Abstract
Many reports have shown that plant growth and yield is superior on mixtures of NO3− and NH4+ compared with provision of either N source alone. Despite its clear practical importance, the nature of this N-source synergism at the cellular level is poorly understood. In the present study we have used the technique of compartmental analysis by efflux and the radiotracer 13N to measure cellular turnover kinetics, patterns of flux partitioning, and cytosolic pool sizes of both NO3− and NH4+ in seedling roots of rice (Oryza sativa L. cv IR72), supplied simultaneously with the two N sources. We show that plasma membrane fluxes for NH4+, cytosolic NH4+ accumulation, and NH4+ metabolism are enhanced by the presence of NO3−, whereas NO3− fluxes, accumulation, and metabolism are strongly repressed by NH4+. However, net N acquisition and N translocation to the shoot with dual N-source provision are substantially larger than when NO3− or NH4+ is provided alone at identical N concentrations.
Although higher plants have the capacity to utilize organic N (Näsholm et al., 1998), the major sources for N acquisition by roots are considered to be NO3− and NH4+ (Haynes and Goh, 1978). Plants vary substantially in their relative adaptations to these two sources of N (Kronzucker et al., 1997). Although NH4+ should be the preferred N source, since its metabolism requires less energy than that of NO3− (Bloom et al., 1992), only a few species actually perform well when NH4+ is provided as the only N source. Among the latter are boreal conifers (Kronzucker et al., 1997), ericaceous species (Pearson and Stewart, 1993), some vegetable crops (Santamaria and Elia, 1997), and rice (Wang et al., 1993; Kronzucker et al., 1998). Most agricultural species develop at times severe toxicity symptoms on NH4+ (Cox and Reisenauer, 1973; Findenegg, 1987); thus, superior growth in these species is seen on NO3− (Rideout et al., 1994). However, when both N sources are provided simultaneously, growth and yield are often enhanced significantly compared with growth on either NH4+ or NO3− alone. The effect is particularly well documented in corn (Below and Gentry, 1987; Smiciklas and Below, 1992; Adriaanse and Human, 1993) and wheat (Cox and Reisenauer, 1973; Heberer and Below, 1989; Chen et al., 1998), but it has also been reported in several other species (Hagin et al., 1990; Cao and Tibbits, 1993; Gill and Reisenauer, 1993), including rice (Ta and Ohira, 1981; Ta et al., 1981). Yield increases of 40% to 70% have been observed in solution culture (Weissman, 1964; Cox and Reisenauer, 1973; Heberer and Below, 1989), although, commonly, somewhat smaller enhancements are obtained in soil culture and under field conditions (Hoeft, 1984; Hagin et al., 1990). Several hypotheses pertaining to the enhanced growth and yield response on mixed N medium have been advanced (Lewis et al., 1982; Findenegg, 1987; Gill and Reisenauer, 1993), but mechanistic examinations of these effects have been lacking. In the present study we have used compartmental analysis with the short-lived radiotracer 13N to examine the reciprocal effects of NH4+ and NO3− on each other in root tissue of intact rice plants with respect to N-flux partitioning and storage capacity at the subcellular level.
MATERIALS AND METHODS
Plant Growth Conditions
Rice (Oryza sativa L. cv IR72) seeds were surface-sterilized in 5% NaOCl for 10 min, rinsed with deionized water, and left to imbibe in aerated deionized water at 30°C in a water bath for 48 h. The partially germinated seeds were then placed onto plastic mesh mounted on Plexiglas discs (Atohaas Americas Inc., Philadelphia, PA) and the discs were transferred to 40-L hydroponic Plexiglas tanks located in walk-in, controlled-environment growth chambers. Growth chambers were maintained at 30°C ± 2°C, 70% RH, and set to a 12-h/12-h photoperiod. A photon flux of approximately 500 μmol m−2 s−1, measured at plant level (with a light meter [LI-189, Li-Cor, Lincoln, NE] and quantum sensor [LI-190SA, Li-Cor]), was provided by fluorescent lamps (1500, F96T12/CW/VHO, 215 W, Philips, Eindhoven, The Netherlands).
Nutrient Solutions
Seedlings were cultivated for 3 weeks in hydroponic medium contained in 40-L Plexiglas tanks. Deionized, distilled water and reagent-grade chemicals were used in the preparation of all nutrient solutions. N was provided either as 100 μm NH4+ (in the form of (NH4)2SO4), as 100 μm NO3− (in the form of Ca(NO3)2), or as 100 μm NH4NO3. Other nutrient salts added were as follows: 1 mm K2SO4, 2 mm MgSO4, 1 mm CaCl2, 300 μm NaH2PO4, 100 μm Fe-EDTA, 9 μm MnCl2, 25 μm (NH4)6Mo7O24, 20 μm H3BO3, 1.5 μm ZnSO4, and 1.5 μm CuSO4. Nutrient solutions in tanks were continuously mixed via electric circulating pumps (model IC-2, Brinkmann). Continuous infusion of nutrient stock solution via peristaltic pumps (Technicon Proportioning Pump II, Technicon Instrument, Tarrytown, NY) allowed steady-state control of nutrient concentrations in the tanks. Solutions were checked daily for [K+] using a spectrophotometer (model 443; Instrumentation Laboratory, Lexington, MA). The solution pH was maintained at 6.5 ± 0.3 by addition of powdered Ca(CO3)2. pH was monitored daily using a microprocessor-based, pocket-size pH meter (pH Testr2 model 59000-20, Cole Parmer, Chicago, IL). [NH4+]o was measured (using a Philips PU 8820 UV/visible spectrophotometer) according to the method described by Solorzano (1969). [NO3−]o was measured spectrophotometrically by the method of Cawse (1967).
Compartmental Analysis
The radiotracer 13N (half-life = 9.98 min) was produced by the cyclotron facility (Tri-University Meson Facility) at the University of British Columbia. Proton irradiation of a water target was used to generate 13N, a procedure that provides chiefly 13NO3− with high radiochemical purity (Kronzucker et al., 1995b). The irradiated solutions were supplied in sealed 20-mL glass vials, with a starting activity of 700 to 740 MBq. At this activity sufficient counts were present in both eluates and plant samples following loading periods of up to 60 min and a total elution period of 22 min (see below). Procedures for the removal of radiocontaminants and conversion of 13NO3− to 13NH4+ were as described in detail elsewhere (Kronzucker et al., 1995a, 1995b, 1995c). A volume of 20 to 100 mL of 13N-containing “stock” solution was prepared in a fume hood and was transferred into the controlled-environment chambers where experiments were carried out. All uptake solutions were premixed and kept behind lead shielding. The chemical composition of the labeling solutions was identical to that of the growth solutions in the hydroponic tanks (see above). The protocol for efflux experiments was essentially as described elsewhere (Kronzucker et al., 1995b, 1995d, 1995e). Roots of intact rice seedlings were immersed for 60 min in 120-mL darkened plastic beakers containing the 13NO3−- or 13NH4+-labeled solution. Steady-state conditions with respect to all nutrients were maintained throughout growth, loading, and elution. The duration of the loading period was chosen on the basis of the half-lives of exchange for the cytoplasmic compartment, i.e. approximately 14 min for NH4+ and 16 min for NO3−. Therefore, 60 min of exposure to tracer should ensure that cytoplasmic specific activity approximate 95% of that in the loading solution (Kronzucker et al., 1995e). Following loading with 13N, seedlings were transferred to efflux funnels (Wang et al., 1993), and the roots were eluted with 20-mL aliquots of nonradioactive solution after varying time intervals. These time intervals ranged from 5 s to 2 min over an experimental duration of 22 min. Eluates from a total of 25 time intervals were collected separately, and the radioactivities of each eluate were determined in a gamma-counter (Minaxi δ, Auto-γ 5000 series, Hewlett-Packard), measuring the 511-keV positron-electron annihilation radiation generated by recombination of ambient electrons and β+ particles emitted from 13N. After the final elution seedling roots were excised from the shoots, the roots were spun in a low-speed centrifuge for 30 s to remove surface liquid, and the fresh weights of roots and shoots were determined. The plant organs were then introduced into 20-mL scintillation vials, and the radioactivities of roots and shoots were determined.
Data Analysis
All experiments were repeated five to eight times, with two replicates per experiment. Data from several experiments were pooled (n ≥ 10) for calculations of means and se. Symbols and calculation of fluxes were as follows: φco, efflux from the cytoplasmic compartment at time 0 divided by the specific activity of 13N in the loading solution; φnet, net flux, obtained from the accumulation of 13N in the plants at the end of the loading period (60 min); φoc, unidirectional influx, calculated from φnet + φco; φxylem, flux of 13N to the shoot at the end of the elution period; and φvac./ass., combined flux to N assimilation and the vacuole, resulting in φnet − φxylem. Half-lives of exchange and pool sizes were determined as described in detail elsewhere (Siddiqi et al., 1991; Kronzucker et al., 1995a, 1995b, 1995c, 1995e).
RESULTS AND DISCUSSION
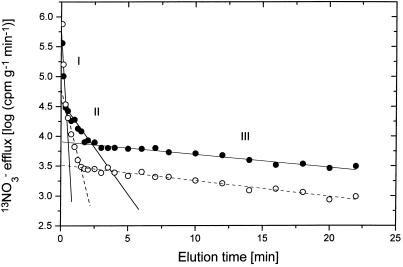
For both NO3− and NH4+, compartmental analyses by efflux revealed exchange with three subcellular compartments (Fig. 1), identified as a surface film (I), a binding component in the cell wall (II), and the cytoplasm (III), in keeping with previous studies in which detailed compartment identity tests were carried out using membrane perturbation, ion-exchange series, and metabolic modifiers (Siddiqi et al., 1991; Kronzucker et al., 1995e). The short isotopic half-life of 13N (9.98 min) made it impossible to trace vacuolar exchange in our study. Half-lives of exchange for the three compartments identified in our study were approximately 2 s, 30 s, and 16 min, respectively, for NO3−, and 2 s, 40 s, and 14 min, respectively, for NH4+ (data not shown). These half-lives were very similar to those reported for N exchange in other studies (Wang et al., 1993; Kronzucker et al., 1995e, 1997), with no significant differences in the presence of the other ion. Cytoplasmic NO3− exchange, however, exhibited half-lives that were about two to three times as long as those observed for other species (compare Devienne et al., 1994; Kronzucker et al., 1995a). The relatively long half-life for cytosolic exchange of NO3− in rice may be seen as an indication of a relatively small negative feedback upon NO3− influx by cytoplasmic NO3−, in keeping with a high cytosolic accumulation capacity and efficiency of uptake for NO3− in this species (H.J. Kronzucker, A.D.M. Glass, M.Y. Siddiqi, and G.J.D. Kirk, unpublished results). It is surprising to find such high capacity and efficiency for NO3− capture in rice, which traditionally has been assumed to prefer NH4+-N (compare Wang et al., 1993; Kronzucker et al., 1998). Notwithstanding the substantial rates of both NO3− influx and net flux, we found a strong inhibitory effect of NH4+ on the latter (Table I). Such repression of NO3− uptake by NH4+ has been documented in many species (Jackson et al., 1976; MacKown et al., 1982; Lee and Drew, 1989; Aslam et al., 1997; Colmer and Bloom, 1998), although there has been an ongoing debate about whether the effect is primarily upon influx or efflux (Kronzucker et al., 1999).
Figure 1.
Representative semilogarithmic plots for the rate of release of 13NO3− [log (cpm released) g−1 h−1] versus time of elution for roots of intact cv IR72 rice seedlings maintained at 100 μm [NO3−]o with NH4+ (○) or without NH4+ (•). Plots include linear regression lines for the three phases of efflux (I, surface film; II, cell wall; III, cytoplasm). Regression lines are dashed for the +NH4+ treatment and solid for the control (phase I overlapped). See text for derivation of compartmental parameters.
Table I.
Component fluxes for NH4+ and NO3− as determined by compartmental analysis
| N Source | N Fluxes
|
||||
|---|---|---|---|---|---|
| φoc | φco | φnet | φvac./ass. | φxylem | |
| μmol g−1 h−1 | |||||
| NO3− | 5.98 ± 0.44 | 0.52 ± 0.04 | 5.46 ± 0.49 | 3.18 ± 0.27 | 2.28 ± 0.32 |
| NO3− (+NH4+) | 2.81 ± 0.23 | 0.32 ± 0.09 | 2.49 ± 0.24 | 1.12 ± 0.08 | 1.37 ± 0.19 |
| NH4+ | 4.08 ± 0.31 | 0.99 ± 0.12 | 3.09 ± 0.33 | 2.04 ± 0.09 | 1.05 ± 0.24 |
| NH4+ (+NO3−) | 5.07 ± 0.38 | 0.51 ± 0.07 | 4.56 ± 0.41 | 1.74 ± 0.11 | 2.82 ± 0.17 |
| ∑ (NH4+ + NO3−) | 7.88 | 0.83 | 7.05 | 2.86 | 4.19 |
Rice plants were grown on 100 μm NO3−, 100 μm NH4+, or 100 μm NH4NO3. The bottom row indicates combined N fluxes in the NH4NO3 treatment. For flux symbols, see Methods. Data are means ± se (n ≥ 10).
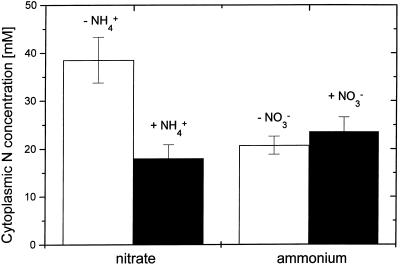
Our present study shows that, under steady-state coprovision of the two N sources, NO3− influx and efflux are both significantly repressed by NH4+, compared with plants fed only with NO3− (Table I). Influx was repressed by approximately 50% (Colmer and Bloom, 1998) and efflux by almost 40%, so that NO3−-net acquisition in the presence of NH4+ was 2.2 times less than with NO3−-only provision. Thus, it is clear that, under steady-state conditions, the principal effect of NH4+ on net NO3− uptake is through its repressive action on influx, not through enhancement of efflux, which supports the conclusions by Lee and Drew (1989) and our own group (Kronzucker et al., 1999; compare Aslam et al., 1997). Also, since NO3− efflux constituted only 8.7% (with NO3−) to 11.4% (with NO3− plus NH4+) of NO3− influx, any effect on efflux could make only a negligible contribution to net NO3− acquisition. The same trend as for NO3− fluxes was observed for cytosolic NO3− accumulation capacity. Figure 1 shows overlaid efflux plots for NO3− in the presence and absence of NH4+, with a significant downward y-axis shift being evident for NO3− efflux from the cytoplasmic compartment in the presence of NH4+. By contrast, half-life for cytoplasmic exchange, as seen in the slope of the regression line for compartment III, was not changed. Given this half-life constancy, the y-axis intercepts for 13N efflux from compartment III in Figure 1 reflect directly the relative sizes of the cytoplasmic NO3− pools. As shown in Figure 2, cytoplasmic [NO3−] was depressed from 36 ± 4.5 mm with NO3−-only provision to 17.8 ± 3.6 mm in the presence of NH4+.
Figure 2.
Cytoplasmic pool sizes (in mm) of NO3− and NH4+ in roots of intact cv IR72 rice seedlings in the presence (black bars) or absence (white bars) of the other N source. Plants were under steady-state conditions with respect to N treatments. Error bars indicate se (n ≥ 10).
In the reverse experimental design, compartmental analysis revealed unexpected effects of NO3− on NH4+ fluxes. Cytoplasmic [NH4+] was not affected significantly by the presence of NO3− (Fig. 2). Due to this, efflux plots for NH4+ with or without NO3− virtually overlapped (data not shown). However, NH4+ influx was increased by almost 25% when NO3− was provided at the same time (Table I). Concurrently, NH4+ efflux was decreased by NO3− almost 2-fold. As a result, net NH4+ acquisition was improved by as much as 50% compared with the NH4+-only control. Under perturbational conditions, since N-deprived plants were resupplied with N, a stimulatory effect of NO3− on NH4+ uptake has been recorded previously for soybean (Rideout et al., 1994; Saravitz et al., 1994). Here we show that NH4+ uptake is stimulated substantially as well under steady-state conditions.
Perhaps even more important, however, is the finding that N-flux partitioning patterns changed significantly when both N sources were provided compared with either NH4+ or NO3− alone. For both NH4+ and NO3−, if supplied alone, approximately 50% of incoming N remained in roots, either channeled to assimilation or to the vacuole, whereas a relatively smaller proportion was translocated to the shoot, approximately 38% of incoming 13N in the case of NO3− and 26% in the case of NH4+. With coprovision of the other N source, xylem-N translocation increased substantially, to approximately 49% on NO3− (in the presence of NH4+) and to approximately 56% on NH4+ (in the presence of NO3−). Our compartmental analyses do not allow us to determine the biochemical profiles of N-translocation compounds, nor can the specific activities of the respective xylem-loading pools of these compounds be known. Hence, the xylem-translocation data presented here include not only the NO3− and NH4+ species, respectively, but also N metabolites and, thus, a fraction of the assimilatory flux.
Whereas in the case of NO3− long-distance N translocation increased only in percentage terms, an absolute increase was seen in the case of NH4+. It has been suggested that the inhibition of NO3− uptake might be accompanied by an inhibition of nitrate reductase in roots (Smith and Thompson, 1971; Radin, 1975; MacKown et al., 1982); therefore, the increased proportion of N translocated to the shoot in the case of NO3− is likely to be accompanied by a decreased rate of N metabolism and hence a lower ratio of N metabolites to free NO3− in the xylem. Since, under most conditions, NH4+ is not transported as such in the xylem of rice at appreciable concentrations (Wang et al., 1993; Kronzucker et al., 1995e), the translocation increase with NH4+ in the presence of NO3− must be due to a stimulation of NH4+ assimilation. A similar NO3−-specific stimulation of NH4+ assimilation has been reported elsewhere for radish plants (Goyal et al., 1982; Ota and Yamamoto, 1989). We propose that the specific induction by NO3− of the proplastidic glutamine synthetase/glutamate synthase pathway (Redinbaugh and Campbell, 1993), in addition to the one localized in the cytoplasm, opens up an assimilatory flux potential that is not available to plants grown on pure NH4+. It is possible that significant portions of N derived from both incoming NO3− and NH4+ could be channeled through this pathway. The increased shoot translocation of N is likely to have important agronomic consequences. In the case of rice, in excess of 70% of N in the grain at harvesting and more than 50% of N in photosynthetically active leaves during grain filling are drawn from N that accumulated in shoot tissue during vegetative growth (Mae et al., 1985, and refs. therein); on the other hand, the rice root system during grain filling is subject to senescence.
In summary, our analyses document distinct changes in the pattern of N-flux partitioning when NO3− and NH4+ are supplied together, compared with provision of either NO3− or NH4+ alone. At least in part, the frequently observed growth and yield maximization on a combined N-source diet (see the introduction) can be attributed to an up-regulation of NH4+ uptake and metabolism by NO3−. Although uptake, metabolism, and cytosolic accumulation of NO3− are depressed by as much as 50% by the simultaneous presence of NH4+, when contributions to the N budget from both NO3− and NH4+ are taken into account (see Table I), a substantially larger N-acquisition rate is achieved than would be possible with either NH4+ or NO3− alone at an identical external N concentration (i.e. 200 μm in our experiments). The Michaelis-Menten saturation kinetics of the individual influx components allow for an increase in influx of approximately only 12% with an increase in external N from 100 to 200 μm (compare Siddiqi et al., 1990; Kronzucker et al., 1998); by contrast, the combined N intake from the NO3−/NH4+ mixture is approximately 20% and 75% larger than individual fluxes at 200 μm in the case of NO3− and NH4+, respectively. It is clear that this benefit from combined N-source provision must be most pronounced at higher concentrations of external N, as influx isotherms are near or at saturation (Siddiqi et al., 1990; Kronzucker et al., 1995d, 1996). In our study with rice, the additive N-budget advantage due to the combined influx components was further enhanced by a reduction in N loss through efflux. In addition, a significant shift in N partitioning was observed in favor of N allocation to the shoot, with agronomic consequences that are likely not trivial.
ACKNOWLEDGMENTS
For technical help and discussion we thank D.T. Britto, M. Okamoto, D. Zhuo, and the staff at the Tri-University Meson Facility for the particle accelerator.
Footnotes
The work reported in this paper was supported by funds from the “New Frontier” project grant to the International Rice Research Institute and by a University of Western Ontario grant to H.J.K.
LITERATURE CITED
- Adriaanse FG, Human JJ. Effect of time of application and nitrate: ammonium ratio on maize grain yield, grain N concentration and soil mineral N concentration in a semi-arid region. Field Crops Res. 1993;34:57–70. [Google Scholar]
- Aslam M, Travis R, Rains DW, Huffaker RC. Differential effect of ammonium on the induction of nitrate and nitrite reductase activities in roots of barley (Hordeum vulgare L.) seedlings. Physiol Plant. 1997;101:612–619. [Google Scholar]
- Below FE, Gentry LE. Effect of mixed N nutrition on nutrient accumulation, partitioning, and productivity of corn. J Fert Issues. 1987;4:79–85. [Google Scholar]
- Bloom AJ, Sukrapanna SS, Warner RL. Root respiration associated with ammonium and nitrate absorption by barley. Plant Physiol. 1992;99:1294–1301. doi: 10.1104/pp.99.4.1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao W, Tibbits TW. Study of various NH4+/NO3− mixtures for enhanced growth of potatoes. J Plant Nutr. 1993;16:1691–1704. doi: 10.1080/01904169309364643. [DOI] [PubMed] [Google Scholar]
- Cawse PA. The determination of nitrate in soil solutions by ultraviolet spectrophotometry. Analyst. 1967;92:311–315. [Google Scholar]
- Chen JG, Cheng SH, Cao WX, Zhou X. Involvement of endogenous plant hormones in the effect of mixed nitrogen source on growth and tillering in wheat. J Plant Nutr. 1998;21:87–97. [Google Scholar]
- Colmer TD, Bloom AJ. A comparison of NH4+ and NO3− net fluxes along roots of rice and maize. Plant Cell Environ. 1998;21:240–246. [Google Scholar]
- Cox WJ, Reisenauer HM. Growth and ion uptake by wheat supplied nitrogen as nitrate, or ammonium, or both. Plant Soil. 1973;38:363–380. [Google Scholar]
- Devienne F, Mary B, Lamaze T. Nitrate transport in intact wheat roots. I. Estimation of cellular fluxes and NO3− distribution using compartmental analysis from data of 15NO3− efflux. J Exp Bot. 1994;45:667–676. [Google Scholar]
- Findenegg GR. A comparative study of ammonium toxicity at different constant pH of the nutrient solution. Plant Soil. 1987;103:239–243. [Google Scholar]
- Gill MA, Reisenauer HM. Nature and characterization of ammonium effects on wheat and tomato. Agron J. 1993;85:874–879. [Google Scholar]
- Goyal SS, Lorenz OA, Huffaker RC. Inhibitory effects of ammoniacal nitrogen on growth of radish plants. I. Characterization of toxic effects of NH4+ on growth and its alleviation by NO3−. J Am Soc Hortic Sci. 1982;107:125–129. [Google Scholar]
- Hagin J, Olson SR, Shaviv A. Review of interaction of ammonium-nitrate and potassium nutrition of crops. J Plant Nutr. 1990;13:1211–1226. [Google Scholar]
- Haynes RJ, Goh KM. Ammonium and nitrate nutrition of plants. Biol Rev. 1978;53:465–510. [Google Scholar]
- Heberer JA, Below FE. Mixed nitrogen nutrition and productivity of wheat grown in hydroponics. Ann Bot. 1989;63:643–649. [Google Scholar]
- Hoeft RG (1984) Current status of nitrification inhibitor use in U.S. agriculture. In RD Hauck, ed, Nitrogen in Crop Production. American Society of Agronomy, Crop Science Society of America, and Soil Science Society of America, Madison, WI, pp 561–570
- Jackson WA, Kwik KD, Volk RJ, Butz RG. Nitrate influx and efflux by intact wheat seedlings: effects of prior nitrate nutrition. Planta. 1976;132:149–156. doi: 10.1007/BF00388896. [DOI] [PubMed] [Google Scholar]
- Kronzucker HJ, Glass ADM, Siddiqi MY. Nitrate induction in spruce: an approach using compartmental analysis. Planta. 1995a;196:683–690. [Google Scholar]
- Kronzucker HJ, Glass ADM, Siddiqi MY (1999) Inhibition of nitrate uptake by ammonium in barley: analysis of component fluxes. Plant Physiol (in press) [DOI] [PMC free article] [PubMed]
- Kronzucker HJ, Kirk GJD, Siddiqi MY, Glass ADM. Effects of hypoxia on 13NH4+ fluxes in rice roots. Kinetics and compartmental analysis. Plant Physiol. 1998;116:581–587. doi: 10.1104/pp.116.2.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronzucker HJ, Siddiqi MY, Glass ADM. Compartmentation and flux characteristics of nitrate in spruce. Planta. 1995b;196:674–682. [Google Scholar]
- Kronzucker HJ, Siddiqi MY, Glass ADM. Compartmentation and flux characteristics of ammonium in spruce. Planta. 1995c;196:691–698. [Google Scholar]
- Kronzucker HJ, Siddiqi MY, Glass ADM. Kinetics of NO3− influx in spruce. Plant Physiol. 1995d;109:319–326. doi: 10.1104/pp.109.1.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronzucker HJ, Siddiqi MY, Glass ADM. Analysis of 13NH4+-efflux in spruce roots. A test case for compartment identification in efflux analysis. Plant Physiol. 1995e;109:481–490. doi: 10.1104/pp.109.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronzucker HJ, Siddiqi MY, Glass ADM. Kinetics of NH4+ influx in spruce. Plant Physiol. 1996;110:773–779. doi: 10.1104/pp.110.3.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronzucker HJ, Siddiqi MY, Glass ADM. Conifer root discrimination against soil nitrate and the ecology of forest succession. Nature. 1997;385:59–61. [Google Scholar]
- Lee RB, Drew MC. Rapid, reversible inhibition of nitrate influx in barley by ammonium. J Exp Bot. 1989;40:741–752. [Google Scholar]
- Lewis OAM, James DM, Hewitt EJ. Nitrogen assimilation in barley (Hordeum vulgare L. cv. Mazurka) in response to nitrate and ammonium nutrition. Ann Bot. 1982;49:39–49. [Google Scholar]
- MacKown CT, Jackson WA, Volk RJ. Restricted nitrate influx and reduction in corn seedlings exposed to ammonium. Plant Physiol. 1982;69:353–359. doi: 10.1104/pp.69.2.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mae T, Hoshino T, Ohira K. Proteinase activities and loss of nitrogen in the senescing leaves of field-grown rice (Oryza sativa L.) Soil Sci Plant Nutr. 1985;31:589–600. [Google Scholar]
- Näsholm T, Ekblad A, Nordin A, Giesler R, Högberg M, Högberg P. Boreal forest plants take up organic nitrogen. Nature. 1998;392:914–916. [Google Scholar]
- Ota K, Yamamoto Y. Promotion of assimilation of ammonium ions by simultaneous application of nitrate and ammonium ions in radish plants. Plant Cell Physiol. 1989;30:365–371. [Google Scholar]
- Pearson J, Stewart GR. The deposition of atmospheric ammonia and its effects on plants. New Phytol. 1993;125:283–305. doi: 10.1111/j.1469-8137.1993.tb03882.x. [DOI] [PubMed] [Google Scholar]
- Radin JW. Differential regulation of nitrate reductase induction in roots and shoots of cotton plants. Plant Physiol. 1975;55:178–182. doi: 10.1104/pp.55.2.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redinbaugh MG, Campbell WH. Glutamine synthetase and ferredoxin-dependent glutamate synthase expression in the maize (Zea mays) root primary response to nitrate. Evidence for an organ-specific response. Plant Physiol. 1993;101:1249–1255. doi: 10.1104/pp.101.4.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rideout JW, Chaillou S, Rape CD, Jr, Morot-Gaudry JF. Ammonium and nitrate uptake by soybean during recovery from nitrogen deprivation. J Exp Bot. 1994;45:23–33. doi: 10.1093/jxb/45.1.23. [DOI] [PubMed] [Google Scholar]
- Santamaria P, Elia A. Producing nitrate-free endive heads: effect of nitrogen form on growth, yield, and ion composition of endive. J Am Soc Hortic Sci. 1997;122:140–145. [Google Scholar]
- Saravitz CH, Chaillou S, Musset J, Raper CD, Jr, Morot-Gaudry J-F. Influence of nitrate on uptake of ammonium by nitrogen-depleted soybean: is the effect located in roots or shoots? J Exp Bot. 1994;45:1575–1584. [Google Scholar]
- Siddiqi MY, Glass ADM, Ruth TJ. Studies of the uptake of nitrate in barley. III. Compartmentation of NO3−. J Exp Bot. 1991;42:1455–1463. [Google Scholar]
- Siddiqi MY, Glass ADM, Ruth TJ, Rufty TW. Studies of the uptake of nitrate in barley. I. Kinetics of 13NO3− influx. Plant Physiol. 1990;93:1426–1432. doi: 10.1104/pp.93.4.1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smiciklas KD, Below FE. Role of cytokinin in enhanced productivity of maize supplied with NH4+ and NO3−. Plant Soil. 1992;142:307–313. [Google Scholar]
- Smith FW, Thompson JF. Regulation of nitrate reductase in excised barley roots. Plant Physiol. 1971;48:219–223. doi: 10.1104/pp.48.2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solorzano L. Determination of ammonia in natural waters by the phenol-hypochlorite method. Limnol Oceanogr. 1969;14:799–801. [Google Scholar]
- Ta TC, Ohira K. Effects of various environmental and medium conditions on the response of Indica and Japonica rice plants to ammonium and nitrate nitrogen. Soil Sci Plant Nutr. 1981;27:347–355. [Google Scholar]
- Ta TC, Tsutsumi M, Kurihara K (1981) Comparative study on the response of Indica and Japonica rice plants to ammonium and nitrate nitrogen. Soil Sci Plant Nutr 27 83–92
- Wang MY, Siddiqi MY, Ruth TJ, Glass ADM. Ammonium uptake by rice roots. I. Fluxes and subcellular distribution of 13NH4+ Plant Physiol. 1993;103:1249–1258. doi: 10.1104/pp.103.4.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman GS. Effect of ammonium and nitrate nutrition on protein level and exudate composition. Plant Physiol. 1964;39:947–952. doi: 10.1104/pp.39.6.947. [DOI] [PMC free article] [PubMed] [Google Scholar]