The relative contribution of corneal SP in regulating the development of severe HSK lesions in a mouse model is demonstrated.
Abstract
Purpose.
To determine whether substance P (SP) in herpes simplex virus-1 (HSV-1) infected cornea regulates the severity of herpetic stromal keratitis (HSK) lesions in a mouse model.
Methods.
C57BL/6 mice were infected ocularly with HSV-1 (RE). The corneas with HSK lesions, on Day 15 postinfection, were grouped on the basis of the corneal opacity as mild (≤2) or severe (>2). The amount of SP was determined in the corneas with mild or severe HSK lesions by enzyme immunosorbent assay (EIA) and confocal microscopy. Subconjunctival inoculation of spantide I, SP receptor antagonist, was carried out during the clinical phase of HSK. ELISA and flow cytometry were used to determine the level of cytokines, chemokines, and influx of immune cell types in the corneal lesions.
Results.
The authors determined a significantly higher level of SP in the corneas with severe HSK lesions in comparison with mild lesions. The corneas with a higher level of SP also exhibited higher amounts of proinflammatory cytokines (IL-6, IFN-γ) and chemokines (CCL3, CXCL2) when compared with the corneas with a lower level of SP. SP receptor NK1R expression was determined in CD45− and CD45+ cells in infected cornea. SP present in the corneal stroma of the eyes with severe HSK lesions colocalized with β-III tubulin+ and IAb+ cell types. Subconjunctival inoculation of spantide I during the clinical phase of HSK resulted in significant reduction in the corneal opacity and angiogenesis.
Conclusions.
Collectively, these results demonstrate the relative contribution of substance P in regulating the clinical severity of HSK lesions in a mouse model.
The corneal infection with herpes simplex virus (HSV)-1 results in inflammation that can persist long after the clearance of the infectious virus. Studies carried out in BALB/c and C57BL/6 mice models have played an important role in determining the pathogenesis of herpetic stromal keratitis (HSK).1,2 HSK in mice models can be categorized into preclinical (Day 0 to 7 postinfection) and clinical phase (Day 7 to 18 postinfection).3 During the preclinical phase, replicating virus is determined in the infected cornea but the damage to the corneal tissue is not evident. On the other hand, the corneal opacity and severe angiogenesis are noted during the clinical phase of disease but intriguingly no replicating virus is determined during this time period. It is reported that dendritic cells, neutrophils, and CD4 T cells are key immune cell types present in the inflamed cornea during the clinical phase of disease and play an important role in the development of severe HSK lesions.2,4,5 However, the molecular mechanisms that regulate chronic inflammation during the clinical phase of HSK are not well understood.
Neuropeptides are well reported to either promote or resolve the inflammation.6,7 Substance P (SP), an eleven amino acid long proinflammatory neuropeptide, is derived from preprotachykinin A (PPTA) protein and is secreted by both neuronal and nonneuronal cell types.8–10 SP can bind to neurokinin (NK1, NK2, and NK3) receptor with highest affinity toward NK1R.11,12 Moreover, SP promotes inflammation by exerting its action on both immune and nonimmune cell types.13,14 Previous studies demonstrated the presence of SP+ nerve termini in the naive cornea by immunohistochemistry and radioimmunoassay.15,16 However, the role of SP in the pathogenesis of HSK is not known.
In the present study, we investigated whether SP in HSV-1–infected cornea defines the severity of HSK lesions in a mouse model. After ocular HSV-1 infection, the eyes of C57BL/6 mice develop mild and severe HSK lesions as determined by the extent of neovascularization and the opacity of the inflamed cornea. While comparing the levels of SP in the infected corneas by enzyme immunosorbent assay (EIA), our results showed approximately a 15-fold higher amount of SP in the corneas with severe HSK lesions in comparison with those with mild HSK lesions. In addition, the corneas with a higher amount of substance P also had significantly higher levels of proinflammatory cytokines (IL-6 and IFN-γ) and chemokines (CCL3 and CXCL2) in comparison with the corneas with mild HSK. SP was present in the corneal stroma during the clinical phase of HSK and colocalized with β-III tubulin+ and IAb+ cell types. Subconjunctival inoculation of spantide I, SP receptor antagonist, during the clinical phase of disease significantly reduced the development of corneal opacity and angiogenesis. Spantide I treatment resulted in lesser influx of neutrophils and CD4 T cells as well as a decreased amount of IL-6 and CCL3 proteins in the cornea. Taken together, our results show the relative contribution of corneal SP in the development of HSK lesion severity.
Methods
Mice
Female, 8- to 10-week-old C57BL/6 (B6) mice were purchased from the Jackson Laboratory (Bar Barbor, ME) and were housed in the animal facility at Oakland University. Special instruction was given to Jackson Laboratory to ensure that mice had no corneal opacity on arrival. Animals were sex- and age-matched for all experiments. All manipulations were performed in a biosafety cabinet. All experimental procedures were in complete agreement with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. In addition, all procedures were carried out in accordance with the rules and regulations of The Institutional Animal Care and Use Committee (IACUC) of the Oakland University.
Virus
HSV-1 (RE) used in the present study was obtained from Robert Hendricks' laboratory at University of Pittsburgh School of Medicine, Pittsburgh, PA. The virus was propagated on a monolayer of Vero cells (CCL81; American Type Culture Collection, Manassas, VA) as described previously.17 Pellets of infected Vero cells were suspended in 2 mL of PBS followed by three cycles of rapid freeze thaw with liquid nitrogen. Virus purified from the supernatant was titrated on Vero cells and stored in aliquots at −80°C until used.
Corneal HSV-1 Infection
To carry out ocular HSV-1 infection, mice were first anesthetized by intraperitoneal injection of avertin (Sigma-Aldrich Co., St. Louis, MO) at a dose of 250 mg/kg in 0.25 mL PBS. The cornea was scarified with trephine (Fine Science Tools, Foster city, CA) while twisting three to four times over the corneal surface. The 1 × 106 or 5 × 106 plaques forming unit (p.f.u.) of HSV-1 virus was then applied to the eye in 3 μL of phosphate buffered solution (PBS) followed by gentle massage of the eyelids.
Clinical Scoring of HSK
The eyes were examined from Day 6 to 15 postinfection, while using a hand-held slit lamp biomicroscope (Kowa, Nagoya, Japan), to determine the extent of the corneal opacity and angiogenesis. A standard scale for opacity, ranging from zero to five, was used and is as follows: 0, normal cornea; 1, mild corneal haze; 2, moderate opacity with iris visible; 3, severe opacity with iris not visible; 4, severe opacity with corneal ulcer; and 5, corneal rupture. The neovascularization (NV) of the cornea was determined by measuring the centripetal growth of newly formed blood vessels in each quadrant of the cornea as described earlier.18 Briefly, the length of blood vessels in each quadrant is graded between zero (no NV) and four (NV reached the central region of the cornea) in increments of approximately ¼ of the corneal radius. The final score from four quadrants is summed to derive the NV index (total range, 0–16) for each eye at a given time point. Corneas with an opacity score >2 and angiogenesis score >4 were considered as severe HSK lesions, whereas the corneas with an opacity score ≤2 and angiogenesis score ≤4 were categorized as mild HSK lesions.
EIA Assay to Determine the Amount of Substance P
To ascertain the amount of SP in the corneas with mild or severe HSK lesions, four infected corneas per sample were pooled on the basis of the corneal lesion severity on Day 15 postinfection in 400 μL of PBS containing protease inhibitor cocktail (Sigma-Aldrich Co.). Each sample was sonicated on ice with a 15-second pulse and an interval of 1 minute between each pulse to allow the sample to cool. The lysates were then clarified by centrifugation at 10,000 rpm for 5 minutes at 4°C. The supernatant was collected and assayed for substance P protein level using a competitive enzyme immunoassay (EIA) kit (R&D Systems, Minneapolis, MN) as per the manufacturer's instructions. While determining the kinetics of SP protein level in the corneas of HSV-1–infected eyes, four corneas were pooled at different time points postinfection irrespective of their clinical score. The samples were processed as described above to determine the amount of SP protein in the corneal lysates obtained at each time point postinfection.
Cytokines and Chemokine ELISA of the Corneal Lysates
On Day 15 post ocular HSV-1 infection, the corneas were excised from HSV-1–infected mice. The corneas were pooled on the basis of mild and severe HSK lesions. Four corneas were pooled to obtain one test sample in 300 μL of PBS containing protease inhibitor cocktail. Samples were sonicated on ice as described above. The lysates were then clarified by centrifugation at 10,000 rpm for 5 minutes at 4°C. The supernatant was collected and assayed for IL-6, IFN-γ, CCL3, and CXCL2 protein levels using ELISA kits (R&D Systems) as per the manufacturer's instructions.
Flow Cytometery
To determine the influx of neutrophils and CD4 T cells, the corneas with mild or severe HSK lesions were excised and pooled respectively on Day 15 postinfection whereas to determine the kinetics of NK1R expression, the corneas were dissected at regular intervals of time post ocular HSV-1 infection. Three corneas were always pooled to obtain one test sample and digested in 2 mg/mL of collagenase type IV (Sigma-Aldrich Co.) and 0.05 mg/mL of DNase I in RPMI only for 90 minutes at 37°C followed by trituration to form a single cell suspension. The suspension was then passed through a 40-μm cell strainer and washed in RPMI medium. Cell suspensions were incubated with anti-mouse CD16/CD32 antibody to block the Fc receptors. The following antibodies were used for cell-surface staining: FITC-conjugated anti-Gr1, anti-CD4 antibodies, and APC-conjugated anti-CD45 antibody (BD Pharmingen, San Diego, CA) in FACS buffer (PBS + 2% FBS + 0.1% sodium azide). Intracellular staining for NK1R is carried out while using a kit (BD Cytofix/Cytoperm; BD Biosciences, San Diego, CA). At the end of the staining, cells were fixed in 1% paraformaldehyde and samples were acquired on a FACS canto II flow cytometer. Data were analyzed using software (Flowjo v8.7.1; TreeStar, Ashland, OR).
Immunofluorescence
Naïve or infected eyes were enucleated and snap frozen in OCT compound. Six- or fifteen-micrometer sections were cut at −20°C and then fixed in ice-cold acetone/methanol solution (1:1 ratio) for 10 minutes at −20°C. Sections were washed in PBS followed by blocking with blocking buffer (PBS + 10% donkey serum + 3% BSA) for 2 hours at room temperature. After 2 hours, sections were washed three times in PBS followed by incubation with either fluorochrome-conjugated or unconjugated primary antibody in the dilution buffer (PBS + 1% BSA + 0.3% Triton-X-100 + 2% donkey serum) overnight at 4°C. The next day, slides were washed three times in PBS and then incubated at 37°C for 90 minutes in the PBS +1% BSA + 0.3% Triton-X-100 solution containing AlexaFluor 546 conjugated secondary antibody. After 90 minutes, sections were washed in PBS and mounted with DAPI containing mounting medium (Vector Laboratories, Burlingame, CA; Nikon Inc., Melville, NY) and visualized under a confocal microscope (Nikon C1; Nikon Inc., Melville, NY). All antibodies were diluted 1:250 in the dilution buffer (PBS + 1% BSA + 0.3% Tween-20). The following antibodies were used for immunofluorescence studies: FITC-conjugated anti-Gr1 and anti-CD4 monoclonal antibody (BD Biosciences), primary goat anti-mouse substance P or an isotype control antibody (Santa Cruz Biotechnology, Santa Cruz, CA) and AlexaFluor 546-labeled anti-goat secondary antibody (Invitrogen, Carlsbad, CA).
Subconjunctival Inoculation of Spantide I
C57BL/6 mice were treated subconjuntivally with 36 μg per eye of spantide I (Sigma-Aldrich Co.) on Days 6, 8, 10, 12, and 14 post ocular infection. Subconjunctival spantide I injections were performed using a 36-gauge stainless steel needle attached to a syringe (Nanofil; World Precision Instruments, Sarasota, FL). The needle was inserted into the subconjunctival space and 3 μL of PBS containing 36 μg spantide I was injected. Control mice were similarly injected with PBS vehicle on the same days post ocular infection. Eyes were monitored daily for disease pathology using a hand held slit lamp microscope. Spantide I-treated and control corneas were then collected on Day 16 post ocular infection and processed for flow cytometery. In separate experiments, corneas from both groups were collected in protease inhibitor cocktail to detect CCL3 and IL-6 using ELISA kits for the respective cytokines (R&D Systems).
Statistical Analysis
Statistical analysis was carried out using software (GraphPad Prism; GraphPad Software, Inc., San Diego, CA). All P values were calculated using an unpaired, two-tailed Student's t-test. P < 0.05 was considered statistically significant. Results are displayed as the mean ± SEM.
Results
Not All HSV-1–Infected Corneas of C57BL/6 Mice Develop Severe HSK Lesions
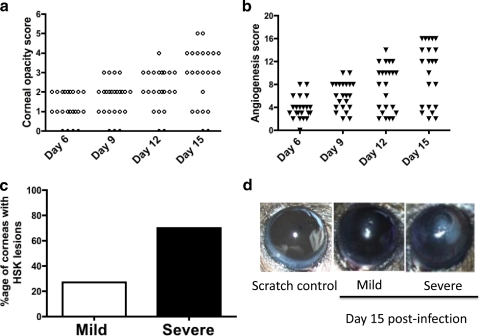
Even though HSV-1-induced herpetic stromal keratitis is a blinding disease of the cornea, not all humans who shed virus at the corneal surface develop severe HSK lesions suggesting a possible regulatory mechanism in the cornea that prevents chronic inflammation in the corneal stroma. In this study, the corneas of C57BL/6 mice were mildly scratched with trephine followed by infection with 1 × 106 plaque forming unit (p.f.u.) of HSV-1 (RE). Opacity and neovascularization of the cornea was scored as stated in Methods, while using a hand-held slit lamp microscope. As is evident in Figures 1a and 1b, on Day 15 postinfection five out of twelve corneas in that particular experiment develop the corneal opacity score ≤2 and angiogenesis score ≤4. We categorized them as the corneas with mild HSK lesions. On the other hand, the corneas that had opacity score >2 and angiogenesis score >4 were categorized as those with severe HSK lesions. The data pooled from two independent experiments (total 24 eyes) determined that approximately 70% of the infected corneas developed severe HSK lesions (corneal opacity >2 and angiogenesis >4) whereas 30% developed mild HSK lesions (corneal opacity ≤2 and angiogenesis ≤4) (Fig. 1c). Taken together, our results determined the development of differential corneal lesion severity, on the basis of the corneal opacity and angiogenesis, after ocular HSV-1 infection of C57BL/6 mice.
Figure 1.
Not all HSV-1–infected corneas develop severe HSK lesions. (a, b) The corneal opacity and angiogenesis was determined at different time points post ocular HSV-1 infection using a hand-held slit lamp microscope. (c) Frequency of corneas with severe and mild HSK lesions on Day 15 postinfection. (d) Representative image of the eye with no disease, mild, and severe HSK lesions. Images were taken 15 days post infection. Data shown is the mean of two independent experiments with similar results. In each experiment 12 eyes were infected with HSV-1.
Viral Load in the Infected Corneas Do Not Affect the Development of Differential Corneal Lesion Severity
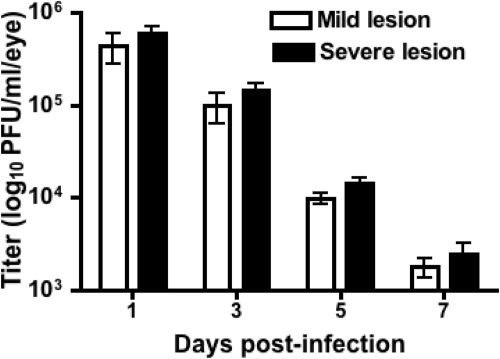
To ascertain if lack of the development of severe HSK lesions in certain corneas was due to a rapid viral clearance, we quantified the viral titers in all infected corneas (n = 12) at four different time points post infection and the viral plaque assay was used to determine the titer of the infectious virus. As is shown in Figure 2, no significant differences in the viral load were seen at the indicated time points postinfection in the corneas that developed either mild or severe HSK lesions suggesting that clinical severity of HSK lesions is independent of the rate of the clearance of infectious virus from the cornea.
Figure 2.
Viral load between the corneas that develop mild or severe HSK lesions were equivalent at the indicated time points postinfection. Eyes were swabbed at the indicated time points postinfection and viral titer was determined by a standard plaque assay. Data shown are indicative of two independent experiments with 12 samples per group. Viral titers from mild and severe lesions at each time point were analyzed using Student's t-test (P > 0.05). Results are reported as the mean titer ± SEM.
Corneas with Severe Lesions Contain a Significantly Higher Amount of Substance P in Comparison with Ones with Mild HSK Lesions
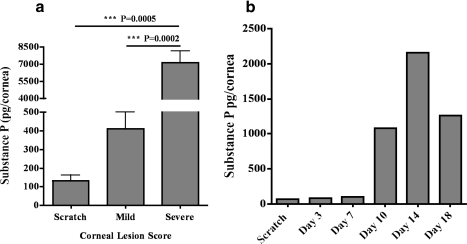
The cornea is highly innervated with sensory nerve endings originating from the neuronal cell bodies present in the trigeminal ganglia (TG).19 Corneal nerve endings are reported to contain neuropeptides like substance P (SP) that can promote inflammation.15,16 Therefore, we next ascertained if there is a differential amount of neuropeptide SP in the corneas with mild and severe HSK lesions. On Day 15 postinfection, the corneal lysate of mild and severe HSK lesions were tested for SP levels while using SP EIA kit from R&D Systems. As is shown in Figure 3a, the corneas with severe HSK lesions have about 15-fold more SP in comparison with those with mild lesions. In another set of experiments, we carried out kinetics to determine the amount of SP in the infected corneas at regular intervals of time post ocular HSV-1 infection. Our data show a heightened amount of SP in HSV-1–infected corneas during the clinical phase of HSK (Fig. 3b).
Figure 3.
Eyes with severe HSK lesions exhibit a higher level of SP in the cornea during the clinical phase of HSK. The corneas were grouped on the basis of the opacity and angiogenesis into mild and severe HSK on Day 15 postinfection. (a) SP levels in the corneal lysates grouped as mild or severe HSK were detected via ELISA. For mild HSK two corneas were pooled to obtain one sample whereas three corneas with severe HSK lesions were pooled to obtain one sample. A total of four to eight samples were obtained from two independent experiments. Results are reported as mean ± SEM. (b) Kinetics of SP amount in naïve and HSV-1–infected corneas were determined at different days postinfection. Six corneas were collected and pooled after infection with HSV-1 at the indicated time points. Graph represents the mean of two independent experiments.
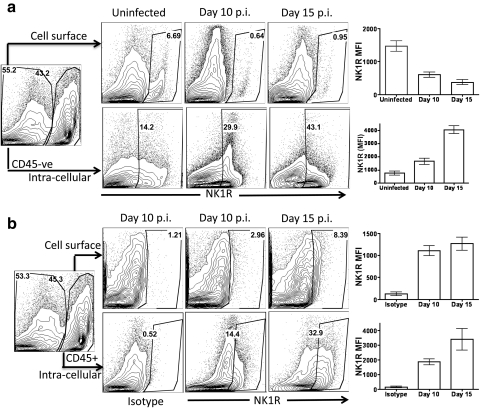
Kinetics of SP Receptor, NK1R Expression on CD45+ and CD45− Cell Types in the Cornea after Ocular HSV-1 Infection
Substance P binds with highest affinity to substance P receptor, NK1R.11 Therefore, we next determined the expression of NK1R on CD45−ve resident cells and CD45+ infiltrating cell types in the corneal tissue during the clinical phase of HSK. Our results showed that both CD45− and CD45+ cells expressed NK1R during the clinical phase of HSK as is evident in Figure 4. Interestingly, the proportion and the level of expression of cell surface NK1R decreases but intracellular NK1R increases in CD45−ve corneal resident cells during the clinical phase of HSK when compared with uninfected control corneas (Fig. 4a). Similarly, the proportion and the level of expression of intracellular NK1R on CD45+ cell types was higher when compared with membrane-bound NK1R in the cornea during the clinical phase of HSK (Fig. 4b).
Figure 4.
SP receptor NK1R is expressed in both CD45− and CD45+ cell types in the cornea during the clinical phase of HSK. The corneas were collagenase-digested and the single-cell suspension obtained was stained for cell surface and intracellular NK1R. Cells were analyzed by flow cytometery. (a, b) Representative FACS plots demonstrating cell surface and intracellular expression of NK1R on gated CD45−ve and CD45+ cells in uninfected and infected corneas. Graphs represent the mean fluorescence intensity at the indicated time points.
Corneas with Higher Levels of SP Also Have an Increased Amount of Proinflammatory Cytokines and Chemokines
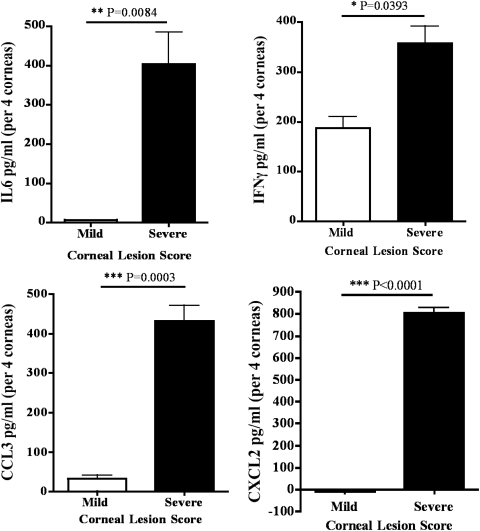
It is well reported that several proinflammatory cytokines like IL-6 and IFN-γ, as well as the chemokines such as CCL3 and CXCL2 play an important role in the development of severe HSK lesions.20,21 Moreover, SP is also documented to regulate the expression of these molecules.22,23 Therefore, we next ascertained the amount of these cytokines and chemokines in the corneas with severe and mild HSK lesions on Day 15 postinfection by ELISA. As is evident in Figure 5, the corneas with severe HSK lesions had significantly higher amounts of IL-6, IFN-γ, CCL3, and CXCL2 proteins when compared with the corneas with mild HSK lesions on Day 15 postinfection. Taken together, our results suggest that the amount of SP in HSV-1–infected cornea directly correlate with the level of the expression of the above-mentioned cytokines and chemokines.
Figure 5.
Amounts of IL-6, IFNγ, CCL3, and CXCL2 proteins are greater in the corneas with severe disease when compared with the corneas with mild HSK. Corneas were removed 15 days post ocular infection with HSV-1. Four corneas with mild or severe lesions were pooled per sample and a total of four to five samples were obtained from three experiments. Samples were sonicated in the protease inhibitor cocktail and assayed for the respective cytokines via ELISA. Results are reported as mean ± SEM (n = 4 per group per cytokine). P values were calculated using Student's t-test (**P < 0.01; ***P < 0.001).
Corneas with Severe HSK Lesions Have a Higher Number of Neutrophils and CD4 T Cells
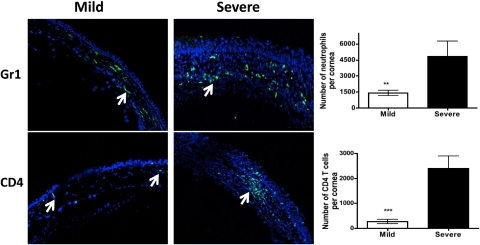
Neutrophils and CD4 T cells are two key immune cell types involved in orchestrating tissue damage to HSV-1–infected cornea.2,5 As is shown in Figure 3a, we determined a significantly higher amount of SP neuropeptide in the cornea with severe HSK lesions when compared with the corneas with mild HSK lesions. Therefore, we next wanted to ascertain if the amount of SP in HSV-1–infected cornea also correlates with the number of infiltrating neutrophils and CD4 T cells. Eyes with mild and severe HSK lesions were either frozen in OCT compound to make 6 μm sections for immunofluorescence or the corneas with mild and severe HSK lesions were excised and collagenase-digested for flow cytometery as described in Methods on Day 15 post ocular HSV-1 infection. As is shown in Figure 6, both confocal microscopy and flow cytometry determined that the corneas with severe HSK lesions had significantly higher numbers of neutrophils and CD4 T cells in comparison with the corneas with mild HSK lesions.
Figure 6.
The corneas with severe HSK demonstrate a significantly higher number of neutrophils and CD4 T cells when compared with those with mild disease. Six-micrometer frozen sections were obtained from the eyes that developed mild or severe HSK lesions as determined on Day 15 postinfection. Sections were stained for neutrophils and CD4 T cells (green) followed by acquisition on a confocal microscope (C1 Nikon; magnification ×20). Arrows denote Gr1+ neutrophils and CD4 T cells in the corneas with mild or severe HSK lesions. The corneas with mild or severe HSK lesions were collagenase-digested on Day 15 postinfection followed by staining for CD4 T cells and neutrophils. The samples were acquired by flow cytometry and absolute numbers were calculated from the proportion of cell types. Three corneas with mild HSK lesions were pooled together to obtain one sample whereas four corneas with severe HSK were pooled to obtain one sample. A total of three to four samples were obtained from two experiments. Graphs indicate the absolute number of cells within the cornea as determined by flow cytometry. Data represents the mean ± SEM of two independent experiments. P values were calculated using Student's t-test (**P < 0.01; ***P < 0.001).
Substance P in the Corneas with Severe HSK Lesions Is Present in the Corneal Stroma and Colocalizes with β-III Tubulin and IAb+ Cell Types
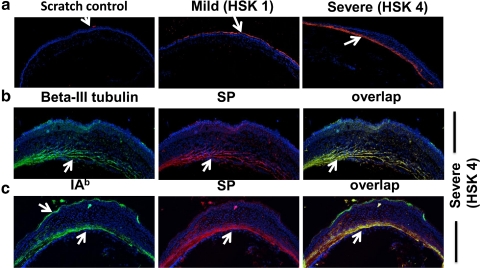
HSK causes tissue damage to the corneal stroma. Therefore, we next ascertained the location of SP in the corneas with severe and mild HSK lesions. As is shown in Figure 7a, the corneas with mild HSK lesions have staining for SP in the corneal epithelium but not in the corneal stroma. On the other hand, SP is largely determined in the posterior part of the corneal stroma but not in the corneal epithelium of the eyes with severe HSK lesions (Fig. 7a). Because SP can be produced by neuronal as well as the nonneuronal cell types,10 we next studied the source of SP in the corneal stroma of the eyes with severe HSK lesions. Our results showed that SP staining in the corneal stroma colocalizes with β-III tubulin, a marker for sensory nerve endings (Fig. 7b). We also determined the colocalization of SP with IAb+ cells in the corneal stroma but not in the corneal epithelium (Fig. 7b). Taken together, our result suggests that SP is produced in the corneal stroma of the eyes by nerve fibers and antigen-presenting cell types.
Figure 7.
Eyes with severe HSK lesions exhibit SP in the corneal stroma and colocalize with β-III tubulin and IA-b+ cell types. (a) Fifteen-micrometer frozen sections obtained from uninfected or the eyes with mild or severe HSK lesions were stained for SP (red) followed by acquisition on a confocal microscope (acquired at magnification ×10). (b, c) Fifteen-micrometer sections obtained from eyes with severe HSK lesions (corneal opacity score 4) on day 15 postinfection were stained with SP (red) and β-III tubulin (green) or IAb (green) obtained on Day 15 postinfection. Images were acquired on a confocal microscope (C1 Nikon; acquired at magnification ×20). Yellow: overlap between SP and β-III tubulin or SP and IAb staining.
Subconjunctival Inoculation of Spantide I Significantly Reduced the Development of Corneal Opacity and Angiogenesis
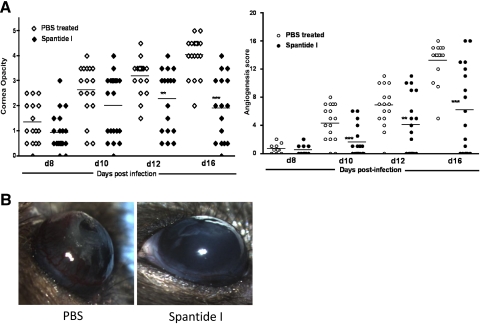
We next ascertained whether presence of SP in the corneal stroma regulates the development of severe HSK lesions. Mice were infected with 5 × 106 (p.f.u.) of HSV-1 (RE). One group of mice received subconjunctival inoculation of spantide I (36 μg per eye) whereas the control group of infected mice received PBS on Days 6, 8, 10, 12, and 14 postinfection. Spantide I is a NK1R antagonist and is well reported to block SP-mediated effects under inflammatory conditions.24 As is shown in Figure 8, spantide I treatment during the clinical phase of HSK resulted in a moderate but significant reduction in the corneal opacity and angiogenesis as determined on Days 12 and 14 postinfection by hand-held slit lamp microscope.
Figure 8.
Subconjunctival inoculation of spantide I during the clinical phase of HSK significantly reduced the corneal opacity and angiogenesis. The corneal opacity and angiogenesis was determined with a hand-held slit lamp microscope. (A) Significant differences in the corneal opacity between PBS- and spantide-treated groups were evident only at Day 12 and Day 16 postinfection whereas significant differences in the angiogenesis were noted on Days 10, 12, and 16 postinfection. Data shown were pooled from two independent experiments. (B) Representative image of the eyes with PBS and spantide treatment. Images were taken 16 days postinfection. P values are calculated using Student's t-test (**P < 0.01; ***P < 0.001).
Spantide I Treatment Reduces the Proportion of Neutrophils and CD4 T Cells in Inflamed Cornea
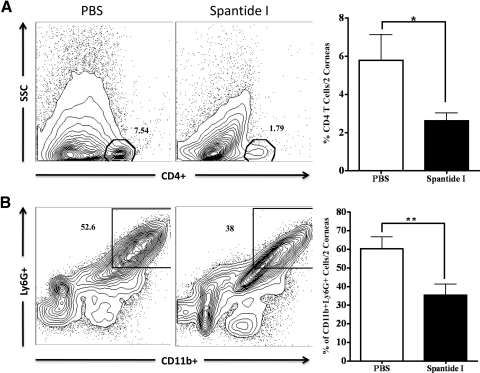
Neutrophils and CD4 T cells are well known to regulate the severity of HSK lesions. Therefore, we next ascertained the influx of CD4 T cells and neutrophils (CD11b+Ly6G+) in the corneas of spantide I and PBS-treated C57BL/6 mice on Day 16 postinfection. Two corneas were pooled to obtain one sample followed by liberase digestion to obtain single-cell suspension for flow cytometery as stated in Methods. Our results demonstrate a significantly reduced proportion of neutrophils and CD4 T cells in spantide I–treated corneas when compared with PBS-injected HSV-1–infected corneas on Day 16 postinfection (Fig. 9).
Figure 9.
Subconjunctival inoculation of spantide I significantly reduced the proportion of CD4 T cells and neutrophils in inflamed corneas. The corneas were obtained from spantide- and PBS-treated groups of mice on Day 16 postinfection. Two corneas were pooled to obtain one sample and liberase-digested. The obtained single-cell suspension was stained for neutrophils and CD4 T cells. Samples were analyzed by flow cytometery. (A, B) Representative FACS plots show cell surface staining for CD4, CD11b, and Ly6G molecules on gated CD45+ cells derived from PBS- and spantide-treated corneas. Graphs represent the mean ± SD of five samples. P values are calculated with two-tailed Student's t-test (*P < 0.05; **P < 0.01).
Spantide I Treatment Reduces the Amount of IL-6 and CCL3 Proteins in HSV-1–Infected Corneas
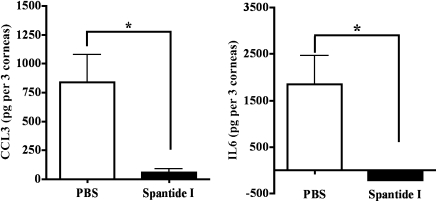
Because IL-6 and CCL3 proteins in HSV-1–infected cornea are involved in the development of severe HSK lesions,25,26 we next compared the levels of these two proteins in the corneas of PBS- and spantide I-treated C57BL/6 mice. The corneas were excised on Day 16 postinfection. Three corneas from each group were pooled in PBS containing protease inhibitor cocktail to obtain one sample. The samples were homogenized and ELISA was used to determine the amounts of IL-6 and CCL3 proteins. Our results determined that subconjunctival inoculation of spantide I resulted in a significant reduction in the amounts of IL-6 and CCL3 proteins in HSV-1–infected corneas when measured on Day 16 postinfection (Fig. 10).
Figure 10.
Significantly reduced amounts of IL-6 and CCL3 in spantide-treated HSV-1–infected corneas. On Day 16 postinfection, the corneas obtained from PBS- and spantide-treated groups of mice were homogenized and centrifuged. Three corneas from each group were pooled to obtain one sample. The amounts of IL-6 and CCL3 proteins were tested in each sample by ELISA. Graphs represent the mean ± SD of five samples. P values are calculated with two-tailed Student's t-test (*P < 0.05).
Discussion
Not all humans who shed virus at the corneal surface get severe HSK lesions suggesting an intricate mechanism involved in the development of HSK. In this report, while using the C57BL/6 mouse model, we demonstrate that not all HSV-1–infected eyes develop severe HSK lesions as determined by the extent of neovascularization and opacity of the infected cornea. The corneas with severe HSK lesions exhibit a significantly higher amount of proinflammatory neuropeptide, substance P, in comparison with the corneas with mild HSK lesions. In addition, the corneas with higher levels of SP also have higher amounts of proinflammatory cytokines (IFN-γ and IL-6) and chemokines (CCL3 and CXCL2), the molecules that are regulated by SP. The SP in the corneal stroma of eyes with severe HSK lesions could come from nerve fibers and/or immune cell types as determined by our results. Most importantly, treatment with SP antagonist, spantide I, during the clinical phase of HSK significantly reduced the severity of the corneal opacity and angiogenesis. To our knowledge this is the first study to demonstrate the relative contribution of corneal SP in regulating the severity of HSK lesions in a mouse model.
Differential rates of viral clearance from infected eyes may cause the development of mild and severe HSK lesions. However, eye swabs taken from infected eyes at different time points postinfection showed no statistically significant difference in viral load in the corneas that developed mild or severe HSK lesions. Our results are in agreement with a recent report where no significant difference in the viral load was determined in the corneal tear film of the eyes that did or did not develop HSK in a BALB/c mouse model.27 It has also been shown that even though replicating virus in the cornea is required to induce immunoinflammatory reactions in the corneal stroma of infected eyes, the virus is not needed to sustain the inflammation that causes stromal tissue damage.28 Therefore, the development of severe HSK lesions might depend on proinflammatory molecules that promote an ongoing inflammation.
Neuropeptides are well reported to either promote or suppress an ongoing inflammation by acting on either immune or nonimmune cell types. SP is a proinflammatory neuropeptide that belongs to a family of bioactive peptides, the tachykinins. It has been shown that elevated levels of SP in the cornea after Pseudomonas aeruginosa infection promotes corneal inflammation and participate in the development of bacterial keratitis.29 Even though earlier studies have shown the level of SP in the cornea after ocular HSV-1 infection,30,31 the relative contribution of SP in regulating the severity of HSK lesions is not known. We determined that the corneas with severe HSK lesions display higher amounts of SP when compared with the corneas with mild HSK lesions. Moreover, the corneas with higher amounts of SP also exhibit higher levels of IL-6, IFN-γ, CCL3, and CXCL2 proinflammatory molecules that are known to orchestrate the corneal stromal tissue damage resulting into the development of severe HSK lesions. Because SP is known to induce the expression of IL-6, IFN-γ, CCL3, and CXCL2,23 it is possible that SP in the inflamed cornea regulates the development of severe HSK lesions by promoting the expression of these proinflammatory molecules in the cornea.
SP exerts its action on both immune and nonimmune cell types by binding to the SP receptors NK1R, NK2R, and NK3R, with highest affinity toward NK1R.12 We determined NK1R expression on both CD45−ve and CD45+ cell types in the inflamed cornea when measured during the clinical phase of HSK suggesting that SP might exert its action on both corneal resident and infiltrating immune cell types. Binding of SP to NK1R results in the internalization of SP-NK1R complex, thereby decreasing the level of expression of membrane-bound NK1R molecules.32 Our studies show higher intracellular level of NK1R expression in both CD45− and CD45+ cells during the clinical phase, a time period when SP is present in abundance in the inflamed cornea. It is possible that an active interaction between SP and NK1R resulted in the internalization of NK1R and thereby increased the intracellular level of NK1R.
Studies carried out in mice models clearly demonstrate that the development of the corneal opacity, and the severe HSK lesions begin during the clinical phase of disease. One can speculate that the molecules that are differentially expressed during preclinical and clinical phases of disease may regulate the extent of the corneal opacity in HSV-1–infected eyes. Our results show a dramatic increase in the amount of SP in the cornea during the clinical phase of HSK demonstrating the possible involvement of SP in the development of severe HSK lesions. SP receptor antagonists have been used in multiple studies to demonstrate the effect of SP in chronic inflammatory conditions.12,24 Our results determined that blocking of SP interaction with its receptor in the cornea during the clinical phase of HSK reduce the corneal opacity and angiogenesis. Antagonist injection resulted in reduced frequency of neutrophils and CD4 T cells in the cornea as well as reduced amounts of IL-6 and CCL3 proteins, thereby demonstrating an active participation of corneal SP in the regulation of cellular and molecular events involved in the development of severe HSK lesions.
HSK is a disease of the corneal stroma where resident stromal keratocytes and/or fibroblasts and infiltrating immune cell types after ocular HSV-1 infection participate in corneal tissue damage by secreting free radicals and matrix-metalloproteinases.33–35 Therefore, studies are carried out to determine whether SP is present in the corneal stroma of eyes with severe or mild HSK lesions. Our results clearly demonstrate that SP is largely localized in the corneal stroma of eyes with severe but not mild HSK lesions and may exert its action on cell types present in the corneal stroma. Previous studies have shown that both neuronal and nonneuronal cell types can produce SP.8,10 The naïve cornea is largely innervated with nerve fibers that originate from the sensory neurons present in the ophthalmic branch of the trigeminal ganglia.19 Moreover, it has been shown that nerve fibers in the naïve cornea exhibit SP.16 In addition to neuronal source, SP is also expressed in immune cell types like macrophages and dendritic cells.36–38 Our data clearly show colocalization of SP with β-III tubulin and IAb+ cells suggesting both nerve fibers as well as antigen-presenting cells as a source of SP in the corneal stroma during the clinical phase of HSK. The presence of SP-exhibiting nerve fibers in the corneal stroma of the eyes with severe HSK lesions is somewhat surprising as a recent study has shown significant but not complete loss of nerve fibers in the cornea during infectious keratitis in humans.39,40 Because nerve fibers can get regenerated after peripheral nerve injury, it is possible that SP-expressing nerve fibers in the posterior stroma is the outcome of the regeneration of damaged nerve fibers. In fact, vascular endothelial growth factor (VEGF), an angiogenic factor that is present in abundance in the corneas with severe HSK, has been shown to participate in the regeneration of the corneal nerve fibers.41 On the basis of the results shown in this study, we propose that SP present in the corneal stroma of the eyes with severe HSK lesions are actively involved in orchestrating stromal tissue damage by exerting its action on both stromal keratocytes and infiltrating immune cell types.
Acknowledgments
The authors thank Shravan K. Chintala for taking the pictures of eyes with severe and mild HSK lesions.
Footnotes
Supported by National Eye Institutes Grant EY020625.
Disclosure: B.S. Twardy, None; R. Channappanavar, None; S. Suvas, None
References
- 1. Biswas PS, Banerjee K, Kim B, Rouse BT. Mice transgenic for IL-1 receptor antagonist protein are resistant to herpetic stromal keratitis: possible role for IL-1 in herpetic stromal keratitis pathogenesis. J Immunol. 2004;172:3736–3744 [DOI] [PubMed] [Google Scholar]
- 2. Niemialtowski MG, Rouse BT. Predominance of Th1 cells in ocular tissues during herpetic stromal keratitis. J Immunol. 1992;149:3035–3039 [PubMed] [Google Scholar]
- 3. Biswas PS, Rouse BT. Early events in HSV keratitis–setting the stage for a blinding disease. Microbes Infect. 2005;7:799–810 [DOI] [PubMed] [Google Scholar]
- 4. Hendricks RL, Janowicz M, Tumpey TM. Critical role of corneal Langerhans cells in the CD4- but not CD8-mediated immunopathology in herpes simplex virus-1-infected mouse corneas. J Immunol. 1992;148:2522–2529 [PubMed] [Google Scholar]
- 5. Thomas J, Gangappa S, Kanangat S, Rouse BT. On the essential involvement of neutrophils in the immunopathologic disease: herpetic stromal keratitis. J Immunol. 1997;158:1383–1391 [PubMed] [Google Scholar]
- 6. Payan DG. Neuropeptides and inflammation: the role of substance P. Annu Rev Med. 1989;40:341–352 [DOI] [PubMed] [Google Scholar]
- 7. Gonzalez-Rey E, Delgado M. Anti-inflammatory neuropeptide receptors: new therapeutic targets for immune disorders? Trends Pharmacol Sci. 2007;28:482–491 [DOI] [PubMed] [Google Scholar]
- 8. Snijdelaar DG, Dirksen R, Slappendel R, Crul BJ. Substance P. Eur J Pain. 2000;4:121–135 [DOI] [PubMed] [Google Scholar]
- 9. Bost KL. Tachykinin-modulated anti-viral responses. Front Biosci. 2004;9:1994–1998 [DOI] [PubMed] [Google Scholar]
- 10. Nelson DA, Bost KL. Non-neuronal mammalian tachykinin expression. Front Biosci. 2004;9:2166–2176 [DOI] [PubMed] [Google Scholar]
- 11. Tuluc F, Lai JP, Kilpatrick LE, Evans DL, Douglas SD. Neurokinin 1 receptor isoforms and the control of innate immunity. Trends Immunol. 2009;30:271–276 [DOI] [PubMed] [Google Scholar]
- 12. Nakanishi S. Mammalian tachykinin receptors. Annu Rev Neurosci. 1991;14:123–136 [DOI] [PubMed] [Google Scholar]
- 13. Janelsins BM, Mathers AR, Tkacheva OA, et al. Proinflammatory tachykinins that signal through the neurokinin 1 receptor promote survival of dendritic cells and potent cellular immunity. Blood. 2009;113:3017–3026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ramnath RD, Bhatia M. Substance P treatment stimulates chemokine synthesis in pancreatic acinar cells via the activation of NF-kappaB. Am J Physiol Gastrointest Liver Physiol. 2006;291:G1113–G1119 [DOI] [PubMed] [Google Scholar]
- 15. Miller A, Costa M, Furness JB, Chubb IW. Substance P immunoreactive sensory nerves supply the rat iris and cornea. Neurosci Lett. 1981;23:243–249 [DOI] [PubMed] [Google Scholar]
- 16. Keen P, Tullo AB, Blyth WA, Hill TJ. Substance P in the mouse cornea: effects of chemical and surgical denervation. Neurosci Lett. 1982;29:231–235 [DOI] [PubMed] [Google Scholar]
- 17. Spear PG, Roizman B. Proteins specified by herpes simplex virus. V. Purification and structural proteins of the herpesvirion. J Virol. 1972;9:143–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dana MR, Zhu SN, Yamada J. Topical modulation of interleukin-1 activity in corneal neovascularization. Cornea. 1998;17:403–409 [DOI] [PubMed] [Google Scholar]
- 19. Mensher JH. Corneal nerves. Surv Ophthalmol. 1974;19:1–18 [PubMed] [Google Scholar]
- 20. Deshpande S, Banerjee K, Biswas PS, Rouse BT. Herpetic eye disease: immunopathogenesis and therapeutic measures. Expert Rev Mol Med. 2004;6:1–14 [DOI] [PubMed] [Google Scholar]
- 21. Lepisto AJ, Frank GM, Hendricks RL. How herpes simplex virus type 1 rescinds corneal privilege. Chem Immunol Allergy. 2007;92:203–212 [DOI] [PubMed] [Google Scholar]
- 22. Gitter BD, Regoli D, Howbert JJ, Glasebrook AL, Waters DC. Interleukin-6 secretion from human astrocytoma cells induced by substance P. J Neuroimmunol. 1994;51:101–108 [DOI] [PubMed] [Google Scholar]
- 23. Sio SW, Puthia MK, Lu J, Moochhala S, Bhatia M. The neuropeptide substance P is a critical mediator of burn-induced acute lung injury. J Immunol. 2008;180:8333–8341 [DOI] [PubMed] [Google Scholar]
- 24. Hazlett LD, McClellan SA, Barrett RP, Liu J, Zhang Y, Lighvani S. Spantide I decreases type I cytokines, enhances IL-10, and reduces corneal perforation in susceptible mice after Pseudomonas aeruginosa infection. Invest Ophthalmol Vis Sci. 2007;48:797–807 [DOI] [PubMed] [Google Scholar]
- 25. Fenton RR, Molesworth-Kenyon S, Oakes JE, Lausch RN. Linkage of IL-6 with neutrophil chemoattractant expression in virus-induced ocular inflammation. Invest Ophthalmol Vis Sci. 2002;43:737–743 [PubMed] [Google Scholar]
- 26. Tumpey TM, Cheng H, Cook DN, Smithies O, Oakes JE, Lausch RN. Absence of macrophage inflammatory protein-1alpha prevents the development of blinding herpes stromal keratitis. J Virol. 1998;72:3705–3710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Divito SJ, Hendricks RL. Activated inflammatory infiltrate in HSV-1-infected corneas without herpes stromal keratitis. Invest Ophthalmol Vis Sci. 2008;49:1488–1495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Babu JS, Thomas J, Kanangat S, Morrison LA, Knipe DM, Rouse BT. Viral replication is required for induction of ocular immunopathology by herpes simplex virus. J Virol. 1996;70:101–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. McClellan SA, Zhang Y, Barrett RP, Hazlett LD. Substance P promotes susceptibility to Pseudomonas aeruginosa keratitis in resistant mice: anti-inflammatory mediators downregulated. Invest Ophthalmol Vis Sci. 2008;49:1502–1511 [DOI] [PubMed] [Google Scholar]
- 30. Tullo AB, Keen P, Blyth WA, Hill TJ, Easty DL. Corneal sensitivity and substance P in experimental herpes simplex keratitis in mice. Invest Ophthalmol Vis Sci. 1983;24:596–598 [PubMed] [Google Scholar]
- 31. Templeton A, Nguyen G, Ash JD, Straub RH, Carr DJ. Chemical sympathectomy increases susceptibility to ocular herpes simplex virus type 1 infection. J Neuroimmunol. 2008;197:37–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bowden JJ, Garland AM, Baluk P, et al. Direct observation of substance P-induced internalization of neurokinin 1 (NK1) receptors at sites of inflammation. Proc Natl Acad Sci U S A. 1994;91:8964–8968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Daheshia M, Kanangat S, Rouse BT. Production of key molecules by ocular neutrophils early after herpetic infection of the cornea. Exp Eye Res. 1998;67:619–624 [DOI] [PubMed] [Google Scholar]
- 34. Lee S, Zheng M, Kim B, Rouse BT. Role of matrix metalloproteinase-9 in angiogenesis caused by ocular infection with herpes simplex virus. J Clin Invest. 2002;110:1105–1111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mistry SK, Zheng M, Rouse BT, Morris SM., Jr Induction of arginases I and II in cornea during herpes simplex virus infection. Virus Res. 2001;73:177–182 [DOI] [PubMed] [Google Scholar]
- 36. Lambrecht BN, Germonpre PR, Everaert EG, et al. Endogenously produced substance P contributes to lymphocyte proliferation induced by dendritic cells and direct TCR ligation. Eur J Iimmunol. 1999;29:3815–3825 [DOI] [PubMed] [Google Scholar]
- 37. Germonpre PR, Bullock GR, Lambrecht BN, et al. Presence of substance P and neurokinin 1 receptors in human sputum macrophages and U-937 cells. Eur Respir J. 1999;14:776–782 [DOI] [PubMed] [Google Scholar]
- 38. Ho WZ, Lai JP, Zhu XH, Uvaydova M, Douglas SD. Human monocytes and macrophages express substance P and neurokinin-1 receptor. J Immunol. 1997;159:5654–5660 [PubMed] [Google Scholar]
- 39. Hamrah P, Cruzat A, Dastjerdi MH, et al. Corneal sensation and subbasal nerve alterations in patients with herpes simplex keratitis: an in vivo confocal microscopy study. Ophthalmology. 2010;117:1930–1936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cruzat A, Witkin D, Baniasadi N, et al. Inflammation and the nervous system: the connection in the cornea in patients with infectious keratitis. Invest Ophthalmol Vis Sci. 2011;52:5136–5143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Yu CQ, Zhang M, Matis KI, Kim C, Rosenblatt MI. Vascular endothelial growth factor mediates corneal nerve repair. Invest Ophthalmol Vis Sci. 2008;49:3870–3878 [DOI] [PMC free article] [PubMed] [Google Scholar]