Abstract
Background:
Memory T cells are well known to have a critical role for host defense in humans. However, their role in actual human cancer remains largely unknown. In this study, we tried to reveal the clinical importance of tumour-infiltrating CD45RO+ memory T cells in renal cell carcinoma (RCC).
Methods:
We analysed 105 patients with RCC, who received radical or partial nephrectomy. Those were 65 in TNM stage I, 7 in stage II, 15 in stage III, and 18 in stage IV, respectively. CD45RO expression was evaluated by immunohistochemistry. CD4 and CD8 expressions were also systematically assessed in the same manner.
Results:
Patients with higher TNM stage or high nuclear grade were found to have higher densities of CD45RO. Furthermore, CD45RO status was positively correlated with preoperative C-reactive protein level. In prognostic analysis, CD45RO+lo patients had a significantly better prognosis than CD45RO+hi patients. There was also a significant difference between CD4+lo and CD4+hi groups, whereas no significant difference was observed in CD8 T-cell status. Finally, multivariate analysis revealed that CD45RO+ status was the independent prognostic factor for patient overall survival.
Conclusion:
CD45RO+ memory T-cell status has a significant independent prognostic value, indicating that the adaptive immune response is functionally critical in human RCC.
Keywords: renal cell carcinoma, memory T cell, CD45RO, tumour-infiltrating lymphocytes, prognosis
Renal cell carcinoma (RCC) is the most common malignancy of the kidney and the incidence has been rising steadily. Due to the lack of symptoms at the early stages, about one third of patients present with advanced disease, either locally advanced or metastatic (Motzer et al, 1999; Cohen and McGovern, 2005). Although the early stage RCC is usually treated by surgery with good prognosis, the therapy for advanced-stage tumours still needs to be improved. Renal cell carcinoma is generally refractory to conventional anticancer treatments including chemotherapy and radiotherapy. It has been long expected that RCC is one of the feasible targets for immunotherapy. Although conventional immunotherapy such as interleukin-2 and interferon-α remains the effective therapy when treating advanced RCC, long-term clinical outcome has been disappointing (Motzer et al, 1999; Yang et al, 2003). Recent another immunotherapy trials including tumour vaccines and allogeneic stem cell transplants have shown a certain effect on the prognosis of patients with advanced RCC (Childs et al, 2000; Holtl et al, 2002; Avigan et al, 2004). New agents targeting vascular endothelial growth factor, platelet-derived growth factor, and mammalian target of rapamycin pathway have been demonstrated to have significant clinical response and prolong patient survival (Escudier et al, 2009; Motzer et al, 2009; Sun et al, 2010). Some of them have been also shown to be superior to cytokine immunotherapy such as interferon. Therefore, those targeted therapies currently represent the first-line standard for metastatic RCC. However, these treatments are not enough to achieve complete response and also have several adverse effects. Therefore, a deeper understanding of biology of RCC is required to develop more effective and less toxic systematic therapies for patients with advanced disease.
CD45 is known as the leukocyte common antigen and function as a tyrosine phosphatase in leukocyte signalling. The expression of different CD45 isoforms is cell type specific and depends on the stage of differentiation and state of activation of cells. In humans, CD45RA and CD45RO are thought to be naive and memory T cells, respectively (Akbar et al, 1988; Merkenschlager et al, 1988). Memory T cells are generated during cell-mediated immunity responses, and survive for many months and years after the antigen is eliminated. These memory T cells are responsible for more rapid and amplified responses to second and subsequent exposures to antigens. Memory T cells have critically important role in host defense for infection in humans. In tumour immunity, recent studies on colorectal and gastric cancer have demonstrated that high density of CD45RO+ tumour-infiltrating lymphocytes (TILs) is correlated with increased survival, and CD45RO+ TILs is independent prognostic factor (Pages et al, 2005, 2009; Galon et al, 2006; Lee et al, 2008). Although those studies have suggested that memory T cells have important role in tumour immunity, the clinical importance of memory T cells in RCC has not been previously addressed. Renal cell carcinoma has been considered to be an immunogenic cancer, with pathological specimens frequently large numbers of TILs (Webster et al, 2006). Several studies have previously reported that high densities of CD4+ or CD8+ T cells have worse impact on the prognosis in RCC, indicating functional importance of TILs (Nakano et al, 2001; Bromwich et al, 2003). However, the significance of each T-cell subset in RCC is still controversial, since results are not always consistent between different studies (Igarashi et al, 2002; Bromwich et al, 2003).
The aim of this study was to systematically investigate the importance of TILs using T-cell subset markers including CD45RO as well as CD4 and CD8, and to clarify the clinical significance of CD45RO+ memory T cells infiltrating in human RCC.
Materials and methods
We analysed 105 patients with RCC, which received radical or partial nephrectomy during 2003 and 2008 at Nara Medical University Hospital. The age of patients ranged from 31 to 84 years (median 65 years) and the male to female ratio was 2.6 : 1.0. None of these patients received preoperative immunotherapy or renal arterial embolisation therapy. They were histopathologically composed of 84 (80%) of clear cell type, 9 (9%) of granular cell type, 5 (5%) of papillary cell type, 4 (4%) of spindle cell type, 2 (2%) of cystic cell type, and 1 (1%) of mixed clear and spindle type. The tumour stage was classified according to the UICC TNM classification of renal tumours (Guinan et al, 1997). Pathological grades were assigned according to the criteria proposed by Fuhrman et al (1982). We measured preoperative serum C-reactive protein (CRP) and defined patients with CRP of >0.5 mg dl−1 as positive group as previously described (Tatokoro et al, 2008; Saito et al, 2009). This study was approved by the Ethical Review Committee of Nara Medical University Hospital.
Immunohistochemistry
Immunohistochemical staining for CD45RO, CD4, and CD8 was performed with a Dako Envision kit (DAKO Cytomation, Tokyo, Japan). Formalin-fixed, paraffin-embedded tissues were cut into 5 mm sections, deparaffinised, and rehydrated in a graded series of ethanols. Antigen retrieval was done by heating tissue sections using a Target Retrieval Solution, pH 9.0 (DAKO). Then, the samples were incubated for 5 min in peroxidase blocking solution (DAKO) to inhibit endogenous peroxidase. The sections were then incubated overnight at 4°C with anti-human CD45RO (UHL1, monoclonal mouse; DAKO), CD4 (1 : 40) (4B12, monoclonal mouse; DAKO), and CD8 (C8/144B, monoclonal mouse; DAKO). A subsequent reaction was carried out using secondary antibodies (DAKO) at 37°C for 30 min. Then, the sections were washed three times with phosphate-buffered saline and subsequently the colour was displayed with DAB (DAKO) for about 5 min. Sections were counterstained with haematoxylin, dehydrated in ethanol, cleared in xylene, and coverslipped.
Evaluation of immunostaining
By immunohistochemistry, we evaluated tumour-infiltrating CD45RO, CD4, and CD8 T cells on RCC tissues as previously reported (Ohigashi et al, 2005; Anraku et al, 2008). We selected different five areas with most abundant positively stained cells in each tissue under 400 magnifications. Positive cells in the selected areas for each T-cell marker were counted independently by two investigators without knowledge of clinical information. In case of disagreement, the slides were re-examined and a consensus was reached by the investigators. Then, we calculated the median number of each sample.
Statistical analysis
Comparisons among the clinical and pathological features were evaluated using χ2 and Fisher's exact tests. Statistical significance between two groups of parametric date was evaluated using an unpaired Student's t-test. Survival curves were estimated using the Kaplan–Meier method, and the significances of differences between survival curves were determined using log-rank test. Multivariate comparisons of survival distributions were made using Cox proportional hazards models. All tests were two-sided and P<0.05 were considered statistically significant.
Results
Density of TILs
We retrospectively analysed 105 patients with RCC without any preoperative anticancer therapy. By immunohistochemistry, CD45RO+, CD4+, and CD8+ T cells infiltrating into RCC tissue were evaluated. These lymphocytes were detected within cancer nests or present in the stroma in contact to cancer cells (Figure 1). The median number of CD45RO-, CD4-, and CD8-positive cells was 49.8, 16.6, and 18.6, respectively. We defined these median numbers as cutoff values. All cases were classified into low- or high-density groups for each marker, that is, CD45RO+lo, CD4+lo, and CD8+lo (low-density groups) and CD45RO+hi, CD4+hi, and CD8+hi (high-density groups).
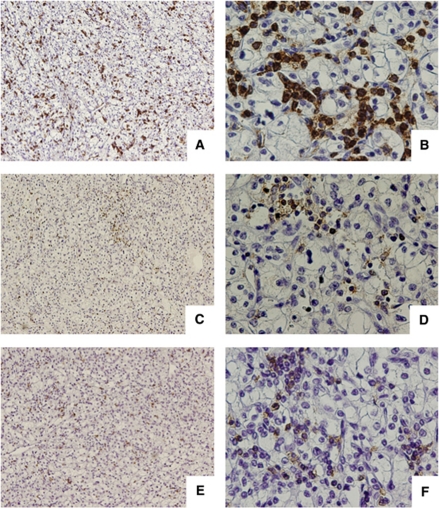
Figure 1.
Immunohistochemical staining of tumour-infiltrating lymphocytes in renal cell carcinoma surgical specimen. CD45RO-, CD4-, and CD8-positive cells were observed in the stromal area outside of tumour cells. Representative case of CD45RO (A and B), CD4 (C and D), and CD8 (E and F). Original magnification (A, C, and E) × 100 and (B, D, and F) × 400.
Associations between the density of tumour-infiltrating T cells and clinicopathological features
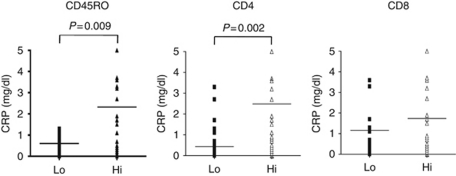
The clinical and pathological characteristics grouped by TIL density with median value cutoffs are summarised in Table 1. The more patients with higher TNM stage and nuclear grade were found in CD45RO+hi and CD4+hi than in CD45RO+lo and CD4+lo. However, this was not observed for CD8. There were no statistically significant associations between all three T-cell markers and patient's age, gender, lymph-node involvement or distant metastases. Preoperative serum CRP levels are known to be prognostic factor and indicate systematic inflammatory response. In this study, 32 patients had positive preoperative CRP and 73 had negative. The positive correlations between preoperative CRP and TNM stage were observed (Table 2). The more patients with higher TNM stage had positive preoperative CRP. Next, we examined the relationship between preoperative CRP levels and T-cell status. As shown in Figure 2, increased preoperative CRP levels were positively correlated with CD45RO and CD4 status, but not with CD8. This indicated that CD45RO+ and CD4+ T cell might have some roles in systematic inflammatory response of RCC patients.
Table 1. Correlation between TIL density and clinicopathological characteristics in the 105 renal cell carcinomas.
|
CD45RO
|
CD4
|
CD8
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Lo | Hi | P | Lo | Hi | P | Lo | Hi | P | |
| Age at surgery (years) | |||||||||
| <60 | 21 | 15 | 0.338 | 18 | 18 | 0.944 | 18 | 18 | 0.944 |
| ⩾60 | 32 | 37 | 35 | 34 | 35 | 34 | |||
| Gender | |||||||||
| Female | 14 | 15 | 0.952 | 12 | 17 | 0.249 | 15 | 14 | 0.874 |
| Male | 39 | 37 | 41 | 35 | 38 | 38 | |||
| T stage | |||||||||
| pT1 | 41 | 28 | 0.038 | 40 | 29 | 0.116 | 34 | 35 | 0.234 |
| pT2 | 5 | 5 | 5 | 5 | 8 | 2 | |||
| pT3 | 6 | 18 | 7 | 17 | 10 | 14 | |||
| pT4 | 1 | 1 | 1 | 1 | 1 | 1 | |||
| Lymph-node involvement | |||||||||
| Absent | 50 | 46 | 0.467 | 49 | 47 | 0.705 | 50 | 46 | 0.282 |
| Present | 3 | 6 | 4 | 5 | 3 | 6 | |||
| Distant metastases | |||||||||
| Absent | 49 | 40 | 0.087 | 49 | 40 | 0.087 | 46 | 43 | 0.559 |
| Present | 4 | 12 | 4 | 12 | 7 | 9 | |||
| TNM stage | |||||||||
| I | 41 | 24 | 0.002 | 40 | 25 | 0.011 | 34 | 31 | 0.379 |
| II | 4 | 3 | 4 | 3 | 5 | 2 | |||
| III | 2 | 13 | 3 | 12 | 5 | 10 | |||
| IV | 6 | 12 | 6 | 12 | 9 | 9 | |||
| Nuclear grade | |||||||||
| 1 | 29 | 15 | 0.011 | 28 | 16 | 0.009 | 26 | 18 | 0.266 |
| 2 | 20 | 25 | 22 | 23 | 21 | 24 | |||
| 3+4 | 4 | 12 | 3 | 13 | 6 | 10 | |||
Abbreviations: TIL=tumour-infiltrating lymphocyte; TNM=tumour-node-metastasis.
Table 2. Correlation between CRP and TNM stage.
|
CRP
|
|||
|---|---|---|---|
| Negative | Positive | P | |
| TNM stage | |||
| I | 57 | 8 | <0.001 |
| II | 6 | 1 | |
| III | 6 | 9 | |
| IV | 4 | 14 | |
Abbreviations: CRP=C-reactive protein; TNM=tumour-node-metastasis.
Figure 2.
Relationship between preoperative CRP levels and expression of T-cell subset marker (CD45RO, CD4, and CD8). Increased preoperative CRP levels were positively correlated with CD45RO and CD4 status (P=0.009 and P=0.002, respectively). There was no significant correlation between CRP and CD8 status.
Clinical outcome
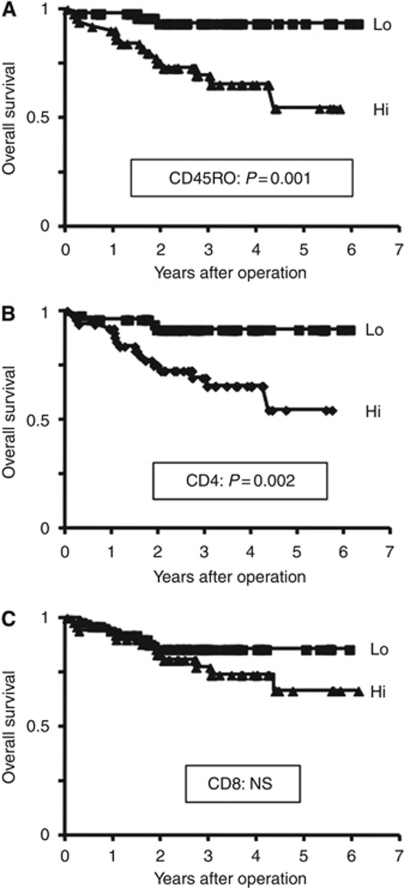
At the time of analysis, 20 of the 105 patients studied had died at median of 15.9 months following surgery (range, 2–52.5). Among the 85 patients who were still alive at last follow-up, the median duration of follow-up was 34.5 months (range, 0.4–73.4). For all subjects estimated overall survival rate 3 and 5 years after surgery was 80.6% and 73.7%, respectively. Univariately, the patients with CD45RO+hi had a significantly worse postoperative prognosis than the CD45RO+lo patients in overall survival rate (hazard ratio, 6.22; 95% confidence interval, 1.88–11.47; P=0.001; Figure 3A). In addition, there was also a significant difference between CD4+hi and CD4+lo patients in overall survival rate (hazard ratio, 4.72; 95% confidence interval, 1.64–9.55; P=0.002; Figure 3B). By contrast, no significant difference was observed in tumour-infiltrating CD8 T-cell status (hazard ratio, 1.73; 95% confidence interval, 0.69–4.21; P=0.243; Figure 3C). In addition, the clinicopathological variables such as T stage, lymph-node involvement, and presence of metastasis, nuclear grade and preoperative CRP levels were all shown to have a significant prognostic impact on overall survival.
Figure 3.
Overall survival of 105 patients with renal cell carcinoma according to tumour-infiltrating T cells. (A) CD45RO+hi patients had a significantly poor prognosis than CD45RO+lo patients (P=0.001). (B) CD4+hi patients had a significantly poor prognosis than CD4+lo patients (P=0.002). (C) There was no significant difference between CD8+hi and CD8+lo patients (P=0.243).
A multivariate model was used to identify independent prognostic factors. The model included histopathological variables, T-cell markers, and preoperative CRP levels. This analysis revealed that distant metastases and CD45RO+ status were the markers to show independent prognostic significance (hazard ratio, 9.09 and 5.57; P=0.002 and P=0.033, respectively; Table 3).
Table 3. Multivariate Cox proportional hazards models for the predictors of overall survival.
| Variable | HR | 95% CI | P |
|---|---|---|---|
| CD45RO+ status | 0.033 | ||
| Lo | 1 | Referent | |
| Hi | 5.57 | 1.15–27.12 | |
| CD4+ status | 0.213 | ||
| Lo | 1 | Referent | |
| Hi | 2.37 | 0.61–9.17 | |
| CD8+ status | 0.276 | ||
| Lo | 1 | Referent | |
| Hi | 0.28 | 0.07–1.14 | |
| Primary tumour | 0.213 | ||
| ⩽T2 | 1 | Referent | |
| >T2 | 2.1 | 0.65–6.76 | |
| Lymph-node involvement | 0.384 | ||
| Negative | 1 | Referent | |
| Positive | 2.6 | 0.65–10.52 | |
| Distant metastases | 0.002 | ||
| Negative | 1 | Referent | |
| Positive | 9.09 | 2.24–35.71 | |
| Nuclear grade | 0.254 | ||
| 1 | 1 | Referent | |
| 2 | 5.52 | 1.55–20 | |
| 3+4 | 6.94 | 0.63–71.42 | |
| Preoperative CRP | 0.265 | ||
| Negative | 1 | Referent | |
| Positive | 0.45 | 0.12–1.74 | |
Abbreviations: HR=hazard ratio; 95% CI=95% confidence interval; CRP=C-reactive protein.
Discussion
Renal cell carcinoma is considered to be an immunogenic tumour associated with a number of TILs. Previous studies have reported the correlation between TILs and clinical outcome in patients with RCC. In an early small study, increased T-cell infiltration was suggested to be associated with an increased risk for tumour recurrence (Kolbeck et al, 1992). More recently, in a larger study, increased mononuclear lymphocyte infiltration in the tumour has been reported to be correlated with poor survival in RCC patients (Webster et al, 2006). Nakano et al (2001) reported that high densities of CD8+ T cells have worse impact on the prognosis in RCC. Similarly, Igarashi et al (2002) also suggested increased CD8+ T cells may be a poor prognostic factor in advanced RCC. However, Bromwich et al (2003) reported no significant correlation between CD8+ TIL and overall survival. Our study also indicated no association between CD8+ TIL and survival. Furthermore, several studies have shown more significant impact of CD4+ TIL rather than CD8+ on the prognosis in patients with RCC. Bromwich et al (2003) found that increased levels of CD4+ T cells in tumours correlated with poor patient survival that is consistent with our finding. By sharp contrast, the other study reported that increased CD4+ TIL was related with a good prognosis in patients with advanced disease status (Igarashi et al, 2002). Taken together, the precise role and differential function of each T-cell subset in RCC tumours remain controversial. In this study, we systematically investigated clinical importance of T-cell subsets infiltrating into human RCC tissues and found that the high density of tumour-infiltrating CD45RO+ as well as CD4+ T cells have negative impact on patient survival. Most importantly, CD45RO status among T-cell subsets was only independent postoperative prognostic marker in RCC as demonstrated by multivariate analysis.
CD45RO is the most suitable single marker for memory T-cell population in human, which could finely represent the activation status of T cell. Therefore, these cells include both CD4+ and CD8+ lymphocytes that have been exposed to antigen. To our best knowledge, none of studies have previously addressed the role of CD45RO+ T cell in RCC. The analysis indicated the positive significant correlations between CD45RO status and advanced pathological features including higher nuclear grade and TNM stage. In addition, besides the presence of distant metastasis, CD45RO status was an independent prognostic marker for RCC patients. These data suggest that CD45RO+ T-cell status has important prognostic value independently of conventional TNM classification in RCC.
C-reactive protein is an indicator of systemic inflammatory response. Previous reports suggested that increased CRP levels predict poor survival in patients with both localised and metastatic RCC (Casamassima et al, 2005; Lamb et al, 2006; Karakiewicz et al, 2007). Furthermore, CRP could be an informative biomarker that reflects disease progression and efficacy of therapeutic intervention. Our data also demonstrated the patients with high CRP level had a significantly poor prognosis than patients with normal CRP level. Furthermore, preoperative CRP levels were positively correlated with CD45RO and CD4 status. This suggests that local inflammatory and immune responses in tumour tissues functionally influence systematic immunological response, thereby leading to clinical outcome in RCC patients. Interestingly, multivariate analysis indicated that CRP status did not reach a significant level for the independent predictors of overall survival in this study. Thus, data further emphasise the importance of CD45RO as a prognostic marker in RCC. However, there are some limitations to draw a definitive conclusion. Approximately 69% of patients evaluated in this study were classified in early stage (stage I or II). Due to this subject imbalance, our results may not have universal validity. Therefore, larger confirmatory studies would be required to validate our data interpretation.
Recent studies have reported that CD45RO+ TILs were associated with better disease outcome for several human cancers. In colorectal cancer, high density of CD45RO+ cells within the tumour was associated with decreased invasiveness, lower stage, and prolonged survival (Pages et al, 2005, 2009; Galon et al, 2006). Furthermore, in gastric cancer, high numbers of CD45RO+ T cells in tumour tissue were significantly correlated with lower frequencies of lymph-node metastasis or longer survival, and further CD45RO+ TILs were independent prognosis factor (Lee et al, 2008). However, this study shows that CD45RO+ T-cell infiltration was associated with poor survival in patients with RCC. This paradoxical phenomenon has been also observed in other T-cell subtypes as previously reported (Nakano et al, 2001; Bromwich et al, 2003). Although this unique feature seems to be found mostly in RCC but not in other types of cancer, the fundamental reasons are still unknown. There may be complex inhibitory mechanisms including apoptosis, cytokine inhibition, angiogenesis, and other regulatory functions on TILs. Several reports indicate that infiltrating lymphocytes within RCC tumours are often impaired and incapable of mediating tumour rejection. CTL effector function has been reported to be lost following tumour infiltration in murine RCC model (Janicki et al, 2008). T cells isolated from TILs of human RCC expressed low granzyme B mRNA levels and did not upregulated its expression upon activation in vitro (Kudoh et al, 1997). It was also reported that RCC could induce apoptosis in activated tumour-infiltrating T cells by virtue of augmented expression of FasL (Uzzo et al, 1999). In addition, the inhibitory interaction of PD-L1/PD-1 has been implicated in RCC (Thompson et al, 2007). Furthermore, RCC tumours and TILs produced several cytokines, which inhibit CTL function, including IL-8, TGF-β, and IL-10 (Wang et al, 1995; Lahn et al, 1999). Taken together, RCC tumours possess local mechanisms to inhibit TILs and undermine antitumour immunity. Further studies on memory T cells in correlation with the above potential inhibitory mechanisms are warranted to develop novel therapeutic strategy.
In conclusion, we have found that CD45RO+ memory T-cell status has a significant independent prognostic value, indicating that the adaptive immune response is functionally critical in human RCC.
Acknowledgments
This work was supported by the following grants: Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan, No. 21591648; Research Grant from Foundation for Promotion of Cancer Research in Japan; Research Grant from Takeda Science Foundation (MS).
Footnotes
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
References
- Akbar AN, Terry L, Timms A, Beverley PC, Janossy G (1988) Loss of CD45R and gain of UCHL1 reactivity is a feature of primed T cells. J Immunol 140: 2171–2178 [PubMed] [Google Scholar]
- Anraku M, Cunningham KS, Yun Z, Tsao MS, Zhang L, Keshavjee S, Johnston MR, de Perrot M (2008) Impact of tumor-infiltrating T cells on survival in patients with malignant pleural mesothelioma. J Thorac Cardiovasc Surg 135: 823–829 [DOI] [PubMed] [Google Scholar]
- Avigan D, Vasir B, Gong J, Borges V, Wu Z, Uhl L, Atkins M, Mier J, McDermott D, Smith T, Giallambardo N, Stone C, Schadt K, Dolgoff J, Tetreault JC, Villarroel M, Kufe D (2004) Fusion cell vaccination of patients with metastatic breast and renal cancer induces immunological and clinical responses. Clin Cancer Res 10: 4699–4708 [DOI] [PubMed] [Google Scholar]
- Bromwich EJ, McArdle PA, Canna K, McMillan DC, McNicol AM, Brown M, Aitchison M (2003) The relationship between T-lymphocyte infiltration, stage, tumour grade and survival in patients undergoing curative surgery for renal cell cancer. Br J Cancer 89: 1906–1908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casamassima A, Picciariello M, Quaranta M, Berardino R, Ranieri C, Paradiso A, Lorusso V, Guida M (2005) C-reactive protein: a biomarker of survival in patients with metastatic renal cell carcinoma treated with subcutaneous interleukin-2 based immunotherapy. J Urol 173: 52–55 [DOI] [PubMed] [Google Scholar]
- Childs R, Chernoff A, Contentin N, Bahceci E, Schrump D, Leitman S, Read EJ, Tisdale J, Dunbar C, Linehan WM, Young NS, Barrett AJ (2000) Regression of metastatic renal-cell carcinoma after nonmyeloablative allogeneic peripheral-blood stem-cell transplantation. N Engl J Med 343: 750–758 [DOI] [PubMed] [Google Scholar]
- Cohen HT, McGovern FJ (2005) Renal-cell carcinoma. N Engl J Med 353: 2477–2490 [DOI] [PubMed] [Google Scholar]
- Escudier B, Eisen T, Stadler WM, Szczylik C, Oudard S, Staehler M, Negrier S, Chevreau C, Desai AA, Rolland F, Demkow T, Hutson TE, Gore M, Anderson S, Hofilena G, Shan M, Pena C, Lathia C, Bukowski RM (2009) Sorafenib for treatment of renal cell carcinoma: final efficacy and safety results of the phase III treatment approaches in renal cancer global evaluation trial. J Clin Oncol 27: 3312–3318 [DOI] [PubMed] [Google Scholar]
- Fuhrman SA, Lasky LC, Limas C (1982) Prognostic significance of morphologic parameters in renal cell carcinoma. Am J Surg Pathol 6: 655–663 [DOI] [PubMed] [Google Scholar]
- Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages C, Tosolini M, Camus M, Berger A, Wind P, Zinzindohoue F, Bruneval P, Cugnenc PH, Trajanoski Z, Fridman WH, Pages F (2006) Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science 313: 1960–1964 [DOI] [PubMed] [Google Scholar]
- Guinan P, Sobin LH, Algaba F, Badellino F, Kameyama S, MacLennan G, Novick A (1997) TNM staging of renal cell carcinoma: Workgroup No. 3. Union International Contre le Cancer (UICC) and the American Joint Committee on Cancer (AJCC). Cancer 80: 992–993 [DOI] [PubMed] [Google Scholar]
- Holtl L, Zelle-Rieser C, Gander H, Papesh C, Ramoner R, Bartsch G, Rogatsch H, Barsoum AL, Coggin Jr JH, Thurnher M (2002) Immunotherapy of metastatic renal cell carcinoma with tumor lysate-pulsed autologous dendritic cells. Clin Cancer Res 8: 3369–3376 [PubMed] [Google Scholar]
- Igarashi T, Takahashi H, Tobe T, Suzuki H, Mizoguchi K, Nakatsu HO, Ito H (2002) Effect of tumor-infiltrating lymphocyte subsets on prognosis and susceptibility to interferon therapy in patients with renal cell carcinoma. Urol Int 69: 51–56 [DOI] [PubMed] [Google Scholar]
- Janicki CN, Jenkinson SR, Williams NA, Morgan DJ (2008) Loss of CTL function among high-avidity tumor-specific CD8+ T cells following tumor infiltration. Cancer Res 68: 2993–3000 [DOI] [PubMed] [Google Scholar]
- Karakiewicz PI, Hutterer GC, Trinh QD, Jeldres C, Perrotte P, Gallina A, Tostain J, Patard JJ (2007) C-reactive protein is an informative predictor of renal cell carcinoma-specific mortality: a European study of 313 patients. Cancer 110: 1241–1247 [DOI] [PubMed] [Google Scholar]
- Kolbeck PC, Kaveggia FF, Johansson SL, Grune MT, Taylor RJ (1992) The relationships among tumor-infiltrating lymphocytes, histopathologic findings, and long-term clinical follow-up in renal cell carcinoma. Mod Pathol 5: 420–425 [PubMed] [Google Scholar]
- Kudoh S, Redovan C, Rayman P, Edinger M, Tubbs RR, Novick A, Finke JH, Bukowski RM (1997) Defective granzyme B gene expression and lytic response in T lymphocytes infiltrating human renal cell carcinoma. J Immunother 20: 479–487 [DOI] [PubMed] [Google Scholar]
- Lahn M, Fisch P, Kohler G, Kunzmann R, Hentrich I, Jesuiter H, Behringer D, Muschal B, Veelken H, Kulmburg P, Ikle DN, Lindemann A (1999) Pro-inflammatory and T cell inhibitory cytokines are secreted at high levels in tumor cell cultures of human renal cell carcinoma. Eur Urol 35: 70–80 [DOI] [PubMed] [Google Scholar]
- Lamb GW, McMillan DC, Ramsey S, Aitchison M (2006) The relationship between the preoperative systemic inflammatory response and cancer-specific survival in patients undergoing potentially curative resection for renal clear cell cancer. Br J Cancer 94: 781–784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HE, Chae SW, Lee YJ, Kim MA, Lee HS, Lee BL, Kim WH (2008) Prognostic implications of type and density of tumour-infiltrating lymphocytes in gastric cancer. Br J Cancer 99: 1704–1711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merkenschlager M, Terry L, Edwards R, Beverley PC (1988) Limiting dilution analysis of proliferative responses in human lymphocyte populations defined by the monoclonal antibody UCHL1: implications for differential CD45 expression in T cell memory formation. Eur J Immunol 18: 1653–1661 [DOI] [PubMed] [Google Scholar]
- Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Oudard S, Negrier S, Szczylik C, Pili R, Bjarnason GA, Garcia-del-Muro X, Sosman JA, Solska E, Wilding G, Thompson JA, Kim ST, Chen I, Huang X, Figlin RA (2009) Overall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cell carcinoma. J Clin Oncol 27: 3584–3590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motzer RJ, Mazumdar M, Bacik J, Berg W, Amsterdam A, Ferrara J (1999) Survival and prognostic stratification of 670 patients with advanced renal cell carcinoma. J Clin Oncol 17: 2530–2540 [DOI] [PubMed] [Google Scholar]
- Nakano O, Sato M, Naito Y, Suzuki K, Orikasa S, Aizawa M, Suzuki Y, Shintaku I, Nagura H, Ohtani H (2001) Proliferative activity of intratumoral CD8(+) T-lymphocytes as a prognostic factor in human renal cell carcinoma: clinicopathologic demonstration of antitumor immunity. Cancer Res 61: 5132–5136 [PubMed] [Google Scholar]
- Ohigashi Y, Sho M, Yamada Y, Tsurui Y, Hamada K, Ikeda N, Mizuno T, Yoriki R, Kashizuka H, Yane K, Tsushima F, Otsuki N, Yagita H, Azuma M, Nakajima Y (2005) Clinical significance of programmed death-1 ligand-1 and programmed death-1 ligand-2 expression in human esophageal cancer. Clin Cancer Res 11: 2947–2953 [DOI] [PubMed] [Google Scholar]
- Pages F, Berger A, Camus M, Sanchez-Cabo F, Costes A, Molidor R, Mlecnik B, Kirilovsky A, Nilsson M, Damotte D, Meatchi T, Bruneval P, Cugnenc PH, Trajanoski Z, Fridman WH, Galon J (2005) Effector memory T cells, early metastasis, and survival in colorectal cancer. N Engl J Med 353: 2654–2666 [DOI] [PubMed] [Google Scholar]
- Pages F, Kirilovsky A, Mlecnik B, Asslaber M, Tosolini M, Bindea G, Lagorce C, Wind P, Marliot F, Bruneval P, Zatloukal K, Trajanoski Z, Berger A, Fridman WH, Galon J (2009) In situ cytotoxic and memory T cells predict outcome in patients with early-stage colorectal cancer. J Clin Oncol 27: 5944–5951 [DOI] [PubMed] [Google Scholar]
- Saito K, Tatokoro M, Fujii Y, Iimura Y, Koga F, Kawakami S, Kihara K (2009) Impact of C-reactive protein kinetics on survival of patients with metastatic renal cell carcinoma. Eur Urol 55: 1145–1153 [DOI] [PubMed] [Google Scholar]
- Sun M, Lughezzani G, Perrotte P, Karakiewicz PI (2010) Treatment of metastatic renal cell carcinoma. Nat Rev Urol 7: 327–338 [DOI] [PubMed] [Google Scholar]
- Tatokoro M, Saito K, Iimura Y, Fujii Y, Kawakami S, Kihara K (2008) Prognostic impact of postoperative C-reactive protein level in patients with metastatic renal cell carcinoma undergoing cytoreductive nephrectomy. J Urol 180: 515–519 [DOI] [PubMed] [Google Scholar]
- Thompson RH, Dong H, Lohse CM, Leibovich BC, Blute ML, Cheville JC, Kwon ED (2007) PD-1 is expressed by tumor-infiltrating immune cells and is associated with poor outcome for patients with renal cell carcinoma. Clin Cancer Res 13: 1757–1761 [DOI] [PubMed] [Google Scholar]
- Uzzo RG, Rayman P, Kolenko V, Clark PE, Bloom T, Ward AM, Molto L, Tannenbaum C, Worford LJ, Bukowski R, Tubbs R, Hsi ED, Bander NH, Novick AC, Finke JH (1999) Mechanisms of apoptosis in T cells from patients with renal cell carcinoma. Clin Cancer Res 5: 1219–1229 [PubMed] [Google Scholar]
- Wang Q, Redovan C, Tubbs R, Olencki T, Klein E, Kudoh S, Finke J, Bukowski RM (1995) Selective cytokine gene expression in renal cell carcinoma tumor cells and tumor-infiltrating lymphocytes. Int J Cancer 61: 780–785 [DOI] [PubMed] [Google Scholar]
- Webster WS, Lohse CM, Thompson RH, Dong H, Frigola X, Dicks DL, Sengupta S, Frank I, Leibovich BC, Blute ML, Cheville JC, Kwon ED (2006) Mononuclear cell infiltration in clear-cell renal cell carcinoma independently predicts patient survival. Cancer 107: 46–53 [DOI] [PubMed] [Google Scholar]
- Yang JC, Sherry RM, Steinberg SM, Topalian SL, Schwartzentruber DJ, Hwu P, Seipp CA, Rogers-Freezer L, Morton KE, White DE, Liewehr DJ, Merino MJ, Rosenberg SA (2003) Randomized study of high-dose and low-dose interleukin-2 in patients with metastatic renal cancer. J Clin Oncol 21: 3127–3132 [DOI] [PMC free article] [PubMed] [Google Scholar]