Parasympathetic neurotransmitters affect signaling pathways and proliferation of immortalized human meibomian gland epithelial cells.
Abstract
Purpose.
A striking characteristic of the human meibomian gland is its rich sensory, sympathetic, and parasympathetic innervation, yet the functional relevance of these nerve fibers remains unknown. Acting on the hypothesis that neurotransmitters are released in the vicinity of the gland, act on glandular receptors, and influence the production, secretion, and/or delivery of meibomian gland secretions to the ocular surface, the goal in this study was to begin to determine whether neurotransmitters influence the meibomian gland.
Methods.
Immortalized human meibomian gland epithelial (SLHMG) cells were examined for the presence of vasoactive intestinal peptide (VIP) and muscarinic acetylcholine (mACh) receptor transcripts and proteins. Cells were also exposed to VIP, carbachol, forskolin, and/or 3-isobutyl-1-methylxanthine (IBMX) to determine whether these agents, alone or in combination, modulate the adenylyl cyclase pathway, the accumulation of intracellular free calcium ([Ca2+]i), or cell proliferation.
Results.
Results demonstrate that SLHMG cells transcribe and translate VIP and mACh receptors; VIP, with either IBMX or forskolin, activates the adenylyl cyclase pathway, and the effect of VIP and forskolin together is synergistic; both VIP and carbachol increase intracellular [Ca2+] in SLHMG cells; and VIP with forskolin stimulates SLHMG cell proliferation.
Conclusions.
This study shows that parasympathetic neurotransmitters and their agonists influence the function of human meibomian gland epithelial cells. It remains to be determined whether this action alters the production, secretion, and/or delivery of meibum to the ocular surface.
Optimal meibomian gland function is essential for protecting the health and integrity of the ocular surface.1–4 This gland, through its synthesis and secretion of lipids, promotes stability and prevents evaporation of the tear film.1–4 Conversely, meibomian gland dysfunction (MGD), and the resulting lipid insufficiency, destabilizes the tear film, heightens its evaporation and osmolarity,3–9 and is very likely the most frequent cause of evaporative dry eye disease throughout the world.4,10–14
One of the most striking characteristics of the meibomian gland is its rich sensory, sympathetic, and parasympathetic innervation.15 Indeed, this tissue is the only human sebaceous gland that has such innervation, with adjacent nerve fibers reactive for acetylcholinesterase, substance P, vasoactive intestinal peptide (VIP), dopamine β-hydroxylase, nitric oxide synthase, tyrosine hydroxylase, somatostatin, neuropeptide Y (NPY), and calcitonin gene-related peptide (CGRP).16–32 Furthermore, as recently reported,4,33 the mouse meibomian gland contains mRNAs of receptors for serotonin, adrenergic, CGRP, cholinergic, dopamine, γ-aminobutyric acid, glutamate, NPY, neurotensin, and somatostatin. It is quite possible that these nerves and, if translated, neurotransmitter receptors play a significant role in the regulation of the meibomian gland. However, this possibility is completely speculative. It is unknown whether neurotransmitters are released into the vicinity of the meibomian gland, act on glandular receptors, or induce a physiological effect.
We hypothesize that neurotransmitters are released in the vicinity of the gland; act on glandular receptors; and influence the production, secretion, and/or delivery of meibomian gland secretions to the ocular surface. If correct, this would indicate an important role for the nervous system in maintaining the tear film lipid layer and, thus, the health of the ocular surface. Our goal in this study was to begin to determine whether neurotransmitters do influence the meibomian gland. Toward that end, we focused on the role of VIPergic and cholinergic neurotransmitters, because innervation of the meibomian gland appears to be largely parasympathetic in origin.18,19,30 We investigated whether VIPergic and cholinergic receptors are transcribed and translated in human meibomian gland epithelial cells and whether corresponding ligands induce cellular physiological responses.
Materials and Methods
Cell Culture Procedures
Immortalized human meibomian gland epithelial (SLHMG) cells, recently generated in our laboratory,34 were cultured in tissue-culture-treated flasks (Corning Inc., Corning, NY) in keratinocyte serum-free medium (KSFM; Invitrogen, Carlsbad, CA) supplemented with 50 μg/mL bovine pituitary extract and 5 μg/mL epithelial growth factor. Cells were subcultured for propagation and used for experimentation at 90% confluence.
Molecular Biological Techniques
To determine whether human meibomian gland epithelial cells express genes for VIP and cholinergic receptors, we examined total RNA samples prepared from human primary and immortalized meibomian gland epithelial cells, as well as from human meibomian glands, for the presence of corresponding receptor mRNAs. The generation of these cellular and tissue samples, their processing by Asuragen (Austin, TX; with HumanHT-12 v3 Expression BeadChips; Illumina, Inc., San Diego, CA), and their analyses (BeadStudio; Illumina, Inc.) have been described.34
SDS-PAGE and Immunoblots
To examine whether SLHMG cells translate transcripts for VIP and cholinergic receptors, cultured cells were trypsinized, centrifuged, and resuspended in 2X reducing sample buffer (BioRad, Hercules, CA). Samples were heated at 95°C for 10 minutes, separated by SDS-PAGE on 10% Tris/glycine precast gels (Invitrogen) and transferred to nitrocellulose. Membranes for VIP receptor blots were blocked with 3% bovine serum albumin (BSA) in phosphate buffered saline (PBS) containing 0.01% Tween-20, followed by incubation with a mouse monoclonal antibody specific for VIP receptor 1 (VPAC1; GenWay Biotech, San Diego, CA) or VIP receptor 2 (VPAC2; Millipore Corp., Billerica, MA) and HRP-conjugated, Fc-specific goat anti-mouse IgG (Sigma-Aldrich, St. Louis, MO). Membranes for muscarinic acetylcholine (mACh) receptor blots were blocked with 5% nonfat dry milk in PBS with 0.01% Tween-20, followed by incubation with rabbit polyclonal anti-mAChR M2 or M3 antibody (GenWay Biotech) and HRP-conjugated goat anti-rabbit IgG (Sigma-Aldrich). Proteins were visualized with chemiluminescent detection reagents (West Pico; Pierce Biotechnology, Rockford, IL). Human heart extract (Imgenex, San Diego, CA) and A431 human skin epidermal cell lysate (Santa Cruz Biotechnology, Santa Cruz, CA) were processed for immunoblot analyses as positive controls for the mACh and VIP receptors, respectively.
cAMP ELISA
To assess the ability of cells to produce adenosine 3′,5′-cyclic monophosphate (cAMP) in response to secretagogues, SLHMG cells were plated in 96-well tissue-culture-treated plates at a density of 3 × 104 cells per well. One day later, cells were treated for varying time intervals, at concentrations determined by dose-response studies, with forskolin (Sigma-Aldrich), 3-isobutyl-1-methylxanthine (IBMX; Sigma-Aldrich), carbachol (CCh; EMD Chemical, Gibbstown, NJ), and/or VIP (EMD Chemical) in fresh KSFM. Cells were then washed and lysed; cAMP levels in this extract were measured using a nonacetylation protocol and a cAMP enzyme-linked immunoassay, according to the manufacturer's recommendations (GE Health Care, Buckinghamshire, United Kingdom). A standard curve in duplicate was run in each assay.
Intracellular Free Calcium Quantification
To monitor intracellular calcium ([Ca2+]i), SLHMG cells were cultured in 35-mm glass-bottom dishes (MatTek Corp., Ashland, MA) at a sparse density. One day later, plates were washed with Kreb's Ringer buffer with HEPES (KRBH: 119 mM NaCl, 4.7 mM KCl, 2.5 mM CaCl2, 1.2 mM MgCl2, 1.2 mM KH2PO4, 2 mM glucose, 10 mM HEPES; pH 7.4) and incubated in KRBH supplemented with 250 μM sulfinpyrazone (Sigma-Aldrich), 1.2 mM pluronic F127 (Sigma-Aldrich), and 6 μM Fura-2AM (Invitrogen) for 1 hour at 37°C. Cells were then washed and exposed to KRBH supplemented with 250 μM sulfinpyrazone for an additional 45 minutes to allow for ester conversion. Intracellular Ca2+ measurements were obtained using a ratio imaging system (InCyt IM2; Intracellular Imaging, Inc., Cincinnati, OH). Excitation wavelengths were 340 and 380 nm and emission wavelength was 505 nm. At least 8 individual cells were selected in each field of view and experiments were repeated a minimum of 3 times. Data were collected in real-time as cells were treated with parasympathetic agonists and are presented as [Ca2+]i over time for each cell monitored. Optimal concentrations of Fura-2AM and agonists for these studies were determined by conducting dose-response experiments.
Cell Proliferation
To assess the capacity of secretagogues to induce SLHMG cell proliferation, cells were plated at a subconfluent density of 3 × 104 cells per well in 24-well plates. Cells were then cultured in KSFM containing 10−6 M forskolin, 10−8 M VIP, or 10−3 M IBMX, either alone or in combination, for up to 7 days at 37°C. When indicated, cells were trypsinized, resuspended, and counted using a hemocytometer. All proliferation experiments were repeated in triplicate.
Statistical Analysis
Analysis of variance, post-hoc analysis, and Student's t-test were performed using statistical software (Prism 5; GraphPad Software, Inc., La Jolla, CA).
Results
SLHMG Cells Express VIP and Cholinergic Receptors
To determine whether human meibomian gland epithelial cells express VIP and mACh receptor transcripts and proteins, we processed samples for molecular biological and immunoblot procedures as outlined in Materials and Methods.
Our results demonstrate that human primary and immortalized meibomian gland epithelial cells, as well as human meibomian glands, contain mRNAs for VPAC1, mACh receptor M2 (M2R) and a species (LOC730413) predicted to be similar to mACh receptor M3 (M3R) (data not shown). In addition, we were able to detect very low levels of VPAC2 mRNA in some of these cellular and glandular samples (data not shown). We were unable to detect mRNA transcripts for mACh receptors M1, M4, or M5 in these samples.
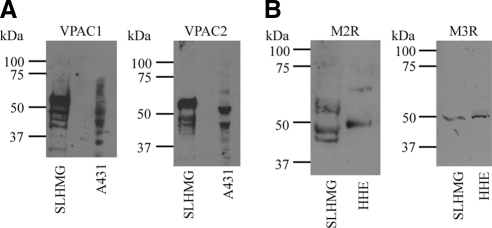
Our immunoblot data show that these neurotransmitter receptor transcripts are translated. As demonstrated in Figure 1A, monoclonal antibodies specific to VPAC1 or VPAC2 react predominantly with a 57 kDa protein species in SLHMG cell lysates. Less abundant and smaller isoforms are also present and may represent deglycosylated or degraded forms of VPAC receptors. Some differences in molecular weights and degradation patterns were found for VPAC1 and VPAC2 immunoreactive bands in human epidermal epithelial cell (A431) lysates, which served as positive control (Fig. 1A).
Figure 1.
SLHMG cells express neuropeptide receptors. Cells were lysed in reducing sample buffer, boiled, and separated on a 10% tris/glycine gel, followed by transfer to nitrocellulose. Immunoblots for four neuropeptide receptors, each representative of 3 to 5 individual blots, are shown. (A) Representative immunoblots for high-affinity VIP receptors, VPAC1 and VPAC2. A431 human skin epidermal cell line serves as positive control. (B) Representative immunoblots for mACh receptor proteins M2R and M3R. Human heart extract lysate (HHE) serves as positive control.
Both M2R and M3R proteins are also expressed by SLHMG cells (Fig. 1B). A polyclonal M2R antibody reacted with a 45 kDa protein in the SLHMG cell lysate and with a slightly larger peptide (50 kDa) in human heart extract lysate (Fig. 1B, left). In addition, a polyclonal antibody specific for M3R identified a 50 kDa protein in both SLHMG cell and human heart tissue extract lysates (Fig. 1B, right). Similar results were obtained with other antibodies specific for these receptors (data not shown).
Activation of the Adenylyl Cyclase Pathway in SLHMG Cells
Parasympathetic neurotransmitters often act by binding to specific transmembrane receptors, altering the activity of the adenylyl cyclase signaling pathway, and causing changes in the generation of cAMP.35,36 To help determine whether such a mechanism may be operative in SLHMG cells, we first conducted studies to optimize the detection of cAMP levels in these cells.
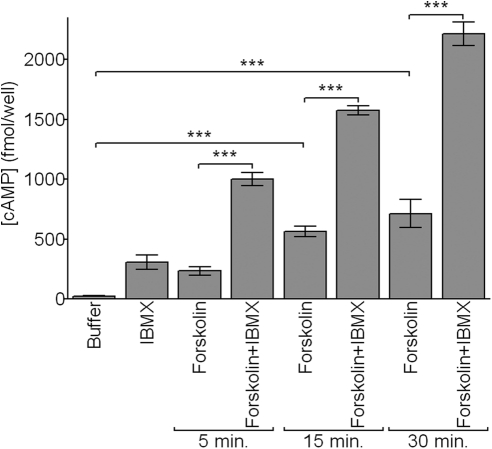
Our results demonstrate that forskolin (10−4 M), a secretagogue known to activate adenylyl cyclase in other cell types,37 induces a significant, time- and dose-dependent increase in cAMP levels in SLHMG cells (Fig. 2). This forskolin effect is significantly amplified in the presence of IBMX (10−3 M; Figs. 2, 3), a phosphodiesterase 4 inhibitor.38 By contrast, IBMX treatment alone has a minimal impact on cellular cAMP content (Figs. 2, 3). Subsequent cAMP experiments were performed with 100-fold less forskolin (10−6 M) to remain within the dynamic range of the assay system.
Figure 2.
Forskolin activates adenylyl cyclase in SLHMG cells. Cells were treated with forskolin (Forsk; 10−4 M), alone or in combination with IBMX (10−3 M), for 5, 15, or 30 minutes. Cells treated with buffer or IBMX alone were incubated 30 minutes. Intracellular cAMP was measured by enzyme-linked immunoassay. Results are representative of three independent experiments. ***P < 0.001.
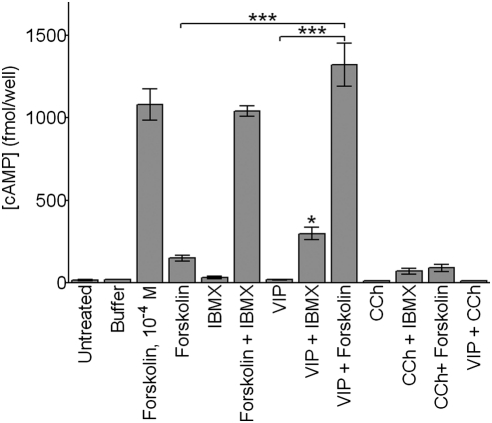
Figure 3.
VIP, but not CCh, promotes cAMP accumulation in combination with IBMX or forskolin. Cells were untreated or incubated with buffer, forskolin (10−6 M, unless otherwise noted), IBMX (10−3 M), VIP (10−8 M), and CCh (10−4 M), alone or in combinations, for 10 minutes. The experimental times and doses used in this study were predetermined in preliminary investigations. Cells were then lysed and analyzed for intracellular cAMP levels. *P < 0.05, compared with either agent alone; ***P < 0.001.
Given this background, we analyzed the influence of VIP and CCh, in the presence or absence of IBMX or forskolin, on cAMP levels in SLHMG cells. As shown in Figure 3, VIP, in combination with either IBMX or forskolin, caused a significant elevation in intracellular cAMP content. This effect was most pronounced with VIP and forskolin together, which led to a synergistic rise in cAMP concentrations that was 10-fold higher than expected (i.e., based on simple additivity) (Fig. 3). By contrast, exposure of SLHMG cells to VIP alone, or to CCh with or without IBMX or forskolin, did not generate a detectable change in cAMP levels.
Effect of Neurotransmitters on Intracellular Free Calcium Concentration
Parasympathetic neurotransmitters may also act on cells by associating with G-protein-coupled receptors that activate other second messengers, such as intracellular Ca2+.35,36 To test this pathway, SLHMG cells were incubated with the calcium indicator Fura-2 and imaged during treatment with either VIP or CCh.
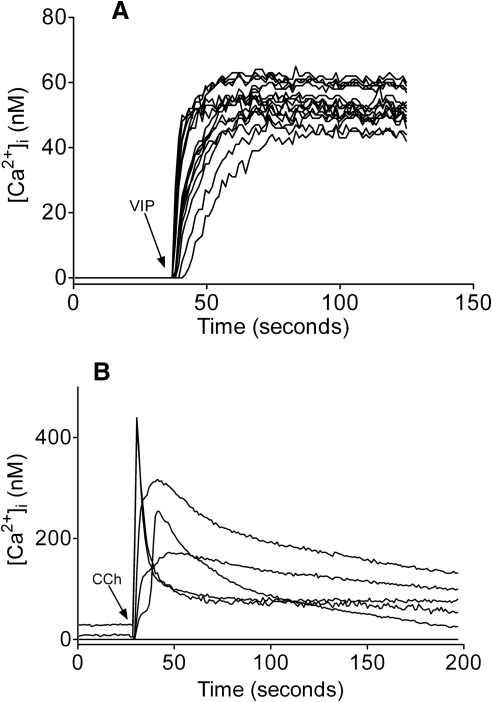
As demonstrated in Figure 4A, cellular exposure to VIP (10−6 M) prompted a rapid increase in [Ca2+]i, followed by a plateau phase lasting several minutes. Of > 75 cells monitored during a series of VIP treatments, 98% responded with increased [Ca2+]i within minutes (Fig. 4A and data not shown).
Figure 4.
VIP and CCh promote intracellular free calcium accumulation in SLHMG cells. Cells were loaded with Fura-2 (6 × 10−6 M) before stimulation with VIP (10−6 M) or CCh (10−3 M) and imaged in real time. Preliminary dose-response experiments were used to determine the treatment concentrations used for Fura-2 and each agonist. Individual traces represent a single cell and graphs shown are representative of three to five plates imaged independently. The y axes have been extended below zero to clearly illustrate the presence or absence of nonresponsive cells. (A) Cells treated with VIP at time indicated by arrow. (B) Cells treated with CCh at time indicated by arrow.
Similarly, CCh (10−3 M) induced an increased [Ca2+]i in SLHMG cells (Fig. 4B). This response, which occurred in approximately 10% to 15% of cells, was far more robust (e.g., up to eightfold greater) than that observed in VIP-treated cells.
Impact of VIP Treatment on SLHMG Cell Proliferation
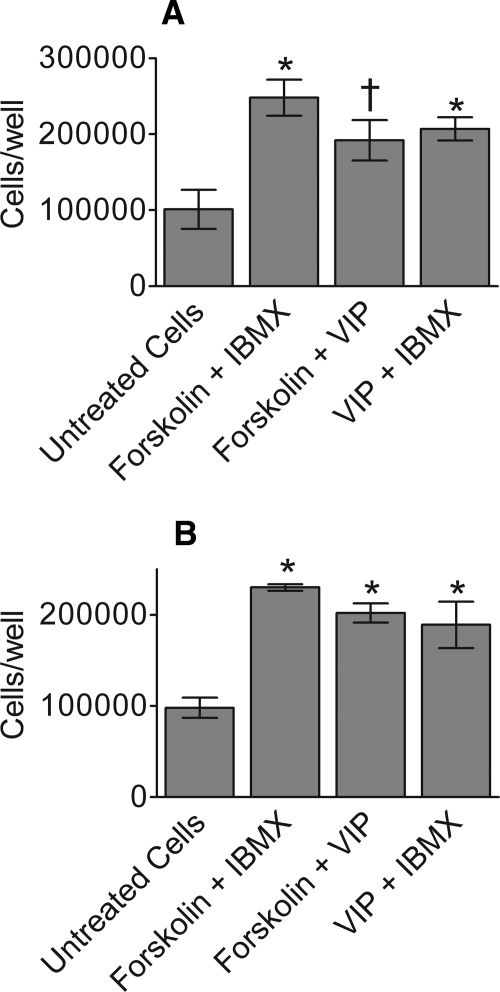
To examine whether a parasympathetic neurotransmitter might stimulate the proliferation of SLHMG cells, we exposed cells to VIP (10−8 M), forskolin (10−6 M), or IBMX (10−3 M), alone or in combination, for up to 7 days. Cells were then harvested and counted.
Our results show that VIP in combination with forskolin or IBMX significantly increased SLHMG cell proliferation, compared with untreated controls (Fig. 5). This effect was duplicated by treatment with forskolin and IBMX (Fig. 5), as well as with IBMX alone (data not shown). In contrast, VIP or forskolin treatment alone did not result in consistent changes in cell number. There was no significant effect of vehicle on cell proliferation, relative to untreated controls.
Figure 5.
Treatment with VIP in combination with forskolin or IBMX promotes SLHMG proliferation. Cells were treated with VIP (10−8 M), forskolin (10−6 M), or IBMX (10−3 M), alone or in combination, for 7 days. Data are shown for two separate experiments (A) and (B) and each column represents the mean of three wells ± SEM. *P < 0.05, two-tail, compared with untreated cells. †P < 0.05, one-tail, compared with untreated cells.
Discussion
The present study indicates that the parasympathetic nervous system may exert a significant influence on the meibomian gland. Our findings show that SLHMG cells transcribe and translate VIP and mACh receptors and that corresponding receptor ligands activate the adenylyl cyclase pathway, increase intracellular [Ca2+], and/or stimulate cell proliferation. These results support our hypothesis that neurotransmitters released in the vicinity of the meibomian gland act on glandular receptors and induce changes in epithelial cell physiology.
Our research identified the presence of VPAC1, VPAC2, M2R, and M3R transcripts and proteins in SLHMG cells. These results are consistent with the findings of other investigators, who reported expression, by meibomian gland epithelial cells, of VIP receptor 1 and all five mACh receptor subtypes in mouse39 as well as M3R in monkey.40 The molecular weights of these SLHMG receptors are analogous (e.g., to VPAC1 in mouse,39 and M2R in human bladder41), or different from (e.g., M3R in mouse39), those found in other studies. The variability reported in the literature, as well as differences in apparent molecular weights observed in our own immunoblots, may relate to differences in posttranslational processing, protein degradation, or antibody selectivity between species and tissue types.39
Of particular importance, our results demonstrate that these VIP and mACh receptors are functional. Cellular exposure to VIP in combination with either IBMX or forskolin led to a marked synergistic rise in cAMP concentrations. Such synergistic activation has been found in a variety of tissues42–46 and was most pronounced with combined VIP and forskolin treatment. Researchers have proposed that this greater-than-additive effect may be due to activation of multiple adenylyl cyclase isoforms, each interacting with a subset of G proteins and/or intermediate activators.43 In contrast to these combinatorial actions, VIP alone did not induce a detectable increase in cAMP levels in SLHMG cells. This lack of a measurable response, which does occur in other cell types,44,46–48 may reflect a rapid breakdown of cAMP within minutes after stimulation.
Both VIP and CCh elicited a significant increase in free [Ca2+]i in SLHMG cells. This second messenger reaction to VIPergic and cholinergic input has also been observed in other cells.35,36 Moreover, the ability of VIP to activate both calcium and adenylyl cyclase signaling pathways appears to be a widespread characteristic of this neuropeptide.48–50 Investigators have speculated that this capacity to trigger different second messenger systems may be the result of VIP receptor coupling to multiple G-protein isoforms.49 In addition to mACh receptor activation, it is possible that nicotinic acetylcholine receptors may also play a role in the response to CCh observed in SLHMG cells.
An intriguing observation was that VIP combined with forskolin or IBMX stimulated SLHMG cell proliferation. This finding suggests that VIP may play a role in the generation of acinar epithelial cells in the meibomian gland. This tissue secretes by a holocrine mechanism, which involves disintegration of the whole cell and release of the cell components into the connecting ductile.4,51 This process requires that the basal layer of epithelial cells in the periphery of the acinus serves as a progenitor cell population, which constantly gives rise to new epithelial cells.4,52 Given that numerous nerve fibers near meibomian gland acini contain VIP,18,29 it is possible that release of this neuropeptide could promote epithelial cell proliferation in vivo. Such an ability, however, remains to be shown.
Another possibility is that the neurotransmitters VIP and acetylcholine may influence the production, secretion, and/or delivery of meibomian gland secretions to the ocular surface. These compounds are known, for example, to stimulate epithelial cell secretion by the lacrimal gland.29,45,48,53,54 If this meibomian gland activity occurs, it would suggest that the parasympathetic nervous system may protect against the development of evaporative dry eye. Consistent with this hypothesis is the finding that administration of the parasympathetic inhibitor scopolamine promotes the generation of dry eye.55–59
Overall, our results indicate a potential role for the parasympathetic nervous system in maintaining the tear film lipid layer and the health of the ocular surface.
Footnotes
Supported by grants from NIH (EY05612) and Alcon Research, Ltd.
Disclosure: W.R. Kam, None; D.A. Sullivan, None
References
- 1. McCulley JP, Shine WE. Meibomian gland function and the tear lipid layer. Ocul Surf. 2003;1:97–106 [DOI] [PubMed] [Google Scholar]
- 2. Bron AJ, Tiffany JM, Gouveia SM, Yokoi N, Voon LW. Functional aspects of the tear film lipid layer. Exp Eye Res. 2004;78:347–360 [DOI] [PubMed] [Google Scholar]
- 3. 2007 Report of the Dry Eye WorkShop. Ocul Surf. 2007;5:65–204 [Google Scholar]
- 4. Knop E, Knop N, Millar T, Obata H, Sullivan DA. The International Workshop on Meibomian Gland Dysfunction: report of the Subcommittee on Anatomy, Physiology, and Pathophysiology of the Meibomian Gland. Invest Ophthalmol Vis Sci. 2011;52:1938–1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Craig JP, Tomlinson A. Importance of the lipid layer in human tear film stability and evaporation. Optom Vis Sci. 1997;74:8–13 [DOI] [PubMed] [Google Scholar]
- 6. Driver PJ, Lemp MA. Meibomian gland dysfunction. Surv Ophthalmol. 1996;40:343–367 [DOI] [PubMed] [Google Scholar]
- 7. Foulks GN, Bron AJ. Meibomian gland dysfunction: a clinical scheme for description, diagnosis, classification, and grading. Ocul Surf. 2003;1:107–126 [DOI] [PubMed] [Google Scholar]
- 8. Lemp MA, Nichols KK. Blepharitis in the United States 2009: a survey-based perspective on prevalence and treatment. Ocul Surf. 2009;7:S1–S14 [DOI] [PubMed] [Google Scholar]
- 9. Mathers W. Evaporation from the ocular surface. Exp Eye Res. 2004;78:389–394 [DOI] [PubMed] [Google Scholar]
- 10. Green-Church KB, Butovich I, Willcox M, et al. The international workshop on meibomian gland dysfunction: report of the subcommittee on tear film lipids and lipid-protein interactions in health and disease. Invest Ophthalmol Vis Sci. 2011;52:1979–1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Horwath-Winter J, Berghold A, Schmut O, et al. Evaluation of the clinical course of dry eye syndrome. Arch Ophthalmol. 2003;121:1364–1368 [DOI] [PubMed] [Google Scholar]
- 12. Nelson JD, Shimazaki J, Benitez-del-Castillo JM, et al. The international workshop on meibomian gland dysfunction: report of the definition and classification subcommittee. Invest Ophthalmol Vis Sci. 2011;52:1930–1937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Schaumberg DA, Nichols JJ, Papas EB, Tong L, Uchino M, Nichols KK. The international workshop on meibomian gland dysfunction: report of the subcommittee on the epidemiology of, and associated risk factors for, MGD. Invest Ophthalmol Vis Sci. 2011;52:1994–2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shimazaki J, Sakata M, Tsubota K. Ocular surface changes and discomfort in patients with meibomian gland dysfunction. Arch Ophthalmol. 1995;113:1266–1270 [DOI] [PubMed] [Google Scholar]
- 15. Thody AJ, Shuster S. Control and function of sebaceous glands. Physiol Rev. 1989;69:383–416 [DOI] [PubMed] [Google Scholar]
- 16. Aisa J, Lahoz M, Serrano P, et al. Acetylcholinesterase-positive and paraformaldehyde-induced-fluorescence-positive innervation in the upper eyelid of the sheep (Ovis aries). Histol Histopathol. 2001;16:487–496 [DOI] [PubMed] [Google Scholar]
- 17. Chanthaphavong RS, Murphy SM, Anderson CR. Chemical coding of sympathetic neurons controlling the tarsal muscle of the rat. Auton Neurosci. 2003;105:77–89 [DOI] [PubMed] [Google Scholar]
- 18. Chung CW, Tigges M, Stone RA. Peptidergic innervation of the primate meibomian gland. Invest Ophthalmol Vis Sci. 1996;37:238–245 [PubMed] [Google Scholar]
- 19. Fan Q, Smith PG. Decreased vasoactive intestinal polypeptide-immunoreactivity of parasympathetic neurons and target innervation following long-term sympathectomy. Regul Pept. 1993;48:337–343 [DOI] [PubMed] [Google Scholar]
- 20. Hartschuh W, Reinecke M, Weihe E, Yanaihara N. VIP-immunoreactivity in the skin of various mammals: immunohistochemical, radioimmunological and experimental evidence for a dual localization in cutaneous nerves and merkel cells. Peptides. 1984;5:239–245 [DOI] [PubMed] [Google Scholar]
- 21. Kirch W, Horneber M, Tamm ER. Characterization of Meibomian gland innervation in the cynomolgus monkey (Macaca fascicularis). Anat Embryol (Berl). 1996;193:365–375 [DOI] [PubMed] [Google Scholar]
- 22. LeDoux MS, Zhou Q, Murphy RB, Greene ML, Ryan P. Parasympathetic innervation of the meibomian glands in rats. Invest Ophthalmol Vis Sci. 2001;42:2434–2441 [PubMed] [Google Scholar]
- 23. Lorber M. Somatostatin-like immunoreactivity (SLIR) in rat harderian and meibomian glands and glands of Zeis. Adv Exp Med Biol. 2002;506:81–89 [DOI] [PubMed] [Google Scholar]
- 24. Luhtala J, Palkama A, Uusitalo H. Calcitonin gene-related peptide immunoreactive nerve fibers in the rat conjunctiva. Invest Ophthalmol Vis Sci. 1991;32:640–645 [PubMed] [Google Scholar]
- 25. Luhtala J, Uusitalo H. The distribution and origin of substance P immunoreactive nerve fibres in the rat conjunctiva. Exp Eye Res. 1991;53:641–646 [DOI] [PubMed] [Google Scholar]
- 26. Montagna W, Ellis RA. Cholinergic innervation of the Meibomian glands. Anat Rec. 1959;135:121–127 [DOI] [PubMed] [Google Scholar]
- 27. Perra MT, Serra A, Sirigu P, Turno F. Histochemical demonstration of acetylcholinesterase activity in human Meibomian glands. Eur J Histochem. 1996;40:39–44 [PubMed] [Google Scholar]
- 28. Seifert P, Spitznas M. Immunocytochemical and ultrastructural evaluation of the distribution of nervous tissue and neuropeptides in the meibomian gland. Graefes Arch Clin Exp Ophthalmol. 1996;234:648–656 [DOI] [PubMed] [Google Scholar]
- 29. Seifert P, Spitznas M. Vasoactive intestinal polypeptide (VIP) innervation of the human eyelid glands. Exp Eye Res. 1999;68:685–692 [DOI] [PubMed] [Google Scholar]
- 30. Simons E, Smith PG. Sensory and autonomic innervation of the rat eyelid: neuronal origins and peptide phenotypes. J Chem Neuroanat. 1994;7:35–47 [DOI] [PubMed] [Google Scholar]
- 31. Smith PG, Reddy H. Reorganization of cranial sympathetic pathways following neonatal ganglionectomy in the rat. J Comp Neurol. 1990;301:490–500 [DOI] [PubMed] [Google Scholar]
- 32. Sokolov VE, Shabadash SA, Zelikina TI. Innervation of specific skin glands in the rabbit: the cholinergic terminal reticulum [in Russian]. Dokl Akad Nauk SSSR. 1984;277:1245–1249 [PubMed] [Google Scholar]
- 33. Schirra F, Suzuki T, Richards SM, et al. Androgen control of gene expression in the mouse meibomian gland. Invest Ophthalmol Vis Sci. 2005;46:3666–3675 [DOI] [PubMed] [Google Scholar]
- 34. Liu S, Hatton MP, Khandelwal P, Sullivan DA. Culture, immortalization, and characterization of human meibomian gland epithelial cells. Invest Ophthalmol Vis Sci. 2010;51:3993–4005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dickson L, Finlayson K. VPAC and PAC receptors: from ligands to function. Pharmacol Ther. 2009;121:294–316 [DOI] [PubMed] [Google Scholar]
- 36. Andersson KE. Muscarinic acetylcholine receptors in the urinary tract. Handb Exp Pharmacol. 2011;319–344 [DOI] [PubMed] [Google Scholar]
- 37. Seamon KB, Padgett W, Daly JW. Forskolin: unique diterpene activator of adenylate cyclase in membranes and in intact cells. Proc Natl Acad Sci U S A. 1981;78:3363–3367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Spina D. PDE4 inhibitors: current status. Br J Pharmacol. 2008;155:308–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zhu HY, Riau AK, Barathi VA, Chew J, Beuerman RW. Expression of neural receptors in mouse meibomian gland. Cornea. 2010;29:794–801 [DOI] [PubMed] [Google Scholar]
- 40. Liu S, Li J, Tan DT, Beuerman RW. The eyelid margin: a transitional zone for 2 epithelial phenotypes. Arch Ophthalmol. 2007;125:523–532 [DOI] [PubMed] [Google Scholar]
- 41. Arrighi N, Bodei S, Peroni A, et al. Detection of muscarinic receptor subtypes in human urinary bladder mucosa: age and gender-dependent modifications. Neurourol Urodyn. 2008;27:421–428 [DOI] [PubMed] [Google Scholar]
- 42. Boige N, Amiranoff B, Munck A, Laburthe M. Forskolin stimulates adenylate cyclase in human colonic crypts: interaction with VIP. Eur J Pharmacol. 1984;101:111–117 [DOI] [PubMed] [Google Scholar]
- 43. Fukayama S, Tashjian AH, Jr, Bringhurst FR. Forskolin-induced homologous desensitization via an adenosine 3′,5′-monophosphate-dependent mechanism(s) in human osteoblast-like SaOS-2 cells. Endocrinology. 1992;131:1770–1776 [DOI] [PubMed] [Google Scholar]
- 44. Guild S, Drummond AH. Vasoactive-intestinal-polypeptide-stimulated adenosine 3′,5′-cyclic monophosphate accumulation in GH3 pituitary tumour cells. Reversal of desensitization by forskolin. Biochem J. 1984;221:789–796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mauduit P, Herman G, Rossignol B. Newly synthesized protein secretion in rat lacrimal gland: post-second messenger synergism. Am J Physiol. 1987;253:C514–C524 [DOI] [PubMed] [Google Scholar]
- 46. Sato K, Sato F. Effect of VIP on sweat secretion and cAMP accumulation in isolated simian eccrine glands. Am J Physiol. 1987;253:R935–R941 [DOI] [PubMed] [Google Scholar]
- 47. Laburthe M, Rousset M, Boissard C, Chevalier G, Zweibaum A, Rosselin G. Vasoactive intestinal peptide: a potent stimulator of adenosine 3′:5′-cyclic monophosphate accumulation in gut carcinoma cell lines in culture. Proc Natl Acad Sci U S A. 1978;75:2772–2775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sreedharan SP, Patel DR, Xia M, Ichikawa S, Goetzl EJ. Human vasoactive intestinal peptide1 receptors expressed by stable transfectants couple to two distinct signaling pathways. Biochem Biophys Res Commun. 1994;203:141–148 [DOI] [PubMed] [Google Scholar]
- 49. Luo X, Zeng W, Xu X, et al. Alternate coupling of receptors to Gs and Gi in pancreatic and submandibular gland cells. J Biol Chem. 1999;274:17684–17690 [DOI] [PubMed] [Google Scholar]
- 50. Yoshimura K, Nezu E. Interaction between the calcium and cyclic AMP messenger systems in perifused rat parotid acinar cells. Possible mechanism for potentiation of amylase secretion. Biochem Pharmacol. 1992;43:1031–1041 [DOI] [PubMed] [Google Scholar]
- 51. Sirigu P, Shen RL, Pinto da Silva P. Human meibomian glands: the ultrastructure of acinar cells as viewed by thin section and freeze-fracture transmission electron microscopies. Invest Ophthalmol Vis Sci. 1992;33:2284–2292 [PubMed] [Google Scholar]
- 52. Olami Y, Zajicek G, Cogan M, Gnessin H, Pe'er J. Turnover and migration of meibomian gland cells in rats' eyelids. Ophthalmic Res. 2001;33:170–175 [DOI] [PubMed] [Google Scholar]
- 53. Hodges RR, Dartt DA. Regulatory pathways in lacrimal gland epithelium. Int Rev Cytol. 2003;231:129–196 [DOI] [PubMed] [Google Scholar]
- 54. Hodges RR, Dicker DM, Rose PE, Dartt DA. Alpha 1-adrenergic and cholinergic agonists use separate signal transduction pathways in lacrimal gland. Am J Physiol. 1992;262:G1087–G1096 [DOI] [PubMed] [Google Scholar]
- 55. Dursun D, Wang M, Monroy D, et al. A mouse model of keratoconjunctivitis sicca. Invest Ophthalmol Vis Sci. 2002;43:632–638 [PubMed] [Google Scholar]
- 56. Rashid S, Jin Y, Ecoiffier T, Barabino S, Schaumberg DA, Dana MR. Topical omega-3 and omega-6 fatty acids for treatment of dry eye. Arch Ophthalmol. 2008;126:219–225 [DOI] [PubMed] [Google Scholar]
- 57. El Annan J, Chauhan SK, Ecoiffier T, Zhang Q, Saban DR, Dana R. Characterization of effector T cells in dry eye disease. Invest Ophthalmol Vis Sci. 2009;50:3802–3807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Scifo C, Barabino S, De Pasquale G, Blanco AR, Mazzone MG, Rolando M. Effects of a new lipid tear substitute in a mouse model of dry eye. Cornea. 2010;29:802–806 [DOI] [PubMed] [Google Scholar]
- 59. Wei Y, Epstein SP, Fukuoka S, Birmingham NP, Li XM, Asbell PA. sPLA2-IIa amplifies ocular surface inflammation in the experimental dry eye (DE) BALB/c mouse model. Invest Ophthalmol Vis Sci. 2011;52:4780–4788 [DOI] [PMC free article] [PubMed] [Google Scholar]