Abstract
Respiratory syncytial virus (RSV) bronchiolitis is the leading cause of infant hospitalization in the United States. Prophylaxis with palivizumab is effective in reducing RSV hospitalizations in premature infants and in infants or children with chronic lung disease or congenital heart disease. Patients with CF or those who are immunocompromised may be at increased risk for RSV infection–related complications; hence, prophylaxis may prove beneficial to these populations. The extent of palivizumab use in the CF and immunocompromised populations is variable. Palivizumab appears to be safe and may be effective in infants and young children with CF and immunocompromise. However, well-designed, randomized, controlled trials published in peer-reviewed journals are lacking, and its routine use can therefore not be recommended at this time. If used in patients with CF or those who are immunocompromised, RSV prophylaxis should be restricted to peak outbreak months in order to optimize the cost benefit of palivizumab.
INDEX TERMS: cystic fibrosis, immunosuppression, palivizumab, respiratory syncytial virus
INTRODUCTION
Respiratory syncytial virus (RSV), a common cause of serious respiratory tract infections in infants and young children, is a leading cause of viral death among infants.1,2 Although RSV prophylaxis has been shown to be effective in reducing RSV hospitalizations in select premature infants and infants or children with chronic lung disease (CLD) or congenital heart disease (CHD),3–6 there exist limited data supporting its use in the cystic fibrosis (CF)7–11 and immunocompromised populations.12 Citing insufficient evidence, the American Academy of Pediatrics (AAP) stated13 in its recently released RSV prophylaxis guideline that specific recommendations for RSV prophylaxis cannot be made in these populations. This review will discuss the impact of RSV infection in patients with CF and immunocompromise and will focus on the safety and efficacy of RSV prophylaxis with palivizumab in these populations.
RESPIRATORY SYNCYTIAL VIRUS INFECTION AND PROPHYLAXIS
In the United States, the RSV season typically begins in November or December and ends in March or April, although the precise onset and offset is regionally dependent.14 Most infants and children infected with RSV will develop mild upper respiratory tract symptoms. However, a subset of patients will progress to severe disease presenting as bronchiolitis and/or pneumonia.1,15
Prevention of RSV during seasonal peaks is critical to reducing RSV-related hospitalization.
The AAP recommends RSV prophylaxis be considered in patients at highest risk for severe RSV disease: premature infants, infants or children <24 months of age with CLD, infants or children ≤24 months of age with hemodynamically significant CHD, and premature infants with congenital abnormalities of the airway or neuromuscular disease that compromises the handling of respiratory secretions.13 In these high-risk populations, RSV prophylaxis has been shown to reduce the incidence of RSV-hospitalization 41–63% in prospective, randomized, double-blind, placebo-controlled trials.3–6 Palivizumab, a humanized murine monoclonal antibody, given once monthly during RSV season (typically 5 doses), is the preferred agent for RSV prophylaxis.13
CYSTIC FIBROSIS
The airway of an individual with CF is exposed to a vicious cycle of obstruction, infection, and inflammation. As a result, patients with CF experience acute pulmonary exacerbations and a decline in pulmonary function throughout their lifetime.16 The airway is infected with both viruses and bacteria, the latter of which chronically colonize the airway.16 Some patients with CF may be at increased risk of RSV infection, and it has been suggested17 that RSV infection may exacerbate the CLD of CF. Based on the potential utility of RSV prophylaxis in this population, the CF Foundation has recommended that palivizumab be considered for infants and children younger than 2 years of age.18
Respiratory Syncytial Virus Infection in Cystic Fibrosis
RSV infection has been shown19–22 to augment airway inflammation. In infants with CF, a marked inflammatory response in the lower airway during viral lower respiratory tract infection (LRTI) has been observed.22 Viral infection of the CF airway may also favor bacterial colonization through promotion of bacterial adherence to the respiratory epithelium and modulation of the patient's innate immune response.21,23,24 This has been evidenced by an increased risk for early acquisition of Pseudomonas aeruginosa among infants with CF who are hospitalized for viral LRTI.22
Viral infection (including RSV) is associated with acute pulmonary exacerbations of CF in infants and young children aged <3 years17,22,25 and in older children and adolescents.26 The RSV-related hospitalization rate among infants and young children with CF aged <3 years has been reported to range from 8.75% to 14.6%,17,22,25 while among older children and adolescents with CV, the rate has been reported to be approximately 12.5%.27 This compares to rates of 3% to 37% in other high-risk populations (i.e., those with prematurity, CLD, CHD);1,15,28–31 and exceeds the reported incidence of <1% among low-risk patients aged <3 years.28 In contrast, the RSV-related hospitalization rate among adults with CF is reported to be 0.5%, much less than that observed in the younger CF population.32 Infants and young children with CF who are hospitalized for RSV often have prolonged hospital admissions and may necessitate mechanical ventilation for respiratory failure. Upon discharge home, they often require continuous home oxygen for persistent hypoxemia.25 The decline in lung function among infants and young children with CF aged <2 years with an RSV-LRTI can persist for several months after resolution of the infection.17 At 2-year follow-up, infants and young children with CF aged <3 years with a history of RSV-related hospitalization more commonly had chronic respiratory signs and worse chest x-ray scores than did those not admitted with an RSV-LRTI.25 Similarly, the annual incidence of viral LRTI (RSV accounting for 19% of symptomatic infections) among older children and adolescents with CF has been significantly correlated with a decline in clinical score (based on the patient's general activity, nutritional status, physical examination, and chest x-ray findings), a lower weight-to-height ratio, and pulmonary deterioration, as depicted by decreases in forced vital capacity, forced-expiratory volume in 1 second, forced expiratory flow in mid-expiration (25%-75%), and the frequency and duration of hospitalizations for acute pulmonary exacerbations.26 Prophylaxis against RSV may be beneficial in the CF population.
Respiratory Syncytial Virus Prophylaxis in Cystic Fibrosis
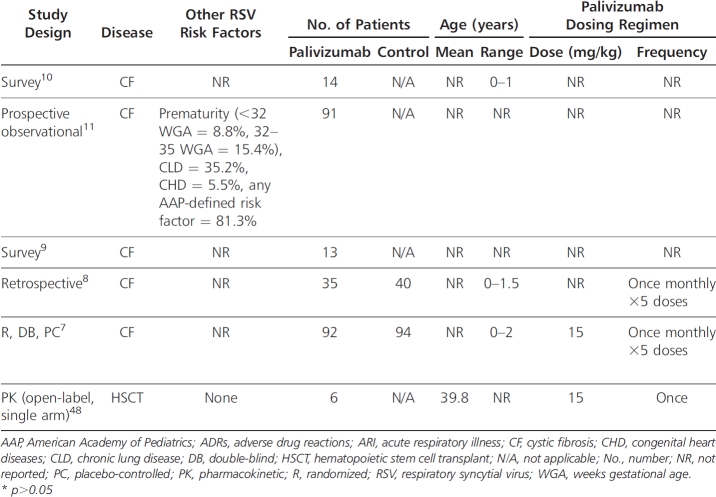
The extent of palivizumab use in the CF population is not widespread, likely owing to its high acquisition cost and the limited evidence supporting its use. In a UK survey study10 conducted during the 2005–2006 RSV season, only 3 of 34 (8.8%) surveyed centers reported using palivizumab in their CF population. Across these 34 centers, 14 of 143 (9.8%) infants with CF aged <1 year received palivizumab. In the United States, the palivizumab outcomes registry, which prospectively collected data on the usage of palivizumab during 4 consecutive RSV seasons (2000–2004), identified 91 of 19,548 (0.47%) infants and children with a diagnosis of CF.11 A survey study9 of North American CF centers conducted during the 2006–2007 RSV season assessed the usage of palivizumab within 83 CF centers across the United States (n=73) and Canada (n=10). The percentage of centers that prescribed palivizumab was 73.5% (United States=75%, Canada=60%), although only 38% of centers reported routinely prescribing the medication for all infants with CF. Palivizumab treatment during the second RSV season was less common, with 39.8% reporting its use (United States=40%, Canada=40%). It was the opinion of 41% of CF center directors (United States=43%, Canada=30%) that palivizumab prophylaxis was the standard of care for infants with CF.
The impact of RSV prophylaxis with palivizumab in the CF population is limited to 2 survey studies, 1 retrospective analysis, data collected via the palivizumab outcomes registry, and 1 double-blind, placebo-controlled study that was published only in abstract form (Table).7–11
Table.
Effectiveness and Safety of Palivizumab Prophylaxis in Cystic Fibrosis and Immunocompromise
Table.
(extended)
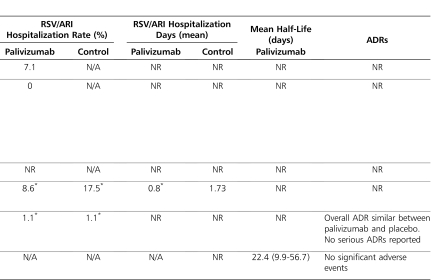
A survey study10 conducted during the 2005–2006 RSV season aimed to assess the incidence of hospitalization and mortality due to RSV infection in infants with CF across 42 UK CF centers. The definition for RSV hospitalization was not stated. Of the 143 infants aged <1 year with CF that were identified across the 34 responding CF centers (81% survey response rate), 14 (9.8%) received palivizumab (dosing regimen not reported). Hospitalization due to RSV infection was reported in 16 of 143 (11.2%) infants, none of whom required admission to the pediatric intensive care unit (ICU) or died. Among the 14 infants who received palivizumab, one (7.1%) was hospitalized (other risk factors for this infant were not reported), which is similar to the population hospitalization rate.
The palivizumab outcomes registry prospectively collected data on 91 infants and children with CF who received ≥1 dose of palivizumab during 4 consecutive RSV seasons (2000–2004) with the aim of identifying hospitalization rates in pediatric CF.11 This patient population consisted of 8 patients (8.8%) born <32 weeks gestational age (WGA), 14 (15.4%) born between 32 and 35 WGA, and 69 (75.8%) born >35 WGA, with 22 (24.2%) patients born weighing <2500 g. CLD and CHD were also present in 32 (35.2%) and 5 (5.5%) patients, respectively. At least one 2006 AAP-defined risk factor (CLD, CHD, multiple births, tobacco smoke exposure, daycare attendance) was present in 74 (81.3%) patients. Eight patients (8.8%) were aged 0 to 3 months, 12 (13.2%) were aged 3 to 6 months, 29 (31.9%) were aged 6 to 12 months, and 42 (46.2%) were >12 months of age. RSV hospitalization was defined as any hospitalization for ≥24 hours during which RSV infection was confirmed by virology testing (rapid antigen detection or viral culture). Infants who were hospitalized within 24 hours of their first palivizumab dose were excluded. The majority of infants (86.8%) were compliant with the palivizumab regimen. Of the 91 infants, many of whom had risk factors for RSV hospitalization in addition to their diagnosis of CF, none were hospitalized with an RSV-LRTI.
A survey study9 conducted during the 2006–2007 RSV season aimed to assess the incidence of RSV infection and RSV-related hospitalization among infants with CF across 139 North American CF centers. Of the 435 infants with CF who were identified across the 83 responding CF centers (60% survey response rate), 74 infants (16%) had a documented RSV infection. Of these RSV-infected infants, 31 (7.1%) responded to outpatient management, while 43 (9.2%) required hospitalization, 3 (0.7%) of whom were admitted to the ICU, requiring intubation. Although the total number of infants who received palivizumab was not reported, among the 74 infants with a documented RSV infection, 14 (18.9%) received palivizumab, while 61 (82.4%) did not.
A recent retrospective analysis8 aimed to assess the impact of palivizumab on hospitalization rate for acute respiratory illness (ARI) in young children with CF during their first RSV season following diagnosis. An ARI was defined as a hospital admission resulting from any acute change in respiratory status (increased cough, wheezing, respiratory rate, etc) with or without systemic signs (fever, decreased oral intake, fatigue, etc) or acute changes in the chest x-ray. RSV infection was defined as an ARI that was associated with a positive enzyme-linked immunosorbent assay and/or viral culture of nasopharyngeal aspirates. The medical records of 75 patients diagnosed with CF before the age of 18 months were included; 40 of the infants did not receive palivizumab (control group) and 35 received palivizumab once monthly (November-March) during RSV season (prophylaxis group). At the onset of RSV season, 29 of 40 (72.5%) patients in the control group and 28 of 35 (80%) patients in the prophylaxis group were <12 months of age (mean age not reported). At baseline, 12.5% of patients in the control group and 5.7% of patients in the prophylaxis group were colonized with Pseudomonas aeruginosa, while 5% of patients in the control group and 22.9% of patients in the prophylaxis group were colonized with Staphylococcus aureus. Compliance with palivizumab was good; 33 of 35 (94%) patients received all 5 scheduled doses, with the remaining 2 patients receiving 4 doses. All patients received influenza vaccinations. In the control group, 7 of 40 (17.5%) patients were hospitalized for an ARI, compared with 3 of 35 (8.6%) patients in the prophylaxis group (p>0.05). Among the hospitalized patients, only 1 patient (control group) had a positive bacterial sputum culture, which was significant for Pseudomonas aeruginosa. There was no significant difference in antibiotic use between the treatment groups. The duration of hospitalization among these patients was similar between the control (median 11 days; range, 3-14 days) and prophylaxis (median 13 days; range, 2-13 days) groups. One patient in the control group required mechanical ventilation. Among hospitalized patients, 3 of 4 patients in the control group (3 patients not tested) and 0 of 3 patients in the prophylaxis group who were screened for RSV infection tested RSV-positive. A non–statistically significant decrease in ARI-related days in the hospital for palivizumab recipients (compared with those patients who did not receive prophylaxis) was observed (mean 0.8 ± 3.07 vs 1.73 ± 4.27 days; p>0.05). Based on multivariable Poisson regression analysis, the number of hospital days was reduced 54% among palivizumab recipients, although no association was attributed based on the confidence interval. Although this study was not able to show a statistically significant difference in hospitalizations, a power analysis was not conducted, and the sample size may have been too small to detect a difference if one existed. The major limitations of this study are its retrospective design, the small number of patients, and the lack of data detailing RSV infection status among hospitalized patients. The primary endpoint, hospitalizations for ARI, is nonspecific for RSV and conflicts with endpoints used in previous efficacy/safety studies.4,5 All of the patients in the control group were not tested for RSV infection, and the actual incidence of hospitalization secondary to RSV infection may, therefore, have been lower than the reported incidence of hospitalization for ARI in this group.
A randomized, double-blind, placebo-controlled, multicenter, phase-IV study7 of 186 infants (<2 years of age) with CF was conducted over the course of 3 consecutive RSV seasons to assess the efficacy and safety of palivizumab in this population. Ninety-two infants with CF received palivizumab (15 mg/kg intramuscularly once monthly for 5 months during RSV season, November-March), and 94 infants with CF received placebo. Overall, compliance with the treatment was good, with 96% of patients in each group receiving all 5 injections. The percentage of patients with RSV-positive antigen tests was less among palivizumab recipients (13% vs 23%; p-value not reported). However, the rate of hospitalization was similar between infants who received and infants who did not receive prophylaxis. Although specific data were not provided, the authors reported there were no meaningful differences for other outcomes: weight-to-height ratio, colonization with Pseudomonas aeruginosa, wheezing episodes, usage of pulmonary medications, and duration of corticosteroid treatment. There were no deaths reported in either group. The overall incidence of adverse effects was similar between the treatment and placebo groups, and no serious adverse effects relating to palivizumab were reported. The results of this study were reported in abstract form only. Therefore, critical assessment of the study methodology and results is difficult, and definitive conclusions cannot be made, as the study was not published in a peer-reviewed journal.
IMMUNOCOMPROMISE
RSV is a major cause of disease among immunocompromised children and adults, including bone marrow and solid-organ transplant recipients, patients with severe combined immunodeficiency syndrome, and patients receiving chemotherapy.33–35 Respiratory viral infections in the immunocompromised patient population are often characterized by a higher frequency of nosocomial infection acquisition, prolonged persistence of the infection, prolonged viral shedding, and a high frequency of LRTI and related mortality.35,36 Since treatment options for RSV are limited, prevention plays a vital role in the management of respiratory viral infections in immunocompromised patients.37,38 The utility of RSV prophylaxis in the immunocompromised population has therefore been considered.
Respiratory Syncytial Virus Infection in Immunocompromised Patients
In the adult bone marrow transplant (BMT) population, the incidence of RSV has been reported to be 31 of 199 (16%), with 23 of 31 (74%) RSV-infected patients having a recent history of an inpatient hospital stay of longer than 7 days.39 The incidence of RSV-LRTI has been reported to be 61% (20/33), with 16 of 21 (76%) cases occurring <1 month posttransplantation.39,40 The mortality rate among a small population of BMT patients with an RSV-LRTI was significant, ranging from 78% to 100%.39,40
RSV has also been identified as an emerging pathogen among solid-organ transplant recipients.41–44 An RSV infection rate of 3.4% (17/493) has been observed in the pediatric liver transplant population, with nosocomial infection during recovery from transplant being responsible for 76% (13/17) of cases. In one study, among 17 patients infected with RSV, 2 died from progressive pulmonary disease. Two risk factors for severe RSV disease have been identified: preexisting pulmonary disease and early acquisition (<20 days) of RSV after transplantation, indicating that the risk may be related to the higher degree of immunocompromise associated with this time period.44
Patients with severe combined immunodeficiency syndrome have profound and long-lasting immunocompromise requiring BMT and are therefore susceptible to severe, recurrent pulmonary infections with RSV. In this patient population, it has been reported that 4.1% (3/73) of children acquire an RSV infection before or at the time of transplantation. In one study45 of RSV infected children, 33% (1/3) died from an RSV-LRTI, despite treatment with a combination of inhaled ribavirin and intravenous immune globulin.
Large hospital outbreaks of RSV among this high-risk population have resulted in significant mortality rates.35,46 Since the early symptoms of RSV infection often begin with mild upper respiratory tract symptoms, the opportunity to detect and initiate therapy may be delayed. In addition, progression to LRTI may often resemble other opportunistic infections common to this population, making a clinical diagnosis difficult and further delaying treatment.33 Prophylaxis against RSV may thus be of benefit in the immunocompromised patient population.
Respiratory Syncytial Virus Prophylaxis in Immunocompromised Patients
Currently, the AAP states that specific recommendations regarding the use of palivizumab for prevention of RSV in immunocompromised children cannot be made. However, the guidelines do recognize that individuals with severe immunodeficiency (i.e., severe combined immunodeficiency or advanced acquired immunodeficiency syndrome) may benefit from RSV prophylaxis.13
In order to gauge and understand current practices for the prevention of RSV infection in the immunocompromised population, a survey study41 of 108 pediatric liver, heart, lung, intestinal, and heart-lung transplant programs in the United States was conducted. Among the 62% (67/108) of institutions that completed the survey, 49% (33/67) reported using RSV prophylaxis in this patient population, and 32 (97%) of these 33 centers administered palivizumab. Palivizumab was more frequently used in children aged 0 to 12 months (compared to the extended age group of 0–24 months; 93% vs 79%, respectively). Three centers reported using palivizumab between the ages of 2 and 4 years, and 2 centers reported its use in children over the age of 4 years. A lack of randomized, controlled trials in the immunocompromised patient population has prevented the routine use of palivizumab.
The role of palivizumab among the immunocompromised population has been evaluated in both animal and phase-I clinical trials. In an animal study,47 immunocompromised rats (receiving intraperitoneal injections of cyclophosphamide for ≥21 days) were given a single 15 mg/kg dose of palivizumab and were subsequently infected with RSV. Marked leukopenia, defined as a >80% reduction in absolute neutrophil and lymphocyte counts, was observed at day 21 in all immunocompromised rats. On day 4 post–RSV infection, viral titers in palivizumab recipients were significantly reduced in both immunocompromised and control rats who received palivizumab (p<0.001). However, multiple doses of palivizumab were required at 4-day intervals in order to prevent “rebound” viral replication in continually immunocompromised rats.
The safety, tolerability, pharmacokinetics, and immunogenicity of intravenous palivizumab (15 mg/kg) as a single infusion was studied in a phase-I study48 involving hematopoietic stem cell transplant (HSCT) recipients (Table). The study included 6 HSCT recipients without active RSV disease, 5 of whom were autologous HSCT recipients. The mean serum half-life of palivizumab was 22.4 days (range, 9.9-56.7 days). The mean serum palivizumab trough concentration at 30 days was 41.9 mg/L (range, 22.4–58.6 mg/L), which is similar to the mean serum concentration of 37 mcg/ml deemed effective in the IMpact study.4 The serum palivizumab concentration associated with significant anti-RSV activity was maintained for 7 days in all patients and for 21 days in 83% of patients. No significant adverse effects were noted.
The significance of respiratory infections among immunocompromised patients and the lack of data from randomized, double-blind, controlled trials for RSV prophylaxis prompted a decision tree analysis.12 This analysis evaluated the mortality from RSV-related lung disease in children who received palivizumab after BMT within the past year and who were now approaching RSV season. The following assumptions were made to simplify the model: 1) the effectiveness of palivizumab in BMT patients is similar to that of other high-risk populations that have already been evaluated, 2) the rate of exposure to RSV in the immunocompromised population is similar to that of the general population, 3) the criteria for hospitalization of an infected individual would be the same regardless of whether palivizumab was administered or not, and 4) children who were never hospitalized would not experience an RSV-related death (Figure). Using probabilities from currently available medical literature, researchers concluded there is a 10% increase in survival in BMT patients who receive palivizumab, with an absolute survival rate increase from 83% to 92%. Based on these survival rates, it was reported that 12 children would need to be treated to save 1 post-BMT patient from dying from RSV-related lung disease. In addition, a sensitivity analysis concluded that palivizumab demonstrated improvement in mortality that was robust over a range of biologically plausible values. The major limitation of this decision analysis was the assumption that the risk of hospitalization in the BMT population is equivalent to that of the CLD and CHD populations, which may or may not be true. Although the use of a decision tree model is not an ideal study methodology when compared to a randomized, controlled, double-blinded study, it may provide assistance during clinical decision-making when adequate trials are lacking.
Figure.
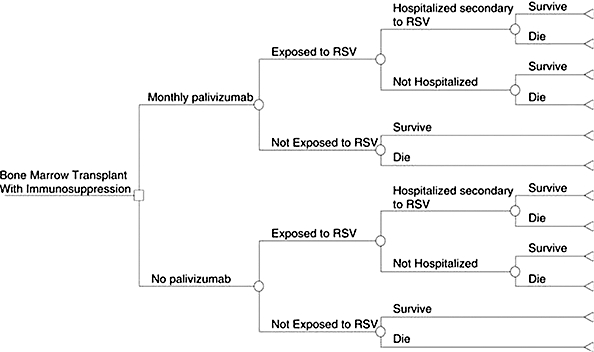
Decision tree analysis model (from Thomas et al. [12]; used with permission). Square, “Decision Node”; Circle, “Chance Node”; Triangle, “Terminal Node.”
COST-EFFECTIVENESS OF PALIVIZUMAB
Data collected between 1997 and 2002 indicated the economic burden of severe disease necessitating hospital admission among infants to be quite substantial, with admissions costing between US$469 million and US$1.1 billion annually.49–51 Palivizumab is supplied preservative-free in 50-mg and 100-mg single-use vials at an average wholesale price of approximately US$1018 and US$1923 per vial, respectively.52 A 5-dose regimen of palivizumab at 15 mg/kg per dose will therefore cost the average 4.8-kg infant US$9615 per season.53 The acquisition cost of palivizumab may outweigh the potential economic benefits of prophylaxis, and extensive studies into the costs and benefits associated with use of this agent in patients with prematurity, CLD, and CHD, have therefore been conducted.53 While acceptable cost-effectiveness ratios with palivizumab prophylaxis have been reported in some analyses, the majority have failed to show cost-savings or cost-effectiveness ratios below commonly accepted thresholds.53 To date, no studies have assessed the cost-effectiveness of palivizumab in the CF or immunocompromised populations, and further study is therefore indicated. While cost-effectiveness is not the only determining factor, it must be considered in light of the clinical effectiveness and safety of palivizumab.
CONCLUSION
RSV prophylaxis has been shown to reduce the incidence of hospitalization related to severe RSV disease in high-risk infants. The definition of high risk is controversial, and CF and immunocompromised patients have been suggested to represent populations worthy of consideration when determining allocation of RSV prophylaxis.
Although the CF Foundation has recommended that RSV prophylaxis with palivizumab be considered for infants and children younger than 2 years of age, the extent of palivizumab use in the CF population is variable, with 8.8% to 73.5% of CF centers reporting its use. Pharmacokinetic studies in this population justifying the recommended 15 mg/kg dose are lacking, which is of potential importance given the altered pharmacokinetic profile of many medications in the CF population. Available data indicate that palivizumab may be safe and of possible benefit in infants and young children <2 years of age with CF, theoretically mitigating airway inflammation and lessening the risk for early acquisition of bacteria. However, statistically significant differences in the rates of hospitalization for RSV-LRTI and ARI between infants and young children who received and who did not receive RSV prophylaxis have not been reported, indicating a lack of benefit. Definitive conclusions regarding the efficacy of palivizumab in preventing RSV-related hospitalization can therefore not be made, since well-designed studies have not been published in the peer-reviewed literature.
RSV prophylaxis is utilized by 49% of solid-organ transplant centers. It is more frequently used among infants, although its use in children older than 4 years of age has been reported. Data indicate that the immunocompromised population is at high risk for RSV-related complications. Pharmacokinetic studies in this population indicate that adequate palivizumab serum concentrations for prophylaxis can be attained with the recommended 15 mg/kg dose. In addition, the results from an animal study, a phase-I trial, and a decision tree analysis indicate a possible benefit of palivizumab in this population. However, well-designed clinical trials have not been conducted, and consensus recommendations can therefore not be made.
Although it would not account for the long-term impact of RSV in these patient populations, published, randomized, controlled, prospective clinical trials involving study of large patient populations with the primary endpoint of hospitalization due to RSV are needed in order to determine the safety and effectiveness of palivizumab in both the CF and immunocompromised patient populations before its routine use can be recommended. Given the substantial acquisition cost of palivizumab, pharmacoeconomic studies are also needed, particularly in light of the current status of health care and the national economic crisis. If palivizumab is utilized in the CF or immunocompromised populations, its use should be restricted to peak outbreak months in order to optimize the cost benefit of palivizumab.
ACKNOWLEDGMENT
We would like to thank Dr Luke Probst for his critical review of our manuscript.
ABBREVIATIONS
- AAP
American Academy of Pediatrics
- ARI
acute respiratory illness
- BMT
bone marrow transplant
- CF
cystic fibrosis
- CHD
congenital heart disease
- CLD
chronic lung disease
- HSCT
hematopoietic stem cell transplant
- ICU
intensive care unit
- LRTI
lower respiratory tract infection
- RSV
respiratory syncytial virus
- WGA
weeks gestational age
Footnotes
DISCLOSURE The authors declare no conflicts or financial interest in any product or service mentioned in the manuscript, including grants, equipment, medications, employment, gifts, and honoraria.
see related editorial page 74
REFERENCES
- 1.Black CP. Systematic review of the biology and medical management of respiratory syncytial virus infection. Respir Care. 2003;;48(3):209–231. 31–33. discussion. [PubMed] [Google Scholar]
- 2.Thompson WW, Shay DK, Weintraub E, et al. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA. 2003;;289(2):179–186. doi: 10.1001/jama.289.2.179. [DOI] [PubMed] [Google Scholar]
- 3.The PREVENT Study Group. Reduction of respiratory syncytial virus hospitalization among premature infants and infants with bronchopulmonary dysplasia using respiratory syncytial virus immune globulin prophylaxis. Pediatrics. 1997;;99(1):93–99. doi: 10.1542/peds.99.1.93. [DOI] [PubMed] [Google Scholar]
- 4.Palivizumab, a humanized respiratory syncytial virus monoclonal antibody, reduces hospitalization from respiratory syncytial virus infection in high-risk infants. The IMpact-RSV Study Group. Pediatrics. 1998;;102((3 Pt 1)):531–537. [PubMed] [Google Scholar]
- 5.Feltes TF, Cabalka AK, Meissner HC, et al. Palivizumab prophylaxis reduces hospitalization due to respiratory syncytial virus in young children with hemodynamically significant congenital heart disease. J Pediatr. 2003;;143(4):532–540. doi: 10.1067/s0022-3476(03)00454-2. [DOI] [PubMed] [Google Scholar]
- 6.Groothuis JR, Simoes EA, Levin MJ, et al. Prophylactic administration of respiratory syncytial virus immune globulin to high-risk infants and young children. The Respiratory Syncytial Virus Immune Globulin Study Group. N Engl J Med. 1993;;329(21):1524–1530. doi: 10.1056/NEJM199311183292102. [DOI] [PubMed] [Google Scholar]
- 7.Cohen A, Boron M, Dingivan C. A phase IV study of the safety of Synagis® (palivizumab) for prophylaxis of respiratory syncytial virus disease in children with cystic fibrosis [abstract] American Thoracic Society Abstracts, 2005 International Conference; 2005. p. A178. p. [Google Scholar]
- 8.Giebels K, Marcotte JE, Podoba J, et al. Prophylaxis against respiratory syncytial virus in young children with cystic fibrosis. Pediatr Pulmonol. 2008;;43(2):169–174. doi: 10.1002/ppul.20751. [DOI] [PubMed] [Google Scholar]
- 9.Giusti R. North American synagis prophylaxis survey. Pediatr Pulmonol. 2009;;44(1):96–98. doi: 10.1002/ppul.20922. [DOI] [PubMed] [Google Scholar]
- 10.McCormick J, Southern KW. A survey of palivizumab for infants with cystic fibrosis in the UK. Arch Dis Child. 2007;;92(1):87–88. doi: 10.1136/adc.2006.0105338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Speer ME, Fernandes CJ, Boron M, Groothuis JR. Use of palivizumab for prevention of hospitalization as a result of respiratory syncytial virus in infants with CF. Pediatr Infect Dis J. 2008;;27(6):559–561. doi: 10.1097/INF.0b013e3181673c15. [DOI] [PubMed] [Google Scholar]
- 12.Thomas NJ, Hollenbeak CS, Ceneviva GD, et al. Palivizumab prophylaxis to prevent respiratory syncytial virus mortality after pediatric bone marrow transplantation: a decision analysis model. J Pediatr Hematol Oncol. 2007;;29(4):227–232. doi: 10.1097/MPH.0b013e3180437ded. [DOI] [PubMed] [Google Scholar]
- 13.Committee on Infectious Diseases. Modified recommendations for use of palivizumab for prevention of respiratory syncytial virus infections. Pediatrics. 2009;;124(6):1694–1701. doi: 10.1542/peds.2009-2345. [DOI] [PubMed] [Google Scholar]
- 14.Forbes M. Strategies for preventing respiratory syncytial virus. Am J Health Syst Pharm. 2008;;65((23 suppl 8)):S13–S19. doi: 10.2146/ajhp080440. [DOI] [PubMed] [Google Scholar]
- 15.Checchia P. Identification and management of severe respiratory syncytial virus. Am J Health Syst Pharm. 2008;;65((23 suppl 8)):S7–S12. doi: 10.2146/ajhp080439. [DOI] [PubMed] [Google Scholar]
- 16.Cystic Fibrosis Foundation. Cystic Fibrosis Foundation Patient Registry, 2006 Annual Data Report to the Center Directors. Bethesda, MD: The Foundation; 2007. [Google Scholar]
- 17.Hiatt PW, Grace SC, Kozinetz CA, et al. Effects of viral lower respiratory tract infection on lung function in infants with cystic fibrosis. Pediatrics. 1999;;103(3):619–626. doi: 10.1542/peds.103.3.619. [DOI] [PubMed] [Google Scholar]
- 18.Borowitz D, Robinson KA, Rosenfeld M, et al. Cystic Fibrosis Foundation evidence-based guidelines for management of infants with cystic fibrosis. J Pediatr. 2009;;155((6 suppl)):S73–S93. doi: 10.1016/j.jpeds.2009.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miller AL, Bowlin TL, Lukacs NW. Respiratory syncytial virus-induced chemokine production: linking viral replication to chemokine production in vitro and in vivo. J Infect Dis. 2004;;189(8):1419–1430. doi: 10.1086/382958. [DOI] [PubMed] [Google Scholar]
- 20.Stark JM, Godding V, Sedgwick JB, Busse WW. Respiratory syncytial virus infection enhances neutrophil and eosinophil adhesion to cultured respiratory epithelial cells. Roles of CD18 and intercellular adhesion molecule-1. J Immunol. 1996;;156(12):4774–4782. [PubMed] [Google Scholar]
- 21.Groskreutz DJ, Monick MM, Powers LS, et al. Respiratory syncytial virus induces TLR3 protein and protein kinase R, leading to increased double-stranded RNA responsiveness in airway epithelial cells. J Immunol. 2006;;176(3):1733–1740. doi: 10.4049/jimmunol.176.3.1733. [DOI] [PubMed] [Google Scholar]
- 22.Armstrong D, Grimwood K, Carlin JB, et al. Severe viral respiratory infections in infants with cystic fibrosis. Pediatr Pulmonol. 1998;;26(6):371–379. doi: 10.1002/(sici)1099-0496(199812)26:6<371::aid-ppul1>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 23.Avadhanula V, Rodriguez CA, Devincenzo JP, et al. Respiratory viruses augment the adhesion of bacterial pathogens to respiratory epithelium in a viral species- and cell type-dependent manner. J Virol. 2006;;80(4):1629–1636. doi: 10.1128/JVI.80.4.1629-1636.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Van Ewijk BE, Wolfs TF, Aerts PC, et al. RSV mediates Pseudomonas aeruginosa binding to cystic fibrosis and normal epithelial cells. Pediatr Res. 2007;;61(4):398–403. doi: 10.1203/pdr.0b013e3180332d1c. [DOI] [PubMed] [Google Scholar]
- 25.Abman SH, Ogle JW, Butler-Simon N, et al. Role of respiratory syncytial virus in early hospitalizations for respiratory distress of young infants with cystic fibrosis. J Pediatr. 1988;;113(5):826–830. doi: 10.1016/s0022-3476(88)80008-8. [DOI] [PubMed] [Google Scholar]
- 26.Wang EE, Prober CG, Manson B, et al. Association of respiratory viral infections with pulmonary deterioration in patients with cystic fibrosis. N Engl J Med. 1984;;311(26):1653–1658. doi: 10.1056/NEJM198412273112602. [DOI] [PubMed] [Google Scholar]
- 27.Garcia DF, Hiatt PW, Jewell A, et al. Human metapneumovirus and respiratory syncytial virus infections in older children with cystic fibrosis. Pediatr Pulmonol. 2007;;42(1):66–74. doi: 10.1002/ppul.20546. [DOI] [PubMed] [Google Scholar]
- 28.Boyce TG, Mellen BG, Mitchel EF, Jr, et al. Rates of hospitalization for respiratory syncytial virus infection among children in Medicaid. J Pediatr. 2000;;137(6):865–870. doi: 10.1067/mpd.2000.110531. [DOI] [PubMed] [Google Scholar]
- 29.Holman RC, Shay DK, Curns AT, et al. Risk factors for bronchiolitis-associated deaths among infants in the United States. Pediatr Infect Dis J. 2003;;22(6):483–490. doi: 10.1097/01.inf.0000069765.43405.3b. [DOI] [PubMed] [Google Scholar]
- 30.Thomas M, Bedford-Russell A, Sharland M. Prevention of respiratory syncytial virus infection with palivizumab. Monaldi Arch Chest Dis. 2000;;55(4):333–338. [PubMed] [Google Scholar]
- 31.Subcommittee on Diagnosis and Management of Bronchiolitis. Diagnosis and management of bronchiolitis. Pediatrics. 2006;;118(4):1774–1793. doi: 10.1542/peds.2006-2223. [DOI] [PubMed] [Google Scholar]
- 32.Clifton IJ, Kastelik JA, Peckham DG, et al. Ten years of viral and non-bacterial serology in adults with CF. Epidemiol Infect. 2008;;136(1):128–134. doi: 10.1017/S0950268807008278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meissner HC. Selected populations at increased risk from respiratory syncytial virus infection. Pediatr Infect Dis J. 2003;;22((2 suppl)):S40–S44. doi: 10.1097/01.inf.0000053884.21238.13. ; discussion S4-S5. [DOI] [PubMed] [Google Scholar]
- 34.Whimbey E, Englund JA, Couch RB. Community respiratory virus infections in immunocompromised patients with cancer. Am J Med. 1997;;102((3A)):10–8. 25–26. doi: 10.1016/S0002-9343(97)80004-6. discussion. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Couch RB, Englund JA, Whimbey E. Respiratory viral infections in immunocompetent and immunocompromised persons. Am J Med. 1997;;102((3A)):2–9. 25–26. doi: 10.1016/S0002-9343(97)00003-X. discussion. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lujan-Zilbermann J, Benaim E, Tong X, et al. Respiratory virus infections in pediatric hematopoietic stem cell transplantation. Clin Infect Dis. 2001;;33(7):962–968. doi: 10.1086/322628. [DOI] [PubMed] [Google Scholar]
- 37.Bredius RG, Templeton KE, Scheltinga SA, et al. Prospective study of respiratory viral infections in pediatric hemopoietic stem cell transplantation patients. Pediatr Infect Dis J. 2004;;23(6):518–522. doi: 10.1097/01.inf.0000125161.33843.bb. [DOI] [PubMed] [Google Scholar]
- 38.Chavez-Bueno S, Mejias A, Merryman RA, et al. Intravenous palivizumab and ribavirin combination for respiratory syncytial virus disease in high-risk pediatric patients. Pediatr Infect Dis J. 2007;;26(12):1089–1093. doi: 10.1097/INF.0b013e3181343b7e. [DOI] [PubMed] [Google Scholar]
- 39.Harrington RD, Hooton TM, Hackman RC, et al. An outbreak of respiratory syncytial virus in a bone marrow transplant center. J Infect Dis. 1992;;165(6):987–993. doi: 10.1093/infdis/165.6.987. [DOI] [PubMed] [Google Scholar]
- 40.Whimbey E, Champlin RE, Couch RB, et al. Community respiratory virus infections among hospitalized adult bone marrow transplant recipients. Clin Infect Dis. 1996;;22(5):778–782. doi: 10.1093/clinids/22.5.778. [DOI] [PubMed] [Google Scholar]
- 41.Michaels MG, Fonseca-Aten M, Green M, et al. Respiratory syncytial virus prophylaxis: a survey of pediatric solid organ transplant centers. Pediatr Transplant. 2009;;13(4):451–456. doi: 10.1111/j.1399-3046.2008.01017.x. [DOI] [PubMed] [Google Scholar]
- 42.Liu M, Worley S, Arrigain S, et al. Respiratory viral infections within one year after pediatric lung transplant. Transplant Infect Dis. 2009;;11(4):304–312. doi: 10.1111/j.1399-3062.2009.00397.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Blanchard SS, Gerrek M, Siegel C, Czinn SJ. Significant morbidity associated with RSV infection in immunosuppressed children following liver transplantation: case report and discussion regarding need of routine prophylaxis. Pediatr Transplant. 2006;;10(7):826–829. doi: 10.1111/j.1399-3046.2006.00583.x. [DOI] [PubMed] [Google Scholar]
- 44.Pohl C, Green M, Wald ER, Ledesma-Medina J. Respiratory syncytial virus infections in pediatric liver transplant recipients. J Infect Dis. 1992;;165(1):166–169. doi: 10.1093/infdis/165.1.166. [DOI] [PubMed] [Google Scholar]
- 45.Crooks BN, Taylor CE, Turner AJ, et al. Respiratory viral infections in primary immune deficiencies: significance and relevance to clinical outcome in a single BMT unit. Bone Marrow Transplant. 2000;;26(10):1097–1102. doi: 10.1038/sj.bmt.1702656. [DOI] [PubMed] [Google Scholar]
- 46.Small TN, Casson A, Malak SF, et al. Respiratory syncytial virus infection following hematopoietic stem cell transplantation. Bone Marrow Transplant. 2002;;29(4):321–327. doi: 10.1038/sj.bmt.1703365. [DOI] [PubMed] [Google Scholar]
- 47.Ottolini MG, Curtis SR, Mathews A, et al. Palivizumab is highly effective in suppressing respiratory syncytial virus in an immunosuppressed animal model. Bone Marrow Transplant. 2002;;29(2):117–120. doi: 10.1038/sj.bmt.1703326. [DOI] [PubMed] [Google Scholar]
- 48.Boeckh M, Berrey MM, Bowden RA, et al. Phase 1 evaluation of the respiratory syncytial virus-specific monoclonal antibody palivizumab in recipients of hematopoietic stem cell transplants. J Infect Dis. 2001;;184(3):350–354. doi: 10.1086/322043. [DOI] [PubMed] [Google Scholar]
- 49.Leader S, Kohlhase K. Recent trends in severe respiratory syncytial virus (RSV) among US infants, 1997 to 2000. J Pediatr. 2003;;143((5 suppl)):S127–S132. doi: 10.1067/s0022-3476(03)00510-9. [DOI] [PubMed] [Google Scholar]
- 50.McLaurin KK, Leader S. Growing impact of RSV hospitalizations among infants in the US, 1997–2002. Abstract 936. The Pediatric Academic Societies Annual Meeting; Washington: 2005. [Google Scholar]
- 51.Pelletier AJ, Mansbach JM, Camargo CA., Jr Direct medical costs of bronchiolitis hospitalizations in the United States. Pediatrics. 2006;;118(6):2418–2423. doi: 10.1542/peds.2006-1193. [DOI] [PubMed] [Google Scholar]
- 52.Redbook for Windows [computer program]. Version 61127. Greenwood Village, CO: Thompson PDR; 2009. (updated February 2009) [Google Scholar]
- 53.Prescott WA, Jr, Doloresco F, Brown J, Paladino JA. Cost effectiveness of respiratory syncytial virus prophylaxis: a critical and systematic review. Pharmacoeconomics. 2010;;28(4):279–293. doi: 10.2165/11531860-000000000-00000. [DOI] [PubMed] [Google Scholar]