Abstract
Background:
Serum levels of a secreted glycoprotein YKL-40 are elevated in patients with a wide range of cancers including breast, colorectal, and ovarian cancers. Furthermore, these increased levels correlate with poorer survival of cancer patients, suggesting that serum levels of YKL-40 might be a prognostic biomarker. However, the tissue expression of YKL-40 and its relationship with clinical outcomes and other potential markers are poorly understood.
Methods:
Tissue samples from invasive breast cancers, breast ductal carcinoma in situ (DCIS), and cancer-free reduction mammoplasty were enrolled. YKL-40 expression was measured using immunohistochemistry and evaluated by a semi-quantification assay. Statistical analyses explored the relationship of YKL-40 with clinical outcome and other breast cancer biomarkers.
Results:
Breast ductal carcinoma in situ expressed low and moderate levels of YKL-40. In the subset of 203 patients with invasive cancer, YKL-40 levels were positively correlated with tumour grade (P<0.0001) and Her2/neu (P<0.01), but negatively correlated with oestrogen (P<0.0001) and progesterone receptor (P<0.0001). YKL-40 levels were inversely correlated with expressions of GATA3 (P=0.0137) and E-cadherin (P=0.0417).
Conclusion:
These data demonstrate that expression levels of YKL-40 are associated with tumour grade, poor differentiation, and other breast cancer markers, highlighting that tissue levels of YKL-40 serve as a valuable biomarker for breast cancer diagnosis and prognosis.
Keywords: YKL-40, breast cancer biomarker, clinical outcome, invasive ductal carcinoma, breast ductal carcinoma in situ
Breast cancer is the most incident cancer among women in the United States. Over the past three decades, we have come a long way towards understanding better how to prevent and treat breast cancer. As a result, fewer women now die from this disease (Jatoi and Miller, 2003; Blackman and Masi, 2006). However, we still have about 180 000 new cases diagnosed with breast cancer each year. While early treatment by surgical resection, chemotherapy, and radiotherapy can effectively prevent cancer progression and help >95% of patients survive longer than 5 years, this disease continues to be devastating for its suffers. Thus, identifying key molecules that may serve as reliable biomarkers for the diagnosis and as therapeutic targets is of paramount importance.
YKL-40 (also named human cartilage glycoprotein-39 or chitinase-3-like-1) is a phylogenetically conserved heparin- and chitin-binding glycoprotein and is classified in the family of mammalian chitinase-like proteins (Renkema et al, 1998; Fusetti et al, 2003). However, YKL-40 lacks chitinase/hydrolase activity due to mutation of an essential glutamic acid to leucine in the chitinase-3-like catalytic domain (Renkema et al, 1998; Fusetti et al, 2003). YKL-40 is expressed in several mammal types including human (Hakala et al, 1993), porcine (Shackelton et al, 1995), cow (Rejman and Hurley, 1988), mouse (Lian et al, 2006), rabbit (De Ceuninck et al, 2001), and goat (Mohanty et al, 2003). In humans, the expression of YKL-40 is restricted to a few cell types including chondrocytes (Hu et al, 1996), synoviocytes (Nyirkos and Golds, 1990), vascular smooth muscle cells (Shackelton et al, 1995), macrophages (Rehli et al, 1997), and neutrophils (Kzhyshkowska et al, 2007). The biophysiological activity of YKL-40 is poorly understood, however, even though its crystal structure has been illustrated (Fusetti et al, 2003). It is currently known that YKL-40 mediates proliferation of chondrocytes and fibroblasts (De Ceuninck et al, 2001; Recklies et al, 2002). It is also capable of promoting macrophage differentiation (Rehli et al, 1997) and regulating extracellular matrix remodelling (Recklies et al, 2002; Badariotti et al, 2006; Bigg et al, 2006), suggesting that YKL-40 functions as a tissue remodelling factor.
Several independent studies have shown that YKL-40 in serum is elevated in a wide range of human carcinomas, including breast (Jensen et al, 2003), colorectum (Cintin et al, 1999), ovary (Hogdall et al, 2003), prostate (Kucur et al, 2008), brain (Pelloski et al, 2005), and blood (Bergmann et al, 2005). These increased levels have also been observed to be associated with poorer survival of cancer patients (Cintin et al, 1999, 2002; Hogdall et al, 2003; Jensen et al, 2003; Johansen et al, 2003; Bergmann et al, 2005; Pelloski et al, 2005), suggesting that serum levels of YKL-40 are a prognostic cancer biomarker. In breast cancer, increased serum levels of YKL-40 were found in 19% of patients with primary cancer (Johansen et al, 2003) and 30% of patients with metastatic cancer (Jensen et al, 2003), supporting the notion that YKL-40 is associated with cancer aggressiveness. We have recently identified that the expression levels of YKL-40 are associated with tumour vascular formation in breast cancer and in brain tumours, demonstrating its angiogenic properties in cancer development (Shao et al, 2009; Francescone et al, 2011). It remains to be determined whether YKL-40 is an independent biomarker or associated with other potential breast cancer biomarkers, and whether expression of YKL-40 is closely associated with clinical outcomes.
In this study of 203 cases of invasive cancer, we took advantage of a recently established polyclonal antibody specifically recognising YKL-40 to evaluate the expression of YKL-40 in different breast tissues including benign breast tissue, breast ductal carcinoma in situ (DCIS), and invasive ductal carcinoma (IDC) using an immunohistochemical (IHC) assay. We then employed a semi-quantitative approach to quantify the expression levels of YKL-40 and analysed the relationship between expression levels of YKL-40 and clinical outcomes. In addition, we investigated the association of YKL-40 with other two potential biomarkers GATA-binding protein 3 (GATA3) and E-cadherin in breast cancer, both of which correlate with ductal differentiation and a favourable prognosis of breast cancer (Bertucci et al, 2000; Graff et al, 2000; Garcia-Closas et al, 2007; Kouros-Mehr et al, 2008a). GATA3 is a zinc-finger transcription factor that has been shown to drive E-cadherin expression (Ko and Engel, 1993; Yan et al, 2010). E-cadherin is expressed in the cell membrane; it regulates cell–cell tight contacts and inhibits tumour cell motility as a tumour suppressor (Cheng et al, 2001; Wong and Gumbiner, 2003).
Materials and methods
Collection of cancer samples and clinical data
We retrospectively collected tissue samples from 221 breast cancer patients that underwent surgical therapy at Baystate Medical Center between 1994 and 2008. This random selection was primarily dependent on the availability of follow-up medical record over 10 years. Of these, 203 patients were diagnosed with invasive cancer and 18 patients were diagnosed with DCIS. Cancer samples (59 IDCs) available between year 2001 and 2002 were selected for the IHC analysis of GATA3 and E-cadherin protein expressions. Three normal tissue samples of reduction mammoplasty available at the Department of Pathology were also selected for the study as normal controls. All tissue specimens were maintained at the Department of Pathology and patient medical records were available at Baystate Cancer Center. The medical chart review abstracted information on ages, diagnosis, and pathological data such as tumour stages and grade in addition to status of lymph nodes and distant organ or tissue metastases. Ectopic cancer metastases were mainly diagnosed from CT scan, MRI, or PET-CT, demonstrating existence of tumours that were not anatomically connected to the primary breast cancer such as lung, liver, bone, chest wall, brain or bowel carcinoma. Most of these metastases were diagnosed at the later period of the disease. This study was approved by both the Baystate Medical Center and University of Massachusetts Amherst Institutional Review Board.
IHC staining
Paraffin-embedded human cancer specimens were cut to 6 μm thickness and processed for the staining of YKL-40, GATA3, E-cadherin, ER, PR, and Her2/neu. In brief, the samples were incubated with 3% H2O2 to block endogenous peroxidase activity for 30 min followed by incubation with blocking buffer containing 10% goat serum for 1 h. Then, one of antibodies such as rabbit anti-YKL-40 (1 : 400), ER (clone 6F11), PR (clone 1A6), or Her2/neu (clone CB11) (pre-diluted antibodies, Ventana Inc., Tucson, AZ, USA), mouse anti-GATA3 (1 : 200, Santa Cruz Inc., Santa Cruz, CA, USA), or anti-E-cadherin (1 : 2000, Invitrogen, Carlsbad, CA, USA) antibodies was incubated at room temperature for 2 h. A goat anti-rabbit or anti-mouse secondary antibody (1 : 100) conjugated with HRP was added. Finally, DAB substrate (Dako Inc., Carpinteria, CA, USA) was introduced for several minutes and after washing, methyl green will be used for counterstaining.
YKL-40, GATA3, and E-cadherin scoring
YKL-40 staining was evaluated as sum of two scores based on percent and intensity of positive staining cells as following (1) percent: no staining is 0 points, <10% of cells stained is 1 point, 11–50% of cells stained is 2 points, and >50% of cells stained is 3 points; (2) intensity: no staining is 0 points, weak staining is 1 point, moderate staining is 2 points, and strong staining is 3 points. Thus, the valid range of scores was 0–6 from combined density and intensity analyses. For statistical analysis, YKL-40 scores were further classified into three groups: negative/low (0–2 points), medium (3–4 points), and high (5–6 points) of YKL-40 staining. Finally, in analyses of YKL-40 and its relationship with GATA3 and E-cadherin, 0–2 points and 3–6 points of YKL-40 were designated as negative and positive, respectively. GATA3 and E-cadherin were evaluated using staining intensity as low/weak or high/strong.
Statistical analysis
Fisher's exact was used to assess the statistical significance of apparent co-variations of YKL-40 and each pathological factor and biomarker. We report two-sided significance levels. All analyses were performed using Stata version 10.1 (StataCorp, College Station, TX, USA). A Kaplan–Meier curve was tested for patient survival followed by a log-rank test analysis.
Results
Specificity of an anti-YKL-40 antibody and expression of YKL-40 in benign breast tissue and DCIS
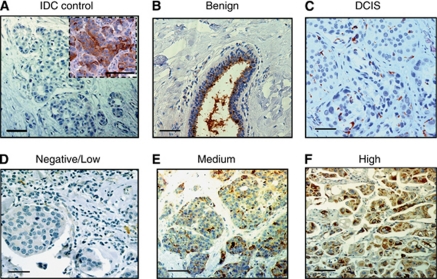
To analyse expression levels of YKL-40 in cancers, we began testing the specificity of an affinity-purified, polyclonal anti-YKL-40 antibody (rAY) in IHC assay. The rAY was pre-incubated with recombinant YKL-40 overnight before applying to breast cancer samples that demonstrated strong expression of YKL-40 in a pretest (Figure 1A, insert). This pre-incubation with YKL-40 recombinant protein fully prevented the binding of rAY with YKL-40 present in the cancer tissue as a result of no YKL-40-specific signal detected (Figure 1A). Pre-immune serum rabbit IgG as a negative control did not recognise any signals in the cancer tissue either (data not shown). We then tested YKL-40 expression in a few cases of both benign mammary tissue and breast cancer tissue at early stages. Benign mammary tissue expressed varied levels of YKL-40 as one of three subjects did not express YKL-40 (data not shown); whereas the other two cases expressed YKL-40 that was considerably accumulated in the apical surface of the epithelial ducts (Figure 1B), suggesting the secreted property of YKL-40 from ductal epithelial cells. In DCIS, YKL-40 immunoreactivity was mainly found in the cytoplasm of cancer cells (Figure 1C). These data indicate that rAY can serve as a powerful tool to evaluate expression of YKL-40 in breast tissue.
Figure 1.
YKL-40 expression in breast cancer. A negative control of IDCs was shown once rAY was pre-incubated with recombinant YKL-40 at a molar ratio of 1 : 1 before application in a cancer specimen (A). Insert indicated the positive staining without pre-incubation with recombinant YKL-40. In benign mammary tissue, YKL-40 secreted from ductal epithelial cells was accumulated in the apical aspect of duct lumen (B). A representative case of DCIS indicated a low level of staining of YKL-40 (C). Representative examples of immunohistochemical staining for negative, medium, and high expression levels of YKL-40 were shown in (D), (E), and (F), respectively. Bars: 100 μm.
In a pretest, we found that YKL-40 was not ubiquitously expressed by cancers in the same density or intensity; instead, its expression pattern commonly displayed diverse levels in each tissue specimen (data not shown). In order to precisely evaluate expression levels of YKL-40 in cancer tissue, a semi-quantification system assaying both density and intensity of the staining was engaged. Quantitative analysis of YKL-40 in 18 cases of DCIS demonstrated that 7 and 11 cases expressed low and medium levels of YKL-40, respectively; but none of cancers exhibited strong expression of YKL-40 (Figure 1C). These data indicate that breast cancer at early stages express low or medium levels of YKL-40.
Characteristics of invasive cancer samples
Study participants were between 23 and 88 years of age with a median of 56.5 years (Table 1). The majority of the cancers were ductal carcinomas (81.3%, IDC) followed by lobular carcinomas (12.3%) and medullary carcinomas (0.5%). The remaining 12 cancers (5.9%) involved infiltrating pleomorphic lobular carcinoma and inflammatory cancer (Table 1). Most were diagnosed between stage I (45.3%) and stage II (43.8%). The median tumour size was ∼2–5 cm (50.2%). Local (e.g., lymphatic nodes) and distant metastases (e.g., lung, liver, or brain) were found in 35.2% and 20.2% of these patients, respectively.
Table 1. Relationship between YKL-40 scores and clinical–pathological factors.
|
No. of YKL-40 scoresa (%)
|
|||||
|---|---|---|---|---|---|
| Factor | No. (%) | Low | Medium | High | P |
| Age (years) | 0.647 | ||||
| <40 | 13 (6.4) | 7 (53.8) | 1 (7.7) | 5 (38.5) | |
| 40–49 | 43 (21.2) | 27 (62.8) | 10 (23.5) | 6 (13.9) | |
| 50–59 | 55 (27.1) | 33 (60) | 11 (20) | 11 (20) | |
| >60 | 92 (45.3) | 54 (58.7) | 17 (18.5) | 21 (22.8) | |
| Total | 203 (100) | 121 (59.6) | 39 (19.2) | 43 (21.2) | |
| Histological subtype | 0.058 | ||||
| Ductal carcinoma | 165 (81.3) | 90 (54.5) | 34 (20.6) | 41 (24.8) | |
| Lobular carcinoma | 25 (12.3) | 20 (80) | 4 (16) | 1 (4) | |
| Medullar carcinoma | 1 (0.5) | 1 (100) | 0 | 0 | |
| Other | 12 (5.9) | 10 (83.3) | 1 (8.3) | 1 (8.3) | |
| Tumour stage | 0.554 | ||||
| I | 92 (45.3) | 58 (63) | 19 (20.6) | 15 (16.3) | |
| II | 89 (43.8) | 51 (57.3) | 16 (17.9) | 22 (24.7) | |
| III | 19 (9.4) | 9 (47.3) | 4 (21) | 6 (31.6) | |
| Unknown | 3 (1.4) | 3 (100) | 0 | 0 | |
| Tumour size (cm) | 0.407 | ||||
| <2 | 84 (41.4) | 55 (65.5) | 15 (17.9) | 14 (16.7) | |
| 2–5 | 102 (50.2) | 58 (56.9) | 21 (20.6) | 23 (22.5) | |
| >5 | 15 (7.4) | 6 (40) | 3 (20) | 6 (40) | |
| Unknown | 2 (1) | 2 (1.6) | 0 | 0 | |
| Tumour grade | <0.0001* | ||||
| I | 27 (13.3) | 22 (81.5) | 3 (11.1) | 2 (7.4) | |
| II | 74 (36.5) | 61 (82.4) | 10 (13.5) | 3 (4) | |
| III | 95 (46.8) | 34 (35.8) | 25 (26.3) | 36 (37.9) | |
| Unknown | 7 (3.4) | 4 (57.1) | 1 (14.3) | 2 (25.6) | |
| Her2/neu | 0.010* | ||||
| Low | 37 (18.2) | 24 (64.9) | 8 (21.6) | 5 (13.5) | |
| Medium | 43 (21.2) | 34 (79) | 7 (16.3) | 2 (4.6) | |
| High | 43 (21.2) | 23 (53.5) | 7 (16.3) | 13 (30.2) | |
| Unknown | 80 (39.4) | 40 (50) | 17 (22.2) | 23 (28.7) | |
| ER status | <0.0001* | ||||
| Positive | 128 (63) | 94 (73.4) | 21 (16.4) | 13 (10.1) | |
| Negative | 59 (29) | 18 (30.5) | 16 (27.1) | 25 (42.4) | |
| Unknown | 16 (7.9) | 9 (56) | 2 (12.5) | 5 (31.2) | |
| PR status | <0.0001* | ||||
| Positive | 101 (49.7) | 78 (77.2) | 10 (9.9) | 13 (12.9) | |
| Negative | 84 (41.4) | 32 (38.1) | 27 (32.1) | 25 (29.8) | |
| Unknown | 18 (8.9) | 11(61.1) | 2 (11.1) | 5 (27.7) | |
| Node status | 0.122 | ||||
| Positive | 81 (35.2) | 48 (59.2) | 15 (18.5) | 18 (22.2) | |
| Negative | 111 (54.7) | 64 (57.6) | 22 (19.8) | 25 (22.5) | |
| Unknown | 11 (5.4) | 11 (100) | 0 | 0 | |
| Distant metastasis b | 0.613 | ||||
| Positive | 41 (20.2) | 24 (58.5) | 11 (26.8) | 6 (14.6) | |
| Negative | 93 (45.8) | 52 (55.9) | 16 (17.2) | 22 (23.6) | |
| Unknown | 69 (34) | 42 (60.9) | 12 (17.4) | 15 (21.7) | |
Abbreviations: ER=oestrogen receptor; PR=progesterone receptor.
YKL-40 low, medium, and high scores from combined intensity and density analysis are 0–2, 3–4, and 5–6 points, respectively.
The status of metastasis was derived from diagnostic imaging tests as described in Materials and methods, in which negative stands for no metastasis identified in distant organs and unknown indicates no these tests were performed. *P<0.05.
Figure 1D–F was representative examples of three different expression levels of YKL-40. In all, 121 of 203 cases (59.6%) exhibited negative or low expression of YKL-40; 82 patients (40.4%) were YKL-40 positive (Table 1). Forty-three patients (21.1%) displayed strong expression of YKL-40 and thirty-nine patients (19.2%) expressed medium levels of YKL-40. Older age was associated with prevalence of invasive cancers, regardless of YKL-40 levels.
Associations of YKL-40 with clinical–pathological factors
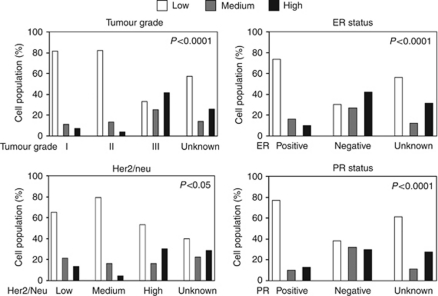
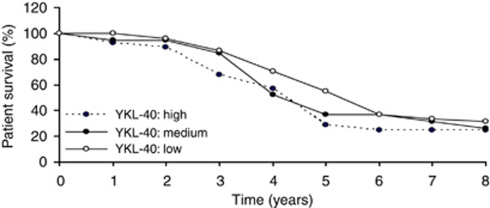
We observed no statistically significant association of YKL-40 with any of age, tumour stage, tumour size, node status, or distant metastasis (Table 1). Histological subtype was marginally statistically associated with YKL-40 (P-value=0.058); this likely reflects the higher prevalence of high YKL-40 expression in the IDC group. YKL-40 expression was strongly associated with tumour grade (Table 1; Figure 2; P<0.0001), reflecting the higher prevalence of high YKL-40 scores in the tumour grade III group, but with no evidence of trend. YKL-40 was also associated with expression of Her2/neu (P<0.01; Figure 2); this similarly reflects the higher prevalence of high YKL-40 scores in Her2/neu subgroup, but without evidence of trend. In contrast, the levels of YKL-40 were inversely correlated with both ER and PR levels (Table 1; Figure 2; P<0.0001). However, there was no significant difference between YKL-40 expression and patient overall survival (Figure 3) or disease-free survival (data not shown) in 8-year follow-up studies. Collectively, these data demonstrate that expression levels of YKL-40 were positively correlated with tumour grade and Her2/neu, but negatively correlated with ER and PR expressions.
Figure 2.
Relationship between YKL-40, tumour grade, and expression levels of ER, PR, or Her2/neu. The data of different expression levels of YKL-40 in tumour grade, ER, PR and Her2/neu listed in Table 2 were analysed graphically. Fisher's exact was used to test the significance and P-values were provided.
Figure 3.
YKL-40 expression is not associated with patient overall survival. Patient survival data available in 8-year follow-up medical record were analysed using a Kaplan–Meier survival curve. A log-rank test did not show a significant difference in groups containing high (n=28), medium (n=19), or low (n=51) levels of YKL-40.
Associations of YKL-40 with GATA3 and E-cadherin
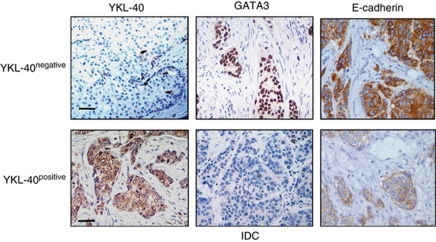
We observed negative associations of YKL-40 with both GATA3 and E-cadherin (Figure 4; Table 2). Samples with low or negative levels of YKL-40 exhibited high expressions of both GATA3 and E-cadherin; whereas cancers with high or positive levels of YKL-40 indicated low or negative levels of GATA3 and E-cadherin, P=0.0137 and P=0.0417, respectively (Table 2). In addition, an analysis of ER with GATA3 and E-cadherin showed a strong correlation between ER and GATA3 or E-cadherin (Table 2). These data support the notion that expression levels of YKL-40 are inversely associated with cancer differentiation.
Figure 4.
An inverse correlation of YKL-40 with GATA3 and E-cadherin. Specimens from 59 IDC patients between year 2000 and 2002 were processed for immunohistochemistry of YKL-40, GATA3, and E-cadherin. Representatives of negative and positive of YKL-40 with corresponding high and low staining of GATA3 and E-cadherin were shown. GATA3 was stained in the nucleus and E-cadherin was located on cell membrane. Bar: 100 μm.
Table 2. Correlation of YKL-40 and ER with GATA3 and E-cadherin in IDC.
|
YKL-40a
|
ERb
|
|||||
|---|---|---|---|---|---|---|
| Negative | Positive | P-value | Negative | Positive | P-value | |
| GATA3 | ||||||
| Low | 15 | 19 | 19 | 14 | ||
| High | 19 | 6 | 0.0137 | 1 | 16 | 0.00030 |
| E-cadherin | ||||||
| Low | 9 | 13 | 15 | 7 | ||
| High | 25 | 12 | 0.0417 | 5 | 23 | 0.00038 |
Abbreviations: ER=oestrogen receptor; IDC=invasive ductal carcinoma.
n=59.
n=50.
Discussion
Accumulating evidence from clinical studies of breast cancers has demonstrated that elevated serum levels of YKL-40 correlate with cancer progression and decreased disease-free survival (Johansen et al, 1995; Jensen et al, 2003). Thus, serum levels of YKL-40 are suggested as a prognostic cancer biomarker. However, little is known concerning the tissue source for YKL-40 production, which may be the determinant of serum levels of YKL-40 in cancer. We currently found that approximately a half (21.1%) of YKL-40-positive cancers (40.4%) expressed strong YKL-40, the population of which may account for the evidence reported previously that 20–24% of patients demonstrate elevated serum levels of YKL-40 in overall breast cancer population (Johansen et al, 1995, 2003). Interestingly, the tissue expression levels of YKL-40 were not directly correlated with patient survival, suggesting that cancer cells may not serve as the only source responsible for serum concentrations of YKL-40. Indeed, YKL-40 is also produced by other cells such as vascular smooth muscle cells (Shackelton et al, 1995), macrophages (Rehli et al, 1997), and neutrophils (Kzhyshkowska et al, 2007). Strong expression of YKL-40 by vascular cells, mast cells, neutrophils, and macrophages associated with cancer cells was found in breast cancer samples (Roslind et al, 2007 and our unpublished data). Thus, all these cells may together contribute to the blood levels of YKL-40, although we currently cannot rule out other sources besides tumour tissue. Nevertheless, we identified the tissue level of YKL-40 is correlated positively with another breast cancer marker Her2/neu and negatively with other markers ER, PR, GATA3, and E-cadherin, highlighting the valuable biomarker of YKL-40 in breast cancer diagnosis.
Here, we used a semi-quantified analysis assaying both staining density and intensity, which can precisely evaluate varied expression levels of YKL-40 in each specimen. Some of the data were consistent with a previous report that showed correlation of increased expression levels of YKL-40 with tumour grade, poor differentiation, and decreased disease-free survival (Kim et al, 2007). Interestingly, another clinical study gave rise to opposite evidence that elevated expression of YKL-40 was associated with positive levels of ER and PR, but did not correlate with patient survival (Roslind et al, 2007). This discrepancy may be attributed to the different quantification analyses by which that study assessed only the expression intensity of YKL-40 in cancers. In addition, different agents engaged for IHC analysis may be another contributor to the inconsistency. For example, different affinity-purified anti-YKL-40 antibodies may underestimate the expression of YKL-40. In the current study, we used multiple staining approaches to validate rAY as an appropriate tool that can specifically recognise YKL-40 in the tissue, before we examined YKL-40 expression in cancer specimens. We substantially analysed this antibody specificity using different positive and negative controls, and pre-incubation of tissue with recombinant YKL-40 that sufficiently prevents the binding of rAY with YKL-40 in cancer. In addition, we also tested the interaction of rAY with recombinant protein YKL-40 or YKL-40 secreted from tumour cells in an immunoblotting assay (e.g., an osteoblastoma line MG-63 and a brain tumour line U87) and we found that its specificity was identical to that observed in IHC (data not shown), confirming the high specificity of rAY.
The traditional evaluation of breast cancer prognosis is mainly dependent on primary tumour size and the status of invaded axillary lymph nodes. Other measurements such as the status of ER and PR, and the degree of tumour differentiation also indicate some value in assisting cancer diagnosis and prognosis. Over the past decades, accumulating studies have demonstrated that elevated expression of transmembrane protein kinase Her2/neu is associated with poorer prognosis (Willsher et al, 1996; Jensen et al, 2003). Testing Her2/neu as the prognostic utility has been directed to devise anti-Her2/neu therapies (Chang, 2010; Junttila et al, 2010). Interestingly, there is strong evidence showing that serum levels of YKL-40 and Her2/neu independently reflect cancer metastasis (Jensen et al, 2003). It is noteworthy that YKL-40 serum levels indicated a stronger predictor of patient survival than Her2/neu or others (e.g., negative ER). This may be attributed to the angiogenic signature of YKL-40 in tumour metastasis, which fuels tumour cell proliferation and invasiveness through facilitating blood vessel formation (Shao et al, 2009). Thus, YKL-40 could not only serve as a prognostic marker, but also as a target for cancer therapy. Apart from these biomarkers for breast cancer, mounting evidence also indicated that expressions of GATA3 and E-cadherin in breast cancer are strongly associated with ductal differentiation and a favourable prognosis, suggestive of new breast cancer biomarkers (Mehra et al, 2005; Eeckhoute et al, 2007; Voduc et al, 2008; Kouros-Mehr et al, 2008b). In the context with these data, here we have identified that YKL-40 expression is negatively associated with GATA3, E-cadherin, ER, and PR; therefore, we demonstrate that YKL-40 together with other markers including Her2/neu, ER, PR, GATA3, and E-cadherin could serve as a powerful tool for the diagnosis and prognosis of breast cancer as well as a potential target for future cancer therapy.
Acknowledgments
This work was supported by NCI R01 CA120659 and DOD W81XWH-06-1-0563 (RS), Collaborative Biomedical Research Program, Baystate Medical Center/University of Massachusetts at Amherst (RS, QJC, and RBA), and Rays of Hope, Baystate Medical Center (QJC and RS). We also thank Dr Xuan Luu for helping collect patient survival data.
Footnotes
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
References
- Badariotti F, Kypriotou M, Lelong C, Dubos MP, Renard E, Galera P, Favrel P (2006) The phylogenetically conserved molluscan chitinase-like protein 1 (Cg-Clp1), homologue of human HC-gp39, stimulates proliferation and regulates synthesis of extracellular matrix components of mammalian chondrocytes. J Biol Chem 281: 29583–29596 [DOI] [PubMed] [Google Scholar]
- Bergmann OJ, Johansen JS, Klausen TW, Mylin AK, Kristensen JS, Kjeldsen E, Johnsen HE (2005) High serum concentration of YKL-40 is associated with short survival in patients with acute myeloid leukemia. Clin Cancer Res 11: 8644–8652 [DOI] [PubMed] [Google Scholar]
- Bertucci F, Houlgatte R, Benziane A, Granjeaud S, Adelaide J, Tagett R, Loriod B, Jacquemier J, Viens P, Jordan B, Birnbaum D, Nguyen C (2000) Gene expression profiling of primary breast carcinomas using arrays of candidate genes. Hum Mol Genet 9: 2981–2991 [DOI] [PubMed] [Google Scholar]
- Bigg HF, Wait R, Rowan AD, Cawston TE (2006) The mammalian chitinase-like lectin, YKL-40, binds specifically to type I collagen and modulates the rate of type I collagen fibril formation. J Biol Chem 281: 21082–21095 [DOI] [PubMed] [Google Scholar]
- Blackman DJ, Masi CM (2006) Racial and ethnic disparities in breast cancer mortality: are we doing enough to address the root causes? J Clin Oncol 24: 2170–2178 [DOI] [PubMed] [Google Scholar]
- Chang HR (2010) Trastuzumab-based neoadjuvant therapy in patients with HER2-positive breast cancer. Cancer 116: 2856–2867 [DOI] [PubMed] [Google Scholar]
- Cheng CW, Wu PE, Yu JC, Huang CS, Yue CT, Wu CW, Shen CY (2001) Mechanisms of inactivation of E-cadherin in breast carcinoma: modification of the two-hit hypothesis of tumor suppressor gene. Oncogene 20: 3814–3823 [DOI] [PubMed] [Google Scholar]
- Cintin C, Johansen JS, Christensen IJ, Price PA, Sorensen S, Nielsen HJ (1999) Serum YKL-40 and colorectal cancer. Br J Cancer 79: 1494–1499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cintin C, Johansen JS, Christensen IJ, Price PA, Sorensen S, Nielsen HJ (2002) High serum YKL-40 level after surgery for colorectal carcinoma is related to short survival. Cancer 95: 267–274 [DOI] [PubMed] [Google Scholar]
- De Ceuninck F, Gaufillier S, Bonnaud A, Sabatini M, Lesur C, Pastoureau P (2001) YKL-40 (cartilage gp-39) induces proliferative events in cultured chondrocytes and synoviocytes and increases glycosaminoglycan synthesis in chondrocytes. Biochem Biophys Res Commun 285: 926–931 [DOI] [PubMed] [Google Scholar]
- Eeckhoute J, Keeton EK, Lupien M, Krum SA, Carroll JS, Brown M (2007) Positive cross-regulatory loop ties GATA-3 to estrogen receptor alpha expression in breast cancer. Cancer Res 67: 6477–6483 [DOI] [PubMed] [Google Scholar]
- Francescone RA, Scully S, Faibish M, Taylor SL, Oh D, Moral L, Yan W, Bentley B, Shao R (2011) Role of YKL-40 in the angiogenesis, radioresistance, and progression of glioblastoma. J Biol Chem 286: 15332–15343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusetti F, Pijning T, Kalk KH, Bos E, Dijkstra BW (2003) Crystal structure and carbohydrate-binding properties of the human cartilage glycoprotein-39. J Biol Chem 278: 37753–37760 [DOI] [PubMed] [Google Scholar]
- Garcia-Closas M, Troester MA, Qi Y, Langerod A, Yeager M, Lissowska J, Brinton L, Welch R, Peplonska B, Gerhard DS, Gram IT, Kristensen V, Borresen-Dale AL, Chanock S, Perou CM (2007) Common genetic variation in GATA-binding protein 3 and differential susceptibility to breast cancer by estrogen receptor alpha tumor status. Cancer Epidemiol Biomarkers Prev 16: 2269–2275 [DOI] [PubMed] [Google Scholar]
- Graff JR, Gabrielson E, Fujii H, Baylin SB, Herman JG (2000) Methylation patterns of the E-cadherin 5′ CpG island are unstable and reflect the dynamic, heterogeneous loss of E-cadherin expression during metastatic progression. J Biol Chem 275: 2727–2732 [DOI] [PubMed] [Google Scholar]
- Hakala BE, White C, Recklies AD (1993) Human cartilage gp-39, a major secretory product of articular chondrocytes and synovial cells, is a mammalian member of a chitinase protein family. J Biol Chem 268: 25803–25810 [PubMed] [Google Scholar]
- Hogdall EV, Johansen JS, Kjaer SK, Price PA, Christensen L, Blaakaer J, Bock JE, Glud E, Hogdall CK (2003) High plasma YKL-40 level in patients with ovarian cancer stage III is related to shorter survival. Oncol Rep 10: 1535–1538 [PubMed] [Google Scholar]
- Hu B, Trinh K, Figueira WF, Price PA (1996) Isolation and sequence of a novel human chondrocyte protein related to mammalian members of the chitinase protein family. J Biol Chem 271: 19415–19420 [DOI] [PubMed] [Google Scholar]
- Jatoi I, Miller AB (2003) Why is breast-cancer mortality declining? Lancet Oncol 4: 251–254 [DOI] [PubMed] [Google Scholar]
- Jensen BV, Johansen JS, Price PA (2003) High levels of serum HER-2/neu and YKL-40 independently reflect aggressiveness of metastatic breast cancer. Clin Cancer Res 9: 4423–4434 [PubMed] [Google Scholar]
- Johansen JS, Christensen IJ, Riisbro R, Greenall M, Han C, Price PA, Smith K, Brunner N, Harris AL (2003) High serum YKL-40 levels in patients with primary breast cancer is related to short recurrence free survival. Breast Cancer Res Treat 80: 15–21 [DOI] [PubMed] [Google Scholar]
- Johansen JS, Cintin C, Jorgensen M, Kamby C, Price PA (1995) Serum YKL-40: a new potential marker of prognosis and location of metastases of patients with recurrent breast cancer. Eur J Cancer 31A: 1437–1442 [DOI] [PubMed] [Google Scholar]
- Junttila TT, Parsons K, Olsson C, Lu Y, Xin Y, Theriault J, Crocker L, Pabonan O, Baginski T, Meng G, Totpal K, Kelley RF, Sliwkowski MX (2010) Superior in vivo efficacy of afucosylated trastuzumab in the treatment of HER2-amplified breast cancer. Cancer Res 70: 4481–4489 [DOI] [PubMed] [Google Scholar]
- Kim SH, Das K, Noreen S, Coffman F, Hameed M (2007) Prognostic implications of immunohistochemically detected YKL-40 expression in breast cancer. World J Surg Oncol 5: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko LJ, Engel JD (1993) DNA-binding specificities of the GATA transcription factor family. Mol Cell Biol 13: 4011–4022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouros-Mehr H, Bechis SK, Slorach EM, Littlepage LE, Egeblad M, Ewald AJ, Pai SY, Ho IC, Werb Z (2008a) GATA-3 links tumor differentiation and dissemination in a luminal breast cancer model. Cancer Cell 13: 141–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouros-Mehr H, Kim JW, Bechis SK, Werb Z (2008b) GATA-3 and the regulation of the mammary luminal cell fate. Curr Opin Cell Biol 20: 164–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucur M, Isman FK, Balci C, Onal B, Hacibekiroglu M, Ozkan F, Ozkan A (2008) Serum YKL-40 levels and chitotriosidase activity as potential biomarkers in primary prostate cancer and benign prostatic hyperplasia. Urol Oncol 26: 47–52 [DOI] [PubMed] [Google Scholar]
- Kzhyshkowska J, Gratchev A, Goerdt S (2007) Human chitinases and chitinase-like proteins as indicators for inflammation and cancer. Biomark Insights 2: 128–146 [PMC free article] [PubMed] [Google Scholar]
- Lian Z, De Luca P, Di Cristofano A (2006) Gene expression analysis reveals a signature of estrogen receptor activation upon loss of Pten in a mouse model of endometrial cancer. J Cell Physiol 208: 255–266 [DOI] [PubMed] [Google Scholar]
- Mehra R, Varambally S, Ding L, Shen R, Sabel MS, Ghosh D, Chinnaiyan AM, Kleer CG (2005) Identification of GATA3 as a breast cancer prognostic marker by global gene expression meta-analysis. Cancer Res 65: 11259–11264 [DOI] [PubMed] [Google Scholar]
- Mohanty AK, Singh G, Paramasivam M, Saravanan K, Jabeen T, Sharma S, Yadav S, Kaur P, Kumar P, Srinivasan A, Singh TP (2003) Crystal structure of a novel regulatory 40-kDa mammary gland protein (MGP-40) secreted during involution. J Biol Chem 278: 14451–14460 [DOI] [PubMed] [Google Scholar]
- Nyirkos P, Golds EE (1990) Human synovial cells secrete a 39 kDa protein similar to a bovine mammary protein expressed during the non-lactating period. Biochem J 269: 265–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelloski CE, Mahajan A, Maor M, Chang EL, Woo S, Gilbert M, Colman H, Yang H, Ledoux A, Blair H, Passe S, Jenkins RB, Aldape KD (2005) YKL-40 expression is associated with poorer response to radiation and shorter overall survival in glioblastoma. Clin Cancer Res 11: 3326–3334 [DOI] [PubMed] [Google Scholar]
- Recklies AD, White C, Ling H (2002) The chitinase 3-like protein human cartilage glycoprotein 39 (HC-gp39) stimulates proliferation of human connective-tissue cells and activates both extracellular signal-regulated kinase- and protein kinase B-mediated signalling pathways. Biochem J 365: 119–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehli M, Krause SW, Andreesen R (1997) Molecular characterization of the gene for human cartilage gp-39 (CHI3L1), a member of the chitinase protein family and marker for late stages of macrophage differentiation. Genomics 43: 221–225 [DOI] [PubMed] [Google Scholar]
- Rejman JJ, Hurley WL (1988) Isolation and characterization of a novel 39 kilodalton whey protein from bovine mammary secretions collected during the nonlactating period. Biochem Biophys Res Commun 150: 329–334 [DOI] [PubMed] [Google Scholar]
- Renkema GH, Boot RG, Au FL, Donker-Koopman WE, Strijland A, Muijsers AO, Hrebicek M, Aerts JM (1998) Chitotriosidase, a chitinase, and the 39-kDa human cartilage glycoprotein, a chitin-binding lectin, are homologues of family 18 glycosyl hydrolases secreted by human macrophages. Eur J Biochem 251: 504–509 [DOI] [PubMed] [Google Scholar]
- Roslind A, Knoop AS, Jensen MB, Johansen JS, Nielsen DL, Price PA, Balslev E (2007) YKL-40 protein expression is not a prognostic marker in patients with primary breast cancer. Breast Cancer Res Treat 112: 275–285 [DOI] [PubMed] [Google Scholar]
- Shackelton LM, Mann DM, Millis AJ (1995) Identification of a 38-kDa heparin-binding glycoprotein (gp38k) in differentiating vascular smooth muscle cells as a member of a group of proteins associated with tissue remodeling. J Biol Chem 270: 13076–13083 [DOI] [PubMed] [Google Scholar]
- Shao R, Hamel K, Petersen L, Cao QJ, Arenas RB, Bigelow C, Bentley B, Yan W (2009) YKL-40, a secreted glycoprotein, promotes tumor angiogenesis. Oncogene 28: 4456–4468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voduc D, Cheang M, Nielsen T (2008) GATA-3 expression in breast cancer has a strong association with estrogen receptor but lacks independent prognostic value. Cancer Epidemiol Biomarkers Prev 17: 365–373 [DOI] [PubMed] [Google Scholar]
- Willsher PC, Beaver J, Pinder S, Bell JA, Ellis IO, Blamey RW, Robertson JF (1996) Prognostic significance of serum c-erbB-2 protein in breast cancer patients. Breast Cancer Res Treat 40: 251–255 [DOI] [PubMed] [Google Scholar]
- Wong AS, Gumbiner BM (2003) Adhesion-independent mechanism for suppression of tumor cell invasion by E-cadherin. J Cell Biol 161: 1191–1203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan W, Cao QJ, Arenas RB, Bentley B, Shao R (2010) GATA3 inhibits breast cancer metastasis through the reversal of epithelial-mesenchymal transition. J Biol Chem 285: 14042–14051 [DOI] [PMC free article] [PubMed] [Google Scholar]