Abstract
Spontaneous orthotopic liver allograft acceptance associated with microchimerism in mice induces tolerance to subsequent skin or heart transplants from the donor but not third-party animals. Despite in vivo hyporesponsiveness, in vitro MLC and CTL assays showed continuing antidonor reactivity. Cells isolated from recipients’ spleens and grafted livers, when tested in MLC and CTL assays, were antidonor reactive out to 3 months to the same degree as splenocytes obtained from either naive or presensitized (with skin or heart) mice. Nevertheless, passive transfer of splenocytes or liver lymphocytes from liver tolerant mice, but not naive or sensitized donor strain mice, were able to prolong skin graft survival significantly in naive irradiated recipients. By using a strain combination in which the donor but not the recipient expressed the stimulatory endogenous super-Ag (Mlsf), it was possible to determine whether super-Ag-reactive T cells bearing Vβ5 and Vβ11 were deleted or anergic. Phenotypic analysis of cells isolated from recipients’ spleens and grafted livers (up to 90 days after transplant), when compared with naive animals, showed no significant difference in Vβ5 and Vβ11 TCR expression. Additionally, when these isolated spleen cells were tested for antibody-mediated stimulation, both anti-Vβ5 and Vβ11 TCR mAb led to marked proliferation of cells obtained from naive and liver-transplanted recipients, but as expected, proliferation was very low in cells from naive donors. These results suggest that liver transplantation induces donor-specific tolerance in vivo, which may not be reflected in in vitro proliferative and cytotoxicity assays (split tolerance). Furthermore, this tolerance does not seem to be induced by clonal deletion or anergy of minor-lymphocyte-stimulating-antigen-reactive T cells in the recipients.
OLT in mice across major and minor histocompatibility barriers results in a high incidence of spontaneous graft acceptance without immunosuppression (1). While some strain combinations (B10 → C3H) fare better (mean survival > 100 days) than others (BALB/c → C3H; mean survival > 47 days), the underlying mechanism(s) for this extemporaneous liver graft acceptance in an unmodified recipient is not clear. However, this property is not unique to the liver, only much stronger, since kidneys (2, 3) and hearts (4) in some strain combinations in mice can also lead to induction of donor-specific tolerance. The present study was designed to analyze the functional status of lymphocytes isolated from various lymphoid and nonlymphoid organs of liver graft recipients, and to investigate the role of minor lymphocyte-stimulating (Mls)* super-Ag and Vβ usage in the induction of donor-specific tolerance after OLT. For the latter study, an I-E/Mlsf-positive liver was grafted into an I-E/Mlsf-negative recipient, which provided us with a direct approach to examine Vβ usage in the periphery.
MATERIALS AND METHODS
Animals
Inbred male mice of the C57BL/10 (B10), B10.BR, B10.D2, C3H/HeJ, and BALB/c strains were obtained from Jackson Laboratory, Bar Harbor, ME. Mice were maintained in pathogen-free facilities, provided with Purina rodent chow and tap water ad libitum, and used at 10–12 weeks of age.
Medium
Dulbecco’s modified Eagle’s medium ([DMEM] GIBCO, Grand Island, NY) supplemented with 2 mM l-glutamine (GIBCO), 0.55 mM l-arginine (GIBCO), 0.3 mM l-asparagine (GIBCO), 13.6 μM folic acid (GIBCO), 100 U/ml penicillin (GIBCO), 100 μg/ml streptomycin (GIBCO), 1 mM sodium pyruvate (Sigma Chemical Co., St. Louis, MO), 10 mM HEPES (Sigma), 5×10−5 M 2-ME (GIBCO), and 0.75% mouse serum were used (complete DMEM) for all cell cultures except cytotoxicity and T cell activation assays, where instead of mouse serum, 10% decomplemented FCS (GIBCO) was used (MLC-DMEM).
Surgical techniques
OLT was performed as described previously (5). Abdominal heterotopic heart transplantation was performed as described previously by Ono and Lindsey (6). Transplanted hearts were monitored daily by direct palpation, and rejection was defined as termination of palpable cardiac contractility. A full-thickness skin graft from the donor tail was placed on the dorsal side of the recipient’s trunk according to a previously described technique (7). The graft was secured by silk sutures, and protected by dressing for 7 to 8 days. The rejection process was monitored by daily inspection until the skin was completely destroyed. All procedures were performed under methoxyflurane anesthesia.
Histology
Animals were killed on days 2, 7, 14, 28, and 84 after transplantation. Tissues were harvested and fixed in neutral buffered formalin, embedded in paraffin, sectioned, and stained with hematoxylin and eosin.
Preparation of cell suspensions
Aseptically harvested spleens and lymph nodes were gently teased with 25-gauge needles in culture medium, and subsequently filtered through a nylon mesh. Erythrocytes were lysed by treatment with 5 ml of red cell lysing buffer (Sigma) for 5 min, and washed twice with complete DMEM.
Free lymphomyeloid cells from the liver were prepared as described previously (8). Briefly, the portal vein was exposed and cannulated with a 21-gauge needle and the inferior vena cava was transected proximal to the renal veins. The liver was then perfused with 40 ml of warm Hank’s solution, excised, and transferred to sterile culture medium. Perfused livers were disrupted on a 50-mesh stainless steel sieve with a sterile 20-ml syringe pestle, and the resulting cell suspension was filtered through a nylon mesh. Further purification was achieved by centrifugation on a continuous 35% Percoll (Pharmacia Diagnostics, Piscataway, NJ) gradient. Cells in the pellet were recovered, treated for 5 min with RBC lysis buffer, and, after 2 washes, resuspended in appropriate volume of complete DMEM.
MLC
All mixed cell culture assays were performed in triplicate in round-bottomed microtiter plates (Corning, Corning, NY) using equal numbers (2×105 cells/well) of responder cells, and γ-irradiated (2000 cGy) stimulator cells in a total volume of 200 μl. Controls included responder cells and irradiated stimulators incubated in medium alone and responders incubated with autologous stimulators. Cultures were incubated at 37°C in a humidified 10% CO2 atmosphere for 2, 3, 4, and 5 days and pulsed with 1 μCi/well of [3H]thymidine (NEN, Boston, MA) 18 hr before termination of the assay. Cells were harvested (Skatron, LKG, Wallac, Norway) onto glass fiber filter mats (Wallac Oy, Turku, Finland) and radioactivity was determined in a liquid scintillation counter (Betaplate 1205; Pharmacia LKB, Gaithersburg, MD). The results are expressed as the mean cpm ± 1 SD of [3H]thymidine incorporation in triplicate cultures.
Cytotoxicity assays
Effector cells from spleens were prepared by incubating equal numbers (4×106 cells/well) of responders and γ-irradiated (2000 cGy) stimulators (syngeneic, donor, and third party) for 4 to 5 days in a 12-well tissue culture plate (Corning). On the contrary, freshly isolated free lymphomyeloid cells from the grafted livers were used as effectors. Target cells were prepared by incubating 4×106 spleen cells for 48 hr in MLC-DMEM containing Con A (Sigma) at 5 μg/ml. The target cells were then labeled with Na2 51CrO4 (NEN) and washed 3 times to remove excess 51Cr, and 4×103 cells/well were placed in V-bottomed 96-well tissue culture plates (Corning). Serial 3-fold dilutions of effector cells were added at a maximum E:T ratio of 100:1 in a total volume of 200 μl/well, and the plates were incubated for 4 to 5 hr at 37°C in 10% CO2. At the end of this incubation, plates were centrifuged at 500×g for 10 min, and 100 μl of supernatant from each well were removed for counting. Spontaneous 51Cr release from target cells was determined by incubating targets with medium alone. The percentage of specific 51Cr release was calculated according to the formula:
The results are expressed as mean cpm ± 1 SD of % specific 51Cr release in triplicate cultures.
Flow cytometric analysis
Cells isolated from spleens and grafted livers were phenotyped by single-color immunofluorescence labeling with the following FITC-conjugated rat anti-mouse mAb (Pharmingen, San Diego, CA): anti-Thy-1 (IgG2c, pan T cell antigen), anti-CD4 (IgG2b, L3T4, Th cells), anti-CD8 (IgG2a, Ly-2, cytotoxic/suppressor T cells), and anti-CD45R (IgG2a, B220, pre-B and B lymphocytes). FITC-conjugated, isotype-matched irrelevant mAb were used as appropriate negative controls. For surface marker analysis, 5×105 cells were incubated with 100 μl (1:100 dilution) of appropriate mAb for 60 min at 4°C. The cells were washed twice, resuspended in PBS containing 1% paraformaldehyde, and analyzed using a FACScan flow cytometer (Becton-Dickinson, Mountain View, CA).
Cells isolated from spleens, lymph nodes, thymus, and grafted livers were also analyzed for relative frequency of various subsets of TCR, by double immunofluorescent staining with the following mAb (Pharmingen): phycoerythrin-conjugated anti-Thy-1 (rat IgG2c) and anti-CD3-ε (hamster IgG), FITC-conjugated or biotinylated anti-Vβ3 TCR (hamster IgG), anti-Vβ5.1, 5.2 TCR (mouse IgG1), anti-Vβ8.1,8.2 TCR (mouse IgG2a), and anti-Vβ11 TCR (rat IgG2b). Cells (5×105 cells/tube) were first incubated for 60 min at 4°C with 100 μl of phycoerythrin-conjugated anti-Thy-1 mAb (1:100 dilution). The cells were then washed twice and 100 μl of FITC-conjugated or biotinylated anti-CD3 or anti-β3 TCR mAb were added at 1:100 dilution. After 60 min of incubation at 4°C, the cells were washed and only those cells that were stained with biotinylated mAb were further incubated for 30 min at 4°C with 100 μl of streptavidin-conjugated FITC (1:100 dilution, Jackson Immunoresearch Laboratory, West Drove, PA). All cells were washed, resuspended in 1% paraformaldehyde, and finally analyzed on a FACScan flow cytometer (Becton-Dickinson). Fluorochrome-conjugated, isotype-matched irrelevant mAb were used as an appropriate negative control.
Adoptive transfer assays
All adoptive transfer assays were performed in the strain combination B10 → C3H. Cells were isolated from spleens of naive animals (C3H), from C3H recipients of B10 liver or skin (4 weeks after transplant), and from transplanted B10 → C3H livers (4 weeks after transplant). They were finally resuspended at 2×107 cells/ml in Hank’s solution and 500 μl (i.e., 107 cells/animal) were infused through the penile vein into sublethally irradiated (650 cGy) naive C3H recipients (syngeneic). These cell transplant recipients were simultaneously challenged with a full-thickness skin graft from a B10 donor.
TCR cross-linking assay
T cell proliferation was induced by cross-linking TCR with mAb directed against T cell surface antigens: CD3, Vβ3, Vβ5, Vβ8, and Vβ11 TCR. The assay was performed as described previously (35). Briefly, 100 μl of relevant antibody solution (10 μg/ml) were incubated overnight at 37°C in 96-well U-bottomed microtiter plates (Corning). At the end of this incubation period, plates were washed with sterile Hank’s solution, and 2 × l05 spleen cells were then added to each well in a final volume of 200 μl of MLC-DMEM. The cells were cultured for 3 to 5 days at 37°C in 10% CO2, with [3H]thymidine (1 μCi/well) added for the last 16–18 hr of culture. Plates were harvested onto glass filter mats and the [3H]thymidine incorporation was determined by liquid scintillation spectrophotometry. Negative controls were cells cultured with MLC-DMEM alone. Positive controls were cells cultured with either Con A (5 μg/ml) or LPS (40 μg/ml). Results are expressed as mean cpm ± 1 SD of triplicate samples.
RESULTS
Characterization of cellular infiltrate
All allotransplanted livers underwent a brisk rejection episode that resolved spontaneously with in 4–5 weeks. However, despite heavy cellular infiltration, little hepatocellular necrosis was observed. Phenotyping of free lymphomyeloid cells isolated from normal (B10 and C3H) and grafted livers (C3H → C3H and B10 → C3H) (2–90 days after transplant) showed a pre-dominance of lymphocytes (Table 1). Normal B10 and C3H livers had approximately equal numbers of B and T cells, where as after transplantation, the majority of isolated cells from allografted but not syngrafted livers were T lymphocytes (Table 1). Of the T cell subsets, CD8+ cells far outnumbered CD4+ cells in the allografted livers (Table 1). On the contrary, the phenotype of splenocytes isolated from liver, heart, or skin recipients was similar to naive controls (data not shown).
Table 1.
Phenotype of cells isolated from livers of naive (C3H and B10) animals, and syngeneic (C3H → C3H) and allogeneic (B10 → C3H) transplanted livers
| Naive (n=3) |
Liver cell yield (×l06) |
B cells (%) |
T cells (%) |
CD4+ (%) |
CD8+ (%) |
CD4:CD8 ratio |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B10 0.7±0.3 |
C3H 0.5±0.4 |
B10 31±4 |
C3H 38±5.1 |
B10 48±6.2 |
C3H 43±3.9 |
B10 41±5.4 |
C3H 29±4.3 |
B10 27±3.5 |
C3H 14±3.1 |
B10 2.07 |
C3H 2.01 |
|
| Time postTxa | syn | allo | syn | allo | syn | allo | syn | allo | syn | allo | syn | allo |
|
|
||||||||||||
| day 2 | 1.4±0.6 | 2.5±1.6 | 26±4.2 | 18±9 | 43±7.5 | 47±8.1 | 37±0.5 | 27±8.8 | 11±1.4 | 20 ±14 | 1.35 | 3.36 |
| 1 wk | 2.8±2.1 | 10±3.6 | 39±5.1 | 13±5 | 41 ±14 | 56±27 | 31±5.7 | 20±9.1 | 13±2.5 | 31±5.7 | 2.38 | 0.67 |
| 4 wk | 0.5±0.3 | 9.8±3.1 | 18±8.5 | 20±7 | 40±3 | 73 ±18 | 33±7.1 | 19±2.5 | 13±2.5 | 53±21 | 2.54 | 0.36 |
| 12 wk | NDb | 3.8±0.9 | ND | 28±9.5 | ND | 61±12 | ND | 18±3.5 | ND | 34±14 | ND | 0.53 |
n=4 at each time point after transplantation.
ND, not done.
Proliferative responses of cells isolated from spleens and grafted livers
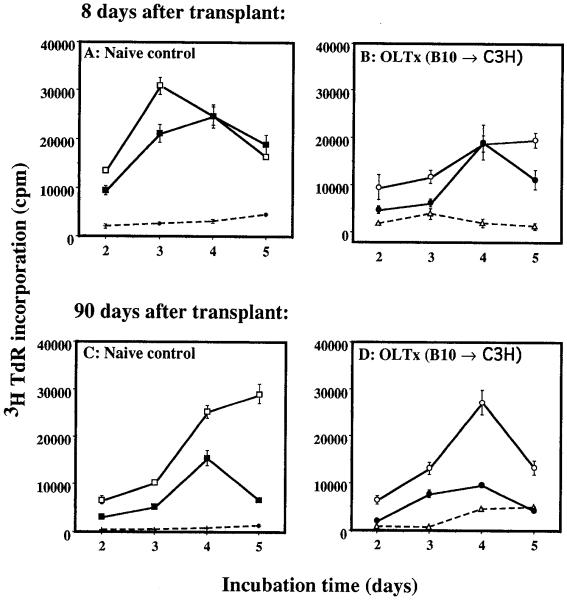
As early as 8 days after liver transplantation, the recipients’ (C3H) spleen cells showed alloreactivity toward γ-irradiated donor (B10) and third-party (BALB/c) splenocytes (Fig. 1B), while sustaining nonresponsiveness to autologous lymphocytes (Fig. 1B). This reactivity was maintained during the entire observation period (tested on days 8, 14, 30, and 90) after liver transplantation (Fig. 1D). A similar lymphoproliferative response was also observed in spleen cells isolated from skin and heart transplant recipients (B10 → C3H, 90 days after transplant) who had rejected their grafts within 14 days (Fig. 2). Spleen cells from long-term liver graft recipients (> 100 days after transplant) in 2 other strain combinations (B10.BR → B10 and B10.BR → B10.D2) also responded normally to donor and third-party, but not to autologous or naive syngeneic lymphocytes (data not shown). Free lymphomyeloid cells isolated from grafted livers (up to 90 days after transplant) were also nonresponsive to self and naive syngeneic lymphocytes, but responded normally to donor and third-party stimulation (data not shown).
Figure 1.
MLC. Spleen cells isolated from either naive C3H (A and C) or C3H recipients of B10 liver, 8 (B) and 90 days (D) after OLT, were used as responders. γ-Irradiated spleen cells from syngeneic (C3H [+,△]), donor (B10 [□,○]), and third-party (BALB/c [■,●) strains were used as stimulators. [3H]Thymidine incorporation is expressed as mean cpm ± 1 SD of triplicate cultures.
Figure 2.
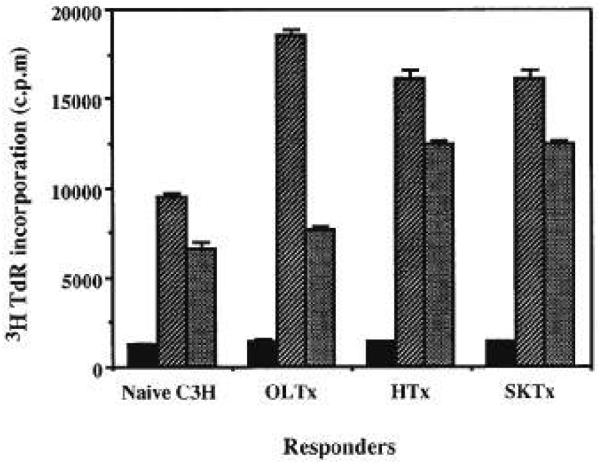
MLC. Responders were isolated from spleens of naive C3H or C3H recipients of B10 liver, heart, and skin (90 days after transplant), and stimulated for 96 hr with γ-irradiated splenocytes from (■) naive syngeneic, ( ) donor, and (
) donor, and ( ) third-party (BALB/c) animals. The results are expressed as mean cpm ± 1 SD of tritiated thymidine incorporation by triplicate cultures.
) third-party (BALB/c) animals. The results are expressed as mean cpm ± 1 SD of tritiated thymidine incorporation by triplicate cultures.
Cytotoxic activity of cells isolated from spleens and grafted livers
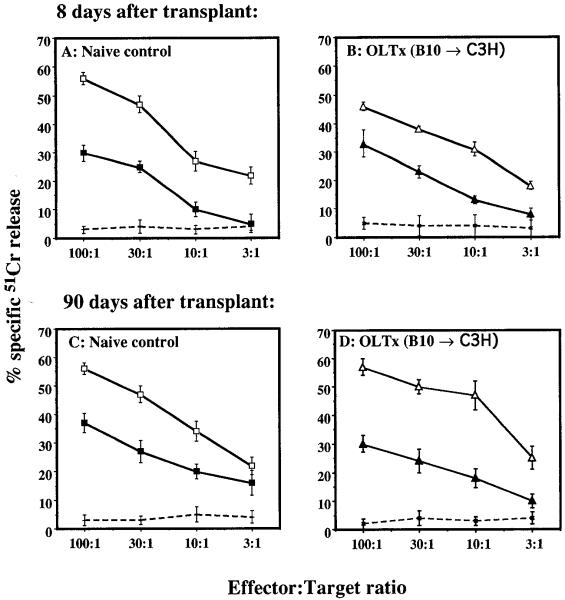
An elevated level of cytotoxic killing was seen in stimulated effectors (C3H spleen cells) on days 8, 14, 30, and 90 after B10 liver transplantation (Fig. 3B and 3D). Lysis of third-party (BALB/c) targets was as effective as donor, whereas killing of syngeneic targets was low, ranging between 2% and 6% (Fig. 3, B and D). Similar observations were also made for spleen cells isolated after skin or heart transplantation (data not shown). Freshly isolated lymphocytes from spleens of liver graft recipients and grafted livers were also tested for their cytotoxic capacity. Cells isolated from grafted livers (8–90 days after transplant), but not spleens, showed high alloreactive cytolysis of both donor and third-party targets, with minimal lysis of syngeneic targets (Table 2).
Figure 3.
CTL assay. Spleen cells (effectors) from naive and transplanted recipients were cultured for 4 days with γ-irradiated splenocytes from syngeneic, donor, or third-party strains. Lytic activity was determined by incubating decreasing doses of stimulated effectors with a constant dose of naive 51Cr-labeled targets. All effectors were stimulated with target-strain-specific naive cells. Experimental groups (E:T): (+) naive C3H:C3H, (□) naive C3H:B10, (■) naive C3H:BALB/c, (X) C3H > OLTx:C3H, (△) C3H > OLTx:B10, and (▲) C3H > OLTx:BALB/c. The results are mean % specific 51Cr release ± 1 SD of triplicate cultures.
Table 2.
Direct cytotoxicity of freshly isolated cells from spleens and livers of liver graft recipients (B10 → C3H), tested against syngeneic (C3H), donor (B10) and third-party (BALB/c) targetsa
| Percent specific 51Cr release (mean cpm ± 1 SD) |
||||||||
|---|---|---|---|---|---|---|---|---|
| Time postTx | Animal | Liver graft-derived cells (E:T ratio) |
Spleen-derived cells (E:T ratio) |
|||||
| C3H |
B10 |
BALB/c |
C3H |
B10 |
BALB/c |
|||
| 100:1 | 100:1 | 30:1 | 100:1 | 100:1 | 100:1 | 100:1 | ||
| 1-2 Weeks | a | 10±3 | 37±3.3 | 16±2.5 | 10±3.2 | 12±2.3 | 1±0 | 15±1.5 |
| b | 2±1.1 | 56±5.4 | 26±4.9 | 10±2.1 | 1±0 | 1±0 | 1±0 | |
| 4 Weeks | a | NDb | 36±2.9 | 20±2.6 | ND | 1±0 | 2±1 | 5±1.8 |
| b | 1±0 | 30±3 | 18±2.9 | 1±0 | 7±2.7 | 3±1.5 | 1±0 | |
| 8-12 Weeks | a | 9 ± 3 | 35±3.9 | 12±4.2 | 8±2.7 | 12±2.6 | 14±1.9 | 4±2 |
| b | 1±0 | 20±3.3 | 10±3.6 | 3±1.3 | 1±0 | 1±0 | 3±1.2 | |
51Cr-labeled Con A blasts were used as targets.
ND, not done.
Effects of transplanting cells from tolerant to naive animals
Unmodified C3H mice rejected B10 skin grafts in 13±0.7 days (Table 3). Skin grafts were rejected in 18±1 days if, at the time of skin transplant, naive spleen cells (107 cells/animal) were infused into γ-irradiated syngeneic mice. Furthermore, infusion of spleen cells from skin-sensitized mice (B10 → C3H) failed to prolong skin graft survival beyond normal controls (17±1 days), whereas reconstitution of γ-irradiated animals with spleen cells from liver recipients (4 weeks > OLT) or cells from grafted livers (4 weeks after transplant) delayed skin graft rejection far beyond normal controls (Table 3).
Table 3.
Adoptive transfer of 107 cells isolated from spleens of either naive, skin, or liver graft recipients (B10 → C3H), or from transplanted livers to naive syngeneic recipients, which were subsequently challenged with B10 skin graft
| Cells transfused | n | γ-Irradiation (650 cGy) |
Skin graft survival mean days ± 1 SD |
|---|---|---|---|
| Control group | |||
| None | 4 | No | 13±0.7 |
| Naive C3H spleen cells | 8 | Yes | 18±1.0 |
| Experimental groupa | |||
| >OLT | |||
| Spleen cells | 7 | Yes | 27±0.5 |
| Liver-derived cells | 3 | Yes | 27±0.6 |
| >Skin transplant | |||
| Spleen cells | 3 | Yes | 17±1.5 |
Thirty days after transplant.
Effect of liver transplantation on Vβ TCR usage in the periphery
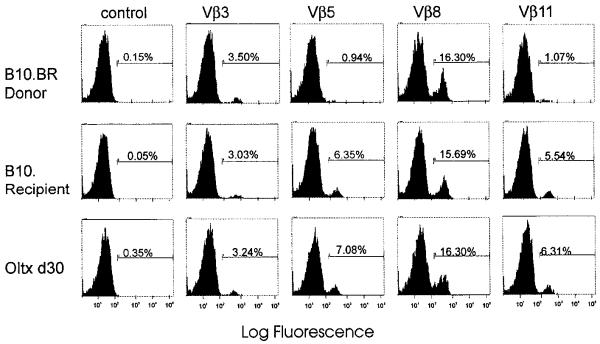
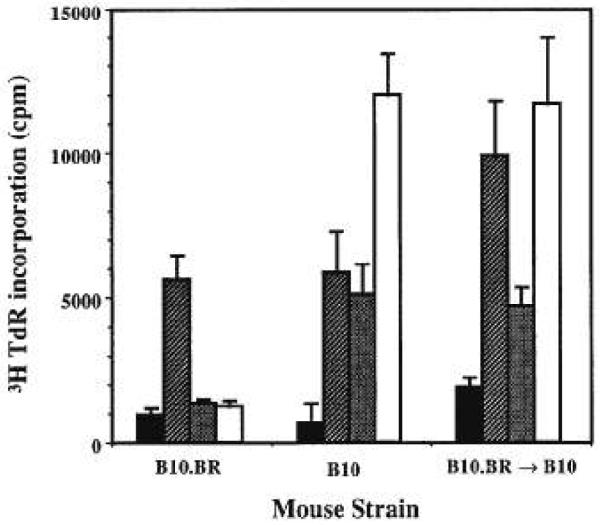
To investigate what role clonal deletion of super-Ag-reactive T cells might play in inducing tolerance after liver transplantation, lymphocytes from spleens, lymph nodes, and thymus of naive and liver-grafted animals were examined for Vβ TCR usage. Peripheral T cells of B10.BR (Mlsf-positive) donors do not express Vβ5 (0.94%) and Vβ11 (1.0%) segments of TCR, whereas T cells of B10 (Mlsf-negative) recipients expressed relatively high levels of Vβ5 (6.4%) and Vβ11 (5.5%) TCR (Fig. 4). However, both animals express comparable levels of Vβ3 (3.25±3%) and Vβ8 (16±1%) TCR, which serve as positive controls. While examining spleen cells from liver transplant recipients (2–90 days after transplant), we found that donor super-Ag-reactive T cells expressing Vβ5 and Vβ11 TCR were not deleted (Fig. 4). Cells isolated from recipients’ lymph nodes, thymus, and grafted liver also expressed normal levels of Vβ5 and Vβ11 TCR (data not shown). Similar results were also obtained in rejecting B10.BR → B10 skin graft recipients (6–15 days after transplant). Expression of Vβ3 and Vβ8 TCR in transplanted animals was comparable to naive controls (Fig. 4).
Figure 4.
FACScan analysis of Vβ usage. The frequency of Vβ3, Vβ5, Vβ8, and Vβ11 TCR was examined in the T cells isolated from the spleens of naive B10.BR (donor), naive B10 (recipient), and recipients of B10.BR livers (30 days after transplant). Isotype-matched irrelevant mAb was used as a negative control.
Antibody-mediated proliferation of peripheral T cells after liver transplantation
If purging of donor super-Ag-reactive T cells is not the basis for tolerance induction, could it be that these cells are rendered functionally inert (clonal “anergy” or “silencing”) by donor cells migrating out of transplanted livers? To test this hypothesis, spleen cells isolated from naive and transplanted recipients were stimulated with a panel of anti-Vβ TCR mAb. Cells isolated from naive B10 (recipient) showed a proliferative response when cultured with anti-Vβ5 and anti-Vβ11 TCR mAb, whereas B10.BR (donor) did not respond beyond background controls. Both B10 and B10.BR responded equally well to anti-CD3-ε (data not shown), anti-Vβ3, and anti-Vβ8 TCR mAb (Fig. 5). Cells isolated from spleens of liver graft recipients (2–90 days after transplant) responded in consonance with naive B10 by proliferating vigorously to anti-CD3-ε, anti-Vβ5, and anti-Vβ11 TCR mAb stimulation (Fig. 5), suggesting that donor super-Ag-reactive T cells in the recipient are functionally active.
Figure 5.
Antibody-mediated T cell activation. Spleen cells isolated from naive B10.BR (donor), naive B10 (recipient), and recipients of B10.BR livers (90 days after transplant), were activated by mAb directed against ( ) Vβ3, (
) Vβ3, ( ) Vβ5, and (□) Vβ11 segments of TCR. Spleen cells cultured in media alone (■) were used as controls. [3H]Thymidine uptake is expressed as mean cpm ± 1 SD of triplicate cultures.
) Vβ5, and (□) Vβ11 segments of TCR. Spleen cells cultured in media alone (■) were used as controls. [3H]Thymidine uptake is expressed as mean cpm ± 1 SD of triplicate cultures.
DISCUSSION
We have demonstrated that liver transplantation in mice across major and minor histocompatibility barriers resulted in spontaneous graft acceptance and induction of donor-specific tolerance (1). Systemic tolerance to the donor was illustrated by the observation that other donor organs, such as heart and skin, were protected from rejection by the grafted liver, whereas third-party organs were rejected within the normal time course (1). Moreover, hepatic tolerogenicity was so robust that an ongoing skin graft rejection was reversed by subsequent liver transplant, thus reverting a state of sensitization to one of tolerance (1). Similar observations have also been made by Kamada et al., who showed that in the DA → PVG rat strain combination, liver was only rejected if the recipient was presensitized against donor antigens (9-12). Despite the liver’s potentially strong tolerogenic capacity, this attribute is not uniquely ascribed to them, since kidneys (2,3) and hearts (4) in some strain combinations in mice can also lead to spontaneous graft acceptance and induction of donor-specific tolerance.
In addition to specific systemic hyporesponsiveness to the donor, these liver graft recipients also exhibited the establishment of microchimerism (1), which we have reported previously is an inevitable outcome of all successful whole organ transplantation (13-18). However, this in vivo nonreactivity to the donor was not maintained in vitro, since cells isolated from the spleens of long-term liver graft recipients (up to 90 days after transplantation) when tested in an MLC, responded vigorously to irradiated donor as well as to third-party stimulators, whereas response to syngeneic stimulators was very low. These cells also exhibited elevated levels of cytotoxicity both toward the donor and third party lymphocytes and cultured bile duct cells. This cytotoxic activity was evident at an E:T ratio as low as 3:1. The autologous cell lysis was very low (> 6%), suggesting that this killing was not due to lymphokine-activated killer cells, but rather due to the generation of specific cytotoxic cells early after liver transplantation. This dichotomy between in vivo hyporesponsiveness and in vitro alloreactivity has been referred to as “split tolerance” (19, 20). These findings are in agreement with those in rats (9,10, 12) and in humans (21), further supplementing our own assertion that in vitro observations are not always a true prediction of the in vivo immune status of the recipient.
Several attempts have been made to associate the phenotypic profile of graft-infiltrating cells with immunological responses to the allografts. Of particular interest are the phenotypes of cells infiltrating the graft and their in vivo and in vitro immunological status. A relative increase in CD8+ T cells with the reversal of the CD4 to CD8 ratio has been demonstrated in the cells infiltrating acutely rejecting rat cardiac allografts (22) and human (23) and rat renal (24) allografts. In our study, we also found a similar reversal of the CD4 to CD8 ratio 1 week after allogeneic but not syngeneic liver transplantation, which was sustained throughout the observation period (12 weeks after transplant). Furthermore, despite significant and continuously persistent donor and third-party MLC and CTL alloreactivity, these liver graft-infiltrating cells appeared to be innocuous in vivo, as there was little morphological evidence of hepatocellular necrosis.
In adoptive transfer assays in rats, Kamada et al. demonstrated that transfusion of thoracic duct lymphocytes from liver-grafted animals into irradiated syngeneic recipients resulted in prolongation of skin grafts of the donor, but not third-party, strains (9-11,25). Similar observations were also made in our experiments in mice, where we found that donor but not third-party skin graft survival was significantly prolonged in naive syngeneic sublethally irradiated mice that were reconstituted with cells isolated from either spleens or livers of long-term liver allograft recipients. On the contrary, donor skin graft survival was not prolonged in irradiated syngeneic animals that were reconstituted with splenocytes from skin-sensitized mice.
Clonal deletion of immature T cells in the thymus bearing Mis-reactive TCR subsets is an established mechanism for the induction of self-tolerance (26-29). The relationship among clonal deletion, chimerism, and donor-specific tolerance induction has also been reported by Streilein and co-workers in an I-E-disparate neonatal transplantation model (30-32). Furthermore, clonal deletion or anergy of mature T cells in the periphery has also been proposed as an alternative mechanism for the induction of tolerance to self or alloantigens (33-37). These findings are in agreement with those of Kamada, who found that liver allograft acceptance and the subsequent induction of donor-specific tolerance in rats was associated with elimination of donor-reactive CD8+ T cells in the recirculating lymphocyte pool of the recipients (38). However, using Mlsf-positive (B10.BR, Vβ5, Vβ11 TCR-negative) mice as donors and Mlsf-negative (B10, Vβ5, Vβ11 TCR-positive) animals as recipients, a strain combination in which liver allografts are accepted spontaneously, resulting in the establishment of multilineage microchimerism (including the seeding of putative super-Ag-expressing donor B cells and dendritic cells in the recipient’s thymus), and the subsequent induction of donor-specific tolerance (1), no difference in Vβ5 and Vβ11 TCR expression and antibody-mediated proliferative responses was observed in splenocytes, lymph node cells, and liver graft-infiltrating cells of naive and experimental animals at any time after transplantation (2–90 days after transplant). These observations are similar to earlier reports by Salaun et al. (39), who showed that the induction of specific tolerance in nude mice that were reconstituted with donor-strain thymic epithelium at birth was not due to clonal deletion or anergy of super-Ag-reactive T cells in the recipients.
In summary, our studies demonstrate that spleen and liver graft-infiltrating cells in recipients of liver allografts, though hyporesponsive in vivo, exhibit a strong donor and third-party alloreactivity in vitro (split tolerance). The complete absence of autologous/syngeneic reactivity suggests that this cytolytic response is not due to engendering of lymphokine-activated killer cells, but probably due to the generation of specific cytotoxic T cells after liver grafting. The significantly prolonged acceptance of donor, but not third-party, skin grafts by irradiated animals that were reconstituted with spleen or liver graft-infiltrating cells obtained from long-term liver allograft recipients is an affirmation that these cells are capable of transferring tolerance to naive syngeneic animals. Furthermore, clonal deletion or anergy of Mis-reactive T cells is not the basis for tolerance induction after liver transplantation in mice. Finally, liver-induced tolerance to the donor is probably not mediated by any suppressor factors in the recipient’s serum (manuscript in preparation). A possible explanation of this paradox might be inherent in the donor bone-marrow-derived cells, which after whole organ transplantation are known to migrate from the allograft into the recipient, leading to the establishment of chimerism, which is thought to be the first step toward subsequent induction of donor-specific tolerance (1, 18).
Acknowledgments
The authors thank Youping Li and Carole Frye for their expert technical assistance.
Footnotes
This work was supported by NIH Grant DK 29961.
Abbreviations: DMEM, Dulbecco’s modified Eagle’s medium; Mls, minor lymphocyte-stimulating antigen.
REFERENCES
- 1.Qian S, Demetris AJ, Murase N, Rao AS, Fung JJ, Starzl TE. Murine liver allograft transplantation: tolerance and donor cell chimerism. Hepatology. 1994;19:916. doi: 10.1002/hep.1840190418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Russell PS, Chase CM, Colvin RB, Plate JMD. Kidney transplants in mice. An analysis of the immune status of mice bearing long-term, H-2 incompatible transplants. J Exp Med. 1978;147(5):1449. doi: 10.1084/jem.147.5.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Inoue K, Niesen N, Albini B, Milgrom F. Studies on immunological tolerance induced in mice by kidney allografts. Int Arch Allergy Appl Immunol. 1991;96:358. doi: 10.1159/000235522. [DOI] [PubMed] [Google Scholar]
- 4.Corry RJ, Winn HJ, Russell PS. Primary vascularized allografts of hearts in mice: the role of H-2D, H-2K and non-H-2 antigens in rejection. Transplantation. 1973;16:343. doi: 10.1097/00007890-197310000-00010. [DOI] [PubMed] [Google Scholar]
- 5.Qian S, Fung JJ, Demetris AJ, Ildstad ST, Starzl TE. Orthotopic liver transplantation in the mouse. Transplantation. 1991;52:562. doi: 10.1097/00007890-199109000-00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ono K, Lindsey ES. Improved technique of heart transplantation in rats. J Thorac Cardiovasc Surg. 1969;7:225. [PubMed] [Google Scholar]
- 7.Billingham RE, Brent L, Medawar PB. Actively acquired tolerance to foreign cells. Nature. 1953;172:603. doi: 10.1038/172603a0. [DOI] [PubMed] [Google Scholar]
- 8.Goossens PL, Jouin H, Marchal G, Milon G. Isolation and flow cytometric analysis of the free lymphomyeloid cells present in murine liver. J Immunol Methods. 1990;132:137. doi: 10.1016/0022-1759(90)90407-m. [DOI] [PubMed] [Google Scholar]
- 9.Davies HffS, Kamada N, Roser BJ. Mechanism of donor-specific unresponsiveness induced by liver grafting. Transplant Proc. 1983;15:831. [PubMed] [Google Scholar]
- 10.Kamada N, Brons G, Davies HffS. Fully allogeneic liver grafting in rats induces a state of systemic nonreactivity to donor transplantation antigens. Transplantation. 1980;29:429. doi: 10.1097/00007890-198005000-00021. [DOI] [PubMed] [Google Scholar]
- 11.Kamada N, Davies HffS, Roser BJ. Fully allogeneic liver grafting and the induction of donor-specific unreactivity. Transplant Proc. 1981;13:837. [PubMed] [Google Scholar]
- 12.Kamada N, Davies HffS, Wight DGD, Culank L, Roser B. Liver transplantation in the rat: biochemical and histological evidence of complete tolerance induction in non-rejector strain. Transplantation. 1983;35:304. [PubMed] [Google Scholar]
- 13.Starzl TE, Demetris AJ, Trucco M, et al. Cell migration and chimerism after whole organ transplantation: the basis for graft acceptance. Hepatology. 1993;17(6):1127. [PMC free article] [PubMed] [Google Scholar]
- 14.Starzl TE, Demetris AJ, Trucco M, et al. Systemic chimerism of human female recipients of male livers. Lancet. 1992;340:876. doi: 10.1016/0140-6736(92)93286-v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Starzl TE, Demetris AJ, Murase N, Ildstad S, Ricordi C, Trucco M. Cell migration, chimerism, and graft acceptance. Lancet. 1992;339:1579. doi: 10.1016/0140-6736(92)91840-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Starzl TE, Demetris AJ, Trucco M, et al. Chimerism after liver transplantation for type IV glycogen storage disease and type I Gaucher’s disease. N Engl J Med. 1993;328:745. doi: 10.1056/NEJM199303183281101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Starzl TE, Demetris AJ, Trucco M, et al. Chimerism and donor specific nonreactivity 27 to 29 years after kidney allotransplantation. Transplantation. 1993;55:1271. doi: 10.1097/00007890-199306000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Demetris AJ, Murase N, Fujisaki S, Fung JJ, Rao AS, Starzl TE. Hematolymphoid cell trafficking, microchimerism, and GVH reactions after liver, bone marrow, and heart transplantation. Transplant Proc. 1993;25:3341. [PMC free article] [PubMed] [Google Scholar]
- 19.Houssaint E, Torano A, Ivanyi J. Split tolerance induced by chick embryo thymic epithelium allografted to embryonic recipients. J Immunol. 1986;136(9):3155. [PubMed] [Google Scholar]
- 20.Mayumi H, Himeno K, Tokuda N, Fan JL, Nomoto K. Augmentation of split tolerance in murine combinations disparate at both H-2 and non-H-2 antigens by the use of spleen cells from donors preimmunized with recipient antigens. Immunobiology. 1987;174:274. doi: 10.1016/S0171-2985(87)80003-7. [DOI] [PubMed] [Google Scholar]
- 21.Roncarlo MG, Touraine JL, Banchereau J. Cooperation between major histocompatibility complex mismatched mononuclear cells from a human chimera in the production of antigen-specific antibody. J Clin Invest. 1986;77:673. doi: 10.1172/JCI112361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Araujo JL, Kupiec-Weglinski JW, Araneda D, et al. Phenotype, activation status, and suppressor activity in host lymphocytes during acute rejection and after cyclosporine-induced unresponsiveness of cardiac allografts. Transplantation. 1985;40:278. doi: 10.1097/00007890-198509000-00012. [DOI] [PubMed] [Google Scholar]
- 23.Platt JL, Lebien TW, Michael AF. Interstitial mononuclear cell populations in renal graft rejection: identification by monoclonal antibodies in tissue sections. J Exp Med. 1982;155:17. doi: 10.1084/jem.155.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wasowska B, Baldwin WM, Howell DN, Sanfilippo F. The effects of donor-specific blood transfusion enhancement of rat renal allografts on cytotoxic activity and phenotypes of peripheral blood lymphocytes, splenocytes, and graft-infiltrating cells. Transplantation. 1991;51:451. doi: 10.1097/00007890-199102000-00036. [DOI] [PubMed] [Google Scholar]
- 25.Kamada N, Wight DGD. Antigen-specific immunosuppression induced by liver transplantation in the rat. Transplantation. 1984;39:93. doi: 10.1097/00007890-198409000-00004. [DOI] [PubMed] [Google Scholar]
- 26.Hengartner H, Odermatt B, Schneider R, et al. Deletion of self-reactive T-cells before entry into the thymus medulla. Nature. 1988;336:35. doi: 10.1038/336388a0. [DOI] [PubMed] [Google Scholar]
- 27.Kappler JW, Roehm N, Marrack P. T cell tolerance by clonal elimination in the thymus. Cell. 1989;49:273. doi: 10.1016/0092-8674(87)90568-x. [DOI] [PubMed] [Google Scholar]
- 28.Kappler JW, Staerz U, White J, Marrack PC. Self-tolerance eliminates T cells specific for Mls-modified products of the major histocompatibility complex. Nature. 1988;332:35. doi: 10.1038/332035a0. [DOI] [PubMed] [Google Scholar]
- 29.MacDonald HR, Schneider R, Lees RK, et al. T-cell receptor Vβ use predicts reactivity and tolerance to Mlsa-encoded antigens. Nature. 1988;332:40. doi: 10.1038/332040a0. [DOI] [PubMed] [Google Scholar]
- 30.Ruiz P, Streilein JW. Evidence that I-E negative mice resistant to neonatal H-2 tolerance induction display ubiquitous thymic clonal deletion of donor reactive T-cells. Transplantation. 1993;55:321. doi: 10.1097/00007890-199302000-00018. [DOI] [PubMed] [Google Scholar]
- 31.Streilein JW, Socarras S, Powell TJ. Influence of I-E expression on induction of neonatal transplantation tolerance. Eur J Immunol. 1991;21:161. doi: 10.1002/eji.1830210204. [DOI] [PubMed] [Google Scholar]
- 32.Streilein JW, Socarras S, Powell TJ. I-E molecules and I-E reactive T cells play a central role in neonatal H-2 tolerance. Transplant Proc. 1991;23:138. [PubMed] [Google Scholar]
- 33.Blackman M, Gerhard-Burgert H, Woodland DL, Palmer E, Kappler JW, Marrack P. A role for clonal inactivation in T cell tolerance to MLs-1a. Nature. 1990;345:540. doi: 10.1038/345540a0. [DOI] [PubMed] [Google Scholar]
- 34.Burkly LC, Lo D, Kanagawa O, Brinster RL, Flavell RA. T cell tolerance by clonal anergy in transgenic mice with nonlymphoid expression of MHC class II I-E. Nature. 1989;342:564. doi: 10.1038/342564a0. [DOI] [PubMed] [Google Scholar]
- 35.Jones LA, Chin LT, Merriam GR, Nelson LM, Kruisbeck A. Failure of clonal deletion in neonatally thymectomized mice: tolerance is preserved through clonal anergy. J Exp Med. 1990;172:1007. doi: 10.1084/jem.172.5.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rammensee HG, Kroschewski R, Frangoulis B. Clonal anergy induced in mature Vβ6+ lymphocytes on immunizing Mis-1b mice with Mls-1a expressing cells. Nature. 1989;339:541. doi: 10.1038/339541a0. [DOI] [PubMed] [Google Scholar]
- 37.Webb S, Morris C, Sprent J. Extrathymic tolerance of mature T-cells: clonal elimination as a consequence of immunity. Cell. 1990;63:1249. doi: 10.1016/0092-8674(90)90420-j. [DOI] [PubMed] [Google Scholar]
- 38.Kamada N, Teramoto K. Cellular basis of transplantation tolerance induced by liver grafting in rat. Transplantation. 1988;46:165. doi: 10.1097/00007890-198807000-00034. [DOI] [PubMed] [Google Scholar]
- 39.Salaun J, Bandeira A, Khazaal I, et al. Transplantation tolerance is unrelated to superantigen-dependent deletion and anergy. Proc Natl Acad Sci USA. 1992;89:10420. doi: 10.1073/pnas.89.21.10420. [DOI] [PMC free article] [PubMed] [Google Scholar]