Abstract
Barley (Hordeum vulgare L.) is a long-day plant whose flowering is enhanced when the photoperiod is supplemented with far-red light, and this promotion is mediated by phytochrome. A chemically mutagenized dwarf cultivar of barley was selected for early flowering time (barley maturity daylength response [BMDR]-1) and was made isogenic with the cultivar Shabet (BMDR-8) by backcrossing. BMDR-1 was found to contain higher levels of both phytochrome A and phytochrome B in the dark on immunoblots with monoclonal antibodies from oat (Avena sativa L.) that are specific to different members of the phytochrome gene family. Phytochrome A was light labile in both BMDR-1 and BMDR-8, decreasing to very low levels after 4 d of growth in the light. Phytochrome B was light stable in BMDR-8, being equal in both light and darkness. However, phytochrome B became light labile in BMDR-1 and this destabilization of phytochrome B appeared to make BMDR-1 insensitive to photoperiod. In addition, both the mutant and the wild type lacked any significant promotion of flowering in response to a pulse of far-red light given at the end of day, and the end-of-day, far-red inhibition of tillering is normal in both, suggesting that phytochrome B is not involved with these responses in barley.
Barley (Hordeum vulgare L.) is a long-day plant, requiring daylengths in excess of some critical minimum to flower (Vince-Prue, 1975). The genetics of its sensitivity to photoperiod are not completely understood, but Yasuda and Hyashi (1980) have identified four recessive (ea, early maturity) and three dominant genes at seven loci that influence flowering in barley. The homozygous recessive easp, eac, eak, and ea7 genotypes located on chromosomes 3, 4, 5, and 6, respectively, individually confer extreme earliness of flowering under short daylengths and relative insensitivity to photoperiod (Gallagher et al., 1991). Genetic studies have indicated that the eak recessive homozygote located on chromosome 5 suppresses other maturity loci that influence the time to heading in the field (Gallagher et al., 1987). In addition, five major genes and eight quantitative trait loci controlling flowering have been mapped by restriction fragment-length polymorphism analysis in a cross between a spring and a winter cultivar of barley (Laurie et al., 1995). The gene Ppd-H1, which is located on the short arm of chromosome 2, controls flowering under long days (>13 h of light), but has no effect under short days (10 h of light). A second gene, Ppd-H2, is located on the long arm of chromosome 5 and controls flowering only under short days. The nomenclature for these genes follows that used for wheat (Law et al., 1993) and it is unknown whether they are related to any of the ea loci. Although it is possible that eak and Ppd-H2 are allelic, this may not be the case, because the Ppd-2 locus in wheat is a dominant gene, whereas eak is recessive.
We have identified an additional early flowering genotype (BMDR-1) that is a single gene recessive mutant derived from an M2 diethyl sulfate-treated dwarf genotype (Deitzer, 1986). It was made isogenic by backcrossing with the photoperiodically sensitive wild-type cv Shabet (BMDR-8, which is a shatter-resistant line of the cv Betzes) and selected over nine generations for normal stature and extreme earliness of flowering. The BMDR-1 mutant is thought to be allelic to the eak genotype (L. Gallagher, personal communication).
The addition of FR during the photoperiod promotes the induction of flowering in barley, which is mediated by phytochrome (Deitzer et al., 1979; Deitzer, 1983). Addition of FR throughout a long photoperiod (24 h) completely overcomes the delay in flowering caused by short days (12 h) in the wild type, allowing it to flower as early as BMDR-1 (Principe et al., 1992). Wild-type plants grown under short photoperiods (12 h) with supplemental FR flower as early as plants grown in long days (24 h) without FR. However, neither flower as early as BMDR-1, which flowers at the same time in all photoperiods with or without supplemental FR.
Phytochrome is a proteinaceous pigment that acts through photointerconversion between an inactive R-absorbing Pr form and a physiologically active FR-absorbing Pfr form (Smith and Whitelam, 1990). Arabidopsis expresses five phytochrome (PHY) genes, PHYA through PHYE (Sharrock and Quail, 1989; Clack et al., 1994). It is well established that the gene product of PHYA (phyA) is necessary for responses mediated by continuous FR (Nagatani et al., 1993; Parks and Quail, 1993) and that phyB is necessary for responses mediated by continuous R in etiolated seedlings (Lopez-Juez et al., 1992; Reed et al., 1993). Seed germination is mediated by phyA under limiting light conditions and by phyB under natural light conditions (Johnson et al., 1994; Shinomura et al., 1994). It has been reported that phyA is involved in daylength perception (Weller et al., 1997) and that phyB is required for enhanced FR level perception in the shade-avoidance and EOD promotion of internode elongation in light-grown plants (Smith and Whitelam, 1990; Johnson et al., 1994; Reed et al., 1994). However, phyB and at least one other species of phytochrome have also been implicated in the detection of photoperiod and the EOD promotion of flowering by FR (Halliday et al., 1994).
Recently, Biyashev et al. (1997) have mapped five phytochrome loci in barley, which were arbitrarily called phy-1, phy-2, phy-3 on chromosomes 7, 4, and 5, respectively, and phy-4, which is represented as duplicate loci on chromosomes 2 and 7. It is unknown which phytochrome genes these encode or whether mutations at any of these loci affect flowering. However, phy-3 maps to the same chromosome as eak (Ppd-H2), which may be allelic to BMDR-1.
Principe et al. (1992) found BMDR-1 to contain about twice the amount of total phytochrome and to differ from BMDR-8 with respect to two other proteins of 26 and 85 kD. However, these two proteins, which are always absent in the mutant genotype, do not decrease in the wild type in response to FR. The increased level of spectrophotometrically detectable total phytochrome was found to be largely accounted for by an increase in phyA (Principe et al., 1992), but nothing was known about the regulation of phyB levels. The hy3 mutant in Arabidopsis (Reed et al., 1993), the ma3R mutant in sorghum (Childs et al., 1992), the ein mutant in Brassica rapa (Devlin et al., 1992), and the lh mutant in cucumber (Lopez-Juez et al., 1992) all flower early and lack a light-stable phyB protein. In Arabidopsis and sorghum it has been shown that the mutation is located in the PHYB gene (Reed et al., 1993; Childs et al., 1997). The goal of our study is to establish the nature of the mutation in BMDR-1 and to determine whether the failure to respond to photoperiod might also be due to a lack of a functional phyB.
MATERIALS AND METHODS
Plant Material
Seeds of barley (Hordeum vulgare L. cv Shabet [BMDR-8]) and the BMDR-1 mutant were obtained as 2 of 10 isogenic lines from a breeding program by Dr. Virgil Smail at the Montana State University (Bozeman) and were maintained by selfing in the greenhouses at the University of Maryland (College Park).
Growth Conditions
Seeds were surface-sterilized by soaking in a 20% solution of commercial bleach (2.5% sodium hypochlorite) for 20 min and washing thoroughly in sterile deionized water. Seeds used for deetiolation measurements were sown at a density of 25 seeds on wet tissue paper (Kimpack) in individual plastic trays before being placed into light-tight aluminum boxes. Seeds used for the flowering experiments were sown at a density of 25 seeds in individual 1-quart plastic freezer boxes filled with coarse vermiculite. They were then subirrigated in plastic trays that held 12 boxes each with full-strength Hoagland no. 1 solution. All of these seeds were then placed in a growth chamber in DD at 20°C ± 0.1°C for at least 4 d to ensure uniform germination before transfer to various light treatments. After 4 d of DD, seedlings were either allowed to remain in DD or transferred to a chamber with either continuous R, continuous FR, or 12-h CW photoperiods with or without supplemental FR. All conditions were maintained at a constant temperature of 20°C ± 0.1°C and 60% ± 5% RH.
CW was supplied by F48T12/CW/VHO fluorescent lamps (GTE-Sylvania, Danver, MA) and the supplemental FR was provided by F48T12/232/VHO single phosphor fluorescent lamps (GTE-Sylvania). The photosynthetic photon flux (400–700 nm) was maintained at 150 μmol m−2 s−1 under all 12-h photoperiods, both with and without supplemental FR, and monitored using a quantum sensor (model L-190SB, Li-Cor, Lincoln, NE). The R was obtained from GTE-Sylvania F48T12/236/VHO lamps filtered through two layers of Roscolux no. 823 red cellophane (Kliegle Bros., New York). FR was produced from F48T12/232/VHO lamps (GTE-Sylvania) filtered through a one-eighth-inch-thick F-700 black Plexiglas filter (Westlake Plastics, Lenni Mills, PA). The levels of both were set at 18 μmol m−2 s−1 and the spectral distribution of these fields was monitored using a spectroradiometer (model Gamma C3, Gamma Scientific, San Diego, CA).
Coleoptile Length and Leaf Opening
The box containing the individual plastic trays with 25 seeds each was covered with a clear plastic wrap, placed in a growth chamber, and covered with black cloth for 4 d. At the end of this germination period, seedlings were exposed to an additional 3 d of continuous CW, R, or FR, or allowed to remain in DD. Seedlings were removed 3 d after transfer to light and coleoptile lengths were measured in millimeters with a ruler from the point of attachment of the scutellum to the tip. Seedling height was measured in millimeters from the attachment of the scutellum to the tip of the primary leaf. Leaf opening was measured as the width of the blade in millimeters at the broadest point of the leaf.
EOD FR Treatment
At the end of the 4-d DD germination period, the trays containing the boxes with 25 seedlings each were placed in a growth chamber set to 12 h of CW and 12 h of darkness. At the end of each photoperiod, plants were given a 10-min pulse of R, FR, R followed immediately by FR, or FR followed immediately by R. Following treatment for 18 d, plants were moved to a cold room (4°C) and kept in darkness until measurement. Plants were gently removed from the vermiculite and the roots and remaining scutellum discarded. We determined the average fresh weight of the shoots and selected 10 plants that were within ±10% of the mean fresh weight. These were then dissected under a dissecting microscope to determine the stage of floral development, the length of the inflorescence, and the number of tillers formed. We repeated all experiments twice and analyzed the variance data using the SAS system, version 6.12 (SAS Institute, Cary, NC).
Protein Extraction and Immunoblot Analysis
Seeds sown for the coleoptile measurements were either left in DD or exposed to an additional 4 d of continuous CW. On the 4th d, 2 g of coleoptile tips or leaves was harvested, frozen immediately, and stored in liquid nitrogen at −80°C. The frozen samples were then extracted according to the method of Wang et al. (1992), using boiling SDS buffer. They were homogenized with 4 mL of hot buffer (125 mm Tris-HCl, 4% [w/v] SDS, 10% [v/v] 2-mercaptoethanol, and 20% [v/v] glycerol, pH 6.8). The homogenate was transferred to a 30-mL centrifuge tube, heated in a boiling water bath for 5 min, and centrifuged at 16,000g for 10 min. The supernatant was recovered, clarified at 16,000g for 10 min, and stored in aliquots at −80°C. We determined the protein content by the Bradford reaction, using BSA as a standard.
SDS-PAGE was performed as described by Pratt et al. (1986). Prestained, high-range Mr standards were obtained from Bio-Rad. The 7.5% gels were transblotted to nitrocellulose according to the manufacturer's instructions and stained, again according to the method of Pratt et al. (1986), except that we used 5-bromo-4-chloro-3-indolyl phosphate/nitroblue tetrazolium as the color substrate. The whole procedure was repeated twice with each sample and MAb. The blots were air dried and scanned into the Adobe PhotoShop 3.05 software program using a color scanner (model CJ 10, Canon, Japan).
Pea 25 (P-25) is a MAb directed against etiolated pea phytochrome that recognizes a highly conserved epitope (Cordonnier et al., 1986) and therefore detects total phytochrome, but does not distinguish between multiple species. Oat (Avena sativa L.) 22 (O-22) is a MAb directed against etiolated oat phytochrome and it specifically recognizes the 124-kD phytochrome apoprotein of phyA (Cordonnier et al., 1983). GO-5 and GO-7 are specific for a 125- and a 123-kD phytochrome molecular species, respectively, and were developed against green oat phytochrome (Pratt et al., 1991). Each lane in the figures represents a separate extract from replicate samples. All MAbs and the nonimmune mouse IgG used as a control were applied at a 1.0 μg mL−1 dilution. Alkaline phosphatase conjugated to an anti-mouse secondary antibody (Sigma) was applied at a 0.2 μg mL−1 dilution.
RESULTS
Deetiolation Responses
Because both phytochromes A and B have been reported to play a role in the photoperiodic induction of flowering (Reed et al., 1993; Childs et al., 1997; Weller et al., 1997), we examined a number of de-etiolation responses in both the mutant and wild-type barley that are known to be mediated by one or the other of these phytochrome species in other plants. Hypocotyl elongation in dicots and coleoptile elongation in monocots is strongly inhibited when etiolated seedlings are exposed to either continuous R, which is mediated primarily by phyB, or to continuous FR, which is mediated by phyA. Therefore, dark-grown plants were allowed to remain in DD or transferred to continuous R, FR, or CW for 3 d (Table I). Coleoptile lengths were not significantly different in BMDR-1 and BMDR-8 after 7 d of growth in the dark, and 3 d of continuous CW inhibited elongation to the same extent in both. Continuous R and FR were less effective than CW; however, both inhibited coleoptile elongation to the same extent in both genotypes. All three light treatments significantly inhibited elongation in both genotypes, but the degree of inhibition was not significantly different in the mutant when compared with the wild type.
Table I.
Responses of etiolated seedlings to 3 d of DD, CW, continuous R (Rc), or continuous FR (FRc)
| Treatment | Coleoptile
Length
|
Seedling Height
|
Leaf Width
|
|||
|---|---|---|---|---|---|---|
| BMDR-1 | BMDR-8 | BMDR-1 | BMDR-8 | BMDR-1 | BMDR-8 | |
| mm | ||||||
| DD | 62.5 ± 3.5 | 60.1 ± 4.7 | 89.0 ± 7.1 | 84.7 ± 9.0 | 1.3 ± 0.1 | 1.1 ± 0.0 |
| CW | 25.2 ± 1.4a | 23.2 ± 1.1a | 82.0 ± 4.8 | 84.0 ± 5.6 | 5.0 ± 0.1b | 4.6 ± 0.1b |
| Rc | 36.6 ± 1.2a | 36.2 ± 3.3a | 96.0 ± 5.3 | 74.1 ± 7.7 | 2.5 ± 0.1b | 2.7 ± 0.1b |
| FRc | 44.8 ± 1.6a | 43.9 ± 3.0a | 107 ± 7.4 | 85.0 ± 10.7 | 1.6 ± 0.1 | 1.8 ± 0.1 |
Seeds were sown and allowed to germinate in the dark for 4 d in a growth chamber at 20°C. Seedlings were then exposed to an additional 3 d of continuous CW, R, FR, or allowed to remain in DD. Coleoptile lengths were measured from the point of attachment of the scutellum to the tip. Seedling height was measured from the attachment of the scutellum to the tip of the primary leaf. Leaf opening was measured as the width of the blade in mm at the broadest point of the leaf. Significance was established by analysis of variance.
Significant at P = 0.01.
Significant at P = 0.05.
Mesocotyl elongation is also affected by R and FR, but in a manner opposite to that of the coleoptile. However, unlike wheat, oats, and sorghum, the mesocotyl in barley does not elongate, and so we examined the effect of light on leaf blade elongation. We found no significant effect of 3 d of CW, R, or FR in either the mutant or the wild type when compared with those left in the dark (Table I, seedling height). This was true even when seedlings were exposed to 6 d of light after germination (data not shown). Although BMDR-1 appeared to be taller than BMDR-8 when grown under continuous R and FR, neither was significantly taller than the dark controls or CW-grown plants, which did not differ significantly between the genotypes. On the other hand, leaf opening was significantly affected by exposure to continuous CW, R, and FR (Table I, leaf width). After 7 d of growth in DD, the leaves of both BMDR-1 and BMDR-8 remained very tightly rolled, whereas growth for 3 d in CW caused these leaves to unroll completely in both genotypes. Continuous R was much less effective in both; however, leaf opening was significantly greater than in the dark controls in both. There was no significant effect of 3 d of continuous FR on either genotype.
Flowering Responses
There was no enhancement of flowering by a 10-min EOD pulse of FR in either BMDR-1 or BMDR-8, whether measured as floral stage or as apex length (Table II). Plants flowered at the same time regardless of the treatment given. However, the difference in flowering time between the genotypes is very significant under the given 12-h photoperiods. BMDR-1 is a very-early flowering genotype and was already in floral stage 4 by d 18, whereas BMDR-8 was still at stage 2 (Principe et al., 1992). Although flowering was not affected by these EOD treatments, the number of tillers formed was significantly affected (Table II). The reduction in the number of tillers following a 10-min pulse of FR was the same in both the mutant and the wild type and was completely red-light/FR reversible.
Table II.
Responses of barley plants grown for 19 d under 12 h photoperiods to 10 min of EOD R, FR, R followed by FR (R/FR), or FR followed by R (FR/R) light
| Treatment | Floral Stage
|
Apex
Length
|
No. of Tillers
|
Dry Wt
|
||||
|---|---|---|---|---|---|---|---|---|
| BMDR-1 | BMDR-8 | BMDR-1 | BMDR-8 | BMDR-1 | BMDR-8 | BMDR-1 | BMDR-8 | |
| mm | g | |||||||
| Control | 3.9 ± 0.5a | 2.0 ± 0.0b | 12.9 ± 3.5a | 1.8 ± 0.1b | 1.3 ± 0.1a | 1.3 ± 0.1a | 0.175a | 0.167a |
| R | 3.8 ± 0.4a | 1.9 ± 0.1b | 13.6 ± 2.5a | 1.5 ± 0.1b | 0.9 ± 0.1a | 1.0 ± 0.1a | 0.134a | 0.149a |
| FR | 4.1 ± 0.4a | 2.0 ± 0.0b | 11.1 ± 2.1a | 1.7 ± 0.1b | 0.4 ± 0.1b | 0.4 ± 0.1b | 0.166a | 0.134a |
| R/FR | 4.0 ± 0.5a | 2.0 ± 0.1b | 8.1 ± 1.9a | 1.6 ± 0.1b | 0.2 ± 0.1b | 0.4 ± 0.1b | 0.146a | 0.150a |
| FR/R | 3.6 ± 0.5a | 2.0 ± 0.1b | 9.5 ± 2.5a | 1.6 ± 0.1b | 0.9 ± 0.1a | 0.7 ± 0.1ab | 0.166a | 0.153a |
Seeds were sown in plastic trays and subirrigated with full-strength Hoagland solution. All seeds were placed in a growth chamber in DD at 20°C ± 0.1°C for 4 d prior to transfer to a growth chamber set to 12 h of CW and 12 h of dark. At the end of each photoperiod plants were given a 10-min pulse of R, FR, R followed immediately by FR, or FR followed immediately by R. Following treatment for 18 d, the average fresh weight of the shoots was determined, and 10 plants that were within ±10% of the mean fresh weight were selected. These were then dissected under a dissecting microscope to determine the stage of floral development, the length of the inflorescence, and the number of tillers formed. Significance was established by analysis of variance. Different letters indicate a significant difference at P = 0.05.
Phytochrome Analysis
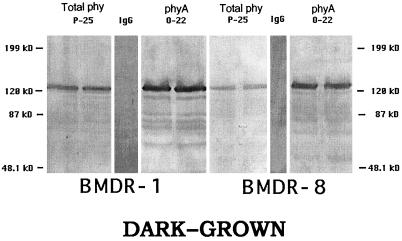
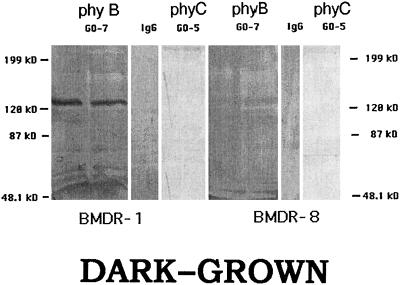
The 124-kD species of phytochrome in oat, which is detected by O-22, is known to be a phyA (Cordonnier et al., 1983, 1986), whereas the 123-kD species, which is detected by GO-7, is now thought to be a phyB (Childs et al., 1997). The 125-kD species, to which GO-5 binds specifically (Wang et al., 1992), and which was thought initially not to be either a phyA, phyB, or phyC (Pratt et al., 1991), seems likely to be a phyC (R. Wikle and M.-M. Cordonnier-Pratt, unpublished data). BMDR-1 has an elevated level of total phytochrome, as detected by P-25 (Fig. 1), and an elevated level of phyA, as detected by O-22 on western blots. Figure 2 shows that BMDR-1 also contains about twice as much phyB, as detected by GO-7, when compared with BMDR-8, but no band was detected by GO-5 in either the mutant or the wild type. Positive controls with extracts of oat tissue showed normal binding to GO-5, indicating that epitope seen by GO-5 is missing from barley.
Figure 1.
Western blots of 4-d-old etiolated shoots of BMDR-1 (left) and BMDR-8 (right) immunostained with the MAbs Pea-25 and O-22. Each lane was loaded with 105 μg (P-25) or 121 μg (O-22) of total protein and each represents a separate extract from replicate samples. IgG, Nonimmune mouse IgG used as the control. Molecular masses of the protein standards are indicated. The original blots were digitized at 400 DPI using a Canon CJ-10 color scanner, labeled with Adobe PhotoShop 4.0, and printed on a Hewlett-Packard LaserJet 4 MV printer. phy, Phytochrome.
Figure 2.
Western blots of 4-d-old-etiolated shoots of BMDR-1 (left) and BMDR-8 (right) probed with the MAbs GO-7 and GO-5. Each lane was loaded with 190 μg of total protein and each represents a separate extract from replicate samples. IgG, Nonimmune mouse IgG used as the control. Molecular masses of the protein standards are indicated. The original blots were digitized as in the Figure 1 legend.
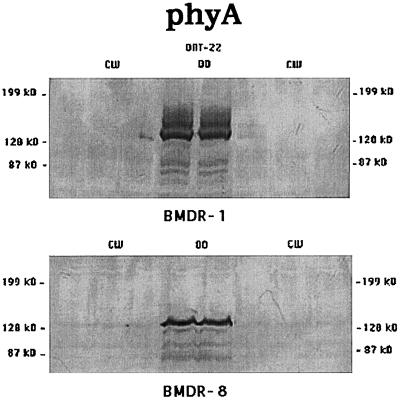
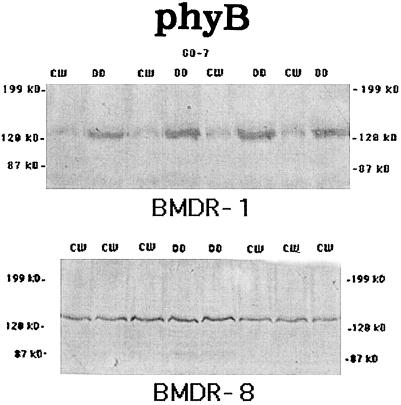
PhyA, although present at high levels in 4-d-old etiolated seedlings, became undetectable in plant extracts that were exposed to 4 d of continuous CW in both BMDR-1 and BMDR-8 (Fig. 3). PhyB, which is a light stable as Pfr in all systems so far examined, was present at equal levels in both the light and the dark in BMDR-8 (Fig. 4, bottom). However, the level of phyB in BMDR-1 (Fig. 4, top) was decreased to barely detectable levels in light-grown plant extracts when compared with extracts from etiolated shoots.
Figure 3.
Western blots of 4-d-old-etiolated shoots or those exposed to 4 d of continuous CW of BMDR-1 (top) or BMDR-8 (bottom) immunostained with O-22. Each lane was loaded with 150 μg of total protein extracted from 4-day-old-etiolated (lanes DD) or deetiolated (lanes CW) shoots, and each lane represents a separate extract from replicate samples. The original blots were digitized as in the Figure 1 legend.
Figure 4.
Western blots of 4-d–old-etiolated shoots or those exposed to 4 d of continuous CW of BMDR-1 (top) or BMDR-8 (bottom) immunoanalyzed with GO-7. Each lane was loaded with 150 μg of total protein extracted from 4-d-old-etiolated (DD) or deetiolated (CW) shoots, and each lane represents a separate extract from replicate samples. The original blots were digitized as in the Figure 1 legend.
DISCUSSION
The early flowering phenotype of BMDR-1 is similar to that in mutants of other systems that lack a functional phyB. It was our hypothesis that BMDR-1 flowered early, regardless of photoperiod, because it also lacked a functional phyB. However, we found that the mutant contained a higher level of phyB than the wild type in the dark (Fig. 2). Levels of different phytochromes in etiolated tissue may reflect the levels present in seeds, which is important for germination (Johnson et al., 1994; Reed et al., 1994; Shinomura et al., 1994). However, it is the level of phytochrome in green tissue that regulates further growth and development. Figure 3 shows that phyA in barley is a typical light-labile species in both BMDR-1 and BMDR-8. PhyB is also expressed normally in the wild type and is a typical light-stable form of phytochrome (Fig. 4, bottom). However, phyB is significantly reduced in BMDR-1 in the light (Fig. 4, top). This may be the consequence of a destabilization of the Pfr form of the phyB protein, resulting in the phyB of BMDR-1 behaving like a phyA in the light. It is unknown whether the apparent destabilization of the phyB protein is a consequence of a mutation in the PHYB gene in BMDR-1 or results from a reduced rate of transcription and/or a decrease in message stability in the light.
Recently, the Ma3 maturity gene in sorghum has been mapped to the PHYB locus, and a premature stop codon has been identified in the PHYB sequence from the mutant ma3R (Childs et al., 1997). This mutant is also insensitive to photoperiod (Childs et al., 1992), flowering very early in a manner similar to that of BMDR-1. This result strongly suggests that phyB is involved in the detection of photoperiod. Therefore, it seems very likely that the instability of phyB in BMDR-1 could be the reason for its lack of photoperiod sensitivity. A similar mapping strategy is currently underway in barley.
PhyA has also been reported to be involved in daylength perception in Arabidopsis (Johnson et al., 1994; Reed et al., 1994) and in peas (Weller et al., 1997). The phyA mutant fhy1 was deficient in sensing an inductive photoperiod, but the phyB mutant hy3, which flowered earlier than both fhy1 and the wild type in all photoperiods tested, still responded to an inductive photoperiod. Although fhy1 seedlings flower at the same time as the wild type under both short and long days, the phyA mutant flowers late under 8-h photoperiods extended by 8 h with dim incandescent light, indicating that phyA is also involved in sensing the photoperiod and that phyA and phyB may have complementary functions in controlling flowering (Johnson et al., 1994). Using phyA and phyB overexpressing transgenic Arabidopsis plants, Bagnall et al. (1995) reported early flowering in the phyA overexpressor in all daylengths, approaching day neutrality. They found that, although phyB mutants lacked the EOD regulation of hypocotyl elongation, they showed normal R/FR reversible EOD regulation of flowering. On the other hand, the phyB overexpressor showed promotion of flowering following EOD-R treatment and inhibition of flowering after an EOD-FR treatment. This is the reverse of what was found for the wild type. They concluded that both light-labile (phyA) and light-stable (phyB) phytochromes interact, and that phytochrome species other than phyA and phyB (physC, D, or E) may also be involved in this regulation.
Although both phyA and phyB are expressed at higher levels in dark-grown mutant plants when compared with the wild type (Figs. 1 and 2), and both decline in the mutant in the light (Figs. 3 and 4), residual levels of phyA may remain higher in BMDR-1, leading to enhanced sensitivity to photoperiod. ELISA measurements following growth for 7 d in the light (Principe et al., 1992) had measurable levels of phyA, which were significantly higher in BMDR-1 than in BMDR-8. In addition, the decrease in phyB levels in the light in BMDR-1 may decrease the levels of a floral inhibitor, which, together with the increase in phyA, would result in complete day neutrality. However, Weller et al. (1997) reported that the phyA-mediated promotion of flowering in peas resulted from a reduction in the synthesis or transport of a floral inhibitor, suggesting that it acts alone to regulate flowering. The fact that BMDR-1 is the only mutation that completely lacks any response to both photoperiod and FR suggests that both phyA and phyB are required for the normal regulation of flowering. It further suggests that they act through distinct, but interrelated pathways.
A marked acceleration of flowering has been noted in Arabidopsis plants in response to a low R-to-FR ratio and even the early flowering hy3 mutant shows a slight acceleration of flowering (Whitelam and Smith, 1991; Robson et al., 1993). Using mutants lacking either phyA or phyB or the double homozygous recessive hy3/hy2, Halliday et al. (1994) and Bagnall et al. (1995) have shown that phyB and at least one other phytochrome species are involved in this response. This accelerated flowering in response to an EOD-FR treatment has also been noted in sorghum (Williams and Morgan, 1979). The product of a gene other than PHYB appears to be required for the perception of the EOD-FR promotion of flowering, which is absent in both the mutant and wild-type barley (Table II). The fact that the EOD-FR inhibition of tillering (Table II) is normal in both the mutant and the wild type indicates that the promotion of flowering and the inhibition of tillering are mediated by distinct phytochrome species operating through separate pathways.
Growth responses, such as the inhibition of coleoptile elongation, seedling height, and the promotion of leaf opening, did not show any significant difference in either BMDR-1 or BMDR-8 under different light conditions (Table I). Although the seedling height was somewhat greater in BMDR-1 than in BMDR-8 in continuous R and FR, neither was significantly different from those in DD and CW. This increase in height was due to elongation of the leaf blade, and not to the elongation of the mesocotyl, as in other grasses such as oats. The mesocotyl in barley did not elongate and there appeared to be no effect on this leaf lade elongation in either genotype. However, there was a slight (but insignificant) increase in BMDR-1, which may reflect the increased phytochrome levels in BMDR-1 relative to BMDR-8. The phyB null mutants in other plant species displayed an elongated growth pattern, had reduced chlorophyll content, and lacked a deetiolation response to high-irradiance R (Childs et al., 1991, 1992; Devlin et al., 1992; Lopez-Juez et al., 1992; Reed et al., 1993). BMDR-1 did not lack a deetiolation response to high-irradiance R and it was not visibly less green. It did have slightly narrower leaves than the wild type, as is the case with the sorghum mutant (Pao and Morgan, 1986). Because the levels of phyB decreased in light-grown BMDR-1, and the response to continuous R was the same or slightly greater than in the wild type, either the enhanced level of phyA was able to compensate for the loss of phyB, or the residual level of phyB was sufficient to mediate this response.
ACKNOWLEDGMENTS
We wish to thank Dr. Harry Swartz for the generous use of his laboratory facilities and Mr. Cen Yiqun and Ms. Jin Ma for their helpful assistance.
Abbreviations:
- BMDR
barley maturity daylength response
- CW
cool-white fluorescent light
- DD
continuous darkness
- EOD
end-of-day
- FR
far-red light
- MAb
monoclonal antibody
- R
red light
Footnotes
This work was supported in part by the Maryland Agricultural Experiment Station (project no. MD-L-97) to G.F.D. and by the U.S. Department of Agriculture National Research Initiative Competitive Grants Program (no. 93-00939) to L.H.P. and M.-M.C.-P.
LITERATURE CITED
- Bagnall DJ, King RW, Whitelam GC, Boylan MT, Wagner D, Quail PH. Flowering responses of phytochrome overexpressors and mutants. Plant Physiol. 1995;108:1495–1503. doi: 10.1104/pp.108.4.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biyashev RM, Ragab RA, Maughan PJ, Saghai Maroof MA. Molecular mapping, chromosomal assignment, and genetic diversity analysis of phytochrome loci in barley (Hordeum vulgare) J Hered. 1997;88:21–26. [Google Scholar]
- Childs KL, Cordonnier-Pratt M-M, Pratt LH, Morgan PW. Genetic regulation of development in Sorghum bicolor. VII. ma3R flowering mutant lacks a phytochrome that predominates in green tissue. Plant Physiol. 1992;99:765–770. doi: 10.1104/pp.99.2.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childs KL, Miller FR, Cordonnier-Pratt M-M, Pratt LH, Morgan PW, Mullet JE. The sorghum photoperiod sensitivity gene, Ma3, encodes a phytochrome B. Plant Physiol. 1997;113:611–619. doi: 10.1104/pp.113.2.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childs KL, Pratt LH, Morgan PW. Genetic regulation of development in Sorghum bicolor. VI. The ma3R allele results in abnormal phytochrome physiology. Plant Physiol. 1991;97:714–719. doi: 10.1104/pp.97.2.714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clack T, Mathews S, Sharrock RA. The phytochrome apoprotein family in Arabidopsis is encoded by five genes: the sequences and expression of PHYD and PHYE. Plant Mol Biol. 1994;25:413–427. doi: 10.1007/BF00043870. [DOI] [PubMed] [Google Scholar]
- Cordonnier M-M, Greppin H, Pratt LH. Phytochrome from green Avena shoots characterized with a monoclonal antibody to phytochrome from etiolated Pisum shoots. Biochemistry. 1986;25:7657–7666. [Google Scholar]
- Cordonnier M-M, Smith C, Greppin H, Pratt LH (1983) Production and purification of monoclonal antibodies to Pisum & Avena phytochrome. Planta 158 369–376 [DOI] [PubMed]
- Deitzer GF (1983) Effect of far-red energy on the photoperiodic control of flowering in Wintex barley (Hordeum vulgare L.). In W Meudt, ed, Strategies of Plant Reproduction. Allenheld-Osmun, Totowa, NJ, pp 99–116
- Deitzer GF. Photoperiodic processes: induction, translocation and initiation. In: Atherton JG, editor. Manipulation of Flowering. London: Butterworths; 1986. pp. 241–253. [Google Scholar]
- Deitzer GF, Hayes R, Jabben M. Kinetics and time dependence of the effect of far-red light on the photoperiodic induction of flowering in Wintex barley. Plant Physiol. 1979;64:1015–1021. doi: 10.1104/pp.64.6.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devlin PF, Rood SB, Somers DE, Quail PH, Whitelam GC. Photophysiology of the elongated internode (ein) mutant of Brassica rapa. The ein mutant lacks a detectable phytochrome B-like polypeptide. Plant Physiol. 1992;100:1442–1447. doi: 10.1104/pp.100.3.1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher LW, Belhardi M, Zahour A. Interrelationships among three major loci controlling heading date of spring barley when grown under short daylength. Crop Sci. 1987;27:155–160. [Google Scholar]
- Gallagher LW, Soliman KM, Vivar H. Interactions among loci conferring photoperiod insensitivity for heading time in spring barley. Crop Sci. 1991;31:256–261. [Google Scholar]
- Halliday KJ, Koornneef M, Whitelam GC. Phytochrome B and at least one other phytochrome mediated the accelerated flowering response of Arabidopsis thaliana L. to low red/far-red ratio. Plant Physiol. 1994;104:1311–1315. doi: 10.1104/pp.104.4.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson E, Bradley M, Harberd NP, Whitelam GC. Photoresponses of light-grown PHYA mutants of Arabidopsis: phytochrome A is required for the perception of daylength extensions. Plant Physiol. 1994;105:141–149. doi: 10.1104/pp.105.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurie DA, Pratchett N, Bezant JH, Snape JW. RFLP mapping of five major genes and eight quantitative trait loci controlling flowering time in a winter × spring barley (Hordeum vulgare L.) cross. Genome. 1995;38:575–585. doi: 10.1139/g95-074. [DOI] [PubMed] [Google Scholar]
- Law CN, Dean C, Coupland G (1993) Genes controlling flowering and strategies for their isolation and characterization. In BR Jordan, ed, The Molecular Biology of Flowering. CAB International, Oxford, UK, pp 47–68
- Lopez-Juez E, Nagatani A, Tomizawa K-I, Deak M, Kern R, Kendrick KE, Furuya M. The cucumber long hypocotyl mutant lacks a light-stable PHYB-like phytochrome. Plant Cell. 1992;4:241–251. [PMC free article] [PubMed] [Google Scholar]
- Nagatani A, Reed JW, Chory J. Isolation and initial characterization of Arabidopsis mutants that are deficient in phytochrome A. Plant Physiol. 1993;102:269–277. doi: 10.1104/pp.102.1.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pao CI, Morgan PW. Genetic regulation of development in Sorghum bicolor. II. Effect of the ma3R allele mimicked by GA3. Plant Physiol. 1986b;82:581–584. doi: 10.1104/pp.82.2.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks BM, Quail PH. hy8, a new class of Arabidopsis long hypocotyl mutants deficient in functional phytochrome A. Plant Cell. 1993;5:39–48. doi: 10.1105/tpc.5.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt LH, McCurdy DW, Shimazaki Y, Cordonnier M-M. Immunodetection of phytochrome: immunocytochemistry, immunoblotting, and immunoquantitation. In: Linskens HF, Jackson JF, editors. Modern Methods of Plant Analysis, Vol 4. New York: Springer-Verlag; 1986. pp. 50–74. [Google Scholar]
- Pratt LH, Stewart SJ, Shimazaki Y, Wang Y-C, Cordonnier M-M. Monoclonal antibodies directed to phytochrome from green leaves of Avena sativa L. cross react weakly or not at all with the phytochrome that is most abundant in etiolated shoots of the same species. Planta. 1991;184:87–95. doi: 10.1007/BF00208241. [DOI] [PubMed] [Google Scholar]
- Principe JM, Hruschka WR, Thomas B, Deitzer GF. Protein differences between two isogenic cultivars of barley (Hordeum vulgare L.) that differ in sensitivity to photoperiod and far-red light. Plant Physiol. 1992;98:1444–1450. doi: 10.1104/pp.98.4.1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed JW, Nagatani A, Elich TD, Fagan M, Chory J. Phytochrome A and phytochrome B have overlapping but distinct functions in Arabidopsis development. Plant Physiol. 1994;104:1139–1149. doi: 10.1104/pp.104.4.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed JW, Nagpal P, Poole DS, Furuya M, Chory J. Mutations in the gene for the red/far-red light receptor phytochrome B alter cell elongation and physiological responses throughout Arabidopsis development. Plant Cell. 1993;5:147–157. doi: 10.1105/tpc.5.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robson PRH, Whitelam GC, Smith H. Selected components of the shade avoidance syndrome are displayed in a normal manner in mutants of Arabidopsis thaliana and Brassica rapa deficient in phytochrome B. Plant Physiol. 1993;102:1179–1184. doi: 10.1104/pp.102.4.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharrock RA, Quail PH. Novel phytochrome sequences in Arabidopsis thaliana: structure, evolution, and differential expression of a plant regulatory photoreceptor family. Genes Dev. 1989;3:1745–1757. doi: 10.1101/gad.3.11.1745. [DOI] [PubMed] [Google Scholar]
- Shinomura T, Nagatani A, Chory J, Furuya M. The induction of seed germination in Arabidopsis thaliana is regulated principally by phytochrome B and secondarily by phytochrome A. Plant Physiol. 1994;104:363–371. doi: 10.1104/pp.104.2.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H, Whitelam GC. Phytochrome, a family of photoreceptors with multiple physiological roles. Plant Cell Environ. 1990;13:695–707. [Google Scholar]
- Vince-Prue D. Photoperiodism in Plants. London: McGraw-Hill; 1975. [Google Scholar]
- Wang Y-C, Cordonnier-Pratt M-M, Pratt LH. Detection and quantities of three phytochromes in unimbibed seeds of Avena sativa L. Photochem Photobiol. 1992;56:709–716. [Google Scholar]
- Weller JL, Murfet IC, Reid JB. Pea mutants with reduced sensitivity to far-red light define an important role for phytochrome A in day-length detection. Plant Physiol. 1997;114:1225–1236. doi: 10.1104/pp.114.4.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitelam GC, Smith H. Retention of phytochrome-mediated shade avoidance responses in phytochrome-deficient mutants of Arabidopsis, cucumber and tomato. J Plant Physiol. 1991;139:119–125. [Google Scholar]
- Williams EA, Morgan PW. Floral initiation in sorghum hastened by gibberellic acid and far-red light. Planta. 1979;145:269–272. doi: 10.1007/BF00454451. [DOI] [PubMed] [Google Scholar]
- Yasuda S, Hyashi J. Linkage and effect of the earliness gene each involved in Chinese cultivars on yield components in barley. Barlet Genet Newsl. 1980;10:74–76. [Google Scholar]