Abstract
Background
Few studies have examined racial/ethnic differences in blood pressure (BP) control over time, especially in an equal access system. We examined racial/ethnic differences in longitudinal BP control in Veterans with type 2 diabetes.
Methods
We collected data on a retrospective cohort of 5,319 Veterans with type 2 diabetes and initially uncontrolled BP followed from 1996 to 2006 at a Veterans Administration (VA) facility in the southeastern United States. The mean blood pressure value for each subject for each year was used for the analysis with BP control defined as <140/<90 mmHg. The primary outcome measure was proportion with controlled BP. The main predictor variable was race/ethnicity categorized as non-Hispanic White (NHW), non-Hispanic Black (NHB), or Hispanic/Other (H/O). Other covariates included age, gender, employment, marital status, service connectedness, and ICD-9 coded medical and psychiatric comorbidities. Generalized linear mixed models were used to assess the relationship between race/ethnicity and BP control after adjusting for covariates.
Results
Mean follow-up was 5.0 years. The sample was 46% NHW, 26% NHB, 19% H/O, and 9% unknown. The average age was 68 years. In the final model, after adjusting for covariates, NHB race (OR = 1.38, 95%CI: 1.2, 1.6) and H/O race (OR = 1.57, 95% CI: 1.3, 1.8) were associated with increased likelihood of poor BP control (>140/>90 mmHg) over time compared to NHW patients.
Conclusion
Ethnic minority Veterans with type 2 diabetes have significantly increased odds of poor BP control over ∼5 years of follow-up compared to their non-Hispanic White counterparts independent of sociodemographic factors and comorbidity patterns.
KEY WORDS: blood pressure control, diabetes, epidemiology, race/ethnicity
INTRODUCTION
Diabetes mellitus affects over 23 million Americans, and it is the 7th leading cause of death in our country.1 Diabetes is also a primary risk factor for cardiovascular disease and stroke, the 1st and 3rd leading causes of death, respectively.2–5 Total medical costs related to diabetes were estimated to exceed $170 billion in 2007.6 Many diabetic patients have comorbid cardiovascular risk factors including hypertension and hyperlipidemia, and current consensus guidelines emphasize the importance of excellent risk factor control in diabetic patients.7,8 Clinical trials strongly support the practice of intensive blood pressure control among diabetic patients to a goal blood pressure of <130/<80 mmHg.9–13
Despite increases in hypertension prevalence, the United States has seen marked improvements in hypertension awareness, treatment, and control over the past three decades with overall control rates reaching 50.1% in the 2007–2008 time period.14,15 However, racial and ethnic disparities in hypertension awareness, treatment and control persist in the general population. For example, McWilliams and colleagues examined data from the National Health and Nutrition (NHANES) surveys between 1999 and 2006, and they found significant improvements in blood pressure control, diabetes control, and lipid control among all racial and ethnic groups. However, while there were no significant differences in the trend between racial/ethnic groups, black individuals were 8.4% less likely to achieve BP control at < 140/<90 mmHg and 16.5% less likely to achieve a hemoglobin A1C value of < 7% in comparison to white individuals in all time periods.16
Improvements in BP control among diabetic patients have not been as robust as for the general population, and racial/ethnic differences also exist for patients with this disease.17 For example, in the Translating Research Into Action for Diabetes (TRIAD) study, a longitudinal study of diabetes treatment in managed-care settings which enrolled over 7,000 diabetic patients, 55.5% of African American patients failed to achieve a goal BP of <140/<90 mmHg as compared to 44.1% of white patients.18 Within the Department of Veterans Affairs (VA), studies have not consistently shown disparities in diabetes processes of care for A1C testing, dilated eye examinations, and diabetic foot exams.19,20 However, African American patients have less often reached adequate blood pressure (<140/<90 mm/Hg) and lipid (LDL < 130 mg/dl) control compared to white patients.19
Prior investigations of hypertension control among diabetics have been cross-sectional in design which limits the ability to draw conclusions regarding the change in BP over time for individual patients.16 Available longitudinal studies have typically examined smaller cohorts of patients, and little is known regarding the contribution of access to care to differences in BP control among diabetics.19,21 The present study was performed in the VA which features equal access to primary medical care and pharmacy benefits. Based on prior investigations, we hypothesized there would be persistent differences in BP control over time among racial/ethnic minority patients including non-Hispanic Black (NHB) patients and Hispanic patients compared to non-Hispanic White (NHW) patients after adjustment for relevant socio-demographic and clinical covariates. The present study was designed to add to the body of knowledge regarding racial/ethnic differences in BP control by analyzing longitudinal blood pressure control in individual patients using a large cohort of over 5,000 diabetic patients with uncontrolled BP cared for in a Southeastern VA medical center with a lengthy average follow up of 5 years.
RESEARCH DESIGN AND METHODS
Study Data Set The Veterans Affairs Health System is the largest integrated health system in the US serving over 5.5 million people at 153 acute care hospitals, 909 ambulatory care and community-based outpatient clinics (CBOCs), and 135 nursing homes.22 Patients receiving care in the VA system experience decreased barriers to primary care and pharmacy benefits in comparison to the general population. Veterans with limited means receive care free of charge, and others receive care using income-based fee schedules. We created a cohort of adults with type 2 diabetes at a Veterans Administration (VA) facility in the Southeastern United States by linking multiple patient and administrative files from the Veterans Health Administration (VHA) Decision Support System (DSS) files using Social Security Number (SSN).23 The study was approved by our institutional review board and our local VA research and development committee.
Longitudinal Data Set Individuals with type 2 diabetes were identified based on having at least two International Classification of Diseases, Ninth Revision (ICD-9) codes for diabetes in either outpatient or inpatient files and having two or more visits each year since diagnosis based on a previously validated algorithm.24 From this group, we included all subjects with BP > 140/>90 mmHg in the first year they appeared in the cohort. Next, we created a person-period data set for each subject from January 1996 to September 2006. Systolic and diastolic blood pressure (BP) measurements for each subject were used for analysis. For subjects with two or more BP values in a given year, the average value for that year was used. Subjects were followed from time of entry into the study until death, loss to follow-up, or September 2006.
Outcome Measures The primary outcome measure was annual odds of uncontrolled BP (i.e. 0 = BP <140/<90, 1 = Otherwise) measured longitudinally over time.
Primary Covariate The main predictor variable was race/ethnicity categorized as non-Hispanic White (NHW), non-Hispanic Black (NHB), Hispanic/other, or missing.
Demographic Variables Age was treated as a continuous variable. Marital status was classified as never married, married, or separated/widowed/divorced. Employment was classified as employed, not employed, or retired. Service connectedness was used as a surrogate for income among Veterans and was classified as a dichotomous variable (yes/no) as higher degrees of disability are associated with lower income levels.
Medical Comorbidity Measures Medical comorbidity variables were defined based on enhanced ICD-9 codes using validated algorithms.25 Hypertension, a key comorbid condition for this analysis was defined according as ICD-9 codes 401–405, but we elected to include all Veterans with type II diabetes and uncontrolled BP at the time of cohort entry with or without an ICD-9 hypertension diagnosis. Cancer was defined as ICD-9 codes 140.x-280.x; congestive heart failure (CHF), was defined as ICD-9 codes 398.91, 402.01, 402.11, 402.91, 404.01, 404.03, 404.11, 404.13, 404.91, 404.93, 425.4–425.9, 428.x; coronary heart disease (CHD), was defined as ICD-9 codes 410–414; and stroke was defined as ICD-9 codes 430–438. All medical comorbidities were dichotomized with presence coded as 1 and absence of the comorbidity as 0.
Psychiatric Comorbidity Measures Six psychiatric comorbidities were recorded as being present (1) or absent (0) and defined based on enhanced ICD-9 codes using validated algorithms3. Bipolar disorder was defined as ICD-9 codes 296.0, 296.4, 296.5, 296.6, and 296.7; generalized anxiety disorder (GAD) was defined as ICD-9 code 300.2; major depressive disorder (MDD) was defined as ICD-9 codes 296.2, and 296.3; post traumatic stress disorder (PTSD) was defined by ICD-9 code 309.81; psychotic disorder was defined as ICD-9 codes 295.1, 295.2, 295.3, 295.4, 295.6, and 295.7; and substance use, which included alcohol abuse was defined by ICD-9 codes 303.9, 304.x, 305.0, and 305.1-305.9.
Statistical Analysis We first examined the characteristics of the sample through univariate analysis. This was followed by pre-model building analysis, which included testing if each covariate was individually associated with the binary outcome BP control (0 = <140/<90, 1 = otherwise). Age was centered to improve computational performance and reduce multi-collinearity. Models for the association between BP control and race/ethnicity were developed with groups of predictors entered into the sequential regression models. Additional regression models were developed for sensitivity analysis using a cutoff for BP control of <130/<80 mmHg. All statistical tests used a two-tailed α = 0.05 level of significance and were performed using SAS® statistical software, version 9.2.We used a generalized linear mixed model (GLMM) approach (PROC GLIMMIX, SAS 9.2) to fit the models assessing the relationship between race and BP after adjusting for potential confounders. GLMM were used to deal with missing data for BP. These methods can be employed since the missing mechanism is assumed missing at random (MAR).26,27 Both unadjusted and covariate adjusted models were fitted in a sequential fashion. The final model was fit to test the effects of race, time, age, gender, marital status, employment status, medical comorbidities and psychiatric comorbidities (bipolar disorder, GAD, MDD, PTSD, psychotic disorder and substance use).
RESULTS
As depicted in Table 1, this cohort consisted of 5,319 Veterans with type 2 diabetes mellitus with elevated blood pressure (>140/90 mmHg) at the time of entry into the cohort. Cohort inception began in 1996 with follow up through 2006. Mean follow-up was 5.0 years (SD = 3.0 years). The racial distribution was 46% non-Hispanic white, 26% non-Hispanic black, 19% Hispanic/Other, and 9% Unknown. The average age was 68 years.
Table 1.
Sample Characteristics of Veterans with Diabetes and Elevated BP by Race/Ethnicity
| Variable | All (n = 5319) | NHW (n = 2451) | NHB (n = 1406) | Hispanic/Other (n = 991) | Unknown (n = 471) |
|---|---|---|---|---|---|
| Age* (years) | 68 (11.1) | 69 (10.2) | 65 (11.9) | 68 (11) | 67 (11.5) |
| Male | 97.9 | 98.0 | 97.3 | 98.3 | 97.7 |
| Female | 2.1 | 2.0 | 2.7 | 1.7 | 2.3 |
| Married | 66.3 | 68.5 | 58.9 | 69.5 | 70.1 |
| Divorced | 27.6 | 26.7 | 30.9 | 26.0 | 26.1 |
| Never Married | 6.0 | 4.7 | 10.2 | 4.3 | 3.8 |
| Unemployed | 46.7 | 47.3 | 51.4 | 40.1 | 43.7 |
| Retired | 34.0 | 35.1 | 29.0 | 37.5 | 35.0 |
| Employed | 19.0 | 17.3 | 19.5 | 21.6 | 21.2 |
| Service Connected Disability | 38.1 | 37.8 | 34.7 | 43.2 | 38.4 |
| Cancer | 5.2 | 4.9 | 8.8 | 2.2 | 2.1 |
| CHD | 13.8 | 19.3 | 13.8 | 5.2 | 3.2 |
| CHF | 8.3 | 9.8 | 11.0 | 3.9 | 1.7 |
| Hypertension | 28.4 | 31.1 | 40.0 | 14.1 | 8.9 |
| Stroke | 3.3 | 4.1 | 4.0 | 1.6 | 0.4 |
| Baseline HbA1c ≥ 8% | 20.5 | 17.3 | 28.9 | 18.1 | 17.8 |
| Baseline HbA1c* (%) | 8 (1.7) | 7 (1.6) | 8 (1.9) | 7 (1.7) | 8 (1.7) |
| Bipolar Disorder | 1.4 | 1.8 | 1.7 | 0.5 | 0.2 |
| Generalized Anxiety Disorder | 2.0 | 3.1 | 1.9 | 0.6 | 0.2 |
| Major Depressive Disorder | 6.6 | 7.8 | 8.9 | 1.9 | 3.2 |
| Post Traumatic Stress Disorder | 4.6 | 3.7 | 7.9 | 2.6 | 3.4 |
| Psychotic Disorder | 1.6 | 1.1 | 3.3 | 0.6 | 0.6 |
| Substance Use Disorder | 13.0 | 13.4 | 19.3 | 6.4 | 5.5 |
| Dead | 15.3 | 16.6 | 17.1 | 11.3 | 12.3 |
| Baseline SBP* (mmHg) | 156 (15.2) | 156 (15.4) | 157 (15.4) | 156 (14.4) | 156 (15.2) |
| Baseline DBP* (mmHg) | 87 (38.3) | 87 (47.3) | 90 (33.5) | 84 (26.1) | 84 (10.4) |
* Data are mean, sd or %
Results of sequential regression models for the primary outcome, the differential odds of poorly controlled average annual BP by race/ethnicity, are depicted in Table 2. Compared to NHW subjects, NHB subjects had significantly higher unadjusted odds of poor BP control (OR = 1.31, 95%CI: 1.2 1.5) as did Hispanic/Other subjects (OR = 1.92, 95%CI 1.6, 2.2). After subsequent inclusion of sociodemographic covariates (Model 2) and medical/psychiatric comorbidities (Model 3), odds ratio estimates were modified with relatively increased odds of poor BP control for NHB subjects and relatively decreased odds for Hispanic/Other subjects.
Table 2.
Unadjusted and Adjusted Odds for Longitudinal BP Control by Race/Ethnicity
| Model | Variable | Odds Ratio | 95% CI | P-Value |
|---|---|---|---|---|
| Model 1: Unadjusted Model* | Non-Hispanic White | 1.00 | NA | NA |
| Non-Hispanic Black | 1.31 | (1.2, 1.5) | < 0.0001 | |
| Hispanic/Other | 1.92 | (1.6, 2.2) | < 0.0001 | |
| Unknown | 2.87 | (2.3, 3.6) | < 0.0001 | |
| Model 2: Model 1 + Demographics† | Non-Hispanic White | 1.00 | NA | NA |
| Non-Hispanic Black | 1.25 | (1.1, 1.4) | 0.0001 | |
| Hispanic/Other | 1.98 | (1.7, 2.3) | < 0.0001 | |
| Unknown | 2.85 | (2.4, 3.4) | < 0.0001 | |
| Model 3: Model 2 + Comorbidities‡ | Non-Hispanic White | 1.00 | NA | NA |
| Non-Hispanic Black | 1.38 | (1.2, 1.6) | < 0.0001 | |
| Hispanic/Other | 1.57 | (1.3, 1.8) | < 0.0001 | |
| Unknown | 2.30 | (1.9, 2.8) | < 0.0001 |
* Reference group of non-Hispanic White race
† Model with time, race, and demographic variables [male (reference: female), age, employment status (reference: unemployed), marital status (reference: married), and service connected disability (reference: no disability)]
‡ Model with time, race, demographic variables, and potential comorbidities (reference levels: no disease; cancer, CHD, CHF, hypertension, stroke, HbA1c (centered at 8%), bipolar disorder, substance use, psychotic, generalized anxiety disorder, major depressive disorder, post traumatic stress disorder)
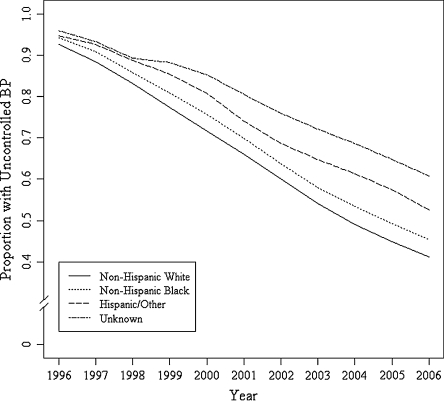
In the final model fully depicted in Figure 1 and Table 3, after adjusting for all confounding variables, the overall odds of uncontrolled BP decreased over time (OR = 0.55, 95%CI: 0.51, 0.59). However, NHB race was associated with 39% (OR = 1.38, 95%CI: 1.2, 1.6) increased odds of poor BP control, and Hispanic/Other race was associated with 57% (OR = 1.57, 95% CI: 1.3, 1.8) increased odds. Among demographic variables included in the final model, there were no significant associations between the odds of adequate BP control and employment status, service connectedness, or marital status. Presence of a hypertension diagnosis at the time of cohort entry appears to be associated with higher odds of achieving BP control over time (OR 0.85, 95% 0.73, 0.98). Among other comorbidities studied, the presence of comorbid cancer, coronary heart disease, and congestive heart failure were all associated with increased odds of good BP control over time. Additionally, substance use was associated with increased odds of good BP control (Table 4).
Figure 1.
Adjusted longitudinal BP control in veterans with diabetes by race/ethnicity, 1996–2006.
Table 3.
Final Adjusted Model for Longitudinal BP Control by Race/Ethnicity (Cutoff <140/90 mmHg)
| Variable | Odds Ratio | Standard Error | P-value | 95% Confidence Interval |
|---|---|---|---|---|
| Time | 0.55 | 0.0367 | < 0.0001 | (0.51, 0.59) |
| Time Squared | 1.02 | 0.0027 | < 0.0001 | (1.01, 1.03) |
| Non-Hispanic Black* | 1.38 | 0.0637 | < 0.0001 | (1.2, 1.6) |
| Hispanic/Other* | 1.57 | 0.0773 | < 0.0001 | (1.3, 1.8) |
| Unknown* | 2.30 | 0.1083 | < 0.0001 | (1.9, 2.8) |
| Male† | 1.15 | 0.1837 | 0.4364 | (0.8, 1.7) |
| Age in years (Centered at mean) | 1.01 | 0.0029 | 0.0024 | (1.00, 1.01) |
| Employed‡ | 1.11 | 0.0751 | 0.1668 | (1.0, 1.3) |
| Retired‡ | 1.12 | 0.0603 | 0.0698 | (1.0, 1.3) |
| Never Married§ | 1.17 | 0.1133 | 0.1627 | (0.9, 1.5) |
| Divorced§ | 1.10 | 0.0600 | 0.0969 | (1.0, 1.2) |
| Service Connected | 0.91 | 0.0538 | 0.0700 | (0.8, 1.0) |
| Cancer║ | 0.63 | 0.1177 | < 0.0001 | (0.5, 0.8) |
| CHD║ | 0.67 | 0.0918 | < 0.0001 | (0.6, 0.8) |
| CHF║ | 0.80 | 0.1033 | 0.0328 | (0.66, 0.98) |
| Hypertension║ | 0.85 | 0.0760 | 0.0295 | (0.73, 0.98) |
| Stroke║ | 1.22 | 0.1419 | 0.1533 | (0.9, 1.6) |
| HbA1c (Centered at 8%) | 1.01 | 0.0121 | 0.6635 | (0.98, 1.03) |
| Bipolar Disorder║ | 0.87 | 0.2241 | 0.5446 | (0.6, 1.4) |
| Substance Use║ | 0.59 | 0.0791 | < 0.0001 | (0.5, 0.7) |
| Psychotic║ | 0.82 | 0.2086 | 0.3422 | (0.5, 1.2) |
| Generalized Anxiety Disorder║ | 0.76 | 0.1811 | 0.1280 | (0.5, 1.1) |
| Major Depressive Disorder║ | 0.81 | 0.1199 | 0.0754 | (0.6, 1.0) |
| Post Traumatic Stress Disorder║ | 0.94 | 0.1308 | 0.6390 | (0.7, 1.2) |
* Reference group: non-Hispanic whites
† Reference group: females
‡ Reference group: unemployed
§ Reference group: married
║ Reference group: absence of condition/disease
Table 4.
Subset Analysis of Longitudinal BP Control by Race/Ethnicity (Cutoff <130/80 mmHg)
| Variable | Odds Ratio | Standard Error | P-value | 95% Confidence Interval |
|---|---|---|---|---|
| Time | 0.57 | 0.0423 | < 0.0001 | (0.5, 0.6) |
| Time Squared | 1.02 | 0.0030 | < 0.0001 | (1.0, 1.1) |
| Non-Hispanic Black* | 1.50 | 0.0672 | < 0.0001 | (1.3, 1.7) |
| Hispanic/Other* | 1.59 | 0.0817 | < 0.0001 | (1.4, 1.9) |
| Unknown* | 1.90 | 0.1120 | < 0.0001 | (1.5, 2.4) |
| Male† | 1.07 | 0.1824 | 0.7045 | (0.7, 1.5) |
| Age in years (Centered at mean) | 1.00 | 0.0030 | 0.5352 | (0.996, 1.008) |
| Employed‡ | 1.23 | 0.0770 | 0.0068 | (1.1, 1.4) |
| Retired‡ | 1.09 | 0.0640 | 0.1709 | (1.0, 1.2) |
| Never Married§ | 1.15 | 0.1215 | 0.2437 | (0.9, 1.5) |
| Divorced§ | 0.99 | 0.0624 | 0.8145 | (0.9, 1.1) |
| Service Connected | 0.97 | 0.0563 | 0.5431 | (0.9, 1.1) |
| Cancer║ | 0.63 | 0.1200 | < 0.0001 | (0.5, 0.8) |
| CHD║ | 0.62 | 0.0949 | < 0.0001 | (0.5, 0.7) |
| CHF║ | 0.69 | 0.1078 | 0.0005 | (0.6, 0.8) |
| Hypertension║ | 0.95 | 0.0796 | 0.4905 | (0.8, 1.1) |
| Stroke║ | 0.93 | 0.1452 | 0.6012 | (0.7, 1.2) |
| HbA1c (Centered at 8%) | 1.03 | 0.0127 | 0.0100 | (1.0, 1.1) |
| Bipolar Disorder║ | 0.88 | 0.2114 | 0.5551 | (0.6, 1.3) |
| Substance Use║ | 0.69 | 0.0815 | < 0.0001 | (0.6, 0.8) |
| Psychotic║ | 0.73 | 0.1976 | 0.1076 | (0.5, 1.1) |
| Generalized Anxiety Disorder║ | 1.02 | 0.1846 | 0.9223 | (0.7, 1.5) |
| Major Depressive Disorder║ | 0.82 | 0.1196 | 0.0986 | (0.6, 1.0) |
| Post Traumatic Stress Disorder║ | 1.06 | 0.1327 | 0.6615 | (0.8, 1.4) |
* Reference group: non-Hispanic whites
† Reference group: females
‡ Reference group: unemployed
§ Reference group: married
║ Reference group: absence of condition/disease
Finally, in the sensitivity analysis using a different cutoff (>130/80 mmHg) value to define poor BP control (N = 7342), odds of poor BP control were higher for each ethnic minority group relative to NHW subjects. Table 4 depicts the results of our sensitivity analysis. Using the more stringent BP cutoff, NHB race was associated with 50% (OR = 1.50, 95%CI: 1.3, 1.7) increased odds of poor BP control, and Hispanic/Other race was associated with 59% (OR = 1.59, 95% CI: 1.4, 1.9) increased odds. However, the relative differences between racial/ethnic groups were similar in direction.
DISCUSSION
In this longitudinal cohort of diabetic Veterans, NHB patients were 38% less likely to be controlled at a goal BP of <140/<90 as compared to NHW patients, and differences of similar magnitude were observed among other racial and ethnic minority groups. This study is important because it represents the largest longitudinal cohort to date to examine the issue of BP control in diabetes over a mean follow up of 5 years. A separate study of the same diabetic cohort found that non-Hispanic black patients had mean hemoglobin A1C measures 0.54% higher over time than non-Hispanic white Veterans. Taken together, these studies add significantly to the body of literature describing racial and ethnic disparities in diabetes disease control.
The results of this study of diabetic patients are similar to recent cross-sectional analyses of the general population.15,16 The proportion of patients with uncontrolled BP decreased in all groups over time. Despite this, the prevalence of uncontrolled BP remained higher in NHB and other minority patients; thus there was not much change in the disparity gap. Several factors have been proposed as potential mediators of these differences. First, differential insurance coverage and access to primary medical care might contribute to these observed differences. However, these differences have been seen within and outside of the Veterans Administration where there is equal access to care; thus efforts are warranted to minimize barriers in access to care in all settings. Second, provider-specific differences in care for diabetic patients have been observed including increased measures of treatment inertia and decreased intensity of management for African American diabetic patients.28–30 Third, a significant proportion of the observed differences have been attributed to patient-specific characteristics including socioeconomic status.28 Recent research has also implicated racial/ethnic differences in medical mistrust and deficiencies in shared decision making as barriers to effective therapeutic relationships between minority patients and their healthcare providers.31–34 Finally, poor medication adherence, which varies by race/ethnicity, has been associated with poorer diabetes disease outcomes in multiple studies.35–37
This study is strengthened by its large sample size and relatively long follow-up. However, the analysis does have some limitations to bear in mind. First, it is possible that unmeasured factors may have confounded the relationship between race/ethnicity and blood pressure control. However, we did employ sequential multivariable models that included relevant socio-demographic and clinical factors. Additional factors to consider that were not available in this data set include information on medication usage and adherence, health literacy, disease beliefs, and trust in healthcare providers. Second, this cohort was largely male (97.3%), limiting the conclusions that can be drawn regarding female diabetic patients. However, our findings are consistent with two other non-VA cohorts with much larger proportions of women.17,18 Finally, this data set had a relatively low proportion of Hispanic individuals and a relatively high proportion of missing self-reported race/ethnicity data. However, our multivariable analyses had significant statistical power to detect differences of the magnitude observed in our study. In addition, we used validated statistical approaches for imputation of missing values.26,27
In conclusion, a large proportion of patients with diabetes mellitus fail to achieve target blood pressure control levels over time, and there appear to be significant racial and ethnic differences in BP control which warrant further investigation and intervention. Specific interventions that target inter-provider variability in care as well as patient-specific characteristics may be useful targets for interventions designed to narrow these gaps in disease outcomes.
Acknowledgements
This study was supported by Grant # REA 08–261, Center for Disease Prevention and Health Interventions for Diverse Populations funded by Veterans Affairs Health Services Research and Development (PI – Leonard Egede).
Conflict of Interest None of the authors have any financial disclosure or conflict of interest to report.
REFERENCES
- 1.National Diabetes Fact Sheet: General Information and Estimates on Diabetes in the United States, 2007. Atlanta, GA: U.S. Department of Health and Human Services; 2007. [Google Scholar]
- 2.Haffner SM, Lehto S, Ronnemaa T, Pyorala K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med. 1998;339:229–234. doi: 10.1056/NEJM199807233390404. [DOI] [PubMed] [Google Scholar]
- 3.Coutinho M, Gerstein HC, Wang Y, Yusuf S. The relationship between glucose and incident cardiovascular events. A metaregression analysis of published data from 20 studies of 95,783 individuals followed for 12.4 years. Diabetes Care. 1999;22:233–240. doi: 10.2337/diacare.22.2.233. [DOI] [PubMed] [Google Scholar]
- 4.Bell DS. Stroke in the diabetic patient. Diabetes Care. 1994;17:213–219. doi: 10.2337/diacare.17.3.213. [DOI] [PubMed] [Google Scholar]
- 5.Biller J, Love BB. Diabetes and stroke. Med Clin North Am. 1993;77:95–110. doi: 10.1016/s0025-7125(16)30274-7. [DOI] [PubMed] [Google Scholar]
- 6.Economic Costs of Diabetes in the U.S. in 2007. Diabetes Care. 2007;31:596–615. [DOI] [PubMed]
- 7.Chobanian AV, Bakris GL, Black HR, Cushman WC, Izzo JL. Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation and Treatment of High Blood Pressure. National High Blood Pressure Education Program Coordinating Committee. Hypertension. 2003;42:1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 8.Expert Panel on Detection E, Treatment of High Blood Cholesterol in Adults. Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA. 2001;285:2486–97. [DOI] [PubMed]
- 9.Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. UK Prospective Diabetes Study Group. Bmj. 1998;317:703–13. [PMC free article] [PubMed]
- 10.Curb JD, Pressel SL, Cutler JA, et al. Effect of diuretic-based antihypertensive treatment on cardiovascular disease risk in older diabetic patients with isolated systolic hypertension. Systolic Hypertension in the Elderly Program Cooperative Research Group. JAMA. 1996;276:1886–1892. doi: 10.1001/jama.276.23.1886. [DOI] [PubMed] [Google Scholar]
- 11.Davis BR, Langford HG, Blaufox MD, Curb JD, Polk BF, Shulman NB. The association of postural changes in systolic blood pressure and mortality in persons with hypertension: the Hypertension Detection and Follow-up Program experience. Circulation. 1987;75:340–346. doi: 10.1161/01.CIR.75.2.340. [DOI] [PubMed] [Google Scholar]
- 12.Hansson L, Zanchetti A, Carruthers SG, et al. Effects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) randomised trial. HOT Study Group. Lancet. 1998;351:1755–1762. doi: 10.1016/S0140-6736(98)04311-6. [DOI] [PubMed] [Google Scholar]
- 13.Tuomilehto J, Rastenyte D, Birkenhager WH, et al. Effects of calcium-channel blockade in older patients with diabetes and systolic hypertension. Systolic Hypertension in Europe Trial Investigators. N Engl J Med. 1999;340:677–684. doi: 10.1056/NEJM199903043400902. [DOI] [PubMed] [Google Scholar]
- 14.Ostchega Y, Yoon SS, Hughes J, Louis T. Hypertension Awareness, Treatment, and Control-Continued Disparities in Adults: United States, 2005–2006. NCHS Data Brief. 2008;3:1–8. [PubMed] [Google Scholar]
- 15.Egan BM, Zhao Y, Axon RN. US trends in prevalence, awareness, treatment, and control of hypertension, 1988–2008. JAMA. 2010;303:2043–2050. doi: 10.1001/jama.2010.650. [DOI] [PubMed] [Google Scholar]
- 16.McWilliams JM, Meara E, Zaslavsky AM, Ayanian JZ. Differences in control of cardiovascular disease and diabetes by race, ethnicity, and education: U.S. trends from 1999 to 2006 and effects of Medicare coverage. Ann Intern Med. 2009;150:505–515. doi: 10.7326/0003-4819-150-8-200904210-00005. [DOI] [PubMed] [Google Scholar]
- 17.Cummings DM, Doherty L, Howard G, et al. Blood pressure control in diabetes. Diabetes Care. 2010;33:798–803. doi: 10.2337/dc09-1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brown AF, Gregg EW, Stevens MR, et al. Race, ethnicity, socioeconomic position, and quality of care for adults with diabetes enrolled in managed care: the Translating Research Into Action for Diabetes (TRIAD) study. Diabetes Care. 2005;28:2864–2870. doi: 10.2337/diacare.28.12.2864. [DOI] [PubMed] [Google Scholar]
- 19.Heisler M, Smith DM, Hayward RA, Krein SL, Kerr EA. Racial disparities in diabetes care processes, outcomes, and treatment intensity. Med Care. 2003;41:1221–1232. doi: 10.1097/01.MLR.0000093421.64618.9C. [DOI] [PubMed] [Google Scholar]
- 20.Halanych JH, Wang F, Miller DR, et al. Racial/ethnic differences in diabetes care for older veterans: accounting for dual health system use changes conclusions. Med Care. 2006;44:439–445. doi: 10.1097/01.mlr.0000207433.70159.23. [DOI] [PubMed] [Google Scholar]
- 21.Wendel CS, Shah JH, Duckworth WC, Hoffman RM, Mohler MJ, Murata GH. Racial and ethnic disparities in the control of cardiovascular disease risk factors in Southwest American veterans with type 2 diabetes: the Diabetes Outcomes in Veterans Study. BMC Health Serv Res. 2006;6:58. doi: 10.1186/1472-6963-6-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Facts about the Department of Veterans Affairs. 2009. (Accessed May 19, 2011, at http://www.va.gov/opa/publications/factsheets/fs_department_of_veterans_affairs.pdf.)
- 23.Maynard C, Chapko MK. Data resources in the Department of Veterans Affairs. Diabetes Care. 2004;27(Suppl 2):B22–B26. doi: 10.2337/diacare.27.suppl_2.B22. [DOI] [PubMed] [Google Scholar]
- 24.Miller DR, Safford MM, Pogach LM. Who has diabetes? Best estimates of diabetes prevalence in the Department of Veterans Affairs based on computerized patient data. Diabetes Care. 2004;27(Suppl 2):B10–B21. doi: 10.2337/diacare.27.suppl_2.B10. [DOI] [PubMed] [Google Scholar]
- 25.Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43:1130–1139. doi: 10.1097/01.mlr.0000182534.19832.83. [DOI] [PubMed] [Google Scholar]
- 26.Diggle PJ, Heagerty P, Liang K-Y, Zeger SL. Analysis of Longitudinal Data. 2. Oxford, England: Oxford University Press; 2002. [Google Scholar]
- 27.Ibrahim JG, Molenberghs G. Missing data methods in longitudinal studies: A review. Test. 2009;18:1–43. doi: 10.1007/s11749-009-0138-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sequist TD, Fitzmaurice GM, Marshall R, Shaykevich S, Safran DG, Ayanian JZ. Physician performance and racial disparities in diabetes mellitus care. Arch Intern Med. 2008;168:1145–1151. doi: 10.1001/archinte.168.11.1145. [DOI] [PubMed] [Google Scholar]
- 29.Hicks LS, Shaykevich S, Bates DW, Ayanian JZ. Determinants of racial/ethnic differences in blood pressure management among hypertensive patients. BMC Cardiovasc Disord. 2005;5:16. doi: 10.1186/1471-2261-5-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Samuels TA, Bolen S, Yeh HC, et al. Missed opportunities in diabetes management: a longitudinal assessment of factors associated with sub-optimal quality. J Gen Intern Med. 2008;23:1770–1777. doi: 10.1007/s11606-008-0757-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Benkert R, Peters R, Tate N, Dinardo E. Trust of nurse practitioners and physicians among African Americans with hypertension. J Am Acad Nurse Pract. 2008;20:273–280. doi: 10.1111/j.1745-7599.2008.00317.x. [DOI] [PubMed] [Google Scholar]
- 32.Doescher MP, Saver BG, Franks P, Fiscella K. Racial and ethnic disparities in perceptions of physician style and trust. Arch Fam Med. 2000;9:1156–1163. doi: 10.1001/archfami.9.10.1156. [DOI] [PubMed] [Google Scholar]
- 33.Peek ME, Odoms-Young A, Quinn MT, Gorawara-Bhat R, Wilson SC, Chin MH. Race and shared decision-making: perspectives of African-Americans with diabetes. Soc Sci Med. 2010;71:1–9. doi: 10.1016/j.socscimed.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wexler R, Elton T, Pleister A, Feldman D. Barriers to blood pressure control as reported by African American patients. J Natl Med Assoc. 2009;101:597–603. doi: 10.1016/s0027-9684(15)30947-0. [DOI] [PubMed] [Google Scholar]
- 35.Cramer JA. A systematic review of adherence with medications for diabetes. Diabetes Care. 2004;27:1218–1224. doi: 10.2337/diacare.27.5.1218. [DOI] [PubMed] [Google Scholar]
- 36.Egede LE, Mueller M, Echols CL, Gebregziabher M. Longitudinal differences in glycemic control by race/ethnicity among veterans with type 2 diabetes. Med Care. 2010;48:527–533. doi: 10.1097/MLR.0b013e3181d558dc. [DOI] [PubMed] [Google Scholar]
- 37.Egede LE, Gebregziabher M, Hunt KJ, et al. Regional, geographic, and racial/ethnic variation in glycemic control in a national sample of veterans with diabetes. Diabetes Care. 2011;34:938–943. doi: 10.2337/dc10-1504. [DOI] [PMC free article] [PubMed] [Google Scholar]