Abstract
Background:
A family history of prostate cancer (PrCa) is a strong risk factor for the disease, indicating that inherited factors are important in this disease. We previously estimated that about 2% of PrCa cases diagnosed ⩽55 years harbour a BRCA2 mutation and PrCa among BRCA2 carriers has been shown to be more aggressive, with poorer survival.
Methods:
To further evaluate the role of BRCA2 in PrCa predisposition, we screened 1864 men with PrCa aged between 36 and 88 years. We analysed the BRCA2 gene using a novel high-throughput multiplex fluorescence heteroduplex detection system developed for the ABI3130xl genetic analyzer.
Results:
We identified 19 protein-truncating mutations, 3 in-frame deletions and 69 missense variants of uncertain significance (UV) in our sample set. All the carriers of truncating mutations developed PrCa at ⩽65 years, with a prevalence of BRCA2 mutation of 1.20% for cases in this age group.
Conclusion:
Based on the estimated frequency of BRCA2 mutations in the United Kingdom we estimate that germline mutations in the BRCA2 gene confer an ∼8.6-fold increased risk of PrCa by age 65, corresponding to an absolute risk of ∼15% by age 65. These results suggest that routine testing of early onset PrCa cases for germline BRCA2 mutations will further help to refine the prevalence and risk associated with BRCA2 mutations and may be useful for guiding management options.
Keywords: prostate cancer, BRCA2 gene, mutation screening, cancer risk
Prostate cancer (PrCa) is the most common cancer in men living in the Western world, with a lifetime risk of ∼1 in 8 for men in Europe and the United States (http://info.cancerresearchuk.org/cancerstats/types/prostate/). Its aetiology remains poorly understood and although many men will not develop a clinically relevant disease, it is recognised that some PrCa cases have a particularly poor prognosis. Although there are some histological and stage predictors of prognosis (Ross et al, 2002), multiple aetiologies, both hereditary and environmental, have been proposed to contribute to the development of PrCa. There is strong evidence that inherited genetic factors are important due to the significant familial aggregation of the disease in some men, particularly those affected at a young age (Edwards and Eeles, 2004). Some family studies have found an increased risk of PrCa among the relatives of breast cancer patients, suggesting a common genetic basis (Thiessen, 1974; Anderson and Badzioch, 1992; Tulinius et al, 1992). Clearer evidence has emerged of an increased risk of PrCa in carriers of BRCA1 and BRCA2 mutations ascertained via family history of breast cancer (Sigurdsson et al, 1997; Ford et al, 1994). Analyses of male mutation carriers in breast cancer families from the Breast Cancer Linkage Consortium (BCLC) found a relative risk (RR) of 4.65 (95% CI 3.48–6.22) of PrCa in male BRCA2 mutation carriers rising to 7.33 below the age of 65 years and RR 1.07 (0.75–1.54) in BRCA1 mutation carriers with a RR rising to 1.82 (1.01–3.29) for men under 65 years old (Thompson and Easton, 2001; Thompson and Easton, 2002). Furthermore, through an analysis of early onset PrCa cases, we previously estimated that the RR of PrCa in BRCA2 mutation carriers was ∼23-fold below age 56 (Edwards et al, 2003). Studies have been conducted in the Ashkenazi population investigating the association of the BRCA2 6174delT founder mutation with PrCa (Hamel et al, 2003). These have reported conflicting data and the majority of these studies have been limited by relatively small sample sizes and did not look for mutations other than the 6174delT founder mutation.
A study from Iceland reported that PrCa occurring in BRCA2 mutation carriers were more aggressive than those in non-carriers, and had poorer survival (Sigurdsson et al, 1997). These individuals all carried a common founder mutation (999del5). Narod et al (2008) reported that survival from PrCa in BRCA2 mutation carriers is shorter (median survival from diagnosis: 4 years) when compared with PrCa survival in BRCA1 carriers (median survival from diagnosis: 8 years). In PrCa where BRCA2 germline mutation status was unknown, allelic loss at the BRCA2 locus has been shown to be a prognostic factor for survival on univariate analysis (Edwards et al, 1998) implying a tumour suppressor mechanism for BRCA2 and thus predisposition to this disease in BRCA2 mutation carriers, but at that time it was not known if this is a surrogate for high tumour grade or due to the mutation per se (Willems et al, 2008). Recent data have shown that it is the mutation per se that is an independent prognostic factor for PrCa survival (Edwards et al, 2010b). This was also supported by an Australian study reporting a very similar finding (Thorne et al, 2011). Here we report a large study of nearly 2000 PrCa cases with varying ages of diagnosis to validate the potential association between germline BRCA2 mutation and increased risk of disease and to refine RR for PrCa among BRCA2 mutation carriers.
Materials and methods
Samples
A series of men with PrCa were recruited from the UK Genetic Prostate Cancer Study (UKGPCS) as reported previously (Eeles et al, 1997) and about 90% of these patients had clinically presenting (non-screen-detected) disease at diagnosis. Case selection for this study was based predominantly on age of disease onset ⩽65 (1621 cases; range 36–65 years), with a further cohort aged >65 but with a family history of one or more first-degree relatives with PrCa (243 cases; range 66–88 years) selected to delineate the contribution of germline BRCA2 mutations to PrCa (Table 1).
Table 1. Age range and family history of prostate cancer samples screened for BRCA2 germline mutation.
| Age range, years | Number of samples | With family history of PrCa (%) | With family history of Br/OvCa (%) | Percentage of samples deceased | Number of mutation carriers | Percentage of mutation carriers | Percentage of mutation carriers deceased |
|---|---|---|---|---|---|---|---|
| 36–55 | 632 (34.5%) | 34.5 | 25.3 | 15.3 | 8 | 1.27 | 37.5 |
| 56–65 | 957 (52.2%) | 50.6 | 24.3 | 12.4 | 11 | 1.15 | 81.8 |
| 66–88 | 243 (13.3%) | 100.0 | 29.6 | 29.8 | 0 | – | – |
| Total | 1832 | 51.6 | 25.4 | 15.7 | 19 | 1.03 | 63.2 |
Abbreviations: BrCa=breast cancer; OvCa=ovarian cancer; PrCa=prostate cancer.
A total of 1832 samples (age range 36–88) passed quality control, of which 1589 were aged ⩽65years at diagnosis. Protein-truncating mutations were enriched at younger age of diagnosis. Family history of BrCa and OvCa is also shown.
Mutation detection
Germline DNA was obtained from peripheral blood samples and extracted as reported in previous articles (Edwards et al, 1997). The full coding region and exon–intron boundaries of BRCA2 were analysed using a high-throughput multiplex fluorescent heteroduplex analysis method. The PCR reactions were performed using the three-primer system described by NGRL Wessex (http://www.ngrl.co.uk/Wessex/downloads) and labelled with one of four dyes (FAM, VIC, NED, ROX). The PCR fragments were diluted 1:50 in H2O and pooled robotically into multiplex groups of up to 8 before mutation screening. These multiplexes were designed using a range of fragment sizes to prevent overlap of tags with interfering absorbance spectra. The BRCA2 PCR primer set used was a modification of that described by NGRI Wessex with alterations to enhance high throughput. This panel consisted of 46 PCR fragments, with larger exons covered by multiple overlapping fragments to ensure mutation detection within the primer regions. The primer sequences, dyes assigned to each fragment, PCR conditions and multiplexing information are available on request.
Multiplexed, dye-tagged PCR fragments were run on an ABI3130xl Genetic Analyzer in 1 × TTE running buffer, using a polymer comprising of 4.5% POP Conformational Analysis Polymer (CAP–Applied Biosystems, Carlsbad, CA, USA, P/N 4340379), 4 M urea and 1% TTE buffer. Polymer was filtered through a 0.5-μm filter before use and stored at 4 °C for up to 1 month. Fragments were analysed for peak shifts corresponding to putative mutations in Genemapper v4.0 (Applied Biosystems) by visual inspection and using Bionumerics (Applied Maths, Sint-Martens-Latem, Belgium).
After confirmation of a peak shift, the samples were re-amplified by PCR from stock DNA and the genetic alteration was characterised by sequencing on an ABI3730 Genetic Analyzer with both forward and reverse PCR primers.
Statistical methods
Confidence intervals for the prevalence of BRCA2 mutations among PrCa cases were computed using an exact binomial procedure.
Estimates for BRCA2 prevalence in the general population were derived from previous studies and used to obtain indirect estimates of the RR of PrCa in BRCA2 mutation carriers (Antoniou et al, 2004). Age-specific cumulative risks of PrCa to age 65 in BRCA2 mutation carriers were computed based on the RR estimated from this study and age-specific pooled incidence rates from five cancer registries in England for the year 2002 as reported in (Ferlay et al, 2007).
Test of differences between carriers and non-carriers in the family history and age cohorts were performed using Fisher's exact test. All statistical analyses were performed using STATA v.10 statistical software (STATA Corp., College Station, TX, USA).
Results
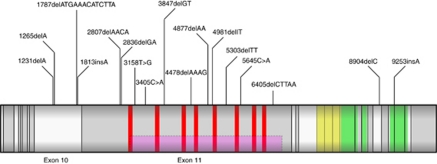
We screened 1864 PrCa cases for germline mutations in the BRCA2 gene. After quality control we excluded 32 samples (all aged ⩽65) from the analysis. In the 1832 samples analysed we have identified 19 protein-truncating mutations, of which 16 were frameshift and 3 were nonsense mutations (Table 1). Of these deleterious mutations, 16 have been reported previously in breast and ovarian cases and these are listed on the Breast Cancer Information Core (BIC) database (http://research.nhgri.nih.gov/bic/), whereas 3 mutations were novel. In addition, we identified 3 in-frame deletions (1 novel) and 69 missense variants (13 novel) of uncertain significance (UV) (Supplementary Table 2), one common nonsense mutation at the 3′ end of the protein (rs11571833), which is considered to be benign, 31 synonymous substitutions (5 novel) and 35 intronic variants. All the deleterious mutations were present in younger onset cases with age of disease diagnosis at ⩽65 years; no truncating mutations were identified among the cohort diagnosed with PrCa at >65 years old. The deleterious mutations were found throughout the gene, in exons 10 (4 mutations), 11 (13 mutations), 22 (1 mutation) and 24 (1 mutation) (Figure 1). DNA samples from other family members were also available for three of the mutation carriers and the appropriate fragments were sequenced from these relatives. One case had two affected brothers of whom only one carried the same mutation (age of onset: 69), one case had one affected brother who also carried the mutation (age of onset: 54) and in the third family the case had one affected brother who was not a carrier for the same mutation (age of onset: 66). Twelve of the deleterious mutation carriers (12/19) have died with relatively short survival (1–11 years), eight of these deaths were confirmed to be PrCa related, the rest are unknown, seven patients are still alive (Table 2). The majority of the mutation carriers had significant disease with Gleason score ⩾8 (12 cases, 63%), four cases had Gleason score ⩽7 and for three patients no data were available (Table 2) indicating a more aggressive clinical course for the majority of the BRCA2 mutation carriers.
Figure 1.
Schematic diagram of the positions of the deleterious mutations found in this study within the BRCA2 transcript. The grey rectangle represents the BRCA2-coding sequence with exon boundaries marked by vertical black lines. The locations of the BRC repeats are marked in red, the helical domain in yellow and the three OB domains in green. The ovarian cancer cluster region is represented as a pink rectangle.
Table 2. Patient information for protein-truncating mutation carriers.
| Sample ID | Nucleotide change | Age at diagnosis | Tumour stage | Node stage | Metastases | Final Gleason | Years to death | Prostate cancer | Breast or ovarian cancer | Other cancers in the family |
|---|---|---|---|---|---|---|---|---|---|---|
| PR1 | 1231delA | 65 | T3b | Nx | M0 | 4+5 | Alive (8) | Twin brother | No | No |
| PR2 | 1265delA | 58 | Tx | Nx | Mx | – | 2 | Father, uncle | Sister (Ov), grandmother (Br) | Bladder |
| PR3 | 1787delATGAAACATCTTA | 55 | T2 | N0 | M0 | 4+4 | 4 | No | No | Stomach |
| PR4 | 1813insA | 60 | T2b | N0 | M0 | 4+4 | 5 | Father, grandfather | Sister (Br) | Bowel, spine, pancreas |
| PR5 | 2807delAACA | 59 | T2b | N0 | M0 | 3+5 | 9 | Brother | Sister (Br), Sister (Br) | Leukaemia, head |
| PR6 | 2836delGA | 51 | T1c | N0 | M0 | 3+3 | Alive (6) | No | No | Pancreas, stomach |
| PR7 | 3158T>G | 46 | T1c | N1 | M0 | 3+3 | Alive (10) | Father | Grandmother (Br) | Lung |
| PR8 | 3405C>A | 62 | T1c | N0 | M0 | – | 8 | Brother | No | Stomach x2, bone, uterus, throat |
| PR9 | 3847delGT | 56 | T4 | Nx | M0 | 4+5 | 2 | Uncle | No | Bowel |
| PR10 | 4478delAAAG | 54 | T2c | N0 | M0 | 4+5 | Alive (18) | Brother, father | Sister (Br), aunt (Ov), daughter (Br) | Thyroid, myeloma |
| PR11 | 4877delAA | 54 | T3b | N1 | M1 | 4+4 | Alive (7) | Father, uncle 4x, first cousin | Aunt (Br) | No |
| PR12 | 4877delAA | 55 | T2a | N0 | M0 | 4+3 | Alive (8) | No | Mother (Br), aunt (Ov) | No |
| PR13 | 4981delT | 62 | Tx | Nx | Mx | 3+3 | Alive (14) | Two brothers | Sister (Br), aunt (Br), grandmother (Br) | Lung x2, bowel |
| PR14 | 5303delTT | 56 | T4 | N0 | M0 | – | 1 | Brother, father | No | No |
| PR15 | 5645C>A | 57 | T2a | N0 | M0 | 4+4 | 11 | No | No | Bowel |
| PR16 | 6405delCTTAA | 53 | T2a | N1 | M0 | 4+4 | 3 | No | Sister (Br), mother (Br), aunt (Br) | Pancreas, leukaemia, skin, glioma |
| PR17 | 6405delCTTAA | 48 | T3 | N0 | M0 | 4+5 | 5 | No | No | Bowel x2 |
| PR18 | 8904delC | 56 | T3a | N0 | M0 | 3+5 | 4 | Father | No | Carcinomatosis |
| PR19 | 9253insA | 57 | T4 | Nx | M1 | 5+5 | 2 | No | No | Bowel |
All 19 germline mutations were identified in patients diagnosed at ⩽65 years old. In all, 12 of 19 carriers had died with relatively short survival periods (1–11 years, mean 4.75, median 4.5). In all, 12 mutation carriers had ⩾1 recorded first or second degree relative with prostate cancer, 9 carriers had ⩾1 known first or second degree relative with breast (Br) or ovarian (Ov) cancer. Clinical and family history data are detailed here if available. The tumour stage, node stage and metastases are given as Tx, Nx and Mx, respectively, if their status is unknown.
Discussion
We have analysed the entire coding region and exon–intron boundaries of the BRCA2 gene from blood DNA of 1864 men diagnosed with prostate cancer. After quality control we included 1832 DNA samples in our analysis. Of these men, 1589 were diagnosed at ⩽65 years (Table 1) and the majority presented with clinical symptoms (data available for 1374 patients, of whom 1218 were clinically detected; 88.6%). The remaining 243 men (PrCa diagnosed >65 years) had a family history of the disease (at least one affected first degree relative with PrCa). In the 1832 samples analysed we identified 19 protein-truncating mutations, which is likely to be an underestimate of the mutation frequency, as the mutation detection method we have used (as with other high through-put methods) would have not been able to detect large deletions/rearrangements (no MLPA was conducted for this set of samples). It is also possible that a proportion of the UVs will be determined to be pathogenic in the future. All 19 deleterious mutation carriers were affected at ⩽65 years and no mutations were found in the older onset group with family history, which is concordant with a previous study (Agalliu et al (2007) where no association was found between BRCA2 mutation status and high familial risk of PrCa. Twelve mutation carriers had family history of PrCa among first- or second-degree relatives (63.2%). This represents a slight but not statistically significant excess (P=0.24) as in the whole study 48.6% of cases ⩽65 had some family history of PrCa. Nine patients had family history of breast or ovarian cancer among first- or second-degree relatives (47.4%) and this is significantly higher than for non-carriers in the study (21.3%, P=0.024). Based on these data, the strongest predictors for the presence of a germline BRCA2 mutation are a young age of onset of PrCa and a family history of breast and/or ovarian cancer. The proportion of high-grade PrCa (Gleason score ⩾8) was 63%, significantly higher than in non-carriers, 15%, (P<0.0001) and similar results were reported previously by our group and by others (Edwards et al, 2010a; Gallagher et al, 2010). The clinical and statistical evaluation of a much larger set of BRCA2 mutation carrier and non-carrier PrCa cases is underway (Castro et al, manuscript in preparation). The prevalence of BRCA2 mutations in this study is 1.27% (8/632) for cases diagnosed ⩽55 years, 1.20% (19/1589) for cases diagnosed ⩽65 years and 0% (0/243) diagnosed >65 years; P=0.14. Based on the previously estimated frequency of BRCA2 mutations in the United Kingdom of 0.16%, we estimated that germline mutations in the BRCA2 gene confer an increased RR of PrCa of ∼8.6-fold (95% CI 5.1–12.6) by age 65 corresponding to an absolute risk of ∼15% by age 65 years based on incidence rates in England from 2002. The estimate of RR from this study is similar to the 7.3-fold RR from the BCLC study for patients diagnosed before age 65 years (Thompson and Easton, 2001). Our previous study reported a higher (∼23-fold) RR (Edwards et al, 2003); although this was calculated from a much smaller cohort of cases all diagnosed at ⩽55 years old and had a very wide confidence interval (95% CI 9–57), which overlaps that of this study. This current study provides more accurate BRCA2 mutation frequencies for PrCa cases and relative and absolute PrCa risk estimates for BRCA2 mutation carriers.
The protein-truncating mutations identified are situated between nucleotides 1231 and 9253, predominantly within exons 10 and 11 but not restricted to any specific domain (Figure 1). The majority, however, are within either the BRC repeat region (nucleotides 3006–6255), which is responsible for RAD51 binding or the OB DNA-binding domain (nucleotides 8007–9570), both of which have an important role in DNA damage repair (Kote-Jarai and Eeles, 1999; Venkitaraman, 2002).
In addition to the protein-truncating mutations, we identified 3 in-frame deletions and 69 missense UVs (Supplementary Table 1). We evaluated the missense variants using various predictive tools (SIFT, polyphen, Align GVGD, pMUT and PANTHER) and combined these with data available from previous studies on functional effects of these sequence variants. Although many of these variants were considered potentially damaging by one or more programme, but benign by another, the vast majority of the UVs identified in this study appear to be of little clinical significance. However, although we cannot rule out a small proportion being pathogenic, we prefer to take a conservative approach and only include clearly pathogenic mutations in our analysis. As a larger number of variants become classified through the efforts of groups such as the BIC and ENIGMA (http://enigmaconsortium.org/) consortia, we can re-examine their role (if any) in PrCa susceptibility.
In conclusion, we have shown that the frequency of germline mutation in BRCA2 in PrCa patients is ∼1.20% at ⩽65 years. No mutations were found in cases diagnosed >65 in our series, suggesting that germline BRCA2 mutation is far more closely linked to a younger age of PrCa onset than to a family history of PrCa. With the advent of PARPi drugs, which preferentially target tumours with a BRCA null phenotype (Fong et al, 2009), germline testing of patients diagnosed at ⩽65 years would be warranted as part of their cancer care pathway once testing becomes faster and cheaper.
Acknowledgments
This work was supported by The Prostate Cancer Research Foundation (now Prostate Action) and Cancer Research UK (Grant numbers C5047/A7357, C1287/A10118, C1287/A5260, C5047/A3354, C5047/A10692, C16913/A6135 and C16913/A6835). DFE is a Principal Research Fellow of Cancer Research UK. We would also like to thank the following for funding support: The Institute of Cancer Research and The Everyman Campaign, Prostate Research Campaign UK, The Orchid Cancer Appeal, The National Cancer Research Network UK, The National Cancer Research Institute (NCRI) UK. We acknowledge NHS funding to the NIHR Biomedical Research Centre at The Institute of Cancer Research and The Royal Marsden NHS Foundation Trust. We would like to acknowledge the NCRN nurses and consultants for their work in the UKGPCS study.
Footnotes
Supplementary Information accompanies the paper on British Journal of Cancer website (http://www.nature.com/bjc)
Supplementary Material
References
- Agalliu I, Kwon EM, Zadory D, McIntosh L, Thompson J, Stanford JL, Ostrander EA (2007) Germline mutations in the BRCA2 gene and susceptibility to hereditary prostate cancer. Clin Cancer Res 13: 839–843 [DOI] [PubMed] [Google Scholar]
- Anderson DE, Badzioch MD (1992) Breast cancer risks in relatives of male breast cancer patients. J Natl Cancer Inst 84: 1114–1117 [DOI] [PubMed] [Google Scholar]
- Antoniou AC, Pharoah PP, Smith P, Easton DF (2004) The BOADICEA model of genetic susceptibility to breast and ovarian cancer. Br J Cancer 91: 1580–1590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards SM, Dearnaley DP, Ardern-Jones A, Hamoudi RA, Easton DF, Ford D, Shearer R, Dowe A, Eeles RA (1997) No germline mutations in the dimerization domain of MXI1 in prostate cancer clusters. The CRC/BPG UK Familial Prostate Cancer Study Collaborators. Cancer Research Campaign/British Prostate Group. Br J Cancer 76: 992–1000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards SM, Dunsmuir WD, Gillett CE, Lakhani SR, Corbishley C, Young M, Kirby RS, Dearnaley DP, Dowe A, Ardern-Jones A, Kelly J, Spurr N, Barnes DM, Eeles RA (1998) Immunohistochemical expression of BRCA2 protein and allelic loss at the BRCA2 locus in prostate cancer. CRC/BPG UK Familial Prostate Cancer Study Collaborators. Int J Cancer 78: 1–7 [DOI] [PubMed] [Google Scholar]
- Edwards SM, Eeles RA (2004) Unravelling the genetics of prostate cancer. Am J Med Genet C Semin Med Genet 129C: 65–73 [DOI] [PubMed] [Google Scholar]
- Edwards SM, Evans DG, Hope Q, Norman AR, Barbachano Y, Bullock S, Kote-Jarai Z, Meitz J, Falconer A, Osin P, Fisher C, Guy M, Jhavar SG, Hall AL, O’Brien LT, Gehr-Swain BN, Wilkinson RA, Forrest MS, Dearnaley DP, Ardern-Jones AT, Page EC, Easton DF, Eeles RA, Oncology UGPCSCaBSo (2010a) Prostate cancer in BRCA2 germline mutation carriers is associated with poorer prognosis. Br J Cancer 103: 918–924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards SM, Evans DG, Hope Q, Norman AR, Barbachano Y, Bullock S, Kote-Jarai Z, Meitz J, Falconer A, Osin P, Fisher C, Guy M, Jhavar SG, Hall AL, O’Brien LT, Gehr-Swain BN, Wilkinson RA, Forrest MS, Dearnaley DP, Ardern-Jones AT, Page EC, Easton DF, Eeles RA, Oncology UGPCSCaBSo (2010b) Prostate cancer in BRCA2 germline mutation carriers is associated with poorer prognosis. Br J Cancer 103: 918–924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards SM, Kote-Jarai Z, Meitz J, Hamoudi R, Hope Q, Osin P, Jackson R, Southgate C, Singh R, Falconer A, Dearnaley DP, Ardern-Jones A, Murkin A, Dowe A, Kelly J, Williams S, Oram R, Stevens M, Teare DM, Ponder BA, Gayther SA, Easton DF, Eeles RA (2003) Two percent of men with early-onset prostate cancer harbor germline mutations in the BRCA2 gene. Am J Hum Genet 72: 1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eeles RA, Dearnaley DP, Ardern-Jones A, Shearer RJ, Easton DF, Ford D, Edwards S, Dowe A (1997) Familial prostate cancer: the evidence and the Cancer Research Campaign/British Prostate Group (CRC/BPG) UK Familial Prostate Cancer Study. Br J Urol 79(Suppl 1): 8–14 [DOI] [PubMed] [Google Scholar]
- Ferlay J, Autier P, Boniol M, Heanue M, Colombet M, Boyle P (2007) Estimates of the cancer incidence and mortality in Europe in 2006. Ann Oncol 18: 581–592 [DOI] [PubMed] [Google Scholar]
- Fong PC, Boss DS, Yap TA, Tutt A, Wu P, Mergui-Roelvink M, Mortimer P, Swaisland H, Lau A, O’Connor MJ, Ashworth A, Carmichael J, Kaye SB, Schellens JH, de Bono JS (2009) Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers. N Engl J Med 361: 123–134 [DOI] [PubMed] [Google Scholar]
- Ford D, Easton DF, Bishop DT, Narod SA, Goldgar DE (1994) Risks of cancer in BRCA1-mutation carriers. Breast Cancer Linkage Consortium. Lancet 343: 692–695 [DOI] [PubMed] [Google Scholar]
- Gallagher DJ, Gaudet MM, Pal P, Kirchhoff T, Balistreri L, Vora K, Bhatia J, Stadler Z, Fine SW, Reuter V, Zelefsky M, Morris MJ, Scher HI, Klein RJ, Norton L, Eastham JA, Scardino PT, Robson ME, Offit K (2010) Germline BRCA mutations denote a clinicopathologic subset of prostate cancer. Clin Cancer Res 16: 2115–2121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamel N, Kotar K, Foulkes WD (2003) Founder mutations in BRCA1/2 are not frequent in Canadian Ashkenazi Jewish men with prostate cancer. BMC Med Genet 4: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kote-Jarai Z, Eeles RA (1999) BRCA1, BRCA2 and their possible function in DNA damage response. Br J Cancer 81: 1099–1102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narod SA, Neuhausen S, Vichodez G, Armel S, Lynch HT, Ghadirian P, Cummings S, Olopade O, Stoppa-Lyonnet D, Couch F, Wagner T, Warner E, Foulkes WD, Saal H, Weitzel J, Tulman A, Poll A, Nam R, Sun P, Danquah J, Domchek S, Tung N, Ainsworth P, Horsman D, Kim-Sing C, Maugard C, Eisen A, Daly M, McKinnon W, Wood M, Isaacs C, Gilchrist D, Karlan B, Nedelcu R, Meschino W, Garber J, Pasini B, Manoukian S, Bellati C, Group HBCS (2008) Rapid progression of prostate cancer in men with a BRCA2 mutation. Br J Cancer 99: 371–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross PL, Gerigk C, Gonen M, Yossepowitch O, Cagiannos I, Sogani PC, Scardino PT, Kattan MW (2002) Comparisons of nomograms and urologists’ predictions in prostate cancer. Semin Urol Oncol 20: 82–88 [DOI] [PubMed] [Google Scholar]
- Sigurdsson S, Thorlacius S, Tomasson J, Tryggvadottir L, Benediktsdottir K, Eyfjörd JE, Jonsson E (1997) BRCA2 mutation in Icelandic prostate cancer patients. J Mol Med 75: 758–761 [DOI] [PubMed] [Google Scholar]
- Thiessen EU (1974) Concerning a familial association between breast cancer and both prostatic and uterine malignancies. Cancer 34: 1102–1107 [DOI] [PubMed] [Google Scholar]
- Thompson D, Easton DF (2001) Variation in cancer risks, by mutation position, in BRCA2 mutation carriers. Am J Hum Genet 68: 410–419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson D, Easton DF (2002) Cancer incidence in BRCA1 mutation carriers. J Natl Cancer Inst 94: 1358–1365 [DOI] [PubMed] [Google Scholar]
- Thorne H, Willems AJ, Niedermayr E, Hoh IM, Li J, Clouston D, Mitchell G, Fox S, Hopper JL, Bolton D (2011) Decreased prostate cancer-specific survival of men with BRCA2 mutations from multiple breast cancer families. Cancer Prev Res (Phila) 4: 1002–1010 [DOI] [PubMed] [Google Scholar]
- Tulinius H, Egilsson V, Olafsdóttir GH, Sigvaldason H (1992) Risk of prostate, ovarian, and endometrial cancer among relatives of women with breast cancer. BMJ 305: 855–857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkitaraman AR (2002) Cancer susceptibility and the functions of BRCA1 and BRCA2. Cell 108: 171–182 [DOI] [PubMed] [Google Scholar]
- Willems AJ, Dawson SJ, Samaratunga H, De Luca A, Antill YC, Hopper JL, Thorne HJ, kConFab Investigators (2008) Loss of heterozygosity at the BRCA2 locus detected by multiplex ligation-dependent probe amplification is common in prostate cancers from men with a germline BRCA2 mutation. Clin Cancer Res 14: 2953–2961 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.