Abstract
Solitary fibrous tumor is a rare tumor type and has an unpredictable course. Local recurrence rate varies between 9 and 19%, and rate of metastatic involvement between 0 and 36 %. It is characterized by a typical architecture and immuno-histochemistry tests. The most important prognostic factor is the complete resection of primary tumor. Treatment of recurrences is not clearly established. If a solitary fibrous tumor is too advanced to allow surgical resection, radiotherapy and chemotherapy may be used. The most often used drugs are doxorubicine and\or ifosfamide. We report the case of man with metastatic solitary fibrous tumor treated with trabectedin, administered at a dose of 1.5 mg/m² every 3 weeks. After 3 cycles, metastases had significantly decreased. Recurrence of the disease was demonstrated 8 months after the start of trabectedin. This case shows that trabectedin is a possible treatment option.
Key words: metastatic solitary fibrous tumor, trabectedin.
Introduction
Solitary fibrous tumor is a rare tumor type and has an unpredictable course. Treatment of recurrences is poorly codified. The surgery remains probably the reference. In the forms too advanced for a surgical solution, it is usual to use radiotherapy and chemotherapy. We describe a case report of a 39-year-old man with metastatic solitary fibrous tumor treated with trabectedin.
Case Report
Diagnosis
On July 2007, a 39-year old man without any relevant medical history had an assessment for chronic cough. Chest radiography revealed a left retro-cardiac thoracic mass. CT-scan found a voluminous solid, heterogeneous, well-encapsulated mass, with benign aspect, developed on lower half of left thorax. Lung biopsy concluded on solitary fibrous tumor (SFT) of the pleura. Surgical resection was performed on October 2007. Histological examination confirmed the diagnosis of STF and surgical margins appeared free. In spite that this subtype of mesenchymatous tumor is widely considered as benign, most cases have the potential for local recurrence even after wide surgical excision. Typically, patient received regular follow-up examination including thoracic CT-scan. In February 2008, CT-scan revealed a unique 8 mm-lesion in the left lung and three other lesions on the right lung. Physical exam had not revealed any significant abnormalities associated with a good performance status. No complaints or functional limitations were described. These micronodules were totally nonspecific and asymptomatic. Their exact nature was uncertain. Subsequent CT-scan performed in Mars 2008 showed an increase in size and number of lung lesions, and doubtful liver images.
Lung biopsies were performed shortly afterwards. Pathological examination showed several very small-sized metastatic nodes in lung parenchyma (Figure 1). The round to spindle-shaped cells had little cytoplasm and round, large-sized nuclei with relatively thin chromatin and small nucleoli. Vascular component was prominent with dilated vessels. Mitotic activity was 4 out of 10 high-power fields. Tumors cells were immunoreactive and immuno-histochemistry testing showed heterogeneous immunoreactivity for CD34, CD99 and bcl2 (Figure 2). Focal and limited immunoreactivity was observed for P S100 and Cytokeratins. Less than 2% of cells expressed the proliferation marker Ki67. These examinations confirmed the diagnosis of SFT with metastatic evolution, based on the shape of lung micronodes and of doubtful liver images observed on CT-scan.
Figure 1.
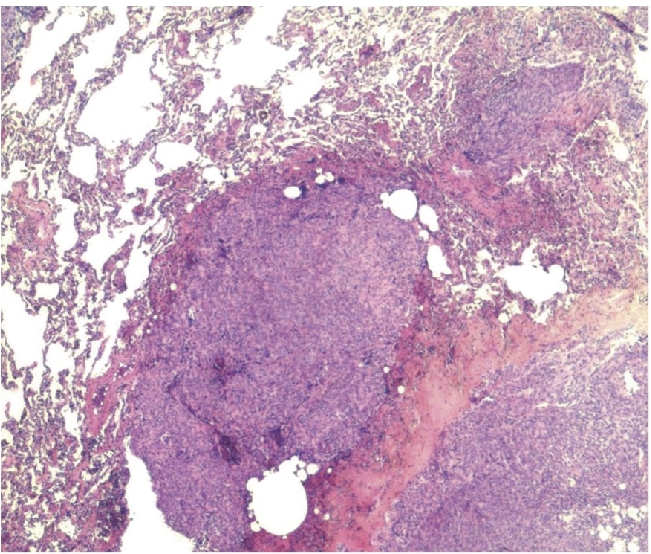
Lung biopsy; several metastatic micronodules of SFT (HES × 10).
Figure 2.

Biopsy; SFT is characterized by spindle cells with vescisular nuclei (HES × 40). All the tumoral cells are immunoreactive with CD34 (×40).
Treatment
Chemotherapy has been started according to the MAID (Mesna, Doxorubicine, Ifosfamide, Deticene) regimen, within framework of the PALSAR protocol from the FNCLCC and the French Sarcoma Group.1 After 6 cycles of MAID, best response was stable disease.
In January, 2009, CT-scan showed disease progression on lung metastases, which had increased in number and size. The biggest metastases were located in lingula and in left lower lobe of the lung (Figure 3). The hepatic lesions had become more visible. A second line of chemotherapy by trabectedin has been initiated with a 24-hour infusion at the dose of 1.5 mg/m2 every 3 weeks, in a compassionnate use program. Premedication consisted on a 30-minute IV infusion of 20 mg of dexamethasone before administration of trabectedin, as anti-emetic prophylaxis and for liver protection, in accordance with good practice recommendations.2 After 3 cycles, lung metastases had significantly decreased (Figure 4). In addition, liver metastases had decreased in size, particularly at the segment VI level. After cycle 4 administration, trabectedin was definitively terminated due to a septic shock in the context of a grade 4 neutropenia. Subsequent CT-scan has been done in June 2009, i.e. 2 months after discontinuation of all chemotherapy agents. The pulmonary lesions were still in partial remission, particularly at the level of the right hilus (Figure 5). However, second recurrence of the disease was demonstrated in September of the same year, i.e. 8 months after the start of trabectedin and 5 months after the end of the chemotherapy.
Figure 3.
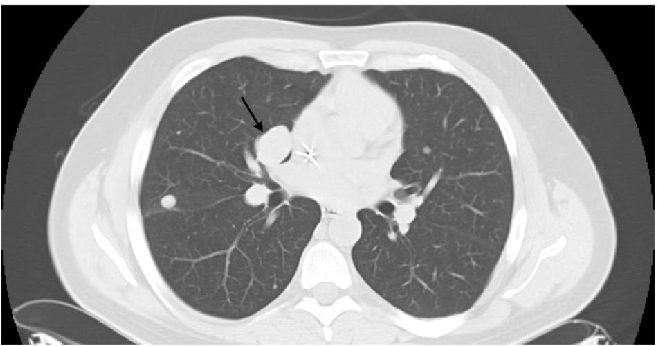
Computed tomografy scan on January 2009, before trabectedin.
Figure 4.
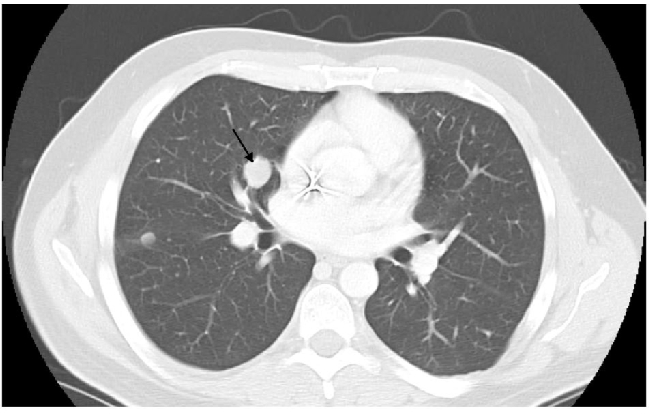
Computed tomografy scan on March 2009, after 3 cycles of trabectedin.
Figure 5.
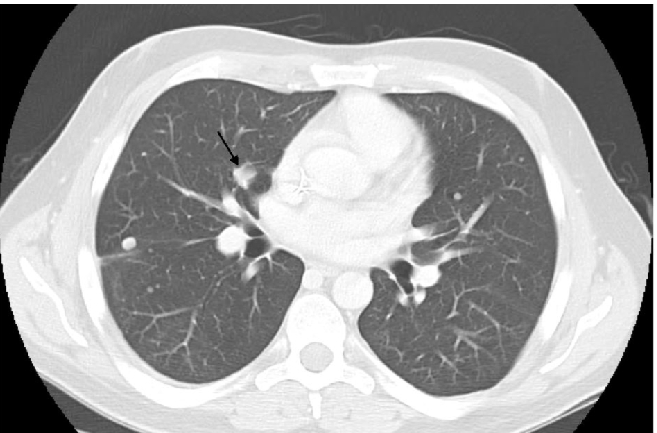
Computed tomografy scan on June 2009, 2 months later the end of chemoterapy.
Discussion
Solitary fibrous tumor was first described on 1931 by P. Klemperer and C. Rabin. SFT is a rare tumor type and often discovered in a fortuitous way.3 Initially observed in the pleura, SFT has also been described at many other sites, including soft tissue of orbit, of liver, thyroid, breast, gastro-intestinal tract, and finally of almost all organs.4,5 SFT can occur at all ages with the highest incidence being between 60 and 70 years without evidence of unbalanced sex ratio.
As this tumor type has a slow growth, it often remains asymptomatic for a long time and can then become very voluminous.
For pathologists, SFT is well circumscribed, often partially encapsulated, of firm consistency, and has a pearly or yellowish-white color.6–10 It is described as a patternless architecture of spindle-shaped cells mimicking sometimes typical sarcomas like leiomyosarcoma, or synovial sarcoma. Moreover, it is characterized by a hemangiopericytoma-like architecture consisting of proliferation of medium-sized, well-formed vessels in the thick wall and hyaline, branching out. Immunohistochemically, SFT neoplastic tumor cells show expression of vimentin, CD34, CD99, bcl2, and less frequently EMA and smooth muscle actin. Focal and limited reactivity for protein S100, cytokeratins, and desmin has also been reported. Histological criteria to classify a SFT as malignant (indicating a risk of recurrence) include more than 4 mitoses out of 10 high-power fields, hypercellularity, nuclear atypia and presence of necrosis. Cytogenetic data on SFT are sparse and not coherent.
SFT has an unpredictable course. Local recurrence rates vary between 9 and 19 %, metastases rates (intra-thoracic, osseous, hepatic, cutaneous) vary between 0 and 36 %. The recurrences justify a long-term follow-up as some recurrences have appeared very lately (until 17 years after initial complete resection). The most important prognostic factor remains the complete resection of primary tumor. Treatment of recurrences is not clearly established and requires discussion at multi-disciplinary medical meetings. Surgery probably remains the standard treatment. If an SFT is too advanced to allow surgical resection, a parallel with soft tissue sarcomas is usually drawn. Radiotherapy and chemotherapy may be used. The most often used drugs are usually doxorubicine and\or ifosfamide. In our case, we have shown that trabectedin may be a possible option. Trabectedin has allowed tumor response for 8 months.
Trabectedin (Yondelis®) is a synthetized molecule, initially extracted from a marine animal (Ecteinascidia turbinata). It binds on the minor groove of the DNA and alkylates N2 of guanines. It may interfere with the DNA repair machinery and blocks cells in G2-M phase.11,12
The European Commission delivered a Marketing Authorization in September 2007. It is indicated for the treatment of patients with two types of cancers: i) Advanced soft tissue sarcoma, after failure of anthracyclines and ifosfamide, or who are unable to receive these agents. Trabectedin has been studied in one main study involving 266 patients with progressive advanced or metastatic soft tissue sarcoma.13 All of the patients had been treated previously with an anthracycline and ifosfamide. This study compared two different trabectedin regimens: 0.58 mg/m2 every week for 3 weeks out of a 4-week cycle, or 1.5 mg/m2 once every three weeks. Trabectedin was more effective when it was given once every three weeks. Median progression-free survival was 3.3 months versus 2.3 months respectively (hazard ratio, 0.755; 95% CI, 0.574 to 0.992; P=0.0418). Efficacy data are based mainly on liposarcoma and leiomyosarcoma patients. Results suggest that the drug may be particularly effective in patients with myxoid liposarcoma; ii) Platinum-sensitive recurrent ovarian cancer, in association with pegylated liposomal doxorubicin (PLD). Trabectedin (1.1 mg/m2) in combination with PLD (30 mg/m2 every 3 weeks) was compared to PLD alone (50 mg/m2 every 4 weeks) in one main study involving 672 women whose disease had recurred after previous treatment.14 Median progression-free survival was 7.3 months for the combination versus 5.8 months for PLD alone (hazard ratio, 0.79; 95% CI, 0.65 to 0.96; P=0.0190). The effect of trabectedin was more pronounced in the women whose cancer was platinum-sensitive (platinum-free interval of 6–12 months); iii) Others studies are ongoing in sarcoma, ovarian carcinoma, breast carcinoma and melanoma.
Conclusions
Solitary fibrous tumor is a rare tumor type and has an unpredictable course. There are no guidelines for the treatment of recurrences. This case report suggests a new treatment option (trabectedin) and gives hope for this indication. Unfortunately, the absence of clinical study and published observation on this subject does not allow drawing of formal conclusion.
References
- 1.FNCLCC and the French sarcoma group. Consolidation with high dose chemotherapy for responding patients to standard chemotherapy in advanced, metastatic. Not published results [Google Scholar]
- 2. http://www.medicines.org.uk/EMC/medicine/20457/SPC/Yondelis+0.25+mg.
- 3.England DM, Hochholzer L, Mc Carthy MJ. Localized benign and malignant fibrous tumors of the pleura. A clinicopathologic review of 223 cases. Am J Surg Pathol. 1989;13:640–58. doi: 10.1097/00000478-198908000-00003. [DOI] [PubMed] [Google Scholar]
- 4.Guillou L, Gebhard S, Coindre JM. Orbital and extraorbital giant cell angiofibroma: a giant cell-rich variant of solitary fibrous tumor? Clinicopathologic and immunhistochemical analysis of a series of a unifying concept. Am J Surg Pathol. 2000;24:971–9. doi: 10.1097/00000478-200007000-00008. [DOI] [PubMed] [Google Scholar]
- 5.Goodlad JR, Fletcher CDM. Solitary fibrous tumour arising at unusual sites: analysis of a series. Histopathology. 1991;19:515–22. doi: 10.1111/j.1365-2559.1991.tb01499.x. [DOI] [PubMed] [Google Scholar]
- 6.Suster S, Nascimento AG, Miettinen M, et al. Solitary fibrous tumors of soft tissue. A clinicopathologic and immunohistochemical study of 12 cases. Am J Surg Pathol. 1995;19:1257–66. doi: 10.1097/00000478-199511000-00005. [DOI] [PubMed] [Google Scholar]
- 7.Brunnemann R, Ro JY, Ordonez NG, et al. Extrathoracic solitary fibrous tumor: a clinicopathologic study of 24 cases. Mod Pathol. 1999;12:1034–42. [PubMed] [Google Scholar]
- 8.Hasegawa T, Matsuno Y, Shimoda T, et al. Extrathoracic solitary fibrous tumors: their histological variability and potentially aggressive behaviour. Hum Pathol. 1999;30:1464–73. doi: 10.1016/s0046-8177(99)90169-7. [DOI] [PubMed] [Google Scholar]
- 9.Hanau CA, Miettinen M. Solitary fibrous tumor: histological and immunohisto-chemical spectrum of benign and malignant variants presenting at different sites. Hum Pathol. 1995;26:440–9. doi: 10.1016/0046-8177(95)90147-7. [DOI] [PubMed] [Google Scholar]
- 10.Guillou L, Gebhard S, Coindre JM. Lipomatous hemangiopericytoma: a fat-containing variant of solitary fibrous tumor? Clinicopathologic, immunohisto-chemical, and ultrastructural analysis of a series in favor of a unifying concept. Hum Pathol. 2000;31:1108–15. doi: 10.1053/hupa.2000.9777. [DOI] [PubMed] [Google Scholar]
- 11.Scotto KW. ET-743: more than an innovative mechanism of action. Anticancer drugs. 2002;13:S3–6. [PubMed] [Google Scholar]
- 12.Fayette J, Ray-Coquard I, Alberti L, et al. ET-743: a novel agent with activity in soft tissue sarcomas. The Oncologist. 2005;10:827–32. doi: 10.1634/theoncologist.10-10-827. [DOI] [PubMed] [Google Scholar]
- 13.Demitri G D, Chawla SP, Von Mehren M, et al. Efficacy and safety of trabectedin in patients with advanced or metastatic liposarcoma or leiomyosarcome after failure of prior anthracyclines and ifosfamide: results of a randomized phase II study of two different schedules. J Clin Oncol. 2009;27:4188–96. doi: 10.1200/JCO.2008.21.0088. [DOI] [PubMed] [Google Scholar]
- 14.Monk BJ, Herzog TJ, Kaye SB, et al. Trabectedin plus pegylated liposomal doxorubicin in recurrent ovarian cancer. J Clin Oncol. 2010;28:3107–14. doi: 10.1200/JCO.2009.25.4037. [DOI] [PubMed] [Google Scholar]


