Abstract
Bivalirudin is a direct thrombin inhibitor approved for use in adult patients with heparin-induced thrombocytopenia (HIT) undergoing percutaneous coronary intervention. Recently, its use in the pediatric population has increased due to its anti-thrombin-independent mechanism of action. As heparin products produce great inter- and intraindividual variability in pediatric patients, often due to decreased anti-thrombin concentrations in the first year of life, some practitioners have turned to direct thrombin inhibitors, such as bivalirudin, for more predictable pharmacokinetics and effects on bound and circulating thrombin. We report our experience using bivalirudin in a 2-month-old female with recurrent systemic thrombi despite continuous unfractionated heparin infusion. Due to the patient's inability to maintain therapeutic activated partial thromboplastin time (aPTT) values during heparin infusion, bivalirudin was initiated at 0.1 mg/kg/h and increased due to subtherapeutic aPTTs to a maximum of 0.58 mg/kg/h. Therapeutic aPTTs were achieved at the increased dose; however, the patient's worsening renal impairment with resultant drug accumulation and overwhelming sepsis on day 5 of therapy led to discontinuation of the infusion and the initiation of comfort measures.
INDEX TERMS: bivalirudin, infant, pediatrics, thrombin, thromboembolism
INTRODUCTION
Unfractionated heparin administered by continuous infusion is a common pharmacologic therapy for deep venous thrombosis and pulmonary embolisms.1–3 While this medication helps to prevent the formation of additional clots in adults, pediatric patients may possess an intrinsic heparin resistance due to lower concentrations of anti-thrombin III.1,4–6 In addition, hospitalized pediatric patients may be at greater risk for such events due to infection, presence of indwelling catheters that may alter normal blood flow or damage vessel walls, decreased concentrations of C and S proteins, and surgical procedures that may introduce foreign materials.2,3 Therefore, direct thrombin inhibitors may offer additional benefits in this patient population through an anti-thrombin III-independent mechanism of action and inhibition of bound and circulating thrombin.6,7 We report the use of one direct thrombin inhibitor, bivalirudin (Angiomax; The Medicines Company, Parsippany, NJ), in a tertiary care pediatric hospital.
Currently, bivalirudin is approved for anticoagulation therapy in adult patients with heparin-induced thrombocytopenia (HIT) undergoing percutaneous coronary intervention. The decision to use bivalirudin in a pediatric patient did not arise from HIT but from the unpredictable effects of an unfractionated heparin infusion and the hope that a mechanism apart from anti-thrombin would provide more reliable anticoagulation. Because the use of this product in pediatric patients is infrequent, the goal of this report was to share an experience with this medication for anticoagulation in an infant with renal impairment.
CASE REPORT
Our patient was a 2-month-old (3.1 kg), former 39 week, Caucasian female born with Turner syndrome and hypoplastic left heart syndrome. On day 5 of life, the patient underwent surgery for a Norwood procedure with Sano modification and was transferred to the pediatric intensive care unit for postoperative care [creatinine, 0.34 mg/dL; Blood Urea Nitrogen (BUN), 25 mg/dL] On postoperative day 8, a lower extremity venous duplex examination was performed in response to the patient's decreased femoral line function and mottled extremity appearance, which revealed occlusive iliac and femoral deep vein thromboses (DVT). One day prior to these findings, she began receiving prophylactic enoxaparin therapy, 0.75 mg/kg subcutaneously every 12 hours, according to our institution's protocol when central lines are present. While this practice is typically initiated on postoperative day 1 at a therapeutic dosage (1.5 mg/kg every 12 hours for patients ≤2 months of age), it was delayed in our patient due to concern for intracranial bleeding and was started later at a prophylactic dosage.
Following duplex examination results, the patient began receiving a heparin drip, 75 units/kg intravenous bolus, followed by a continuous infusion of 28 units/kg/h. Her baseline activated partial thromboplastin time (aPTT) on the day prior to initiation of heparin therapy was elevated at 69 seconds (institutional control, <29 seconds); reasons for the elevation were unknown, and her goal aPTT was left at the institutional standard of 60 to 85 seconds at that time. During the time she received the heparin infusion, aPTT values ranged from 29 to >200 seconds, with peaks as high as >200 seconds after boluses and later times of <50 seconds. As a result, she was rarely within goal range for prolonged periods and required frequent rate adjustments and boluses.
Repeated duplex examinations and imaging of the abdomen revealed unresolved DVT and a new inferior vena cava thrombus. The heparin infusion was increased to 33 units/kg/h with an aPTT goal of 90 to 105 seconds, a range determined by the intensive care attending physician, which reflects the next tier in the pediatric heparin protocol that would normally require a dosage decrease. In addition, two separate HIT antibody assays were sent for analysis 1 month apart, and both returned negative results. Despite these interventions, all thrombi remained unchanged.
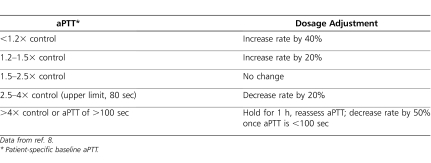
On day 52 of heparin therapy, the decision was made to switch the patient to bivalirudin. Due to the patient's renal impairment (serum creatinine, 0.89 mg/dL), she began receiving bivalirudin, 0.1 mg/kg/h instead of 0.2 mg/kg/h, and was not given a bolus (baseline aPTT, 43 seconds). Her aPTT goal was changed according to institutional direct thrombin inhibitor protocol to 64 to 80 seconds (1.5–2.5× baseline; upper limit, 80 seconds), and aPTTs were collected every 4 hours. Following institutional policy, all aPTT values for patients receiving a direct thrombin inhibitor are assessed by a pharmacist who communicates rate change recommendations to physician and nursing staff, a practice offered 24 hours a day, 7 days a week through an on-call pharmacist program. Values below or above the target goal may result in either a dosage increase or decrease of approximately 20%. The institution's direct thrombin inhibitor protocol is described in Table 1, although patient-specific adjustments differing from the protocol are acceptable following discussions with the medical team.9
Table 1.
Bivalirudin Nomogram
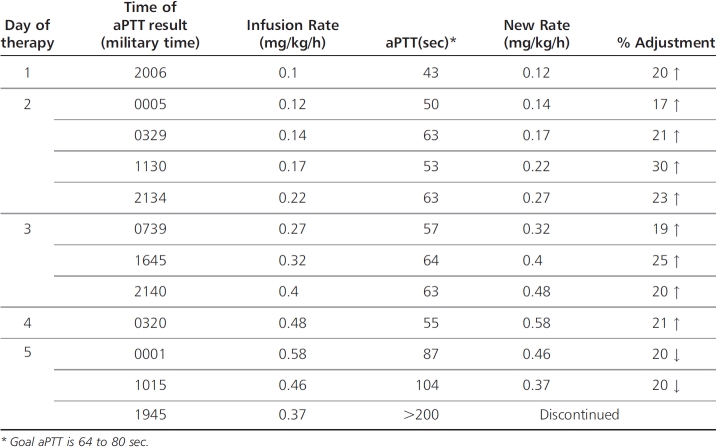
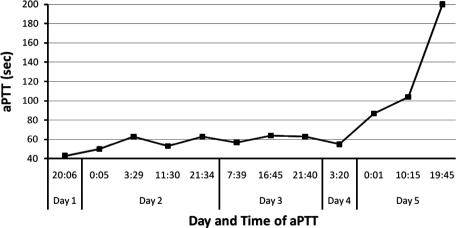
During our patient's time receiving bivalirudin, the infusion rate was steadily increased due to subtherapeutic aPTT values, to a final rate of 0.58 mg/kg/h on day 5 of therapy (Table 2). At that time, the patient's serum creatinine concentration had risen to 0.98 mg/dL from 0.82 mg/dL over a 24-hour period, and aPTT values began to increase concurrently (Figure). As a result, the infusion rate was decreased by 20%. Serum creatinine levels continued to increase the following day to 1.09 mg/dL, and the infusion rate was decreased again by 20%. When aPTT values increased to 104 seconds and then to >200 seconds, it was noted that the patient had increased chest tube output with blood-tinged fluid, and the bivalirudin infusion was turned off. Four days later, the patient began receiving therapeutic enoxaparin, 1.5 mg/kg subcutaneously daily, adjusted for renal impairment. Anti-factor Xa concentrations were found to be 0.45 units/mL at steady state, with the goal of 0.5 to 1 unit/mL. No dosage adjustment was made due to the patient's critical status and worsening renal function.
Table 2.
Bivalirudin Course
Figure.
aPTT trend during bivalirudin infusion. Goal patient aPTT was 64 to 80 seconds.
Nine days after bivalirudin was discontinued, the patient was placed on peritoneal dialysis. She also battled recurrent pneumonia associated with Pseudomonas aeruginosa infection, which later evolved into bacteremia and peritonitis. During her course, she received antibacterial therapy with various treatments including piperacillin/tazobactam, gentamicin, cefepime, tobramycin, meropenem, and levofloxacin, with bacteria displaying greater resistance as her course proceeded. Late in her stay, the patient's peripheral and arterial blood, peritoneal, and bronchial cultures continued to return test results positive for multidrug-resistant P aeruginosa. Soon afterward, increased hemodynamic support was required, and the decision was made to provide comfort measures. Our patient expired on day 79 of life.
DISCUSSION
Direct thrombin inhibitors are often used in adult patients for anticoagulation therapy in the case of HIT. For pediatric patients, there may be additional use for treatment of persistent thrombi or heparin resistance.6,7 Heparin products have unpredictable inter- and intraindividual variability in pediatric patients, especially in patients less than 6 months of age, due to reduced anti-thrombin concentrations. As the mechanism of action of direct thrombin inhibitors is not dependent upon anti-thrombin, pediatric patients with variable anti-thrombin concentrations may benefit from more predictable drug action. Direct effects on bound and circulating thrombin may also provide a small thrombolytic effect.1,4–7
Use of direct thrombin inhibitors has begun to emerge in the pediatric population. A bivalirudin dosage-finding study conducted in 2007 in 16 patients under the age of 6 months with confirmed thrombus. Patients were given 1 of 3 different bolus doses (0.125 mg/kg, 0.25 mg/kg, or 0.5 mg/kg) and 1 of 2 different initial infusion rates (0.125 mg/kg/h or 0.25 mg/kg/h). All but 1 patient achieved a therapeutic aPTT after the initial bolus and infusion rate, and there was no thrombus progression in any patient at 72 hours. Significant hematuria occurred in 2 patients, both of whom were receiving the greatest infusion rates, and resolved after infusion rates were decreased.7
A follow-up study to this report was conducted in Illinois in 16 patients, 1 day to 14 years old.6 The average effective infusion rate was 0.16 mg/kg/h, and the average maximum infusion rate required was 0.25 mg/kg/h, with 0.55 mg/kg/h required in 1 patient.6 Despite the fact that one-fourth of the study group lacked efficacy data due to the retrospective nature of the study, the results of that study differ greatly from ours.
Our patient's condition exceeded previous infusion requirements and did not have consistent aPTT measurements within goal range, as was reported in the previous studies. Our patient was given no bolus, and her therapy was started at an infusion rate of 0.1 mg/kg/h due to renal impairment, an adjustment determined by our institutional protocol. This was very similar to the 0.125 mg/kg/h dosage reported to be effective in the original dosage-finding study. However, we increased the infusion rate to 0.58 mg/kg/h before seeing a significant aPTT increase, an observation likely due to our patient's confounding factors of critical illness, renal impairment, sepsis, and lack of bolus. Bivalirudin is metabolized by blood proteases, with approximately 20% of the drug excreted in the urine, and product labeling calls for dosage reductions at creatinine clearance (CrCl) of <30 mL/min. Our institutional protocol calls for starting infusions of 0.2 mg/kg/h for healthy patients and 0.1 mg/kg/h for critically ill patients, with 0.08 to 0.12 mg/kg/h for CrCl creatinine clearance of 30 to 60 mL/min and 0.05 to 0.08 mg/kg/h for creatinine clearance of <30 mL/min.
Possible explanations for our patient's lack of response are multifactorial. We did not administer a bolus dose to our patient at the initiation of bivalirudin, which may have lengthened her time to reach therapeutic aPTT values. Additionally, according to our institutional protocol, aPTT values are assessed every 4 hours for patients receiving direct thrombin inhibitors. It may be that our patient's renal impairment was such that 4 hours was not enough time in which to see effects from dose modifications until significant accumulation had occurred. Another possibility is that the patient was clearing the medication appropriately but as her severity of illness increased with renal impairment and infection she experienced significant accumulation and response to the bivalirudin. Evidence of this may lie in the increased bloody discharge from her chest tube prior to drug discontinuation. In addition, there is the possibility of Disseminated intravascular coagulation (DIC) during our patient's septic episodes. While a DIC panel was drawn at the initiation of bivalirudin (D-dimer, 1.62 mg/L; aPTT, 43 seconds; prothrombin time [PT], 12.3 seconds; International Normalized. Ratio [INR], 1.2), a repeat panel was not drawn at its discontinuation. Finally, the time lag from the start of unfractionated heparin to the initiation of bivalirudin may have allowed organization of thrombi, making them less susceptible to treatment.
One final consideration, from our bivalirudin experience, is that current dosing recommendations are too conservative for pediatric patients. Similar to emerging data from the use of low-molecular-weight heparin products, increased initial dosing regimens may be required to rapidly achieve therapeutic goals. To avoid a prolonged time to therapeutic goal, as experienced in our patient, it may be beneficial to initiate bivalirudin at 0.2 mg/kg/h and aggressively titrate every 4 hours by 20% to 40% and then decrease if necessary (Table 2). Furthermore, a bolus dose may be beneficial in pediatric patients to achieve therapeutic aPTT values in a reasonable amount of time. As more experience is gained, future studies will likely provide additional insight on appropriate initiation and titration of direct thrombin inhibitor therapy in the pediatric population.
SUMMARY
This report describes the first pediatric patient at our institution to receive a direct thrombin inhibitor. The decision to start bivalirudin therapy was made due to concern for the patient's heparin resistance and occurrence of new thrombi while she received unfractionated heparin therapy. Our patient had multiple risk factors for thromboembolic complications, for example, age <1 year, surgery, central venous catheters, and sepsis. She also may have had an element of heparin resistance, as at 3 months old, her anti-thrombin concentrations may not have reached adult values.2,3 Despite that, it is clear that more studies will be needed to fully elucidate ideal dosage, dosage adjustments, and monitoring requirements.
ABBREVIATIONS
- aPTT
activated partial thromboplastin time
- CrCl
creatinine clearance
- DIC
Disseminated intravascular coagulation
- DVT
deep vein thrombosis
- HIT
heparin-induced thrombocytopenia
Footnotes
DISCLOSURE The authors declare no conflicts or financial interest in any product or service mentioned in the manuscript, including grants, equipment, medications, employment, gifts, and honoraria.
REFERENCES
- 1.Newall F, Johnston L, Ignjatovic V, et al. Unfractionated heparin therapy in infants and children. Pediatrics. 2009;123(3):e510–e518. doi: 10.1542/peds.2008-2052. [DOI] [PubMed] [Google Scholar]
- 2.Schneppenheim R, Greiner J. Thrombosis in infants and children. Hematology Am Soc Hematol Educ Program. 2006. pp. 86–96. [DOI] [PubMed]
- 3.Chalmers E. Epidemiology of venous thromboembolism in neonates and children. Thromb Res. 2006;118(1):3–12. doi: 10.1016/j.thromres.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 4.Newall F, Ignjatovic V, Summerhayes R, et al. In vivo age dependency of unfractionated heparin in infants and children. Thromb Res. 2009;123(5):710–714. doi: 10.1016/j.thromres.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 5.Ignjatovic V, Summerhayes R, Than J, et al. Therapeutic range for unfractionated heparin therapy: age-related differences in response in children. J Thromb Haemost. 2006;4(10):2280–2283. doi: 10.1111/j.1538-7836.2006.02136.x. [DOI] [PubMed] [Google Scholar]
- 6.Young G. New anticoagulants in children. Hematology Am Soc Hematol Educ Program. 2008. pp. 245–250. [DOI] [PubMed]
- 7.Rayapudi S, Torres A, Deshpande G, et al. Bivalirudin for anticoagulation in children. Pediatr Blood Cancer. 2008;51(6):798–801. doi: 10.1002/pbc.21731. [DOI] [PubMed] [Google Scholar]
- 8.Young G, Tarantino M, Wohrley J, et al. Pilot dose-finding and safety study of bivalirudin in infants <6 months of age with thrombosis. J Thromb Haemost. 2007;5(8):1654–1659. doi: 10.1111/j.1538-7836.2007.02623.x. [DOI] [PubMed] [Google Scholar]
- 9.Davis G, Lewis D, Ensom M. Direct Thrombin Inhibitors. Clinical Pharmacokinetics Service and Anticoagulation Guidelines. 31st ed. Lexington, KY: Preston Publishing;; 2009. [Google Scholar]