Abstract
Background
It is unclear if primary care physicians are following guidelines or using other patient characteristics and factors to determine when to perform spirometry in patients at risk for COPD. It is also unclear to what degree a diagnosis of COPD is accurately reflected by spirometry results.
Objectives
To examine characteristics associated with use of spirometry in primary care for patients with increased risk for COPD and to determine the accuracy of COPD diagnosis in patients with spirometry.
Design
Retrospective cohort study.
Subjects
A cohort that met the following criteria was identified: ≥35 years of age; ≥ 2 primary care visits in internal medicine clinic in 2007; at least one respiratory or smoking cessation medication, or diagnosis of COPD or shortness of breath or dyspnea in 2007.
Main Measures
Medical records of all primary care physician visits prior to the time of inclusion in 2007 were reviewed. Data on patient demographics, co-morbidities, respiratory medication use, presence of symptoms, history of tobacco use, and pulmonary function tests were extracted.
Key Results
A total 1052 patients were identified. Dyspnea on exertion (Adjusted odds ratio (AOR) 1.52 [95% CI 1.06–2.18]) and chronic cough (AOR 1.71 [1.07–2.72]) were the only chronic symptoms associated with use of spirometry. Current (AOR 1.54 [0.99–2.40]) or past smoking (AOR 1.09 [0.72–1.65]) status were not associated with use of spirometry. Of the 159 patients with a diagnosis of COPD, 93 (58.5%) met GOLD criteria and 81(50.9%) met lower limit of normal (LLN) criteria for COPD.
Conclusion
Clinicians use spirometry more often among patients with symptoms suggestive of COPD but not more often among patients with current or past tobacco use. For patients who had a spirometry and a diagnosis of COPD, primary care physicians were accurate in their diagnosis only half of the time.
Key words: chronic disease, diagnosis, health care delivery, chronic obstructive pulmonary disease, spirometry, quality of care
INTRODUCTION
The hallmark of chronic obstructive pulmonary disease (COPD) is the presence of airflow obstruction that is not fully reversible and is most commonly assessed by spirometry. Several guidelines provide guidance as to when to suspect COPD and recommend spirometry to confirm diagnosis.1,2 Nonetheless, it has been shown that the use of spirometry is limited with only a third of newly diagnosed individuals with COPD actually having spirometry to confirm the presence of irreversible air flow obstruction.3–5 Although appropriate therapy with inhaled medications have been demonstrated to reduce symptoms, improve health-related quality of life, and decrease the rate of acute exacerbations6–19, there is growing evidence that pharmacotherapy used to treat patients for COPD pose risks.6,7,20–25 Without spirometry, patients who have been diagnosed with COPD may be being medically managed without evidence of airways obstruction, leading to unnecessary exposure to risks and costs associated with COPD therapy.
Appropriate diagnosis of COPD with spirometry is also important as recent research have started to show some benefit of therapy in those with mild or moderate disease. For example, a post hoc analysis examined the effects of salmeterol/fluticasone combination therapy by disease stage focusing on moderate COPD.26 In patients with moderate COPD, this analysis showed significant improvement in post-bronchodilator FEV1 and health status, and a reduction in exacerbation rates compared to placebo. Another post hoc analysis focusing on those with moderate disease, showed better health status and longer time to first exacerbation in the group treated with tiotropium compared to placebo.27 Although the evidence is based on post hoc analysis, both suggest benefits of pharmacotherapy in those with moderate disease. One prospective study evaluated symptomatic COPD patients with mild and moderate disease and efficacy of tiotropium.28 This study showed improvement in airflow limitation in those using tiotropium compared to placebo.
Studies evaluating rates of spirometry in new diagnosis of COPD used the Healthcare Effectiveness Data and Information Set (HEDIS) measure for COPD which recommends spirometry 720 days prior to or 180 days after a new clinician diagnosis of COPD.29 It is unclear if primary care physicians are following guidelines or using other patient characteristics and factors to determine when to perform spirometry. In addition, it is unclear how often spirometry is used to confirm a clinicians’ suspicion of COPD and how often they are correct when compared to spirometric measurement. Previous studies have shown associations between healthcare use, patient characteristics, and the use of spirometry using the Department of Veterans Affairs (VA) database.4,5 A limitation of these studies has been the lack of information about patient symptoms, smoking status, spirometry results to validate a COPD diagnosis and a predominantly male population. In order to improve the use of spirometry for the diagnosis of COPD, it is important to understand what characteristics, including patient symptoms, are associated with its use in primary care. The objectives of this study were to examine the characteristics associated with the use of spirometry in a primary care setting, determine the prevalence of spirometry use in all patients with a diagnosis of COPD, and determine the accuracy of COPD diagnosis in patients with spirometry.
METHODS AND MATERIALS
Study Design and Cohort
Using a retrospective cohort design, we used electronic administrative data from an urban academic medical center to identify patients who were followed in the internal medicine primary care outpatient clinic and possibly at risk for COPD. The Global Initiative for Chronic Obstructive Lung disease (GOLD), American Thoracic Society (ATS) and European Respiratory Society (ERS) guidelines state a diagnosis of COPD should be considered in any patient who has any of the following indicators: 1) dyspnea, 2) chronic cough, 3) chronic sputum, and/or 4) history of exposure to risk factors.1,2 Based on these criteria, we used the presence of a respiratory medication and ICD-9 codes for dyspnea and shortness of breath as indicators of presence of symptoms and the presence of a tobacco cessation medication as a marker of recent tobacco use. As billing data do not always correlate with presence of the disease, we also included ICD-9 codes for COPD, emphysema, and chronic bronchitis which includes “smoker’s cough” to identify those at risk for having chronic airways obstruction. As there is not a consensus on the age for testing across the two guidelines, we used an age cut off of ≥35 as appropriate to capture the majority of patients that may be considered at risk by healthcare providers.
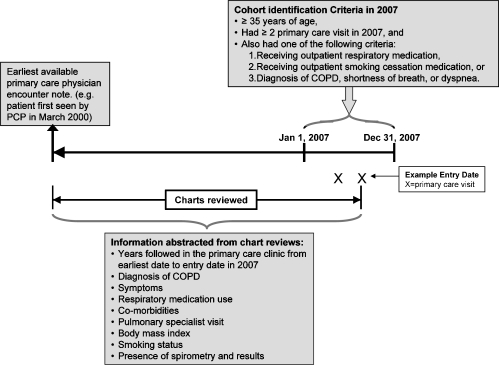
More specifically, from January 1, 2007 to December 31, 2007, we identified patients who were ≥35 years of age and had at least two visits to the general internal medicine outpatient clinic in 2007. To be included, the patient also had to have at least one of the following: 1) an active prescription for a respiratory medication (i.e. inhaled corticosteroids (ICS), long-acting beta-2 agonist (LABA), long acting anticholinergic (LAA), short-acting beta-2 agonist (SABA), short-acting anticholinergic (SAA), or theophylline), 2) an active prescription for a smoking cessation medication (i.e. buproprion, varenicline, or nicotine replacement therapy), 3) a diagnosis of dyspnea or shortness of breath (i.e. International Classification of Diseases, Ninth Revision (ICD-9) 786.0 or 786.05, respectively), or 4) a diagnosis of COPD (i.e. ICD-9 491.x, 492.x, 496). This study was approved by the University of Illinois at Chicago Office for the Protection of Research Subjects (Protocol # 2008–0818). Figure 1 shows a diagram of the timeline of cohort inclusion and data extraction, while Figure 2 represents patients in the cohort who had a spirometry performed.
Figure 1.
A diagram of the timeline of cohort inclusion and data extraction.
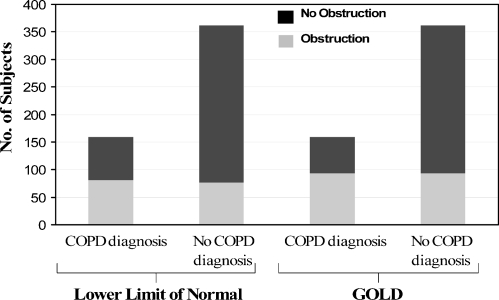
Figure 2.
This figure represents patients in the cohort who had a spirometry performed. Of those who also had a diagnosis of COPD, the proportion of patients with airway obstruction as defined by the lower limit of normal and GOLD criteria is shown. Of those without a diagnosis of COPD, the proportion of patients with airway obstruction as defined by the lower limit of normal and GOLD criteria is shown.
Data Collection
The study period was from the first primary care encounter note to the most recent primary care encounter note in 2007 as shown in Figure 1. The abstraction was performed by the principal investigator (MJ) and a research assistant (RA). Data for the first 200 patients included in the analysis were extracted by MJ and the RA independently. Extracted data was compared after every 25 patients and discrepancies were discussed until a consensus was reached and discrepancies were minimized. After the 200 charts, the RA completed the rest of the chart reviews under the guidance of Dr. Joo. After each 50 charts reviewed thereafter, any questions were discussed and resolved by MJ and the RA.
Demographic information (i.e. age, gender, race) were obtained using electronic administrative data. Electronic medical records used for the review included primary internal medicine care encounter notes with a physician. These encounters notes were used to determine years followed in the primary care clinic up to the inclusion date, co-morbidities, and respiratory medication use (i.e. ICS, LABA, SABA, LAA, SAA). Documentation for a visit with a pulmonary specialist was also noted. Body mass index was calculated from the most recent encounter.
From the primary care encounter notes, we abstracted the presence of chronic symptoms including dyspnea on exertion, shortness of breath, cough, and sputum. We defined chronic symptoms as follows: 1) same symptom noted in two consecutive visits more than 2 months apart and not noted as “resolved” or limited to less than 8 weeks, 2) specifically described as “chronic” and not time limited to less than eight weeks. Dyspnea on exertion was defined when stated as “dyspnea on exertion” or shortness of breath with a defined amount of exertion (e.g. “with three blocks”, “going up one flight of stairs”).
Smoking status was identified as “current” if active smoking was noted, “past” if past smoking was noted or smoking was mentioned without a time frame defined, “never smoker” is noted as a never smoker in the chart, or “not noted” if smoking was not mentioned anywhere in the primary care notes.
Procedure notes as well as scanned records from outside medical care were reviewed to determine whether spirometry was performed and the results were recorded. We recorded the presence of spirometry in the chart and used the most recent results for patients with multiple sets. For those patients who had spirometry, the following characteristics were identified up to the time of the most recent spirometry and used in the adjusted logistic regression analysis: diagnosis of COPD, chronic symptoms, and respiratory medications.
Statistical Analysis
Characteristics of the patients were summarized using percentages to describe categorical variables and means and standard deviations to describe continuous variables. Comparisons between groups were made using Chi squared tests for categorical variable and t tests for continuous variables. Odds ratios and 95% confidence intervals were used to quantify the association with spirometry use using unadjusted and adjusted logistic regression models. The adjusted model included the following covariates: age, gender, race, body mass index, years seen by primary care provider, pulmonary visits, comorbidities, chronic symptoms, tobacco history, and respiratory medications. For patients with available spirometry results in the medical records, the results were compared to the Global Initiative for chronic Obstructive Lung Disease (GOLD) criteria2 and the lower limit of normal(LLN) criteria30,31 for obstructive lung disease. Analysis was performed using STATA® 10. The level of significance used was a p-value of 0.05.
RESULTS
There were 1052 patients identified as being potentially at risk for COPD. A total of 527 (50.0%) had a spirometry performed at any time during their primary care. Table 1 shows the patient characteristics stratified by whether spirometry was performed. At least one chronic symptom was reported by 73.5% of the cohort. Table 1 also shows the adjusted analysis between spirometry use and covariates.
Table 1.
Patient Characteristics Stratified by the Use of Spirometry and Associations between Spirometry and Patient Characteristics
| Total (N = 1052) | Spirometry (N = 527) | No Spirometry (N = 525) | p-value | Adjusted OR (95% CI) | |
|---|---|---|---|---|---|
| Gender, n (%) | |||||
| Female | 769 (73.1) | 383 (72.7) | 386 (73.5) | 0.76 | 1 |
| Male | 283 (26.9) | 144 (27.3) | 139 (26.5) | 0.88 (0.60–1.30) | |
| Age, years, n (%) | |||||
| 35 to <45 | 169 (16.1) | 61 (11.6) | 108 (20.6) | <0.001 | 1 |
| 45 to <55 | 324 (30.8) | 158 (30.0) | 166 (31.6) | 1.37 (0.85–2.21) | |
| 55 to <65 | 281 (26.7) | 138 (26.2) | 143 (27.2) | 1.32 (0.78–2.25) | |
| ≥65 | 278 (26.4) | 170 (32.3) | 108 (20.6) | 2.10 (1.18–3.74) | |
| mean ± SD | 57.0 ± 11.6 | 58.9 ± 11.5 | 55.1 ± 11.4 | <0.001 | -- |
| Race, n (%) | |||||
| Caucasian | 160 (15.2) | 68 (12.9) | 92 (17.5) | 0.02 | 1 |
| African-American | 725 (68.9) | 386 (73.2) | 339 (64.6) | 1.41 (0.87–2.27) | |
| Hispanic | 151 (14.4) | 68 (12.9) | 83 (15.8) | 1.24 (0.68–2.25) | |
| Other | 16 (1.5) | 5 (1.0) | 11 (2.1) | 0.47 (0.11–1.97) | |
| Body mass index, kg/cm2, n (%) | |||||
| <18.5 | 17 (1.6) | 9 (1.7) | 8 (1.5) | 0.49 | 0.99 (0.27–3.58) |
| 18.5 to <25 | 152 (14.5) | 71 (13.5) | 81 (15.4) | 1 | |
| 25 to <35 | 479 (45.5) | 231 (43.8) | 248 (47.2) | 1.11 (0.69–1.81) | |
| 35 to <40 | 153 (14.5) | 80 (15.2) | 73 (13.9) | 1.41 (0.77–2.58) | |
| ≥40 | 251 (23.9) | 136 (25.8) | 115 (21.9) | 1.20 (0.66–2.17) | |
| mean ± SD | 33.9 ± 10.3 | 34.3 ± 10.3 | 33.4 ± 10.3 | 0.94 | -- |
| Years seen by primary care provider, years, n (%) | |||||
| 0 to <2 | 229 (21.8) | 102 (19.4) | 127 (24.2) | 0.01 | 1 |
| 2 to <4 | 173 (16.4) | 106 (20.1) | 67 (12.8) | 1.05 (0.63–1.74) | |
| 4 to <6 | 158 (15.0) | 85 (16.1) | 73 (13.9) | 0.57 (0.33–0.99) | |
| 6 to <8 | 194 (18.4) | 88 (16.7) | 106 (20.2) | 0.33 (0.19–0.58) | |
| ≥8 | 298 (28.3) | 146 (27.7) | 152 (29.0) | 0.31 (0.17–0.52) | |
| mean ± SD | 5.2 ± 2.9 | 5.2 ± 2.8 | 5.2 ± 3.0 | 0.39 | -- |
| Pulmonary visits, n (%) | 333 (31.7) | 295 (56.0) | 38 (7.2) | <0.001 | 16.69 (10.95–25.42) |
| Comorbidities, n (%) | |||||
| COPD | 222 (21.1 ) | 160 (30.4) | 62 (11.8) | <0.001 | 0.91 (0.56–1.48) |
| Asthma | 522 (49.6) | 264 (50.1) | 258 (49.1) | 0.76 | 1.02 (0.69–1.51) |
| Obstructive sleep apnea | 175 (16.6) | 109 (20.7) | 66 (12.6) | <0.001 | 1.28 (0.80–2.06) |
| Diabetes mellitus | 365 (34.7) | 193 (36.6) | 172 (32.8) | 0.19 | 0.89 (0.61–1.28) |
| Hypertension | 758 (72.1) | 405 (76.9) | 353 (67.2) | 0.001 | 1.10 (0.75–1.64) |
| Hyperlipidemia | 547 (52.0) | 288 (54.7) | 259 (49.3) | 0.08 | 0.95 (0.67–1.35) |
| Coronary artery disease | 201 (19.1) | 127 (24.1) | 74 (14.1) | <0.001 | 1.47 (0.94–2.31) |
| Congestive heart failure | 153 (14.5) | 89 (16.9) | 64 (12.2) | 0.03 | 0.69 (0.42–1.13) |
| Depression | 311 (29.6) | 144 (27.3) | 167 (31.8) | 0.11 | 0.85 (0.59–1.23) |
| Bipolar disorder | 26 (2.5) | 9 (1.7) | 17 (3.2) | 0.11 | 0.30 (0.11–0.83) |
| Chronic symptoms, n (%) | |||||
| Dyspnea on exertion | 420 (39.9) | 259 (49.2) | 161 (30.7) | <0.001 | 1.52 (1.06–2.18) |
| Shortness of breath | 511 (48.6) | 295 (56.0) | 216 (41.1) | <0.001 | 0.88 (0.61–1.28) |
| Cough | 589 (56.0) | 317 (60.1) | 272 (51.8) | 0.01 | 1.71 (1.07–2.72) |
| Sputum | 418 (39.7) | 221 (41.9) | 197 (37.5) | 0.14 | 0.60 (0.38–0.96) |
| Tobacco use, n (%) | |||||
| Nonsmoker | 335 (31.8) | 152 (28.8) | 183(34.9) | 0.04 | 1 |
| Tobacco current | 267 (25.4) | 141 (26.8) | 126 (24.0) | 0.31 | 1.54 (0.99–2.40) |
| Tobacco past | 336 (31.9) | 188 (35.7) | 148 (28.2) | 0.01 | 1.09 (0.72–1.65) |
| Tobacco, not noted | 114 (10.8) | 46 (8.7) | 68 (13.0) | 0.03 | 0.50 (0.28–0.89) |
| Medications, n (%) | |||||
| SABA | 641 (60.9) | 380 (72.1) | 261 (49.7) | <0.001 | 2.06 (1.32–3.21) |
| SAA | 216 (20.5) | 162 (30.7) | 54 (10.3) | <0.001 | 1.86 (1.14–3.04) |
| LABA | 375 (35.7) | 235 (44.6) | 140 (26.7) | <0.001 | 1.02 (0.60–1.74) |
| LAA | 79 (7.5) | 70 (13.3) | 9 (1.7) | <0.001 | 2.93 (1.21–7.07) |
| ICS | 472 (44.9) | 278 (52.8) | 194 (37.0) | <0.001 | 0.96 (0.55–1.66) |
SAA = Short-acting β-agonist; SAA = Short-acting anticholinergic, LABA = Long-acting β-agonist, LAA = Long-acting anticholinergic; ICS = Inhaled corticosteroids
Table 1 shows the prevalence of COPD diagnosis prior to the most recent spirometry if a spirometry was performed or COPD diagnosis at any time during the study period if spirometry was not performed (n = 222). Of these patients, 72.1% had spirometry performed during the study period. There were 59 patients who had a new diagnosis of COPD after spirometry. During the study period, of all those with a diagnosis of COPD at any time (n = 281), 77.9% had spirometry during the study period.
Of the 527 patients who had spirometry performed, 521 had results available. Figure 1 shows the accuracy of a clinician diagnosis of COPD based on lower limit of normal and GOLD criteria. Of the 521 patients, 159 (30.5%) had a diagnosis of COPD. Of the 159 patients with a diagnosis of COPD, only 93 (58.5%) met GOLD criteria and 81(50.9%) met LLN criteria for COPD. Of the 362 patients without a diagnosis of COPD, 93 (25.7%) met GOLD criteria and 77 (21.3%) met LLN for COPD. After spirometry was performed 34% who met GOLD criteria for obstruction and 32% who met LLN criteria for obstruction had a diagnosis of COPD by their primary care provider.
A total of 62 patients carried a diagnosis of COPD and did not have a spirometry associated with the diagnosis. Of those 23 also had a diagnosis of asthma; therefore 39 had a diagnosis of COPD without asthma. Of the 62 patients who had COPD, 45 (72.6%) were receiving at least one respiratory medication. Of the 39 who had a COPD diagnosis only, 28 (71.8%) were receiving at least one respiratory medication
DISCUSSION
In our study, those with chronic dyspnea on exertion and chronic cough were more likely to have a spirometry performed, whereas current or past smoking was not associated with the use of spirometry. We found that the rate of spirometry use in those with new and existing diagnosis of COPD was quite high but not optimal. Moreover, clinician diagnosis of COPD compared to spirometry was accurate only about half of the time.
Our study is reflective of an urban medical center with a large proportion of minority patients and high rates of obesity, a population not previously represented in studies evaluating COPD and spirometry use. We found that neither of these characteristics was associated with the use or nonuse of spirometry compared to Caucasians and normal weight individuals, respectively. Previous studies using HEDIS criteria which is 2 years prior to and up to 6 months after the new clinician diagnosis of COPD show that only about a third of those with a new diagnosis of COPD had spirometry. Our study shows that primary care physicians are performing spirometry outside of the HEDIS time frame resulting in more than three-quarters of those with COPD having spirometry performed at some time during their usual care. Although this is an improvement, it shows that nearly a quarter of patients with COPD still have not had spirometry to confirm the diagnosis. This remains important as primary care physicians are only accurate in their clinical diagnosis of COPD about half the time compared to spirometry results. As spirometry testing was available in the outpatient primary care clinic, the rate of spirometry use seen in this study may be inflated compared to other clinics without spirometry in the outpatient setting.
Consistent with ATS/ERS and GOLD guidelines1,2, primary care physicians were more likely to obtain spirometry when there was the presence of chronic dyspnea on exertion or chronic cough. However, they were less likely to obtain spirometry in the presence of chronic sputum and the results were equivocal with chronic shortness of breath. The reason for this is unclear. It is possible that sputum when considered separately may not be considered a respiratory symptom in the same category as dyspnea on exertion and cough with respect to COPD, even though listed as a key indicator for considering a diagnosis of COPD by the above guidelines. Shortness of breath alone without an indicator of any level of exertion may be too nonspecific to warrant a diagnostic test such as spirometry or not pursued in detail as the level of exertion is a way to quantitatively assess any level of shortness of breath. The lack of association of current or past smoking status and spirometry is also not consistent with ATS/ERS and GOLD guidelines, but is consistent with the U.S. Preventative Services Task Force (USPSTF) recommendation against screening for COPD.32 However, physician practice in our study, although reflective of USPSTF recommendations, could not have been in response to the guidelines as they were published in 2008 and our cohort was not followed beyond the year 2007.
Prior to this study, we assumed that the longer a patient was followed in the primary care clinic, the more likely they were to have a spirometry performed. However, that was not the case. Patients who were followed longer were less likely to get spirometry when compared to those who were followed for only up to 2 years. A possible explanation could be the competing demands in a busy practice where COPD may be less of a priority than other acute and chronic medical concerns such as hypertension and diabetes mellitus. As primary care encounters have time constraints, multiple medical conditions, patient concerns, and point of care for other chronic conditions compete for priority during a single visit. Although competing demands have not been studied for COPD, it has been shown to have a negative impact on tobacco cessation counseling.33 Once a patient has been seen, primary care physicians may be less likely to obtain spirometry unless there is a new issue (e.g. new or worsening symptoms). If these patients are being treated with respiratory medications, a new clinical paradigm may be needed to confirm diagnosis in those with pre-existing clinical diagnosis of COPD.
In our study, almost a quarter of patients without a diagnosis of COPD prior to spirometry had airways obstruction and subsequent follow-up did show a new diagnosis of COPD in about a third of these patients. We did not have appropriate follow-up time to observe all physician decision making in regards to the diagnosis of COPD post-spirometry. In future, it would be important to note if spirometry results alter the pre-test diagnosis in clinical practice or if providers are still diagnosing patients based on symptoms, history, and/or response to therapy post-spirometry.
Our study has some limitations. First, this was a single center study which limits its generalizability. However, our cohort was from an academic medical center with outpatient spirometry testing available, so the rate of spirometry use is likely much higher than the general primary care clinic setting. Second, post-bronchodilator values were not available for all spirometry results. This may have misclassified some with reversible airways disease. Third, information garnered from the primary care encounters may not be exactly reflective of the encounter. For example, a patient may complain of a symptom that is not listed in the encounter note. Fourth, we did not delineate why the spirometry was performed. For example, it may have been performed for perioperative risk assessment. Our analysis does not infer causality; however, regardless of the indication for spirometry, once performed, obstruction can be defined based on the results.
In conclusion, the use of spirometry at any time during primary care in the diagnosis of COPD was suboptimal and the symptom indicators associated with COPD and spirometry were chronic dyspnea on exertion and chronic cough which is consistent with guidelines. For patients who had a spirometry and a diagnosis of COPD, primary care physicians were accurate in their diagnosis only half of the time. Our results suggest that recommendations for the use of spirometry should emphasize confirmation of diagnosis not only when the diagnosis is new but also when the diagnosis is pre-existing.
Contributors
The authors would like to thank Rebecca Michaelis, research assistant, who participated in the chart reviews.
Funders This work was supported by the National Heart, Lung, and Blood Institute [K23HL094461]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NHLBI, the National Institutes. The views expressed in this manuscript reflect those of the authors and not necessarily those of the Department of Veterans Affairs.
Prior Presentations An abstract of preliminary data from this study was presented in poster form at that American Thoracic Society meeting in New Orleans, LA in May, 2010 and in Denver, CO in May 2011.
Conflict of Interest None disclosed.
References
- 1.Celli BR, MacNee W. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J. 2004;23:932–46. doi: 10.1183/09031936.04.00014304. [DOI] [PubMed] [Google Scholar]
- 2.Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease. December 2010; http://www.goldcopd.org/Guidelineitem.asp?l1=2&l2=1&intId=989. Accessed June 3, 2011.
- 3.Han MK, Kim MG, Mardon R, et al. Spirometry utilization for COPD: how do we measure up? Chest. 2007;132:403–9. doi: 10.1378/chest.06-2846. [DOI] [PubMed] [Google Scholar]
- 4.Joo MJ, Lee TA, Weiss KB. Geographic variation of spirometry use in newly diagnosed COPD. Chest. 2008;134:38–45. doi: 10.1378/chest.08-0013. [DOI] [PubMed] [Google Scholar]
- 5.Lee TA, Bartle B, Weiss KB. Spirometry use in clinical practice following diagnosis of COPD. Chest. 2006;129:1509–15. doi: 10.1378/chest.129.6.1509. [DOI] [PubMed] [Google Scholar]
- 6.Burge PS, Calverley PM, Jones PW, Spencer S, Anderson JA, Maslen TK. Randomised, double blind, placebo controlled study of fluticasone propionate in patients with moderate to severe chronic obstructive pulmonary disease: the ISOLDE trial. Bmj. 2000;320:1297–303. doi: 10.1136/bmj.320.7245.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Calverley PM, Anderson JA, Celli B, et al. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med. 2007;356:775–89. doi: 10.1056/NEJMoa063070. [DOI] [PubMed] [Google Scholar]
- 8.Donohue JF, Noord JA, Bateman ED, et al. A 6-month, placebo-controlled study comparing lung function and health status changes in COPD patients treated with tiotropium or salmeterol. Chest. 2002;122:47–55. doi: 10.1378/chest.122.1.47. [DOI] [PubMed] [Google Scholar]
- 9.Gartlehner G, Hansen RA, Carson SS, Lohr KN. Efficacy and safety of inhaled corticosteroids in patients with COPD: a systematic review and meta-analysis of health outcomes. Ann Fam Med. 2006;4:253–62. doi: 10.1370/afm.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jones PW, Bosh TK. Quality of life changes in COPD patients treated with salmeterol. Am J Respir Crit Care Med. 1997;155:1283–9. doi: 10.1164/ajrccm.155.4.9105068. [DOI] [PubMed] [Google Scholar]
- 11.Lung Health Study Research Group Effect of inhaled triamcinolone on the decline in pulmonary function in chronic obstructive pulmonary disease. N Engl J Med. 2000;343:1902–9. doi: 10.1056/NEJM200012283432601. [DOI] [PubMed] [Google Scholar]
- 12.Macie C, Wooldrage K, Manfreda J, Anthonisen NR. Inhaled corticosteroids and mortality in COPD. Chest. 2006;130:640–6. doi: 10.1378/chest.130.3.640. [DOI] [PubMed] [Google Scholar]
- 13.Ram FS, Sestini P. Regular inhaled short acting beta2 agonists for the management of stable chronic obstructive pulmonary disease: Cochrane systematic review and meta-analysis. Thorax. 2003;58:580–4. doi: 10.1136/thorax.58.7.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ramirez-Venegas A, Ward J, Lentine T, Mahler DA. Salmeterol reduces dyspnea and improves lung function in patients with COPD. Chest. 1997;112:336–40. doi: 10.1378/chest.112.2.336. [DOI] [PubMed] [Google Scholar]
- 15.Rennard SI, Anderson W, ZuWallack R, et al. Use of a long-acting inhaled beta2-adrenergic agonist, salmeterol xinafoate, in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;163:1087–92. doi: 10.1164/ajrccm.163.5.9903053. [DOI] [PubMed] [Google Scholar]
- 16.Stockley RA, Chopra N, Rice L. Addition of salmeterol to existing treatment in patients with COPD: a 12 month study. Thorax. 2006;61:122–8. doi: 10.1136/thx.2004.033266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stockley RA, Whitehead PJ, Williams MK. Improved outcomes in patients with chronic obstructive pulmonary disease treated with salmeterol compared with placebo/usual therapy: results of a meta-analysis. Respir Res. 2006;7:147. doi: 10.1186/1465-9921-7-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Noord JA, Aumann JL, Janssens E, et al. Effects of tiotropium with and without formoterol on airflow obstruction and resting hyperinflation in patients with COPD. Chest. 2006;129:509–17. doi: 10.1378/chest.129.3.509. [DOI] [PubMed] [Google Scholar]
- 19.Wadbo M, Lofdahl CG, Larsson K, et al. Effects of formoterol and ipratropium bromide in COPD: a 3-month placebo-controlled study. Eur Respir J. 2002;20:1138–46. doi: 10.1183/09031936.02.00301702. [DOI] [PubMed] [Google Scholar]
- 20.Dewan NA, Rafique S, Kanwar B, et al. Acute exacerbation of COPD: factors associated with poor treatment outcome. Chest. 2000;117:662–71. doi: 10.1378/chest.117.3.662. [DOI] [PubMed] [Google Scholar]
- 21.Alsaeedi A, Sin DD, McAlister FA. The effects of inhaled corticosteroids in chronic obstructive pulmonary disease: a systematic review of randomized placebo-controlled trials. Am J Med. 2002;113:59–65. doi: 10.1016/S0002-9343(02)01143-9. [DOI] [PubMed] [Google Scholar]
- 22.Ernst P, Gonzalez AV, Brassard P, Suissa S. Inhaled corticosteroid use in chronic obstructive pulmonary disease and the risk of hospitalization for pneumonia. Am J Respir Crit Care Med. 2007;176:162–6. doi: 10.1164/rccm.200611-1630OC. [DOI] [PubMed] [Google Scholar]
- 23.Kardos P, Wencker M, Glaab T, Vogelmeier C. Impact of salmeterol/fluticasone propionate versus salmeterol on exacerbations in severe chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2007;175:144–9. doi: 10.1164/rccm.200602-244OC. [DOI] [PubMed] [Google Scholar]
- 24.Lee TA, Weiss KB. Fracture risk associated with inhaled corticosteroid use in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2004;169:855–9. doi: 10.1164/rccm.200307-926OC. [DOI] [PubMed] [Google Scholar]
- 25.Joo MJ, Au DH, Fitzgibbon ML, Lee TA. Inhaled corticosteroids and risk of pneumonia in newly diagnosed COPD. Respir Med.104:246–52. [DOI] [PubMed]
- 26.Jenkins CR, Jones PW, Calverley PM, et al. Efficacy of salmeterol/fluticasone propionate by GOLD stage of chronic obstructive pulmonary disease: analysis from the randomised, placebo-controlled TORCH study. Respir Res. 2009;10:59. doi: 10.1186/1465-9921-10-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Decramer M, Celli B, Kesten S, Lystig T, Mehra S, Tashkin DP. Effect of tiotropium on outcomes in patients with moderate chronic obstructive pulmonary disease (UPLIFT): a prespecified subgroup analysis of a randomised controlled trial. Lancet. 2009;374:1171–8. doi: 10.1016/S0140-6736(09)61298-8. [DOI] [PubMed] [Google Scholar]
- 28.Johansson G, Lindberg A, Romberg K, Nordstrom L, Gerken F, Roquet A. Bronchodilator efficacy of tiotropium in patients with mild to moderate COPD. Prim Care Respir J. 2008;17:169–75. doi: 10.3132/pcrj.2008.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.National Committee for Quality Assurance (NCQA). HEDIS 2010. Technical Specifications for Physician Measurement. Washington (DC): National Committee for Quality Assurance (NCQA); 2009:.
- 30.Hansen JE, Sun XG, Wasserman K. Discriminating measures and normal values for expiratory obstruction. Chest. 2006;129:369–77. doi: 10.1378/chest.129.2.369. [DOI] [PubMed] [Google Scholar]
- 31.Pellegrino R, Viegi G, Brusasco V, et al. Interpretative strategies for lung function tests. Eur Respir J. 2005;26:948–68. doi: 10.1183/09031936.05.00035205. [DOI] [PubMed] [Google Scholar]
- 32.U. S. Preventive Services Task Force Screening for chronic obstructive pulmonary disease using spirometry: U.S. preventive services task force recommendation statement. Ann Intern Med. 2008;148:529–34. doi: 10.7326/0003-4819-148-7-200804010-00212. [DOI] [PubMed] [Google Scholar]
- 33.Jaen CR, McIlvain H, Pol L, Phillips RL, Jr, Flocke S, Crabtree BF. Tailoring tobacco counseling to the competing demands in the clinical encounter. J Fam Pract. 2001;50:859–63. [PubMed] [Google Scholar]