Abstract
Systemic sclerosis (SSc) is a multisystem connective tissue disease of unknown etiology. The hallmark of SSc is scleroderma, referring to the presence of thickened, hardened skin. Oral and maxillofacial manifestations of the disease are numerous including masklike appearance, trismus, muscular atrophy, thin atrophied lips, secondary microstomia, xerostomia, rigidity of tongue and lips, widening of the periodontal ligament space, trigeminal neuralgia, and resorption of the mandible. A 35-year-old woman with limited cutaneous SSc presented with bilateral mandibular condylysis, severe class II mandibular deficiency, and large anterior open bite and limited range of mandibular opening at 27 mm. Surgical correction consisted of bilateral total temporomandibular joint reconstruction with stock prostheses combined with Le Fort I maxillary impaction and functional advancement genioplasty. This resulted in a functional occlusion with elimination of her open bite and a more esthetic profile. Her occlusion has remained stable at 7 months. The incidence of mandibular resorption in SSc has been found to be 20% to 33%. The mandibular angles are most commonly involved (37.6%), followed by the condyle (20.8%), coronoid process (20.0%), and the posterior border of the ascending ramus (14.4%). Bilateral condylysis is present in 13.7% of the cases. Very few cases of surgical correction of malocclusion induced by SSc-related condylysis have been reported in the literature. To the best of our knowledge, this is the first case report of bilateral condylysis from SSc where surgical replacement of the resorbed condyles was attempted. Bilateral total temporomandibular joint replacement can give these patients a functional occlusion, improved facial balance, and improved quality of life.
Keywords: Scleroderma, systemic, temporomandibular joint, arthroplasty, replacement, osteolysis
Systemic sclerosis (SSc) is a multisystem connective tissue disease of unknown etiology. The hallmark of SSc is scleroderma, referring to the presence of thickened, hardened skin. SSc can be divided into limited or diffuse cutaneous forms by the extent and distribution of skin involvement.1 In limited cutaneous SSc (lcSSc), skin involvement is limited to the distal extremities or face, or may involve only the fingers (sclerodactyly). In diffuse cutaneous SSc (dcSSc), there is widespread involvement of the skin with thickening proximal to the elbows or knees and often involving the chest or abdominal wall.
Other systemic manifestations are multiple including involvement of the vascular (especially Raynaud's phenomenon), pulmonary, renal, gastrointestinal, musculoskeletal, cardiac, and endocrine systems. LcSSc is commonly associated with the CREST syndrome (calcinosis, Raynaud's phenomenon, esophageal dysmotility, sclerodactyly, and telangiectasias). Raynaud's phenomenon generally precedes other disease manifestations in lcSSc. In dcSSc, Raynaud's phenomenon coincides with or follows the other disease manifestations. Pulmonary involvement includes pulmonary fibrosis (more with dcSSc) and pulmonary hypertension (more with lcSSc). Renal, cardiac, and musculoskeletal involvement is more common with dcSSc.
SSc is a sporadic disease with incidence estimates ranging from 9 to 19 cases/1 million per year, with prevalence rates ranging from 28 to 253 cases/1 million per year in the United States.2,3 Similar to other connective tissue diseases, SSc is more frequent in women than men, with peak onset in the 30- to 50-year age group. African-Americans are more commonly affected, with an earlier age of onset, and are more likely to have diffuse skin involvement compared with caucasians.4
Characteristic pathological findings are a noninflammatory proliferative/obliterative vasculopathy affecting small arteries and arterioles in multiple vascular beds, combined with interstitial and vascular fibrosis in the skin, lungs, and multiple other internal organs.5 The pathogenesis is complex, integrating the three cardinal features of SSc: vascular injury and damage, evidence of autoimmunity, and generalized interstitial and vascular fibrosis.6,7 Antinuclear antibodies are found in nearly every patient (> 95%). Anticentromere antibodies are detected in ~15 to 20% of patients and are associated more commonly with the limited form of the disease. Antitopoisomerase I (anti-Scl-70) antibodies are also found in 15 to 20% of patients, are associated specifically with the diffuse cutaneous form, may correlate with the extent of skin and lung fibrosis, and show fluctuations with disease activity.8 Antibodies to RNA polymerases (4 to 20% of patients) are also associated with diffuse skin changes, cardiac and renal involvement, and increased mortality.
Oral and maxillofacial manifestations of the disease include: masklike appearance, trismus, muscular atrophy, thin atrophied lips, secondary microstomia, xerostomia, rigidity of tongue and lips, widening of the periodontal ligament space, trigeminal neuralgia, and resorption of the mandible.9 This case report describes the surgical management of an lcSSc patient with bilateral mandibular condylysis with severe class II mandibular deficiency and large anterior open bite (AOB) and limited range of mandibular opening at 27 mm.
CLINICAL REPORT
Patient History
A 35-year-old Caucasian woman with lcSSc presented to the Oral and Maxillofacial Surgery Department of the Queen Elizabeth II Hospital, Halifax, Nova Scotia, Canada, with a complaint of facial deformity with open occlusion affecting her mastication. This change in occlusion was noted ~4 years after her diagnosis of lcSSc. Due to progressive worsening over the next 3 years, she decided to seek medical attention. Her past medical history was significant for lcSSc, present for 7 years, with associated Raynaud's phenomenon, mild pulmonary fibrosis, gastroesophageal reflux disease, and hypothyroidism. She also suffered from anxiety. She was treated medically with omeprazole, levothyroxine, nifedipine, naproxen, and domperidone.
Clinical Examination
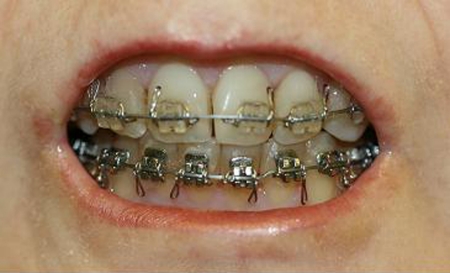
Her scleroderma mainly affected her hands and the soft tissues of her face. Her lips were thin, atrophied, and incompetent. Significant mandibular retrusion was evident. Vertical maxillary excess was present, and her chin was deficient (Fig. 1). Examination of her occlusion revealed a severe class II mandibular deficiency malocclusion with a large AOB (Fig. 2). Her AOB was 10 mm and her overjet was 7 mm. Occlusal contact was limited to her first and second molars bilaterally. Mouth opening was reduced with an interincisal opening of 37 mm (27 mm of movement from an AOB of 10 mm).
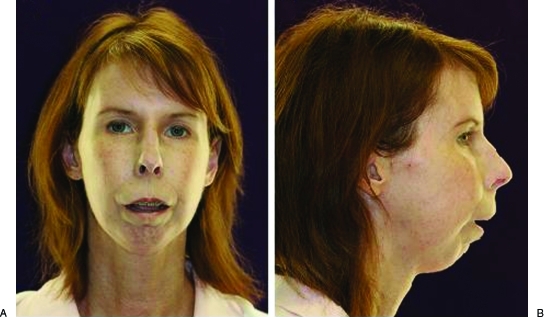
Figure 1.
(A) Preoperative frontal view showing sclerotic skin, masklike appearance, and thin, atrophied, and incompetent lips. (B) Preoperative profile view illustrating the significant mandibular retrusion.
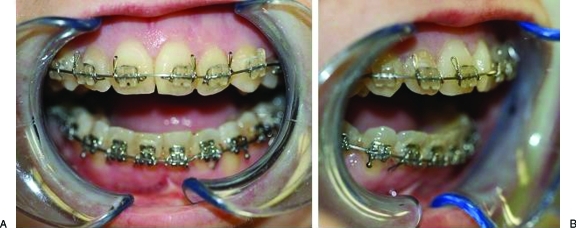
Figure 2.
(A) Bilateral condylysis caused a large anterior open bite. (B) A severe class II mandibular deficiency malocclusion.
Radiological Examination
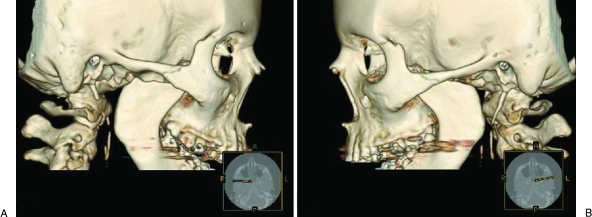
A panoramic radiograph revealed bilateral mandibular condylysis (Fig. 3). The angles of her mandible, the coronoid processes, and the posterior borders of both ascending rami were relatively normal. The lateral cephalometric radiograph showed mandibular retrusion, AOB, and chin deficiency (Fig. 4). Computed tomography with three-dimensional reconstructions also illustrated these findings (Fig. 5).
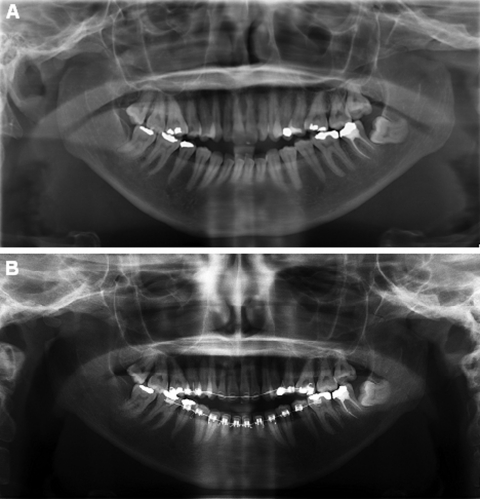
Figure 3.
(A) Panoramic radiograph at presentation revealed bilateral mandibular condylysis. The angles, the coronoid processes, and the posterior borders of the ascending rami were relatively normal. (B) After 2 years of orthodontic treatment, resorption of the condyles was relatively stable.
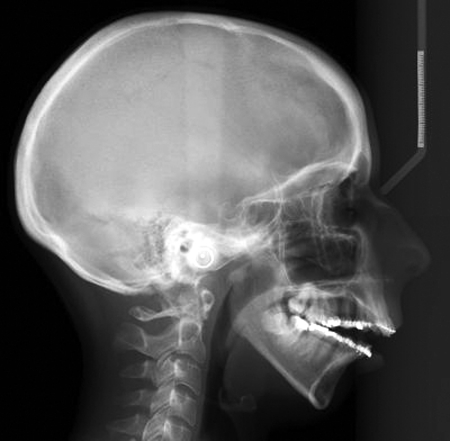
Figure 4.
Lateral cephalometric radiograph showing mandibular retrusion, anterior open bite, and chin deficiency.
Figure 5.
Computed tomography with three-dimensional reconstructions. (A) Right condylar resorption. (B) Left condylar resorption.
Surgical Treatment
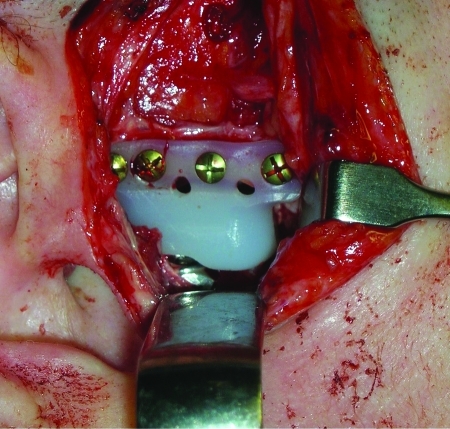
After 2½ years of observation without significant changes in the resorption and 2 years of orthodontic treatment, the patient underwent jaw reconstructive surgery. Bilateral total temporomandibular joint (TMJ) reconstruction was performed using the stock prosthesis Biomet microfixation TMJ replacement system (Walter Lorenz, Jacksonville, FL). The fossa component of this system is made up of ultra-high-molecular-weight polyethylene (Fig. 6). The condylar/mandibular component is made of cobalt-chromium-molybdenum alloy with titanium plasma spray coating on the undersurface. This was combined with a 7-mm advancement functional genioplasty and a Le Fort I maxillary impaction of 5 mm anteriorly and 7 mm posteriorly. The maxilla was fixated with wire osteosythensis at the zygomatic buttress regions and with titanium plates and screws at the piriform rim regions. The functional genioplasty advancement was fixated with wire osteosynthesis. Her third molars (1–8, 2–8, and 3–8) were also extracted. Bilateral labial commissure lacerations occurred during the surgery due to the lack of elasticity of the tissues. These where repaired primarily at the time of surgery. At the end of the case, the patient was kept in maxillomandibular fixation with elastics.
Figure 6.
View from preauricular incision of ultra-high-molecular-weight polyethylene fossa with part of cobalt-chromium-molybdenum alloy condyle.
Results
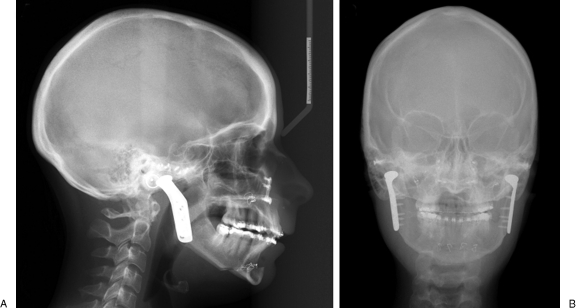
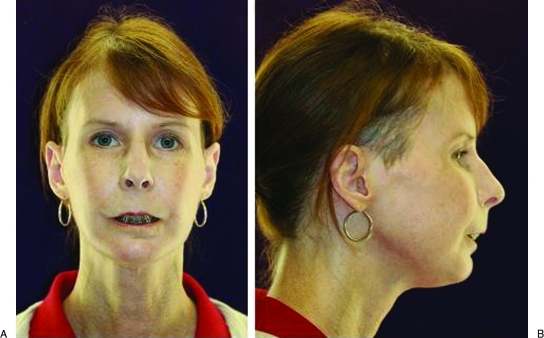
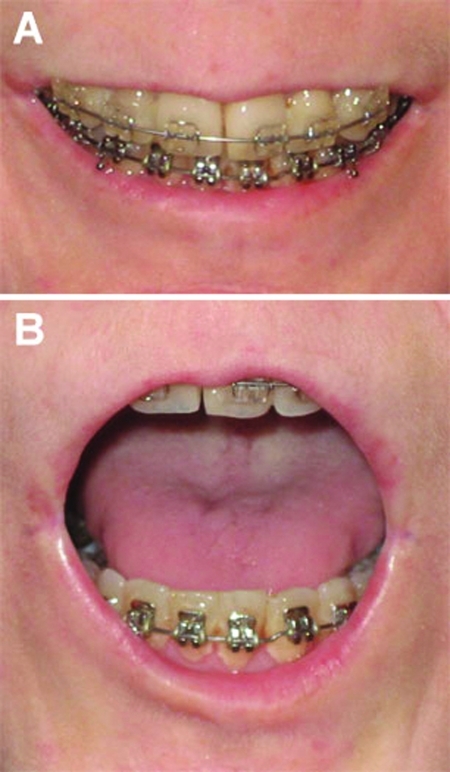
The patient had an uncomplicated postoperative course and was discharged home on day 4. Postoperative radiographs revealed an adequate positioning of the TMJ prostheses and correction of the mandibular deficiency and AOB (Fig. 7). The maxillomandibular fixation was released after 4 weeks. Seven weeks postoperatively, the patient was very pleased with the change in appearance (Fig. 8). Her occlusion was stable with 1 mm of overbite (Fig. 9). Her interincisal opening was limited at 13 mm, and her lips were still incompetent. Lip exercises and jaw-opening exercises were prescribed. At 7 months, her occlusion was still stable and her opening was up to 26 mm (Fig. 10).
Figure 7.
(A) Postoperative lateral (B) and posteroanterior views. Cephalometric radiographs revealed an adequate positioning of the temporomandibular joint prostheses and correction of the mandibular deficiency and anterior open bite.
Figure 8.
(A) Postoperative frontal view demonstrating change in facial appearance with shortening of lower facial third. (B) Postoperative profile view showing improvement facial balance and corrected chin contour.
Figure 9.
Seven weeks postoperatively, the occlusion was stable with 1 mm of overbite. Her lips were still incompetent.
Figure 10.
Seven months postoperatively, her occlusion was still stable (A) and her opening was up to 26 mm (B).
DISCUSSION
Bone resorption is one of the clinical manifestations of SSc. The most frequent radiological findings are resorption of the terminal phalanges of the hands and the distal portions of the radius and ulna.10 Resorption of the ribs and distal clavicle has also been reported.11 Specific mandibular radiographic findings include resorption of the angle, condyle, coronoid process, ascending ramus, and antegonial notch and widening of the periodontal ligament space.
A review by Haers and Sailer9 of 22 publications included 52 affected mandibles with an overall mandibular resorption incidence of 20 to 33%. The mean age of these patients was 40 years (range 14 to 62).9 Females were found to be affected seven times more frequently than males.9 Resorption was detected most frequently between 5 and 7 years after the diagnosis of SSc.9 The mandibular angle is most commonly affected (37.6%).9 The condyle is involved in 20.8%, followed by the coronoid process in 20.0%, and the posterior border of the ascending ramus in 14.4%.9 Other areas were rarely affected. Bilateral condylysis was present in 13.7% of the cases.9 Other studies of mandibular resorption reported lower incidences of 7% (2 of 30)12 and 10% (2 of 21).13
The pathogenesis of mandibular resorption is thought to be due to both pressure ischemia14 and vascular ischemia.15 Pressure ischemia originates from the rigidity and hardening of the overlying skin causing pressure and lack of mobility.14 Vascular ischemia is due to the fact that areas commonly showing resorption are not supplied by the main inferior alveolar artery.15 These areas such as the condyle, coronoid process, and mandibular angle are supplied by small arterial branches of the internal maxillary artery.15 Vasculitis of these small muscular arteries caused by SSc can lead to the formation of these ischemic osteolytic lesions.15
There is no clear correlation between the incidence of the mandibular resorption and the severity, progression, and duration of SSc. Marmary et al16 found no association between resorption of the mandibular angle and age, medication, clinical and laboratory findings, widening of the periodontal ligament, or duration of the disease. In contrast, Wood and Lee17 reported that the mandibular osteolytic lesions were correlated with greater restriction in mouth opening and more widespread organ involvement.
Mandibular resorption from SSc can lead to mandibular fractures. Mugino and Ikemura18 reported a case of spontaneous mandibular angle fracture due to severe resorption from SSc. Cases of bilateral angle fractures from fall19 and osteomyelitis with ramus fracture20 have also been reported. Severe resorption can also lead to trigeminal neuralgia. Retrospective studies have shown that 4 to 13% of SSc patients have trigeminal sensory complaints.21,22 These mainly involved the second and third divisions of the trigeminal nerve (83%).21 A case of painful compression neuropathy of the inferior alveolar nerve from severe mandibular angle resorption has also been reported.23
SSc causes numerous other orofacial manifestations. Vincent et al12 studied 30 consecutive patients with SSc. Of these patients, 93% had skin atrophy (n = 28), 83% had increased peribuccal rhagades (n = 25), 70% had telangiectasia (n = 21), 67% had decreased mouth opening (n = 20), 67% had xerostomia (n = 20), 53% had xerophthalmia (n = 16), 33% had periodontal ligament space widening (n = 10), 30% had TMJ pain (n = 9), 7% had bone resorptions (n = 2), and 3% had trigeminal neuralgia (n = 1).12 This 33% periodontal ligament space widening was low compared with the 70% found in a prior study.24 Xerostomia was considered the most discomforting orofacial symptom (mean visual analog scale (VAS) = 3.8), followed by decreased mouth opening (mean VAS = 2.6).12 Statistically, significant associations were found between xerostomia and lcSSc (p = 0.045), as well as anticentromere antibodies expression (p = 0.002).12 Decreased mouth opening was significantly correlated to esophageal involvement (p = 0.025).12
Very few cases of surgical correction of malocclusion induced by SSc-related condylysis have been reported in the literature.9,25,26 The most recent case reported was a case of bilateral condylysis from SSc that led to a severe class II occlusion with AOB of 8 mm.9 This case was treated with a maxillary intrusion combined with mandibular and functional genioplasty advancements. After 2 years of follow-up, evidence of recurrence of the AOB and class II mandibular deficiency was present. This was felt to be due to gradual opening of the mandibular angle, suggesting ongoing resorption of the cranial part of both rami with subsequent shortening of the ascending ramus.9 Even with the relapse, the surgical result was still considered more functional.
To the best of our knowledge, this is the first case report of bilateral condylysis from SSc where surgical correction of the root of the problem was attempted by replacing the resorbed condyles. By performing bilateral total TMJ replacement, combined with Le Fort I impaction and functional genioplasty advancement, our patient obtained a functional occlusion with elimination of her open bite and a more esthetic profile. Lip competence was not achieved due to the rigidity of the perioral soft tissues. After 7 months, her occlusion remained stable. Ongoing follow-up will determine the long-term stability of the surgical correction.
CONCLUSION
SSc is a multisystem connective tissue disease with numerous oral and maxillofacial manifestations including mandibular resorption. All SSc patients should have regular follow-up with their dentist with a yearly panoramic radiograph looking for signs of mandibular resorption. If identified, these patients should be subsequently referred to an oral and maxillofacial surgeon for evaluation and management. Surgical treatment should be offered to patients presenting with malocclusions from bilateral mandibular condylysis. If traditional orthognathic surgical techniques are employed, it is essential to confirm that the mandibular resorption is relatively stable prior to attempting correction. This is not required for total joint replacement. Also, orthodontic treatment with regular follow-up is necessary to help maintain the stability of the occlusion. Replacement of the resorbed condyles with bilateral total TMJ replacement can give these patients a functional occlusion, improved facial balance, and improved quality of life.
References
- LeRoy E C, Black C, Fleischmajer R, et al. Scleroderma (systemic sclerosis): classification, subsets and pathogenesis. J Rheumatol. 1988;15:202–205. [PubMed] [Google Scholar]
- Steen V D, Oddis C V, Conte C G, Janoski J, Casterline G Z, Medsger T A., Jr Incidence of systemic sclerosis in Allegheny County, Pennsylvania. A twenty-year study of hospital-diagnosed cases, 1963–1982. Arthritis Rheum. 1997;40:441–445. doi: 10.1002/art.1780400309. [DOI] [PubMed] [Google Scholar]
- Mayes M D, Lacey J V, Jr, Beebe-Dimmer J, et al. Prevalence, incidence, survival, and disease characteristics of systemic sclerosis in a large US population. Arthritis Rheum. 2003;48:2246–2255. doi: 10.1002/art.11073. [DOI] [PubMed] [Google Scholar]
- Nietert P J, Mitchell H C, Bolster M B, Shaftman S R, Tilley B C, Silver R M. Racial variation in clinical and immunological manifestations of systemic sclerosis. J Rheumatol. 2006;33:263–268. [PubMed] [Google Scholar]
- D'Angelo W A, Fries J F, Masi A T, Shulman L E. Pathologic observations in systemic sclerosis (scleroderma). A study of fifty-eight autopsy cases and fifty-eight matched controls. Am J Med. 1969;46:428–440. doi: 10.1016/0002-9343(69)90044-8. [DOI] [PubMed] [Google Scholar]
- Jimenez S A, Derk C T. Following the molecular pathways toward an understanding of the pathogenesis of systemic sclerosis. Ann Intern Med. 2004;140:37–50. [PubMed] [Google Scholar]
- Charles C, Clements P, Furst D E. Systemic sclerosis: hypothesis-driven treatment strategies. Lancet. 2006;367:1683–1691. doi: 10.1016/S0140-6736(06)68737-0. [DOI] [PubMed] [Google Scholar]
- Hu P Q, Fertig N, Medsger T A, Jr, Wright T M. Correlation of serum anti-DNA topoisomerase I antibody levels with disease severity and activity in systemic sclerosis. Arthritis Rheum. 2003;48:1363–1373. doi: 10.1002/art.10977. [DOI] [PubMed] [Google Scholar]
- Haers P E, Sailer H F. Mandibular resorption due to systemic sclerosis. Case report of surgical correction of a secondary open bite deformity. Int J Oral Maxillofac Surg. 1995;24:261–267. doi: 10.1016/s0901-5027(95)80025-5. [DOI] [PubMed] [Google Scholar]
- LeRoy E C. Sentinel signs and symptoms of systemic sclerosis. Curr Opin Rheumatol. 1990;2:942–946. doi: 10.1097/00002281-199002060-00013. [DOI] [PubMed] [Google Scholar]
- Bassett L W, Blocka K LN, Furst D E, Clements P J, Gold R H. Skeletal findings in progressive systemic sclerosis (scleroderma) AJR Am J Roentgenol. 1981;136:1121–1126. doi: 10.2214/ajr.136.6.1121. [DOI] [PubMed] [Google Scholar]
- Vincent C, Agard C, Barbarot S, et al. Orofacial manifestations of systemic sclerosis: a study of 30 consecutive patients. Rev Med Interne. 2009;30:5–11. doi: 10.1016/j.revmed.2008.06.012. [DOI] [PubMed] [Google Scholar]
- Rout P G, Hamburger J, Potts A J. Orofacial radiological manifestations of systemic sclerosis. Dentomaxillofac Radiol. 1996;25:193–196. doi: 10.1259/dmfr.25.4.9084272. [DOI] [PubMed] [Google Scholar]
- Seifert M H, Steigerwald J C, Cliff M M. Bone resorption of the mandible in progressive systemic sclerosis. Arthritis Rheum. 1975;18:507–512. doi: 10.1002/art.1780180514. [DOI] [PubMed] [Google Scholar]
- Ramon Y, Samra H, Oberman M. Mandibular condylosis and apertognathia as presenting symptoms in progressive systemic sclerosis (scleroderma). Pattern of mandibular bony lesions and atrophy of masticatory muscles in PSS, presumably caused by affected muscular arteries. Oral Surg Oral Med Oral Pathol. 1987;63:269–274. doi: 10.1016/0030-4220(87)90188-5. [DOI] [PubMed] [Google Scholar]
- Marmary Y, Glaiss R, Pisanty S. Scleroderma: oral manifestations. Oral Surg Oral Med Oral Pathol. 1981;52:32–37. doi: 10.1016/0030-4220(81)90169-9. [DOI] [PubMed] [Google Scholar]
- Wood R E, Lee P. Analysis of the oral manifestations of systemic sclerosis (scleroderma) Oral Surg Oral Med Oral Pathol. 1988;65:172–178. doi: 10.1016/0030-4220(88)90161-2. [DOI] [PubMed] [Google Scholar]
- Mugino H, Ikemura K. Progressive systemic sclerosis with spontaneous fracture due to resorption of the mandible: a case report. J Oral Maxillofac Surg. 2006;64:1137–1139. doi: 10.1016/j.joms.2005.11.037. [DOI] [PubMed] [Google Scholar]
- Weber D D, Blunt M H, Caldwell J B. Fracture of mandibular rami complicated by scleroderma: report of case. J Oral Surg. 1970;28:860–863. [PubMed] [Google Scholar]
- Ilacqua J A, Murphy J B. Management of osteomyelitis and nonunion of the mandible in a patient with progressive systemic sclerosis. J Oral Maxillofac Surg. 1986;44:561–563. doi: 10.1016/s0278-2391(86)80097-0. [DOI] [PubMed] [Google Scholar]
- Farrell D A, Medsger T A., Jr Trigeminal neuropathy in progressive systemic sclerosis. Am J Med. 1982;73:57–62. doi: 10.1016/0002-9343(82)90926-3. [DOI] [PubMed] [Google Scholar]
- Medsger T A, Jr, Masi A T. The epidemiology of systemic sclerosis (scleroderma) among male US veterans. J Chronic Dis. 1978;31(2):73–85. doi: 10.1016/0021-9681(78)90092-9. [DOI] [PubMed] [Google Scholar]
- Fischoff D K, Sirois D. Painful trigeminal neuropathy caused by severe mandibular resorption and nerve compression in a patient with systemic sclerosis: case report and literature review. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;90:456–459. doi: 10.1067/moe.2000.108798. [DOI] [PubMed] [Google Scholar]
- Rowell N R, Hopper F E. The periodontal membrane in systemic sclerosis. Br J Dermatol. 1977;96:15–20. doi: 10.1111/j.1365-2133.1977.tb05179.x. [DOI] [PubMed] [Google Scholar]
- Lanigan D T, Myall R W, West R A, McNeill R W. Condylysis in a patient with a mixed collagen vascular disease. Oral Surg Oral Med Oral Pathol. 1979;48:198–204. doi: 10.1016/0030-4220(79)90002-1. [DOI] [PubMed] [Google Scholar]
- Thaller S R, Cavina C, Kawamoto H K. Treatment of orthognathic problems related to scleroderma. Ann Plast Surg. 1990;24:528–533. doi: 10.1097/00000637-199006000-00010. [DOI] [PubMed] [Google Scholar]