Mice lacking leptin dependent-STAT3 signaling are obese and fertile yet have impaired mammary ductal development.
Abstract
Mice lacking leptin (ob/ob) or its full-length receptor (db/db) are obese and reproductively incompetent. Fertility, pregnancy, and lactation are restored, respectively, in ob/ob mice treated with leptin through mating, d 6.5 post coitum, and pregnancy. Therefore, leptin signaling is needed for lactation, but the timing of its action and the affected mammary process remain unknown. To address this issue, we used s/s mice lacking only leptin-dependent signal transducer and activator of transcription (STAT)3 signaling. These mice share many features with db/db mice, including obesity, but differ by retaining sufficient activity of the hypothalamic-pituitary-ovarian axis to support reproduction. The s/s mammary epithelium was normal at 3 wk of age but failed to expand through the mammary fat pad (MFP) during the subsequent pubertal period. Ductal growth failure was not corrected by estrogen therapy and did not relate to inadequate IGF-I production by the MFP or to the need for epithelial or stromal leptin-STAT3 signaling. Ductal growth failure coincided with adipocyte hypertrophy and increased MFP production of leptin, TNFα, and IL6. These cytokines, however, were unable to inhibit the proliferation of a collection of mouse mammary epithelial cell lines. In conclusion, the very first step of postnatal mammary development fails in s/s mice despite sufficient estrogen IGF-I and an hypothalamic-pituitary-ovarian axis capable of supporting reproduction. This failure is not caused by mammary loss of leptin-dependent STAT3 signaling or by the development of inflammation. These data imply the existence of an unknown mechanism whereby leptin-dependent STAT3 signaling and obesity alter mammary ductal development.
At birth, the mammary gland consists of a small epithelial rudiment embedded in a fatty stroma or mammary fat pad (MFP) (1–3). The epithelial compartment develops into a milk-secreting tissue in a synchronous manner with reproductive function, reflecting the regulation of both processes by a common set of peptides and steroid hormones (2, 4). Major developmental steps include the formation of the ductal network during puberty in response to estrogen and GH, followed during pregnancy by the formation of the lobulo-alveolar apparatus in response to progesterone, prolactin, and placental lactogens (2, 4, 5). These hormonal signals are amplified by reciprocal interactions between epithelial and stromal compartments. For example, GH stimulates stromal IGF-I production, which in turn supports epithelial cell proliferation within terminal end buds (TEBs) and ductal elongation (5). Analogous mechanisms have been described for amplifying the intramammary estrogen, progesterone, and prolactin signals (2, 6).
Mice lacking either leptin (ob/ob) or its receptor (db/db) are infertile and have near-complete absence of mammary development (7–9). Leptin treatment of ob/ob females or transplantation of wild-type (WT) adipose tissue into ob/ob females can rescue fertility (10–13). Restoring expression of the long form of the leptin receptor (Ob-Rb) only in the hypothalamus of db/db mice is also sufficient to rescue the obese phenotype as well as reproduction and lactation (14). These data suggest that absence of mammary development in leptin-deficient animals is due to abnormal function of the hypothalamic-pituitary-ovarian (HPO) axis. Alternatively, obesity itself could induce factors that inhibit epithelial cell proliferation. This process would be facilitated by the proximity of adipocytes and epithelial cells in the developing mammary gland (2, 4, 6). Indeed, obesity in the absence of any genetic defects has been shown to impair mammary development or function in both rodents and humans (15–17). This possibility cannot be evaluated in ob/ob and db/db mice because normalization of leptin signaling not only rescues fertility and lactation but also corrects obesity (10–14).
We have previously developed mice with a targeted mutation of the intracellular tyrosine residue 1138 in Ob-Rb (18). This tyrosine residue is uniquely involved in leptin-dependent recruitment and activation of signal transducer and activator of transcription (STAT)3 (18, 19). Mice homozygous for this mutation (s/s) share many features with db/db mice, including obesity. They differ, however, by retaining the ability to complete all steps of the reproductive cycle with the exception of lactation (18, 20). Given their reproductive competence, we hypothesized that s/s mice had an HPO axis capable of supporting mammary development and that their inability to lactate reflected incomplete differentiation of secretory epithelial cells (20). Surprisingly, s/s mice fail to initiate the very first step of postnatal mammary development consisting of formation of a ductal network. We show that this failure is unaccounted for by inadequate production of estrogen and stromal IGF-I, the loss of leptin-dependent STAT3 signaling in mammary epithelial and stromal cells, or the development of mammary inflammation.
Materials and Methods
Generation of experimental animals
All procedures were approved by Cornell Institutional Animal Care and Use Committee. Mice with replacement of tyrosine 1138 in Ob-Rb with a serine residue (referred to as s mutation) were described previously (18). Male and female mice heterozygous for Leprtm1mgmj (also known as Leprs1138 or the s allele) were intercrossed to generate WT mice and s/s mice. Mice were weaned at 3 wk of age and genotyped as described elsewhere (18). Experimental animals were fed ad libitum levels of a standard rodent chow containing 22% protein and 5% fat (diet no. 8640; Harlan Teklad, Madison WI).
Mammary gland development
Abdominal mammary glands were dissected from subsets of WT and s/s females killed at 3, 4, 6, and 10 wk of age (n = 2 to 5 for each genotype × age combination). The right gland was spread onto a glass plate for whole-mount analysis. Spread glands were fixed overnight in Carnoy's solution (60% ethanol, 30% chloroform, 10% acetic acid), postfixed in 70% ethanol, rehydrated in decreasing concentrations of ethanol, and stained overnight in carmine-alum (21). Slides were then dehydrated in increasing ethanol concentrations, cleared in xylene overnight, and covered with Permount, and coverslips were attached. Whole mounts were photographed with a digital camera mounted on a dissecting microscope. The left abdominal gland was fixed overnight in 10% buffered formalin. Fixed glands were embedded in paraffin and stained with hematoxylin and eosin (H&E) or Masson's trichrome. Histological sections were photographed under ×10 magnification using bright-light microscopy.
Estrogen treatment
Two groups of mice, 21–23 d of age, were used. A first group of intact WT and s/s female mice were treated with 100 μl of corn oil containing 0.03 μg of 17β-estradiol-valerate (Sigma, St. Louis, MO) or corn oil alone (n = 3 to 4 for each genotype × estrogen combination). Treatments were administered every 3 d for 4 consecutive weeks. A second group of WT female mice were ovariectomized and treated similarly (n = 2 for each treatment). At the end of the treatment period, mice were euthanized, and the right abdominal mammary gland was dissected, weighed, and analyzed by whole-mount staining.
Trunk blood was collected from other groups of WT and s/s female mice at 4 and 6 wk of age (n = 8 for each genotype × age combination). Serum was prepared and assayed in duplicate for estrogen concentrations using a RIA kit (DPC Coat-A-Count Estradiol Kit; Diagnostic Products Corp., Los Angeles, CA). The sensitivity of the assay was 5 pg/ml, and the intraassay coefficient of variation was 15.2%.
Mammary transplantation experiments
Epithelial recombination experiments were performed on 20- to 22-d-old female mice of both genotypes as previously described (21). Briefly, the right abdominal mammary gland of recipient mice was cleared of epithelium (EPI) by dissecting the nipple region containing the mammary primordium. The left abdominal gland was left intact to serve as a control. For each transplantation, a 1-mm3 piece of epithelial tissue was prepared from a dissected primordium and transplanted into the center of the cleared mammary gland of a recipient mouse. After each recombination, dissected mammary tissues were evaluated by whole mount to verify that a piece of EPI was isolated from the donor mice and that the entire primordium was removed from the recipient mice. Transplantations experiments failing these criteria were excluded from the analysis. After 6 wk, mice were weighed and euthanized, and both abdominal mammary glands were dissected, weighed, and analyzed by whole-mount staining.
Whole mammary gland transplantations were performed using 25-d-old female mice as described elsewhere (22). Right and left abdominal glands were dissected from euthanized donor females and placed in a sterile saline solution. One whole mammary gland was inserted into a subcutaneous pocket in the subscapular region of a recipient mouse. After 10 wk, recipient mice were weighed and euthanized. Transplanted mammary glands and endogenous abdominal mammary glands were dissected, weighed, and analyzed by whole-mount staining.
Gene expression in the mammary gland
Abdominal glands were collected from WT and s/s female mice at 4 and 6 wk of age. Each gland was dissected into the portion containing only the MFP (portion of the gland that is distal to the lymph node) and the portion containing the EPI (portion of the gland proximal to the nipple). Total RNA was isolated and purified using RNeasy Mini columns and on-column ribonuclease-free deoxyribonuclease treatment (QIAGEN, Chatsworth, CA). Quantity and integrity of RNA were determined using the RNA Nano Lab Chip Kit and BioAnalyzer (Agilent Technologies, Palo Alto, CA). Reverse transcription reactions were performed with 2 μg of RNA, 500 ng of random primers (Invitrogen, Carlsbad, CA), and High Capacity cDNA Synthesis Kit (Applied Biosystems, Foster City, CA) in a 25-μl vol. Real-time PCR assays were performed in duplicate in a 20-μl volume using Power SYBR Mix (Applied Biosystems). Reactions contained 500 nm of each primer (Supplemental Table 1 published on The Endocrine Society's Journals Online web site at http://end.endojournals.org) and reverse-transcribed RNA (25 ng, except 2.5 ng for 18S reactions). Data were analyzed using a relative standard curve prepared from pooled MFP or EPI cDNA. The standard curve consisted of seven serial 2-fold dilution covering a 64-fold range in expression level. Unknown sample expression was determined from the standard curve. Gene expression data were normalized to 18S in the MFP and to keratin 18 (K18) in the EPI.
Mammary epithelial cell proliferation
Cell proliferation was measured using thymidine incorporation (23) in the normal mouse mammary epithelial cell lines HC11, Eph4, CIT3, and Scp2 (24–27). HC11 cells were cultured in RPMI 1640 supplemented with 8% fetal calf serum (FCS), 10 ng/ml epidermal growth factor (EGF), 5 μg/ml insulin, 2.4 nm glutamine, and antibiotics. Eph4 cells were cultured in DMEM (4.5 m glucose) supplemented with 5% FCS, 1 m HEPES, 2.4 nm glutamine, and antibiotics. CIT3 cells were cultured in DMEM/F12 containing 2% FCS, 10 μg/ml insulin, 5 ng/ml EGF, 2.4 nm glutamine, and antibiotics. Scp2 cells were cultured in DMEM/F12 media supplemented with 2% FCS, 5 μg/ml insulin, 2.4 nm glutamine, and antibiotics. For each experiment, cells were plated at 1 × 104 cells/cm2 in 48-well plates. After 48 h, cells were washed twice with PBS and incubated in basal media without serum, insulin, or EGF for 24 h. After this serum-free incubation period, media containing treatments (WT or s/s serum; various combinations of hormones as described in figure legends) and [3H]thymidine were added for 24 h. Each treatment was performed in triplicate. Hormones used included recombinant human TNFα, IL6, and monocyte chemoattractant protein 1 (MCP1) (PeproTech, Rocky Hill, NJ), human IGF-I (National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD) and murine leptin (NIDDK). WT and s/s serum were prepared from 6- to 8-wk-old mice (three to five mice per group).
Statistical analysis
Analyses were performed using SAS statistical software (SAS Institute, Cary, NC). Serum estrogen concentrations were analyzed with a model including the fixed effect of genotype (G; WT or s/s), age (A; 4 or 6 wk), and their interaction (G×A). A similar model was used for gene expression except for the use of a covariate (18S expression for the MFP and K18 expression for the EPI). Data collected from the estrogen treatment experiment were analyzed with a model including the fixed effect of genotype, estrogen treatment (E; oil or estrogen), and their interaction (G×E). Thymidine incorporation data were analyzed with fixed effect of hormone (IGF-I, and TNFα, IL6 or MCP1) and their interaction, and experiment (block), when multiple experiments were performed. All other variables were analyzed by ANOVA with a model including the fixed effect of genotype. Statistical significance was declared at P < 0.05. In the presence of significant interaction, appropriate pairwise comparisons were performed.
Results
Mammary ductal development is impaired in s/s females
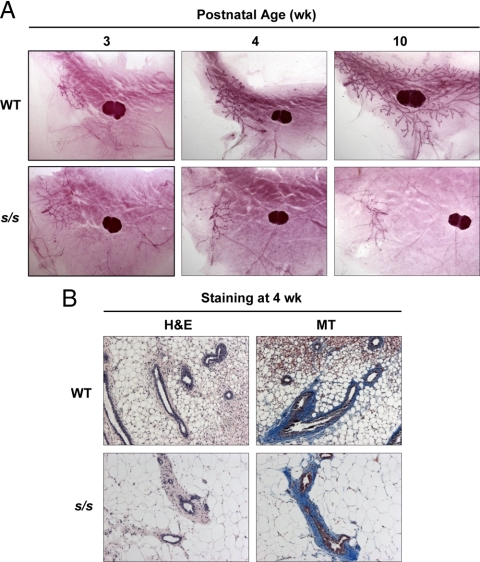
WT and s/s females had similar mammary epithelial rudiment at 3 wk of age (Fig. 1A). In WT mice, the rudiment expanded rapidly as shown by complete ductal invasion of the MFP between 3 and 10 wk of age. This invasion was associated with the presence of TEBs at the tip of the ducts at 4 and 10 wk of age (Fig. 1A). This development pattern was also seen in mice heterozygous for the s mutation (results not shown). In contrast, these indices of mammary epithelial growth were completely absent in s/s females (Fig. 1A). Epithelial cells lining the lumen and the epithelial-stromal interface were visualized by H&E and Masson's trichrome staining, respectively. This analysis did not reveal gross defects in s/s mice at either 4 or 10 wk of age (Fig. 1B and data not shown). These data show that leptin-dependent STAT3 signaling is dispensable for development of the mammary primordium but necessary for ductal growth occurring during the peripubertal period.
Fig. 1.
Effect of lack of leptin-dependent STAT3 signaling on mammary development in peripubertal mice. Mammary glands were collected from female mice lacking leptin-dependent STAT3 signaling (s/s) or their WT counterparts at 3, 4, and 10 wk of age. A, Whole mammary gland mounts were prepared at the indicated ages (n = 2–5 mice for each genotype × age combination). B, Mammary tissue sections were stained at 4 wk of age with H&E or Masson trichrome (MT) (n = 3 WT and 3 s/s). Representative images are shown.
Systemic hormones required for ductal growth are normal in s/s mice
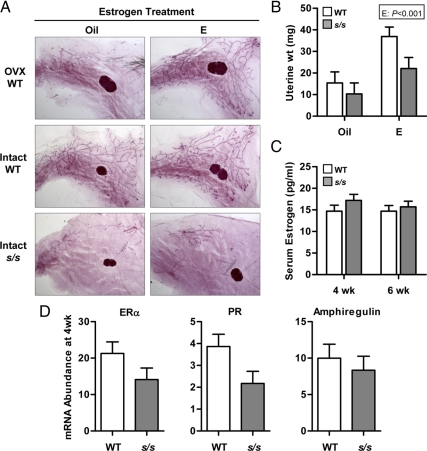
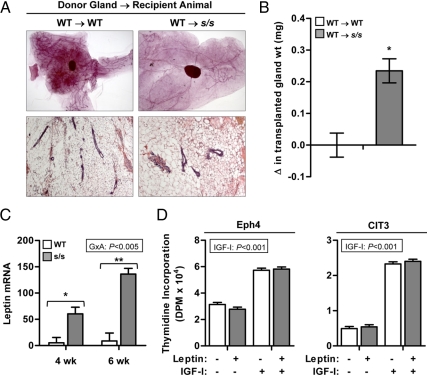
We first considered the possibility that s/s mice lack one or more hormones needed for ductal growth, which in the mouse include estrogen, GH, and IGF-I (2, 5–6). Inadequate estrogen secretion appeared possible because s/s mice experience delayed puberty (18). To test this possibility, WT and s/s mice were injected every 3 d with corn oil or 0.03 μg of 17β-estradiol. Treatments were initiated between d 21 and d 23 and lasted for 4 consecutive weeks. This estrogen administration regimen was devoid of negative effects on the mammary development of intact WT mice and, more importantly, completely restored ductal elongation when they were ovariectomized (Fig. 2A). Estrogen treatment, however, failed to rescue mammary ductal elongation in intact s/s mice (Fig. 2A), even though it increased uterine tissue weight in a similar fashion in s/s and WT females (Fig. 2B; E, P < 0.001; and G×E, P = 0.38). These data indicate that the impaired ductal growth of s/s mice is not caused by a deficit in circulating estrogen. This conclusion was supported by similar serum estrogen concentrations when measured in a second cohort of WT and s/s mice at 4 and 6 wk of age (Fig. 2C).
Fig. 2.
Effect of estrogen on mammary ductal growth in the absence of leptin-dependent STAT3 signaling. A, Female mice lacking leptin-dependent STAT3 signaling (s/s) or their WT counterparts were treated with either corn oil (Oil) or 0.03 μg of 17β-estradiol-valerate (E, n = 3–4 mice for each genotype × estrogen combination). Injections were initiated at 21–23 d of age and were given every 3 d for 4 consecutive weeks. A second group of WT mice were ovariectomized at 22–26 d of age and treated similarly with oil or E (OVX WT, n = 2 per group). The right abdominal mammary gland was analyzed by whole-mount staining. B, Uterine tissue weights were measured at the end of the treatment period. The significant effect of estrogen (E) treatment is shown. Each bar represents the mean ± se of three to four mice. C, Serum was collected from WT and s/s mice at 4 and 6 wk of age and assayed for estrogen concentrations by RIA. Each bar represents the mean ± se of eight mice. D, Mammary tissues containing EPI were collected from female mice lacking leptin-dependent STAT3 signaling (s/s) or their WT counterparts at 4 wk of age. Total RNA was analyzed by real-time PCR for the mRNA abundance of ERα, PR, and amphiregulin. Each bar represents the mean ± se of eight mice.
To assess the possibility that s/s mice have defective epithelial estrogen signaling, expression of progesterone receptor (PR) and amphiregulin (28) was measured in the EPI of 4-wk-old WT and s/s mice (Fig. 2D). In agreement with the whole-mount data, expression of the marker of epithelial cell mass K18 was 4-fold higher in WT than s/s mice [s/s vs. WT, 1.6 vs. 6.5 ± 0.8 (n = 8); P < 0.001]. After K18 normalization, PR expression tended to be reduced by 40% in s/s mice (P = 0.10), whereas amphiregulin expression did not differ. Estrogen receptor-α (ERα) was expressed at a numerically lower level in s/s than in WT mice (Fig 2D, P = 0.20). This analysis was not performed at 6 wk of age because K18 expression was more than 32-fold higher in WT than s/s mice [s/s vs. WT, 1.4 vs. 46.0 ± 5.4 (n = 6), P < 0.001]. These data raise the possibility of lower estrogen signaling in the mammary EPI of s/s mice.
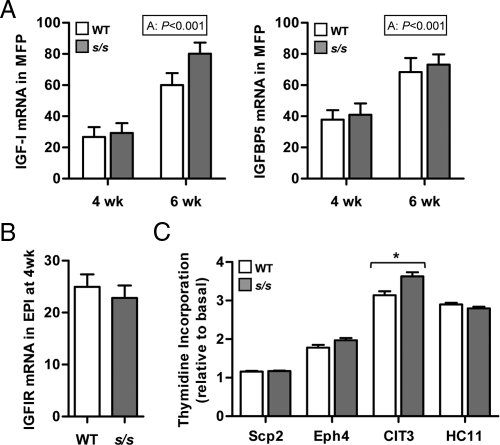
Previous studies showed that s/s mice have slightly elevated plasma IGF-I concentration suggesting adequate GH secretion (29). Ductal growth, however, depends on intramammary, GH-dependent production of IGF-I (5). Moreover, positive actions of IGF-I can be opposed by mammary-specific production of IGF-binding protein (IGFBP)5 (30). To assess the possibility that the local GH-IGF-I system is disturbed in s/s mice, we measured IGF-I and IGFBP5 expression in the MFP. Expression of IGF-I and IGFBP5 increased between 4 and 6 wk of age (Fig. 3A, P < 0.001) but did not differ between WT and s/s mice at either time. Similar results were obtained when expression was measured in EPI tissues (results not shown).
Fig. 3.
Effect of lack of leptin-dependent STAT3 signaling on the intramammary IGF-I system and on the ability of serum to stimulate mammary epithelial cell proliferation. A, MFPs were collected from female mice lacking leptin-dependent STAT3 signaling (s/s) or their WT counterparts at 4 and 6 wk of age. Total RNA was analyzed by real-time PCR for the mRNA abundance of IGF-I and IGFBP5. The significant effect of age (A) is shown. Each bar represents the mean ± se of six to eight mice. B, Mammary tissues containing EPI were collected from female mice lacking leptin-dependent STAT3 signaling (s/s) or their WT counterparts at 4 wk of age. Total RNA was analyzed by real-time PCR for the mRNA abundance of IGFIR. Each bar represents the mean ± se of eight mice. C, Scp2, Eph4, CIT3, and HC11 mouse mammary epithelial cells were incubated in basal media with or without 1% serum obtained from s/s or WT female mice. Thymidine incorporation was measured for each cell line and expressed relative to the incorporation obtained under basal condition. Each bar represents the mean ± se of two to five serum samples per genotype. Within cell lines, s/s differs from WT at P < 0.05 (*).
The IGF-I receptor (IGFIR) is not expressed in adipose tissue (31) and accordingly expression was measured only in EPI tissues. After K18 normalization, IGF-I receptor expression did not differ between 4-wk-old s/s and WT mice (Fig 3B). Finally, s/s serum was as potent as WT serum in increasing the rate of thymidine incorporation in the mouse mammary epithelial cell lines Scp2, Eph4, and HC11, or even more effective in CIT3 cells (Fig. 3C). Overall, these data indicate that the ductal growth failure of s/s mice is not caused by insufficient expression of IGF-I and its receptor or by the presence of a circulating growth inhibitor.
Intramammary leptin signaling via STAT3 is not required for ductal growth
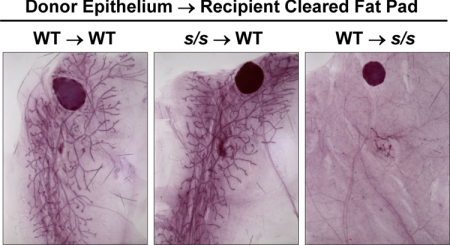
Next, we asked whether the inhibition of ductal growth in s/s mice is related to the absence of leptin-dependent STAT3 signaling in the EPI. A piece of the mammary primordium was dissected from 21-d-old mice of both genotypes (WT and s/s) and transplanted into the cleared MFP of similar age WT or s/s recipient animals. Recipient glands were visualized 6 wk later by whole-mount analysis. The s/s EPI transplanted into a WT recipient (s/s → WT transplantation) filled the MFP in a manner that was undistinguishable from that seen in WT → WT transplantations (Fig. 4). In contrast, the WT primordium failed to expand in the WT → s/s transplantations even though it was visible. These data indicate that absence of leptin-dependent STAT3 signaling in the EPI does not account for the lack of ductal growth in s/s mice.
Fig. 4.
Effect of lack of leptin-dependent STAT3 signaling on the ability of mammary EPI to grow into a cleared fat pad. A 1-mm3 piece of epithelial tissue was dissected from female mice lacking leptin-dependent STAT3 signaling (s/s) or their WT counterparts and transplanted in a cleared MFP of s/s or WT mice. Donor and recipient mice were 20–22 d of age at the time of transplantation. Recombined mammary glands were dissected and analyzed by whole-mount staining 6 wk later. Data are representative of four to six transplantations for each donor-recipient category.
Next, we considered the possibility that ductal growth requires leptin-dependent STAT3 signaling in the stromal compartment. Entire mammary glands were dissected from WT females at 25 d of age and transplanted into the subscapular region of similar age WT or s/s female recipients. The transplanted mammary glands were collected 10 wk later and evaluated by whole-mount analysis. Ducts were visible throughout the transplanted WT gland in the WT → WT transplantations and appeared histologically normal by H&E staining (Fig. 5A). In the case of the WT → s/s transplantations, however, only a few ducts were detected (Fig. 5A). Therefore, integrity of leptin-dependent STAT3 signaling in the stroma cannot rescue ductal growth in s/s mice.
Fig. 5.
Effect of lack of leptin-dependent STAT3 signaling on the intramammary leptin system. A, WT or heterozygous (referred to as WT) mammary glands were transplanted into the subscapular region of female mice lacking leptin-dependent STAT3 signaling (s/s) or their WT counterparts (n = 5–7 transplantations per recipient category). Donor and recipient mice were 25 d of age at the time of transplantation. After 10 wk, the transplanted mammary glands were dissected and analyzed by whole-mount preparations and H&E staining. Representative glands are shown. B, The change (Δ) in the weight of the transplanted gland was calculated as the difference between final and initial weight. Each bar represents the mean ± se of five to seven transplantations. *, P < 0.05. C, MFPs were collected from female s/s mice or their WT counterparts at 4 and 6 wk of age. Total RNA was analyzed by real-time PCR for the mRNA abundance of leptin. The significant interaction between genotype × age (G×A) is shown. Pairwise comparisons performed within each age are significant at P < 0.05 (*) or P < 0.001 (**). Each bar represents the mean ± se of six to eight mice. D, Proliferation of Scp2 and Eph4 mouse mammary epithelial cells was measured using thymidine incorporation. Cells were incubated under basal conditions in the absence or presence of leptin (100 ng/ml) and IGF-I (100 ng/ml). The significant effect of IGF-I is indicated. Each bar represents the mean ± se of three wells.
Intramammary leptin and inflammatory cytokines do not cause ductal growth failure
In the course of these studies, we noted that the ductal growth failure of s/s mice coincided with a rapid increase in the mass of the MFP. At 25 d of age, s/s mammary glands were twice as heavy as WT glands [s/s vs. WT, 100 ± 1.1 mg (n = 7) vs. 52 ± 0.5 mg (n = 10), P < 0.001], even though both genotypes had similar body weights [s/s vs. WT, 10.5 ± 1.2 g (n = 5) vs. 9.9 ± 0.4 g (n = 13)]. The s/s females were first heavier on d 28 [14.5 ± 0.5 g (n = 12) vs. 11.8 ± 0.2 g (n = 12), P < 0.001], and by that time their mammary glands were 3 times heavier than WT glands [183 ± 1.5 mg (n = 12) vs. 53 ± 0.3 mg (n = 12), P < 0.001]. Moreover, when the whole mammary gland was transplanted subscapularly, the mass of the transplanted gland increased nearly 3-fold in the WT → s/s transplantation whereas it did not change in the WT → WT transplantation (Fig. 5B, P < 0.05). This effect was associated with a marked hypertrophy of WT adipocytes present in the MFP, similar to the hypertrophy already present at 4 wk in s/s adipocytes (compare H&E stain, Fig. 5A vs. Fig. 1B).
These data raised the possibility that adipocyte hypertrophy induces the production of a local factor inhibiting ductal growth. We first considered the possibility that leptin itself was this factor by measuring leptin gene expression in the MFP of WT and s/s mice. Expression of leptin in the MFP was 12- and 18-fold higher in s/s than in WT mice at 4 and 6 wk of age, respectively (Fig. 5C, P < 0.001). To assess whether this elevation affected proliferation, mouse mammary epithelia cell lines Eph4, CIT3, and Scp2 were incubated with leptin in the absence or presence of IGF-I. All cells were responsive to IGF-I as evidenced by significant increases in proliferation (P < 0.005, Fig. 5D and data not shown), but proliferation was not repressed by leptin under basal or IGF-I stimulated conditions.
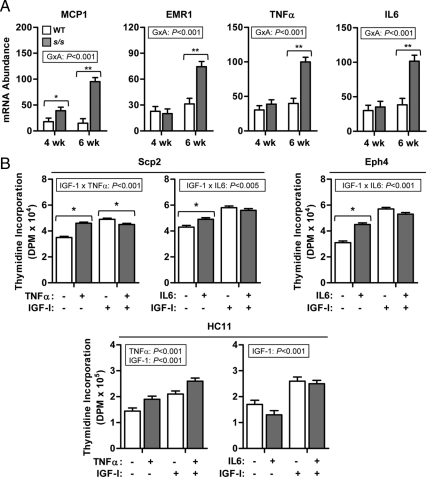
Finally, we considered the possibility that hypertrophy of the MFP in s/s mice leads to macrophage invasion and increased production of proinflammatory cytokines (32, 33). This was assessed by measuring MFP expression of genes indicative of this process. The s/s MFP already had higher expression of the chemoattractant MCP1 at 4 wk of age (Fig. 6A), whereas expression of the marker of macrophage infiltration egf-like module containing, mucin-like, hormone receptor-like 1 (EMR1) (F4/80) and proinflammatory proteins TNFα and IL6 were increased only at 6 wk of age (Fig. 6A; G×A, P < 0.001), after ductal growth failed between 3 and 4 wk of age (Fig. 1A). We also assessed whether these cytokines could inhibit mammary epithelial cell proliferation as they do in human breast cancer cells (34, 35). In Scp2 cells, TNFα inhibited proliferation by only 8% in the presence of IGF-I, but had the opposite effect under basal condition (Fig. 6B; IGF-I × TNFα, P < 0.001). In other cell lines, TNFα was stimulatory (HC11 cells, P < 0.001) or had no effect (Eph4 and CIT3 cells, Fig. 6 and results not shown). IL6 stimulated proliferation under basal conditions (Scp2 and Eph4) or had no effect (HC11), whereas MCP1 was devoid of any effects for all mammary epithelial cell lines (results not shown). Overall, these data do not implicate inflammation of the MFP as the mechanism blocking peripubertal induction of ductal growth in s/s mice.
Fig. 6.
Effect of lack of leptin-dependent STAT3 signaling on the expression of proinflammatory genes in the prepubertal mammary gland. A, MFPs were collected from female mice lacking leptin-dependent STAT3 signaling (s/s) or their WT counterparts at 4 and 6 wk of age. Total RNA was analyzed by real-time PCR for the mRNA abundance of MCP1, EMR1, TNFα, and IL6. Each bar represents the mean ± se of six to eight mice. The significant effect of the genotype × age (G×A) is given. Pairwise comparisons performed within each age are significant at P < 0.05 (*) or P < 0.001 (**). B, Proliferation of Scp2, Eph4, and HC11 mouse mammary epithelial cells was measured using thymidine incorporation. Cells were incubated under basal conditions in the absence or presence of IGF-I (100 ng/ml), TNFα (100 ng/ml), and IL6 (100 ng/ml). The significant effects are indicated. In the case of an interaction, pairwise comparisons performed in the absence or presence of IGF-I are significant at P < 0.05 (*). Each bar represents the mean ± se of three wells.
Discussion
The leptin-signaling deficit of ob/ob and db/db mice suppresses the activity of the HPO axis. Accordingly, ob/ob and db/db female mice are arrested in a permanent prepubertal state that does not support ovulation, conception, pregnancy, and lactation (36). Leptin therapy restores these functions if continued until mating for fertility, d 6.5 post coitum for pregnancy and through pregnancy for lactation (10, 11, 37). Similarly, genetic therapy correcting the leptin-signaling deficit of db/db mice specifically in the hypothalamus is sufficient to restore fertility, pregnancy, and lactation (14). These studies show that leptin signaling is needed for lactation but do not identify the exact aspect of mammary development or function that is impaired by a leptin-signaling deficiency. To start addressing this issue, we used s/s mice lacking only leptin-dependent STAT3 signaling. As db/db mice, s/s mice suffer from hyperphagia, decreased energy expenditure, and obesity but maintain neuroendocrine functions necessary for fertility and pregnancy (18). After parturition, however, they are unable to lactate, a defect associated with insufficient prolactin secretion (20). Unexpectedly, we show that ductal growth, the very first step of postnatal mammary development, is completely arrested in s/s mice even though they are reproductively competent.
At birth, the mouse mammary gland consists of a rudimentary EPI embedded in a much larger MFP (1, 2). The MFP contains predominantly adipocytes and a collection of other mesenchymal cells, including fibroblasts and macrophages (2, 6). Starting around 3 wk of age, areas of extensive proliferation represented by TEBs form at the tips of the ducts and drive ductal invasion of the entire MFP (2, 3, 6). These events are triggered by the onset of estrogen production whereas the other ovarian hormone progesterone is completely dispensable, as shown by normal ductal elongation in progesterone knockout mice (2, 4, 38). An estrogen deficit appeared possible in s/s mice because they experience a delay in first estrus, and STAT3 action in the hypothalamus is important for LH secretion (18, 39). Moreover, absence of estrogen receptor (ER)α in the EPI causes the exact same defects, namely absence of TEBs and ductal growth (40). However, our data rule out this possibility because exogenous estrogen treatment did not rescue these defects in peripubertal s/s females. This conclusion is supported by our observations that peripubertal s/s and WT females have undistinguishable plasma estrogen concentrations.
We also evaluated epithelial estrogen signaling in s/s mice by measuring the expression of ERα, PR, and amphiregulin genes (28). Importantly, this analysis was performed at 4 wk of age when ductal growth failure was already visible. The s/s mice had a numerical reduction in ERα expression. ERα is expressed in both stromal and epithelial cells in the mammary gland (41, 42); therefore, we cannot rule out the possibility that this reduction occurs predominantly in epithelial cells. This possibility, however, is not completely supported by the PR and amphiregulin expression data. Both genes are mostly expressed in the mammary EPI in an estrogen-dependent manner (6, 41, 42). PR expression tended to be reduced by 40% in s/s mice, but amphiregulin was unaffected. The possibility that s/s mice suffer some reduction in estrogen signaling in epithelial cells deserves further study, but this mechanism alone appears unlikely to account for the complete absence of ductal growth in s/s mice.
Estrogen restores TEB formation in ovariectomized and hypophysectomized rodents but only if pituitary extracts or GH is provided (4, 5). IGF-I can substitute for GH in inducing TEB formation and ductal growth whereas prolactin signaling is dispensable (43–45). Later experiments in genetically altered mouse models are consistent with a model whereby GH stimulates IGF-I production, which, in turn, induces epithelial cell proliferation via the IGF-I receptor (46–48). Previous studies indicate that s/s mice are slightly longer (18). They also have 1.5-fold higher circulating IGF-I levels than WT mice (29), suggesting sufficient GH secretion. The biologically relevant IGF-I for ductal growth, however, is derived from the stromal production rather than plasma (46, 49). Moreover, reduced prolactin secretion, such as seen in s/s mice (20), has been shown to induce local IGFBP5 synthesis, leading to sequestration of IGF-I into inactive complexes (50, 51). We found no evidence that these components of the mammary IGF system were altered in s/s mice in a way that would lead to ductal growth failure (i.e. reduced IGF-I and/or increased IGFBP5 expression). This was true whether these variables were measured only in the stromal compartment or in mammary tissues containing both epithelial and stromal compartments. This lack of difference also extended to IGFIR expression, although it will be important in future studies to assess whether downstream signaling events are affected in s/s mice. Finally, s/s plasma stimulated the proliferation of a collection of mouse mammary epithelial cells just as well as WT plasma, arguing against the presence of systemic inhibitor of epithelial cell proliferation in s/s mice. Accordingly, ductal elongation failure in s/s mice does not appear to relate to inadequate levels of GH, presence of a circulating inhibitor, or inadequate intramammary IGF-I production.
Multiple leptin receptor isoforms exist, but Ob-Rb appears to account for most of leptin actions (52). A number of studies have suggested the presence of Ob-Rb and other receptor isoforms in the epithelial and stromal compartments of the mouse mammary gland (17, 53) as well as in various human mammary epithelial cell lines (54–56). In vitro studies have also shown that leptin stimulates the proliferation of the human mammary epithelial cell lines MCF-7 and T47D cells, and this positive action was associated with activation of STAT3 signaling (55–57). Therefore, we considered the possibility that leptin plays a direct role in mammary ductal growth. We performed transplantation of s/s epithelial cells into a WT MFP to assess whether ductal growth required leptin-dependent STAT3 signaling in vivo. This manipulation completely rescued the ability of s/s mammary EPI tissue to invade the MFP. Ductal growth also depends on stromal cues (4, 6), raising the possibility that the requirement for leptin-dependent STAT3 signaling was in the stroma rather than EPI. However, ductal growth failed when entire WT mammary glands were transplanted into s/s mice. Therefore, the ductal growth failure of s/s mice does not relate to the loss of leptin-dependent STAT3 signaling in epithelial or stromal cells.
We also considered the reciprocal possibility that ductal growth failure in s/s mice was caused by excessive leptin production. This inhibition is theoretically possible in s/s mice because mutation of tyrosine residue 1138 in Ob-Rb does not prevent leptin-dependent activation of STAT5 signaling and actually increases ERK signaling (18, 29). The latter reflects the role of STAT3 in reducing the activation of ERK by Ob-Rb. Briefly, leptin-dependent STAT3 activation induces the synthesis of suppressor of cytokine signaling-3 (SOCS3). SOCS3 then binds to phosphorylated tyrosine 985 of the leptin receptor, preventing the recruitment of short heterodimer partner 2 and activation of the ERK cascade (29, 52). Moreover, a recent report showed that diet-induced obesity reduced ductal density and branching frequency in intact mice (17). The authors attributed this effect to excessive leptin signaling because mammary leptin expression was increased in the obese mice, and leptin was able to inhibit proliferation of bovine mammary epithelial cells in vitro (17). We previously reported that the plasma leptin concentration is 16-fold higher in s/s than in WT mice (18) and now show that intramammary leptin production is also increased. In our hands, however, leptin is completely unable to inhibit the growth of a collection of mouse and bovine mammary epithelial cells under both basal and IGF-I-stimulated conditions (data herein and Ref. 58). Moreover, mice lacking tyrosine 985 in Ob-Rb, and thus refractory by SOCS3 inhibition, are not only reproductively competent but also able to lactate (59). Although it remains possible that a leptin excess affects later stages of mammary development such as ductal branching (17), it is unlikely that it is responsible for the complete ductal growth arrest seen in s/s mice.
A near universal feature of obesity is macrophage invasion of adipose tissue followed by production of proinflammatory proteins such as TNFα and IL6 (33, 60). These proteins not only interfere with insulin signaling but they also impair IGF-I-stimulated proliferation of human mammary epithelial cells by attenuating the activity and/or abundance of signaling elements shared by both insulin and IGF-I (e.g. IRS proteins) (34, 60, 61). Accordingly, we assessed the possibility that stromal production of inflammatory-related proteins underlies ductal elongation failure of s/s mice. First, we monitored stromal gene expression at 4 wk of age when ductal growth failure is first detected in s/s mice, and at 6 wk of age when obesity is apparent. Macrophage invasion was monitored by measuring mammary expression of MCP1, a monocyte chemoattractant produced by adipose tissue in parallel with rising adiposity, and the macrophage marker EMR1 (62). MCP1 expression was increased in s/s stroma at 4 wk of age whereas increased expression of EMR1, TNFα, and IL6 occurred only later at 6 wk of age. This latency in production of macrophage-derived proteins suggests that MFP inflammation follows, rather than precedes, ductal growth failure. Moreover, TNFα inhibited cell proliferation weakly and only in Scp2 cells incubated in the presence of IGF-I whereas IL6 was either ineffective or even stimulatory. These results are in contrast with the potent ability of TNFα to inhibit the proliferation of a collection of human and bovine mammary epithelial cells in the presence of IGF-I (23, 34, 35). Our results, however, rule out a primary role for intramammary inflammation because it develops only after ductal growth failure is detected.
In summary, s/s mice suffer from an absolute failure to develop a mammary ductal network after weaning despite being reproductively competent and having sufficient estrogen, GH, and IGF-I. Importantly, this failure is not caused by loss of leptin-dependent STAT3 signaling in epithelila and stromal cells or by the intramammary inflammation that develops with obesity. It is possible that hypertrophy alters some other function of the MFP that is necessary for ductal growth (production of other growth factors, composition of the extracellular matrix, etc.) (4, 6). Our data are also consistent with the possibility that central leptin-dependent STAT3 signaling regulates the synthesis of an unknown systemic signal. This would fit with the observation that rescue of leptin signaling only in the hypothalamus normalizes ductal growth in db/db mice (14).
Supplementary Material
Acknowledgments
We thank Dr. Mina Bissell (Lawrence Berkley Laboratory, Berkeley, CA) for providing the ScP2 cells and Dr. Margaret Neville (University of Colorado, Denver, CO) for providing the CIT3 and Eph4 cells.
Address all correspondence and requests for reprints to: Yves Boisclair, Department of Animal Science, 259 Morrison Hall, Ithaca, New York 14853. E-mail: yrb1@cornell.edu.
This work was supported by the National Research Initiative Competitive Grant 2003-35203-12832 and 2007-35206-17844 from the U.S. Department of Agriculture Cooperative State Research, Education, and Extension Service and by the Cornell University Agricultural Experiment Station.
Disclosure Summary: The authors have nothing to declare.
Footnotes
- EMR1
- Egf-like module containing, mucin-like, hormone receptor-like-1
- EPI
- epithelium
- ER
- estrogen receptor
- FCS
- fetal calf serum
- H&E
- hematoxylin and eosin
- HPO
- hypothalamic-pituitary-ovarian
- IGFBP
- IGF-binding protein
- IGFIR
- IGF-I receptor
- K18
- keratin 18
- MCP1
- monocyte chemoattractant protein 1
- MFP
- mammary fat pad
- Ob-Rb
- long form of the leptin receptor
- SOCS3
- suppressor of cytokine signaling-3
- STAT
- signal transducer and activator of transcription
- TEB
- terminal end bud
- WT
- wild type.
References
- 1. Hovey RC, McFadden TB, Akers RM. 1999. Regulation of mammary gland growth and morphogenesis by the mammary fat pad: a species comparison. J Mammary Gland Biol Neoplasia 4:53–68 [DOI] [PubMed] [Google Scholar]
- 2. Hennighausen L, Robinson GW. 2005. Information networks in the mammary gland. Nat Rev Mol Cell Biol 6:715–725 [DOI] [PubMed] [Google Scholar]
- 3. Richert MM, Schwertfeger KL, Ryder JW, Anderson SM. 2000. An atlas of mouse mammary gland development. J Mammary Gland Biol Neoplasia 5:227–241 [DOI] [PubMed] [Google Scholar]
- 4. Hovey RC, Trott JF, Vonderhaar BK. 2002. Establishing a framework for the functional mammary gland: from endocrinology to morphology. J Mammary Gland Biol Neoplasia 7:17–38 [DOI] [PubMed] [Google Scholar]
- 5. Kleinberg DL, Feldman M, Ruan W. 2000. IGF-I: an essential factor in terminal end bud formation and ductal morphogenesis. J Mammary Gland Biol Neoplasia 5:7–17 [DOI] [PubMed] [Google Scholar]
- 6. Sternlicht MD. 2006. Key stages in mammary gland development: the cues that regulate ductal branching morphogenesis. Breast Cancer Res 8:201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hu X, Juneja SC, Maihle NJ, Cleary MP. 2002. Leptin—a growth factor in normal and malignant breast cells and for normal mammary gland development. J Natl Cancer Inst 94:1704–1711 [DOI] [PubMed] [Google Scholar]
- 8. Cleary MP, Juneja SC, Phillips FC, Hu X, Grande JP, Maihle NJ. 2004. Leptin receptor-deficient MMTV-TGF-α/Lepr(db)Lepr(db) female mice do not develop oncogene-induced mammary tumors. Exp Biol Med (Maywood) 229:182–193 [DOI] [PubMed] [Google Scholar]
- 9. Sainsbury A, Schwarzer C, Couzens M, Jenkins A, Oakes SR, Ormandy CJ, Herzog H. 2002. Y4 receptor knockout rescues fertility in ob/ob mice. Genes Dev 16:1077–1088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Malik NM, Carter ND, Murray JF, Scaramuzzi RJ, Wilson CA, Stock MJ. 2001. Leptin requirement for conception, implantation, and gestation in the mouse. Endocrinology 142:5198–5202 [DOI] [PubMed] [Google Scholar]
- 11. Chehab FF, Mounzih K, Lu R, Lim ME. 1997. Early onset of reproductive function in normal female mice treated with leptin. Science 275:88–90 [DOI] [PubMed] [Google Scholar]
- 12. Mounzih K, Qiu J, Ewart-Toland A, Chehab FF. 1998. Leptin is not necessary for gestation and parturition but regulates maternal nutrition via a leptin resistance state. Endocrinology 139:5259–5262 [DOI] [PubMed] [Google Scholar]
- 13. Klebanov S, Astle CM, DeSimone O, Ablamunits V, Harrison DE. 2005. Adipose tissue transplantation protects ob/ob mice from obesity, normalizes insulin sensitivity and restores fertility. J Endocrinol 186:203–211 [DOI] [PubMed] [Google Scholar]
- 14. de Luca C, Kowalski TJ, Zhang Y, Elmquist JK, Lee C, Kilimann MW, Ludwig T, Liu SM, Chua SC., Jr 2005. Complete rescue of obesity, diabetes, and infertility in db/db mice by neuron-specific LEPR-B transgenes. J Clin Invest 115:3484–3493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Flint DJ, Travers MT, Barber MC, Binart N, Kelly PA. 2005. Diet-induced obesity impairs mammary development and lactogenesis in murine mammary gland. Am J Physiol Endocrinol Metab 288:E1179–E1187 [DOI] [PubMed] [Google Scholar]
- 16. Rasmussen KM, Hilson JA, Kjolhede CL. 2001. Obesity may impair lactogenesis II. J Nutr 131:3009S–3011S [DOI] [PubMed] [Google Scholar]
- 17. Kamikawa A, Ichii O, Yamaji D, Imao T, Suzuki C, Okamatsu-Ogura Y, Terao A, Kon Y, Kimura K. 2009. Diet-induced obesity disrupts ductal development in the mammary glands of nonpregnant mice. Dev Dyn 238:1092–1099 [DOI] [PubMed] [Google Scholar]
- 18. Bates SH, Stearns WH, Dundon TA, Schubert M, Tso AW, Wang Y, Banks AS, Lavery HJ, Haq AK, Maratos-Flier E, Neel BG, Schwartz MW, Myers MG., Jr 2003. STAT3 signalling is required for leptin regulation of energy balance but not reproduction. Nature 421:856–859 [DOI] [PubMed] [Google Scholar]
- 19. Banks AS, Davis SM, Bates SH, Myers MG., Jr 2000. Activation of downstream signals by the long form of the leptin receptor. J Biol Chem 275:14563–14572 [DOI] [PubMed] [Google Scholar]
- 20. Bates SH, Myers MG. 2004. The role of leptin→STAT3 signaling in neuroendocrine function: an integrative perspective. J Mol Med 82:12–20 [DOI] [PubMed] [Google Scholar]
- 21. Ip MM, Asch BB. 2000. Methods in mammary gland biology and breast cancer research. New York: Kluwer Academic/Plenum Publishers [Google Scholar]
- 22. Cases S, Zhou P, Shillingford JM, Wiseman BS, Fish JD, Angle CS, Hennighausen L, Werb Z, Farese RV., Jr 2004. Development of the mammary gland requires DGAT1 expression in stromal and epithelial tissues. Development 131:3047–3055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Thorn SR, Purup S, Vestergaard M, Sejrsen K, Meyer MJ, Van Amburgh ME, Boisclair YR. 2008. Regulation of mammary parenchymal growth by the fat pad in prepubertal dairy heifers: role of inflammation-related proteins. J Endocrinol 196:539–546 [DOI] [PubMed] [Google Scholar]
- 24. Schmidhauser C, Bissell MJ, Myers CA, Casperson GF. 1990. Extracellular matrix and hormones transcriptionally regulate bovine β-casein 5′ sequences in stably transfected mouse mammary cells. Proc Natl Acad Sci USA 87:9118–9122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ball RK, Friis RR, Schoenenberger CA, Doppler W, Groner B. 1988. Prolactin regulation of β-casein gene expression and of a cytosolic 120-kd protein in a cloned mouse mammary epithelial cell line. EMBO J 7:2089–2095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Desprez PY, Hara E, Bissell MJ, Campisi J. 1995. Suppression of mammary epithelial cell differentiation by the helix-loop-helix protein Id-1. Mol Cell Biol 15:3398–3404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Reichmann E, Schwarz H, Deiner EM, Leitner I, Eilers M, Berger J, Busslinger M, Beug H. 1992. Activation of an inducible c-FosER fusion protein causes loss of epithelial polarity and triggers epithelial-fibroblastoid cell conversion. Cell 71:1103–1116 [DOI] [PubMed] [Google Scholar]
- 28. Ciarloni L, Mallepell S, Brisken C. 2007. Amphiregulin is an essential mediator of estrogen receptor α function in mammary gland development. Proc Natl Acad Sci USA 104:5455–5460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dunn SL, Björnholm M, Bates SH, Chen Z, Seifert M, Myers MG., Jr 2005. Feedback inhibition of leptin receptor/Jak2 signaling via Tyr1138 of the leptin receptor and suppressor of cytokine signaling 3. Mol Endocrinol 19:925–938 [DOI] [PubMed] [Google Scholar]
- 30. Flint DJ, Beattie J, Allan GJ. 2003. Modulation of the actions of IGFs by IGFBP-5 in the mammary gland. Horm Metab Res 35:809–815 [DOI] [PubMed] [Google Scholar]
- 31. Clemmons DR. 2006. Involvement of insulin-like growth factor-I in the control of glucose homeostasis. Curr Opin Pharmacol 6:620–625 [DOI] [PubMed] [Google Scholar]
- 32. Hotamisligil GS. 2006. Inflammation and metabolic disorders. Nature 444:860–867 [DOI] [PubMed] [Google Scholar]
- 33. Tilg H, Moschen AR. 2006. Adipocytokines: mediators linking adipose tissue, inflammation, and immunity. Nat Rev Immunol 6:772–783 [DOI] [PubMed] [Google Scholar]
- 34. Shen WH, Zhou JH, Broussard SR, Freund GG, Dantzer R, Kelley KW. 2002. Proinflammatory cytokines block growth of breast cancer cells by impairing signals from a growth factor receptor. Cancer Res 62:4746–4756 [PubMed] [Google Scholar]
- 35. Shen WH, Yin Y, Broussard SR, McCusker RH, Freund GG, Dantzer R, Kelley KW. 2004. Tumor necrosis factor α inhibits cyclin A expression and retinoblastoma hyperphosphorylation triggered by insulin-like growth factor-I induction of new E2F-1 synthesis. J Biol Chem 279:7438–7446 [DOI] [PubMed] [Google Scholar]
- 36. Chehab FF. 1997. The reproductive side of leptin. Nat Med 3:952–953 [DOI] [PubMed] [Google Scholar]
- 37. Mounzih K, Lu R, Chehab FF. 1997. Leptin treatment rescues the sterility of genetically obese ob/ob males. Endocrinology 138:1190–1193 [DOI] [PubMed] [Google Scholar]
- 38. Lydon JP, DeMayo FJ, Funk CR, Mani SK, Hughes AR, Montgomery CA, Jr, Shyamala G, Conneely OM, O'Malley BW. 1995. Mice lacking progesterone receptor exhibit pleiotropic reproductive abnormalities. Genes Dev 9:2266–2278 [DOI] [PubMed] [Google Scholar]
- 39. Buettner C, Pocai A, Muse ED, Etgen AM, Myers MG, Jr, Rossetti L. 2006. Critical role of STAT3 in leptin's metabolic actions. Cell Metab 4:49–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mallepell S, Krust A, Chambon P, Brisken C. 2006. Paracrine signaling through the epithelial estrogen receptor α is required for proliferation and morphogenesis in the mammary gland. Proc Natl Acad Sci USA 103:2196–2201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Haslam SZ, Nummy KA. 1992. The ontogeny and cellular distribution of estrogen receptors in normal mouse mammary gland. J Steroid Biochem Mol Biol 42:589–595 [DOI] [PubMed] [Google Scholar]
- 42. Shyamala G, Chou YC, Louie SG, Guzman RC, Smith GH, Nandi S. 2002. Cellular expression of estrogen and progesterone receptors in mammary glands: regulation by hormones, development and aging. J Steroid Biochem Mol Biol 80:137–148 [DOI] [PubMed] [Google Scholar]
- 43. Vomachka AJ, Pratt SL, Lockefeer JA, Horseman ND. 2000. Prolactin gene-disruption arrests mammary gland development and retards T-antigen-induced tumor growth. Oncogene 19:1077–1084 [DOI] [PubMed] [Google Scholar]
- 44. Brisken C, Kaur S, Chavarria TE, Binart N, Sutherland RL, Weinberg RA, Kelly PA, Ormandy CJ. 1999. Prolactin controls mammary gland development via direct and indirect mechanisms. Dev Biol 210:96–106 [DOI] [PubMed] [Google Scholar]
- 45. Ruan W, Newman CB, Kleinberg DL. 1992. Intact and amino-terminally shortened forms of insulin-like growth factor I induce mammary gland differentiation and development. Proc Natl Acad Sci USA 89:10872–10876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Richards RG, Klotz DM, Walker MP, Diaugustine RP. 2004. Mammary gland branching morphogenesis is diminished in mice with a deficiency of insulin-like growth factor-I (IGF-I), but not in mice with a liver-specific deletion of IGF-I. Endocrinology 145:3106–3110 [DOI] [PubMed] [Google Scholar]
- 47. Bonnette SG, Hadsell DL. 2001. Targeted disruption of the IGF-I receptor gene decreases cellular proliferation in mammary terminal end buds. Endocrinology 142:4937–4945 [DOI] [PubMed] [Google Scholar]
- 48. Ruan W, Kleinberg DL. 1999. Insulin-like growth factor I is essential for terminal end bud formation and ductal morphogenesis during mammary development. Endocrinology 140:5075–5081 [DOI] [PubMed] [Google Scholar]
- 49. Walden PD, Ruan W, Feldman M, Kleinberg DL. 1998. Evidence that the mammary fat pad mediates the action of growth hormone in mammary gland development. Endocrinology 139:659–662 [DOI] [PubMed] [Google Scholar]
- 50. Tonner E, Barber MC, Travers MT, Logan A, Flint DJ. 1997. Hormonal control of insulin-like growth factor-binding protein-5 production in the involuting mammary gland of the rat. Endocrinology 138:5101–5107 [DOI] [PubMed] [Google Scholar]
- 51. Flint DJ, Tonner E, Beattie J, Allan GJ. 2008. Role of insulin-like growth factor binding proteins in mammary gland development. J Mammary Gland Biol Neoplasia 13:443–453 [DOI] [PubMed] [Google Scholar]
- 52. Villanueva EC, Myers MG., Jr 2008. Leptin receptor signaling and the regulation of mammalian physiology. Int J Obes (Lond) 32(Suppl 7):S8–S12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Lin Y, Li Q. 2007. Expression and function of leptin and its receptor in mouse mammary gland. Sci China C Life Sci 50:669–675 [DOI] [PubMed] [Google Scholar]
- 54. Laud K, Gourdou I, Pessemesse L, Peyrat JP, Djiane J. 2002. Identification of leptin receptors in human breast cancer: functional activity in the T47-D breast cancer cell line. Mol Cell Endocrinol 188:219–226 [DOI] [PubMed] [Google Scholar]
- 55. Yin N, Wang D, Zhang H, Yi X, Sun X, Shi B, Wu H, Wu G, Wang X, Shang Y. 2004. Molecular mechanisms involved in the growth stimulation of breast cancer cells by leptin. Cancer Res 64:5870–5875 [DOI] [PubMed] [Google Scholar]
- 56. Dieudonne MN, Machinal-Quelin F, Serazin-Leroy V, Leneveu MC, Pecquery R, Giudicelli Y. 2002. Leptin mediates a proliferative response in human MCF7 breast cancer cells. Biochem Biophys Res Commun 293:622–628 [DOI] [PubMed] [Google Scholar]
- 57. Okumura M, Yamamoto M, Sakuma H, Kojima T, Maruyama T, Jamali M, Cooper DR, Yasuda K. 2002. Leptin and high glucose stimulate cell proliferation in MCF-7 human breast cancer cells: reciprocal involvement of PKC-α and PPAR expression. Biochim Biophys Acta 1592:107–116 [DOI] [PubMed] [Google Scholar]
- 58. Thorn SR, Purup S, Cohick WS, Vestergaard M, Sejrsen K, Boisclair YR. 2006. Leptin does not act directly on mammary epithelial cells in prepubertal dairy heifers. J Dairy Sci 89:1467–1477 [DOI] [PubMed] [Google Scholar]
- 59. Björnholm M, Münzberg H, Leshan RL, Villanueva EC, Bates SH, Louis GW, Jones JC, Ishida-Takahashi R, Bjørbaek C, Myers MG., Jr 2007. Mice lacking inhibitory leptin receptor signals are lean with normal endocrine function. J Clin Invest 117:1354–1360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Wellen KE, Hotamisligil GS. 2005. Inflammation, stress, and diabetes. J Clin Invest 115:1111–1119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Rui L, Yuan M, Frantz D, Shoelson S, White MF. 2002. SOCS-1 and SOCS-3 block insulin signaling by ubiquitin-mediated degradation of IRS1 and IRS2. J Biol Chem 277:42394–42398 [DOI] [PubMed] [Google Scholar]
- 62. Sartipy P, Loskutoff DJ. 2003. Monocyte chemoattractant protein 1 in obesity and insulin resistance. Proc Natl Acad Sci USA 100:7265–7270 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.