three independent experiments. Moreover, cell viability was comparable between the two experimental conditions, indicating that deficiency of SR-BI protein did not adversely affect cells (data not shown).
Figure 1.
Effect of HDL and LDL on progesterone secretion in SR-BI KD cells. A, HGL5 cells were seeded at 1.5 × 105 cells/well in a 12-well format. Scrambled (SCR, lanes 1 and 2) or SR-BI-specific siRNA was added (5 nm, lanes 3 and 4; 10 nm, lanes 5 and 6) for 24, 48, and 72 h to KD SR-BI protein expression. Levels of SR-BI protein were measured by Western blot and normalized to the loading control β-actin. B, Scrambled or SR-BI KD cells were incubated with DMSO or Fo ± HDL (50 μg protein/ml), ± LDL (50 μg protein/ml), ± serum (10%), or serum, HDL, and LDL alone for an additional 24 h. The data represent the mean ± se of three independent experiments, with each experiment performed using duplicate wells. *, P < 0.01 between scrambled and SR-BI KD cells.
We next examined whether there was a differential effect of human HDL or LDL on steroidogenesis in scrambled or SR-BI KD cells. HGL5 cells were first transfected with either scrambled siRNA (10 nm) or SR-BI siRNA (10 nm) for 72 h; the medium was aspirated and then cells were incubated with DMSO or the same medium containing either Fo (10 μm), human serum (10%), HDL (50 μg protein/ml), LDL (50 (μg protein/ml), Fo + serum, Fo + HDL, or Fo + LDL for an additional 24 h. In scrambled cells, progesterone secretion was significantly higher in cells incubated with Fo alone (>2-fold, P < 0.001), Fo + serum (>11-fold, P < 0.001), Fo + HDL (>4.5 fold, P < 0.001), Fo + LDL (>6-fold, P < 0.001), serum alone (>3-fold, P < 0.001), LDL alone (>3-fold, P < 0.001), and HDL alone (>1.4-fold, P < 0.001) as compared with DMSO (Fig. 1B). Compared with scrambled cells, progesterone secretion in SR-BI KD cells was significantly lower in all conditions (P < 0.01).
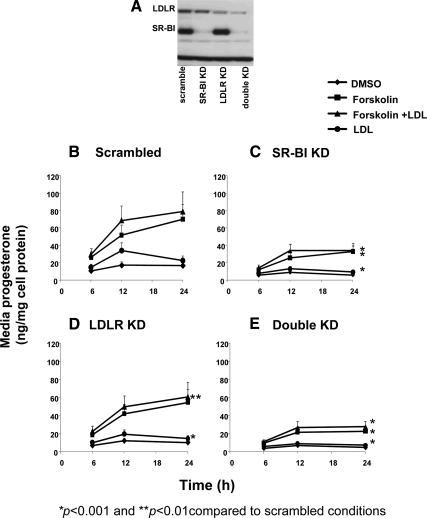
Having shown that knockdown of SR-BI alone significantly reduced progesterone secretion, we next examined the effects of LDLR KD alone and SR-BI–LDLR double KD on progesterone secretion. As shown in Fig. 2A, we first measured SR-BI, LDLR, and double KD protein levels in scrambled and receptor specific siRNA KD cells, and showed that the respective receptor proteins were markedly reduced as compared with scrambled cells (94% lower in SR-BI KD cells, 68% lower in LDLR KD cells, and 96[SR-BI]-86[LDLR]% in the double KD cells, respectively). Scrambled and receptor specific siRNA KD cells were then incubated with control medium (DMSO) or the same medium plus Fo (10 μm), LDL (50 μg protein/ml), or Fo + LDL for 6–24 h. Cells were not incubated with HDL because this lipoprotein does not bind to the LDLR, but LDL does bind to SR-BI and the LDLR. Comparisons of the mean change of progesterone levels over time in the different receptor specific KD conditions (Fig. 2, C–E) were made to the control scrambled cells (Fig. 2B). Thus, the mean change in progesterone secretion over 6–24 h was significantly different in SR-BI KD cells (Fig. 2C) incubated with LDL (81% lower, P < 0.001), Fo (52% lower, P < 0.001), and Fo + LDL (60% lower, P < 0.001). In LDLR KD cells (Fig. 2D), the mean change in progesterone secretion was significantly different in cells incubated with LDL (41% lower, P < 0.001) and Fo + LDL (23% lower, P < 0.05) but not significantly lower in cells incubated with DMSO or Fo alone. In double KD cells (Fig. 2E), the mean change in progesterone secretion was significantly different in cells incubated with LDL (81% lower, P < 0.001), Fo (70% lower, P < 0.001), and Fo + LDL (66% lower, P < 0.001).
Figure 2.
The effect of LDLR and SR-BI KD in HGL5 cells. A, Cells were transfected for 72 h with specific siRNA for either scrambled, SR-BI, LDLR, or both (double KD). Protein expression was determined by Western blot. Each condition was performed in duplicate wells, and the Western blot is representative of at least three independent experiments. B–E, Progesterone secretion (nanogram per milligram of cell protein) was measured in scrambled (B), SR-BI KD (C), LDLR KD (D), or double KD (E) cells. Transfected cells were incubated in the presence of DMSO, Fo (10 μm), Fo + LDL (50 μg of protein/ml), or LDL alone for an additional 6–24 h. Media levels of progesterone were measured by RIA. The data represent the mean ± se of three independent experiments, with each experiment performed using duplicate wells. Error bars not visualized are contained with the symbol. *, P < 0.001 and **, P < 0.01, as compared with scrambled cells.
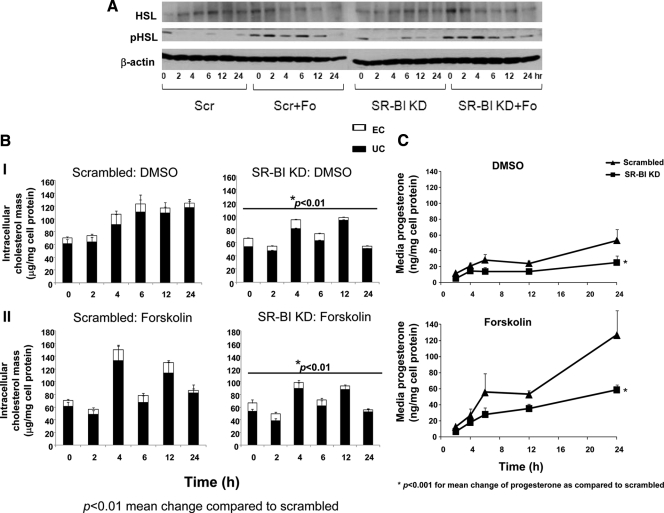
What clearly emerged from the results shown in Fig. 2 was that SR-BI protein deficiency significantly reduced progesterone secretion. What also emerged was the effect of SR-BI KD on lipoprotein-dependent (cells incubated with LDL) and lipoprotein-independent (cells incubated with Fo alone without the presence of any lipoproteins in the culture media) progesterone secretion. Because it would be expected that deficiency of SR-BI or LDLR would be associated with impaired lipoprotein dependent progesterone secretion, we pursued the novel finding that SR-BI KD affected Fo-induced progesterone secretion (which was not observed in LDLR KD cells). A major effect of Fo is the phosphorylation of hormone-sensitive lipase (pHSL), a key intracellular enzyme that stimulates CE hydrolysis with the generation of unesterified cholesterol (UC) needed for steroidogenesis (22). We, therefore, next examined the effect of SR-BI KD on Fo stimulation on total and pHSL, intracellular cholesterol mass, and progesterone secretion (Figs. 3, A–C). Scrambled and SR-BI KD cells were incubated with either DMSO or Fo (10 μm) for time periods varying from 2–24 h. As shown in Fig. 3A, in a representative Western blot, pHSL expression was detectable in both scrambled and SR-BI cells cultured in transfection medium containing serum (time zero, which serves as the baseline for the Fo-stimulated cells). The transfection medium was removed and replaced with medium containing only Fo without serum. The pattern of pHSL expression in scrambled and SR-BI siRNA transfected cells incubated with Fo was similar (Fig. 3A). The expression of total HSL was also similar between scrambled and SR-BI KD cells. In Fig. 3B (panel I), over the entire 24 h period, the mean change in intracellular total cholesterol (TC) mass (EC plus UC) was significantly different between SR-BI KD cells incubated with DMSO as compared with scrambled cells (P < 0.05), with the majority of the affect due to a reduction in UC mass in SR-BI KD cells after 12 h. In comparing the mean change in TC mass in cells incubated with Fo (panel II), we found significantly lower TC mass in SR-BI KD cells (P < 0.05), with the majority of the affect due to a reduction in UC mass after 6 h. The corresponding progesterone levels are shown in Fig. 3C, indicating significantly lower progesterone secretion in SR-BI KD cells incubated with DMSO over the 24 h time period (P < 0.01), as well as significantly lower levels in SR-BI KD cells incubated with Fo (P < 0.01).
Figure 3.
Total and pHSL expression in SR-BI KD cells. HGL5 cells were transfected with scrambled or SR-BI siRNA oligonucleotides for 72 h, and then transfected cells were incubated with DMSO or Fo (10 μm) for an additional 0–24 h. Cell lysates were harvested at each time point, and then total HSL and pHSL were measured by Western blotting using specific monoclonal antibodies. The blot is representative of three independent experiments. B, Intracellular cholesterol mass in SR-BI KD cells under basal and Fo-stimulated conditions. Scrambled (Scr) and SR-BI KD cells were incubated with DMSO or Fo (10 μm) for 0–24 h. At each time point, intracellular lipids were extracted using hexane:isopropanol, then quantified by gas chromatography using stigmasterol as an internal standard, and normalized to milligram of cell protein. The data represent the mean ± se of three independent experiments, with each experiment performed using triplicate wells. *, P < 0.01 of the mean change of total cholesterol mass compared with scrambled cells. Cells designated 0 h for DMSO or Fo were cells after transfection and served as the baseline condition for the treatment phase. Error bars not visualized are contained with the symbol. C, Time course of progesterone secretion in SR-BI KD cells. Scrambled and SR-BI KD cells were incubated with DMSO or Fo (10 μm) for 2–24 h. Media progesterone levels were measured by RIA. The data represent the mean ± se of three independent experiments, with each experiment performed using triplicate wells. Error bars not visualized are contained with the symbol. *, P < 0.001 for the mean progesterone change over 2–24 h compared with scrambled cells.
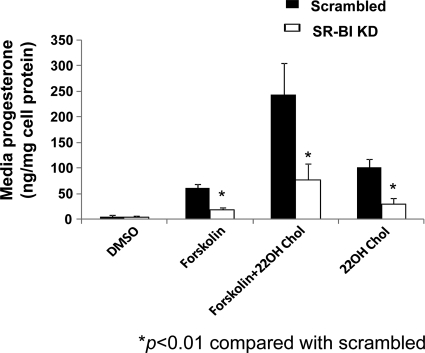
Given that there was no available exogenous source of cholesterol in the culture media, the only other possible source of intracellular cholesterol mass available for progesterone synthesis would have been from de novo cholesterol synthesis. Therefore, we hypothesized that SR-BI protein deficiency might be associated with impaired de novo cholesterol synthesis, and we then performed experiments to determine whether incubating cells with excess 22-OH cholesterol would overcome this possible impairment. Scrambled and SR-BI KD cells were incubated with DMSO, Fo (10 μm), 22-OH cholesterol (20 μm), or Fo + 22-OH cholesterol for 24 h. As shown in Fig. 4, progesterone secretion was significantly lower in SR-BI KD cells incubated with either Fo (P < 0.001), 22-OH cholesterol alone (P < 0.001), or 22-OH plus Fo (P < 0.001) compared with scrambled cells.
Figure 4.
The lack of effect of 22-OH cholesterol (22OH Chol) on progesterone secretion in SR-BI KD cells. HGL5 cells were transfected with scrambled or SR-BI siRNA for 72 h, and then incubated with DMSO, Fo (10 μm), Fo + 22-OH cholesterol (20 μm), or 22-OH cholesterol for an additional 24 h. Media levels of progesterone were measured by RIA. The data represent the mean ± se of three independent experiments, with each experiment performed using duplicate wells. *, P < 0.01 compared with scrambled cells.
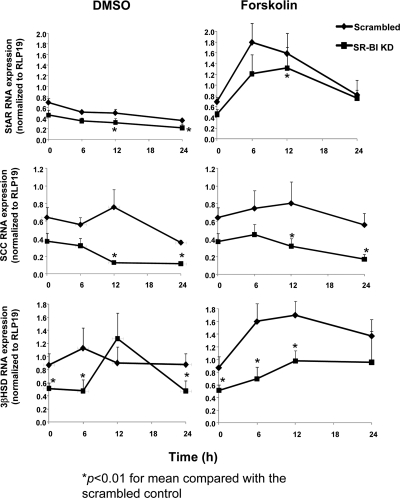
Thus far we have shown that SR-BI KD cells reduced progesterone secretion and that the presence of excess 22-OH cholesterol did not overcome this impairment. We therefore examined the effect of SR-BI deficiency on expression of factors in the steroidogenesis pathway downstream to HSL activation. Scrambled and SR-BI KD cells were incubated with DMSO or Fo (10 μm) for 0–24 h, and then total RNA was extracted from the cells. Baseline RNA expression of StAR and SCC were not significantly different from scrambled cells, whereas the baseline levels of 3βHSD RNA was significantly lower in SR-BI KD cells as compared with scrambled cells (Fig. 5) (P < 0.05). Overall, mean RNA expression at varying time points for the three genes was significantly lower in SR-BI KD cells (P < 0.05), regardless of treatment. In scrambled cells, the RNA expression of StAR and 3βHSD significantly increased in response to Fo stimulation, whereas the response of SCC and 3βHSD, in particular, in SR-BI KD cells to Fo was attenuated (P < 0.05).
Figure 5.
Decreased RNA expression of StAR, SCC, and 3βHSD in SR-BI KD cells. HGL5 cells were transfected with scrambled or SR-BI siRNA for 72 h and then incubated with DMSO or Fo (10 μm) for varying periods of time (0–24 h). Total RNA was extracted at each time point, and each gene target was measured by real-time PCR using RPL19 as the housekeeping gene. The data represent the mean ± se of six independent experiments, with each experiment performed using duplicate wells The 0 h time point reflects the baseline condition prior to the treatment phase with either DMSO or Fo. *, P < 0.01 for the mean progesterone compared with scrambled cells.
In addition, we found that intracellular levels of progesterone were significantly lower in SR-BI KD cells incubated with either DMSO or Fo as compared with scrambled cells (data not shown).
Discussion
While virtually nothing is known regarding the role of SR-BI on human reproduction, whether male or female, there is more known regarding its role in reproductive biology in rodents (5,23). For instance, Miettinen et al. (6) showed that female SR-BI knockout (KO) mice were infertile. These mice were characterized as having normal ovulation but formed abnormal, vacuolated embryos, with poor viability. More recently, Miranda-Jimenez et al. (24) showed that SR-BI KO mice had 50% lower serum progesterone levels as compared with wild-type mice. Our in vitro data are consistent with an adverse effect of SR-BI protein deficiency on progesterone levels. We did not observe any changes in cell viability despite the reduction in progesterone secretion. This might be because we have not observed complete loss of progesterone secretion in the SR-BI KD cells, as other investigators have found that complete loss of progesterone secretion in either monkey or rat granulosa cells was associated with increased atresia or apoptosis (25,26).
The results shown in Fig. 1 demonstrated that LDL cholesterol was more effective in inducing progesterone secretion compared with HDL, regardless of the presence or absence of SR-BI protein under basal or Fo stimulated conditions. These findings are consistent with results we previously reported in rhesus monkeys. In this study, Cherian-Shaw et al. (9) showed that LDL was significantly associated with increased progesterone secretion as compared with monkey granulosa cells incubated with either HDL or VLDL. Other investigators have also shown that granulosa cells use either LDL (11,12,13,14,16,27,28,29) or HDL for steroidogenesis (30,31). None of this is incompatible with the role of SR-BI in human steroidogenesis, as it has been previously shown that SR-BI binds with high affinity to VLDL, LDL, and HDL (1,32).
In rodents, Azhar et al. (10) reported that deficiency of the LDL receptor in mice does not negatively impact ovarian steroidogenesis. These investigators found that LDLR deficiency was not associated with changes in key steroidogenic enzymes or progestin production. It is known that LDLR deficiency does not affect rodent female reproduction, while complete deficiency of murine SR-BI is associated with infertility (5,6). The results in Fig. 2 show that LDLR deficiency can significantly reduce progesterone secretion, especially in cells incubated with LDL or Fo + LDL, but not in cells incubated with either Fo or DMSO alone. In contrast, progesterone secretion was significantly lower in SR-BI KD cells regardless of treatment. This suggested that in LDLR KD cells the expression and/or function of SR-BI remains intact to compensate for the reduced function of LDLR, a finding that is consistent with that reported by Azhar et al. (10) in LDLR-deficient mice.
A novel finding observed in Fig. 2 was the fact that progesterone secretion was significantly lower in SR-BI KD cells stimulated with Fo alone. It was not unexpected that progesterone secretion was lower in SR-BI KD cells incubated with LDL, given that a major function of plasma membrane SR-BI is mediating the uptake of neutral lipids from the core of lipoproteins. It was, however, surprising to observe lower progesterone secretion in SR-BI KD cells treated with Fo alone, which suggested a different, but important, role for SR-BI. It has been observed that in addition to its location in the plasma membrane, SR-BI has been shown to be localized to the peri-nuclear region in cells (2,3), but its function in this location is unclear. Most recently, Ahras et al. (2) conducted a series of experiments in a variety of cells (HeLa [immortalized cervical cancer cells], fibroblasts, and primary hepatocytes) to examine the subcellular localization and purported function of SR-BI. These investigators concluded that SR-BI might participate in intracellular cholesterol trafficking from the late endosomal/lysosomal compartment. While we did not directly assess the different pools of SR-BI on progesterone secretion, we did perform fractionation experiments and found that the SR-BI siRNA oligonucleotides were equally and potently effective in reducing the cytosolic and plasma membrane pools of SR-BI (data not shown). Thus, at this time, we cannot ascribe any particular pool of SR-BI as exerting a major affect on progesterone secretion.
With the knowledge that hydrolysis of stored cholesteryl esters would generate unesterified cholesterol mass needed for newly synthesized progesterone, and that HSL has been shown to exert a major effect on CE hydrolysis, we evaluated the effects of Fo on activation of HSL and intracellular cholesterol mass in scrambled and SR-BI KD cells. Our results indicated that the protein kinase A pathway activated by Fo was intact in the SR-BI KD cells, suggesting that the impairment of progesterone secretion in SR-BI KD cells was likely downstream to pHSL. We had also incubated scrambled and SR-BI KD cells with dibutryl cAMP but this also did not overcome the defect in progesterone secretion, suggesting that the defect was downstream of HSL (data not shown).
The lack of effect of 22-OH cholesterol incubation on progesterone secretion in SR-BI KD cells also suggested that SR-BI deficiency was not directly negatively affecting de novo cholesterol synthesis, as if this was the case then incubating SR-BI KD cells with 22-OH cholesterol would have been associated with restored or nearly restored progesterone secretion. Moreover, if SR-BI deficiency was affecting intracellular cholesterol transport via StAR expression or function, incubating SR-BI KD cells with 22-OH cholesterol should have overcome this defect as well, as the effects of 22-OH cholesterol on progesterone secretion can be StAR independent (33,34). Alternatively, if SR-BI directly exerts an effect on intracellular cholesterol trafficking, then the addition of 22-OH cholesterol in SR-BI KD cells would not be expected to overcome the defect. We did observe that SR-BI deficiency down-regulated StAR, SCC, and 3βHSD RNA expression, especially after Fo stimulation. It is unclear whether there is a direct link between SR-BI expression and regulation of these key steroidogenic genes, but more than likely an indirect link(s) explains this novel association. A link between StAR and SR-BI was observed in studies reported by Rao et al. (35). These investigators found that the rat R2C Leydig tumor cell line constitutively expressed steroids due to increased basal expression of StAR and SR-BI. Lastly, we believe it is less likely that progesterone down-regulated expression of these gene targets, as Chaffin et al. (36) had previously shown that progesterone did not affect expression of these key steroidogenic genes in monkey granulosa cells.
In conclusion, we have shown that SR-BI protein deficiency exerts a major influence on progesterone secretion in human granulosa cells. In addition to its well-known role of mediating uptake of neutral lipids from the core of lipoproteins, SR-BI appears to have a major influence on lipoprotein independent aspects of progesterone secretion, including regulating the expression of StAR, SCC, and 3βHSD, key proteins involved in the steroidogenic pathway.
Footnotes
This work was supported by Burroughs Wellcome Clinical Translation Research Award to Dr. A. Rodriguez and a National Institutes of Health RO1 grant (HD043358) to Dr. C. Chaffin.
Disclosure Summary: The authors have nothing to declare.
First Published Online September 15, 2010
Abbreviations: 22-OH cholesterol, 22-Hydroxycholesterol; 3βHSD, 3β-hydroxysteroid dehydrogenase; CE, cholesteryl esters; DMSO, dimethylsulfoxide; EC, esterified cholesterol; FBS, fetal bovine serum; Fo, forskolin; HDL, high-density lipoprotein; KD, knockdown; LDL, low-density lipoprotein; LDLR, LDL receptor; pHSL, phosphorylation of hormone-sensitive lipase; PVDF, polyvinylidene fluoride; SCC, side-chain cleavage; SR-BI, scavenger receptor class B type I; StAR, steroidogenic acute regulatory protein; TC, total cholesterol; UC, unesterified cholesterol.
References
- Acton S, Rigotti A, Landschulz KT, Xu S, Hobbs HH, Krieger M 1996 Identification of scavenger receptor SR-BI as a high density lipoprotein receptor. Science 271:518–520 [DOI] [PubMed] [Google Scholar]
- Ahras M, Naing T, McPherson R 2008 Scavenger receptor class B type I localizes to a late endosomal compartment. J Lipid Res 49:1569–1576 [DOI] [PubMed] [Google Scholar]
- Tondu AL, Robichon C, Yvan-Charvet L, Donne N, Le Liepvre X, Hajduch E, Ferre P, Dugail I 2005 Dagher G. Insulin and angiotensin II induce the translocation of scavenger receptor class B type I from intracellular sites to the plasma membrane of adipocytes. J Biol Chem 280:33536–33540 [DOI] [PubMed] [Google Scholar]
- Landschulz KT, Pathak RK, Rigotti A, Krieger M, Hobbs H 1996 Regulation of scavenger receptor, class B, type I, a high density lipoprotein receptor, in liver and steroidogenic tissues of the rat. J Clin Invest 98:984–995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trigatti B, Rayburn H, Vinals M, Braun A, Miettinen H, Penman M, Hertz M, Schrenzel M, Amigo L, Rigotti A, Krieger M 1999 Influence of the high density lipoprotein receptor SR-BI on reproductive and cardiovascular pathophysiology. Proc Natl Acad Sci USA 96:9322–9327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miettinen HE, Rayburn H, Krieger M 2001 Abnormal lipoprotein metabolism and reversible female infertility in HDL receptor (SR-BI)-deficient mice. J Clin Invest 108:1717–1722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yesilaltay A, Morales MG, Amigo L, Zanlungo S, Rigotti A, Karackattu SL, Donahee MH, Kozarsky KF, Krieger M 2006 Effects of hepatic expression of the high-density lipoprotein receptor SR-BI on lipoprotein metabolism and female fertility. Endocrinology 147:1577–1588 [DOI] [PubMed] [Google Scholar]
- Velasco M, Alexander C, King J, Zhao Y, Garcia J, Rodriguez A 2006 Association of lower plasma estradiol levels and low expression of scavenger receptor class B type I in infertile women. Fertil Steril 85:1391–1397 [DOI] [PubMed] [Google Scholar]
- Cherian-Shaw M, Puttabyatappa M, Greason E, Rodriguez A, VandeVoort CA, Chaffin CL 2009 Expression of scavenger receptor-BI and low-density lipoprotein receptor and differential use of lipoproteins to support early steroidogenesis in luteinizing macaque granulosa cells. Endocrinology 150:957–965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azhar S, Nomoto A, Leers-Sucheta S, Reaven E 1998 Simultaneous induction of an HDL receptor protein (SR-BI) and the selective uptake of HDL-cholesteryl esters in a physiologically relevant steroidogenic cell model. J Lipid Res 39:1616–1628 [PubMed] [Google Scholar]
- Azhar S, Luo Y, Medicherla S, Reaven E 1999 Upregulation of selective cholesteryl ester uptake pathway in mice with deletion of low-density lipoprotein receptor function. J Cell Physiol 180:190–202 [DOI] [PubMed] [Google Scholar]
- Brannian J, Stouffer R 1993. Native and modified (acetylated) low density lipoprotein-supported steroidogenesis by macaque granulosa cells collected before and after the ovulatory stimulus: correlation with fluorescent lipoprotein uptake. Endocrinology 132:591–597 [DOI] [PubMed] [Google Scholar]
- Brannian J, Shiigi S, Stouffer R 1992 Gonadotropin surge increases fluorescent-tagged low-density lipoprotein uptake by macaque granulosa cells from preovulatory follicles. Biol Reprod 47:355–360 [DOI] [PubMed] [Google Scholar]
- Parinaud J, Perret B, Ribbes H, Chap H, Pontonnier G, Douste-Blazy L 1987 High density lipoprotein and low density lipoprotein utilization by human granulosa cells for progesterone synthesis in serum-free culture: respective contributions of free and esterified cholesterol. J Clin Endocrinol Metab 64:409–417 [DOI] [PubMed] [Google Scholar]
- Volpe A, Coukos G, Uccelli E, Droghini F, Adamo R, Artini PG 1991 Follicular fluid lipoproteins in preovulatory period and their relationship with follicular maturation and progesterone production by human granulosa-luteal cells in vivo and in vitro. J Endocrinol Invest 14:737–742 [DOI] [PubMed] [Google Scholar]
- Carr BR, MacDonald PC, Simpson ER 1982 The role of lipoproteins in the regulation of progesterone secretion by the human corpus luteum. Fertil Steril 38:303–311 [DOI] [PubMed] [Google Scholar]
- Havelock JC, Rainey WE, Carr BR 2004 Ovarian granulosa cell lines. Mol Cell Endocrinol 228:67–78 [DOI] [PubMed] [Google Scholar]
- Brown MS, Ho YK, Goldstein JL 1980 The cholesteryl ester cycle in macrophage foam cells. J Biol Chem 255:9344–9352 [PubMed] [Google Scholar]
- Ishikawa TT, MacGee J, Morrison JA, Glueck CJ 1974 Quantitative analysis of cholesterol in 5 to 20 microliters of plasma. J Lipid Res 15:286–291 [PubMed] [Google Scholar]
- West M, Greason E, Kolmakova A, Jahangiri A, Asztalos B, Pollin TI, Rodriguez A 2009 Scavenger receptor class B type I protein as an independent predictor of HDL cholesterol levels in subjects with hyperalphalipoproteinemia. J Clin Endo Metab 94:1451–1457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fru KN, Vandevoort CA, Chaffin CL 2006 Mineralocorticoid synthesis during the periovulatory interval in macaques. Biol Reprod 75:568–574 [DOI] [PubMed] [Google Scholar]
- Kraemer FB, Shen W-J, Harada K, Patel S, Osuga J-I, Ishibashi S, Azhar S 2004 Hormone-sensitive lipase is required for high-density lipoprotein cholesteryl ester-supported adrenal steroidogenesis. Mol Endo 18:549–557 [DOI] [PubMed] [Google Scholar]
- Trigatti B, Rigotti A 2000 Scavenger receptor class B type I (SR-BI) and high-density lipoprotein metabolism: recent lesions from genetically manipulated mice. Int J Tissue React 22:29–37 [PubMed] [Google Scholar]
- Miranda-Jimenez L, Binelli M, Bertolin K, Pelletier RM, Murphy BD 2010 Scavenger receptor B-I and luteal function in the mouse. J Lipid Res 51:2362–2371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaffin CL, Stouffer RL 2000 Role of gonadotrophins and progesterone in the regulation of morphological remodelling and atresia in the monkey peri-ovulatory follicle. Hum Reprod 15:2489–2495 [DOI] [PubMed] [Google Scholar]
- Friberg PA, Larsson DG, Billig H 2009 Dominant role of nuclear progesterone receptor in the control of rat periovulatory granulosa cell apoptosis. Biol Reprod. 80:1160–1167 [DOI] [PubMed] [Google Scholar]
- Parker Jr CR, Illingworth DR, Bissonnette J, Carr BR 1986 Endocrine changes during pregnancy in a patient with homozygous familial hypobetalipoproteinemia. N Engl J Med 314:557–560 [DOI] [PubMed] [Google Scholar]
- Simpson ER, Rochelle DB, Carr BR, MacDonald PC 1980 Plasma lipoproteins in follicular fluid of human ovaries. J Clin Endocrinol Metab 51:1469–1471 [DOI] [PubMed] [Google Scholar]
- Carr BR, Sadler RK, Rochelle DB, Stalmach MA, MacDonald PC, Simpson ER 1981 Plasma lipoprotein regulation of progesterone biosynthesis by human corpus luteum tissue in organ culture. J Clin Endocrinol Metab 52:875–881 [DOI] [PubMed] [Google Scholar]
- Azhar S, Tsai L, Medicherla S, Chandrasekher Y, Giudice L, Reaven E 1998 Human granulosa cells use high density lipoprotein cholesterol for steroidogenesis. J Clin Endocrinol Metab 83:983–991 [DOI] [PubMed] [Google Scholar]
- Reaven E, Tsai L, Azhar S 1995 Cholesterol uptake by the ’selective’pathway of ovarian granulosa cells: early intracellular events. J Lipid Res 36:1602–1617 [PubMed] [Google Scholar]
- Swarnakar S, Temel RE, Connelly MA, Azhar S, Williams DL 1999 Scavenger receptor class B type I mediates selective uptake of low density lipoprotein cholesteryl ester. J Biol Chem 274:29733–29739 [DOI] [PubMed] [Google Scholar]
- Jefcoate C, Simpson E, Boyd G 1974 Spectral properties of rat adrenal-mitochondrial cytochrome P-450. Eur J Biochem 42:539–551 [DOI] [PubMed] [Google Scholar]
- Meaney S, Bodin K, Diczfalusy U, Bjorkhem I 2002 On the rate of translocation in vitro and kinetics in vivo of the major oxysterols in human circulation: critical importance of the position of the oxygen function. J Lipid Res 43:2130–2135 [DOI] [PubMed] [Google Scholar]
- Rao RM, Jo Y, Leers-Sucheta S, Bose HS, Miller WL, Azhar S, Stocco DM 2003 Differential regulation of steroid hormone biosynthesis in R2C and MA-10 Leydig tumor cells: role of SR-BI mediated selective cholesteryl ester transport. Biol Reprod 68:114–121 [DOI] [PubMed] [Google Scholar]
- Chaffin CL, Dissen GA, Stouffer RL 2000 Hormonal regulation of steroidogenic enzyme expression in granulosa cells during the peri-ovulatory interval in monkeys. Mol Hum Reprod 6: 11–18 [DOI] [PubMed] [Google Scholar]